Abstract
Oestradiol and progesterone act in the brain to elicit profound effects on behaviour and physiology. One physiological function of oestradiol is the induction of progesterone receptor (PR) expression in a variety of behaviourally relevant brain regions, including the ventromedial nucleus of the hypothalamus (VMN), the medial preoptic nucleus of the preoptic area (MPOA), the arcuate nucleus (ARC) and the medial central grey (MCG). Ligand-dependent transcriptional activity of steroid receptors, including oestrogen receptors (ER) and PR, is dramatically influenced by nuclear receptor coactivators. In previous studies, we have found that two of these nuclear receptor coactivators, steroid receptor coactivator-1 (SRC-1) and CREB-binding protein (CBP), are important in ER-mediated induction of PR in the VMN and in steroid-dependent behaviours. For nuclear receptor coactivators to function in hormone-dependent transcription in the brain and regulate behaviour, both receptor and coactivator must be expressed in the same cell. In the present study, we used a dual-label immunohistochemical technique to investigate if individual cells in behaviourally relevant brain regions coexpress nuclear receptor coactivators and steroid receptors. Confocal analysis revealed that in oestrogen-primed rats, most of the E-induced PR cells in the VMN (89.6%), MPOA (63%), ARC (82.6%), and many in the MCG (39%), also express SRC-1. In addition, the majority of the cells containing E-induced PR in the VMN (78.3%), MPOA (83.1%), ARC (83.6%), and MCG (60%) also express CBP. These results, taken together with the findings that virtually all oestradiol-induced PR containing cells in the brain express ER, suggest that these neurones represent sites of functional interaction of nuclear receptor coactivators with ovarian steroid receptors in the brain. The present findings provide neuroanatomical evidence that nuclear receptor coactivators are integral in mediating steroid hormone action in behaviourally relevant brain regions.
Keywords: oestrogen receptors, progestin receptors, SRC-1, CBP, reproductive behaviour
The ovarian steroids, oestradiol and progesterone, act in the brain to regulate a variety of behavioural and physiological processes (1, 2). These steroids act in the hypothalamus and other brain regions to regulate female reproductive behaviour and physiology in rodents (1-3). In addition, animal and human studies reveal that these hormones act in the hippocampus to influence memory and cognition (4-6).
Many of the effects of oestradiol and progesterone are mediated by their respective intracellular receptors, oestrogen (ER) and progestin receptors (PR), which are members of a large superfamily of nuclear transcription factors (7-9). Upon binding hormone, these receptors undergo a conformational change that causes the dissociation of heat shock proteins and immunophilins, transforming the receptor into the active form (10). The activated receptors dimerise and preferentially bind to steroid response elements of steroid-target genes to influence gene transcription (7-9). A classic example of a steroid-responsive gene is the induction of the PR gene by oestradiol in a variety of hormone-responsive tissues, including brain (11-14). This ER-dependent induction of PR gene expression is thought to occur via an oestrogen response element in the promoter of the PR gene (15-17). Oestradiol induces PR expression in behaviourally relevant brain regions, including the ventromedial nucleus of the hypothalamus (VMN), the medial preoptic area (MPOA), the arcuate nucleus (ARC) and the midbrain central grey (MCG) (13, 14, 18-26). In addition, virtually all hypothalamic oestradiol-induced PR cells also express ERα (27, 28). Finally, this ER-mediated induction of PR gene expression in the VMN is important for progesterone-facilitated reproductive behaviour (18).
Nuclear receptor coactivators dramatically enhance the transcriptional activity of nuclear receptors, including ER and PR (29-31). It has been proposed that nuclear receptor coactivators influence nuclear receptor transcription through a variety of mechanisms, including acetylation, methylation, phosphorylation and chromatin remodelling (30). Steroid receptor coactivator-1 (SRC-1, also known as NCoA-1), was the first coactivator of steroid receptors to be identified and cloned, and belongs to a larger p160 family of nuclear receptor coactivators (32). The p160 family also includes SRC-2 (also known as NCoA-2, TIF-2, GRIP-1; 33, 34), and SRC-3 (AIB1, TRAM-1, p/CIP, ACTR, RAC3; 35-37). These p160 coactivators enhance the transcriptional activity of steroid, retinoid and thyroid hormone receptors through direct ligand-dependent interactions (29-31). CREB binding protein (CBP), and its homolog p300, are coactivators for a variety of transcription factors and have been proposed to be integrators of nuclear receptors with other signalling pathways, including CREB and AP-1 (38, 39). CBP enhances the activity of nuclear receptors (39-41) and is thought to be recruited to the coactivator complex by the SRC family (42). In support, SRC-1 and CBP physically associate with each other (43) and act synergistically to enhance the activity and function of ER and PR in vitro (44-47).
Although much is known about the molecular mechanisms of nuclear receptor coactivators from a variety of studies performed in vitro (29-31), we are just beginning to understand their role in hormone action in vivo. SRC-1 knockout mice, although fertile, have decreased hormone-dependent growth and development in a variety of steroid target organs, including the uterus, mammary gland, prostate and testis (48). However, it should be noted that SRC-1 knockouts have a two-fold increase in TIF-2 mRNA, which may partially compensate for the loss of SRC-1 (48). SRC-1 is expressed in a variety of hormone-responsive tissues, including the brain (49). SRC-1 mRNA and protein are expressed at high levels in the cortex, hypothalamus, and hippocampus of rodents (50-55) and birds (56, 57). SRC-1 expression in the brain appears to be regulated by a variety of factors, including hormones (58-61), day length (62) and stress (60, 63; for a review, see 64). Immunohistochemical studies reveal that CBP is expressed throughout the brain, including high levels in the hypothalamus, preoptic area, thalamus, amygdala, hippocampus and cortex (55, 65-67).
More recently, the function of these nuclear receptor coactivators with respect to hormone action in the brain and behaviour has been investigated (49, 68, 69). Our laboratory has found that SRC-1 and CBP function in the VMN to modulate ER-mediated transactivation of the behaviourally relevant PR gene (55). Others studies provide support for these findings, and extend them to include a role for SRC-2 in this hormone-dependent event (70). In addition, SRC-1 and CBP function in the VMN to regulate both ER and PR-dependent aspects of female sexual behaviour (71), providing further support for these nuclear receptor coactivators being involved in hormone action in the brain. In the developing rodent brain, SRC-1 (72) and CBP (66) are expressed differentially and are important in hormone-dependent sexual differentiation of the sexually dimorphic nucleus and development of reproductive behaviour (54, 66). In addition, SRC-1 modulates hormone-dependent gene expression, brain plasticity and behaviour in adult quail brain (73). Finally, the p160 coactivators function in glucocorticoid receptor action in glial cells (74). These findings indicate that SRC-1 and CBP have profound effects on hormone action in the brain and the regulation of behaviour.
For nuclear receptor coactivators to function with steroid receptors in vivo, both coactivator and receptor must be expressed in the same cell. As discussed above, SRC-1 is involved in ER-mediated transactivation of the PR gene in the VMN (55, 70). However, in mammary epithelium, SRC-1 was not detected in oestradiol-induced PR cells, suggesting that SRC-1 is not necessary for ER-mediated induction of PR gene expression in this tissue (75). In the present study, we used dual-label immunofluorescence to test the hypothesis that oestradiol-induced PR containing cells express the nuclear receptor coactivators SRC-1 and CBP in behaviourally relevant brain regions. Identification of these coexpressing neurones would represent potential sites of functional interaction between ovarian steroid receptors and nuclear receptor coactivators in hormone-dependent gene expression in brain and behaviour.
Materials and methods
Animals
Female Sprague-Dawley rats, weighing 175-200 g, were obtained from Charles River Breeding Laboratories, Inc. (Wilmington, MA, USA) and group-housed for 1 week under a 14 : 10 h light/dark cycle with food and water freely available. One week after arrival, animals were ovariectomised under ketamine/xylazine (100 mg ketamine and 18 mg xylazine/0.75 ml/kg in saline). Ten days following ovariectomy, rats were anaesthetised with sodium pentobarbital (89 mg/kg) and chloral hydrate (425 mg/kg) dissolved in saline prior to sacrifice. All animal procedures were approved by the Institutional Animal Care and Use Committee of Skidmore College (Saratoga Springs, NY, USA).
Western blot analysis of SRC-1 and CBP
Animals were sacrificed by decapitation and hypothalamic brain tissue was excised, placed in chilled microfuge tubes, snap frozen on dry ice and stored at -80 °C. Tissue was homogenised in TEDG (consisting of 10 mM Tris-base, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol 400 mM NaCl, pH = 7.4) and protease inhibitors (1 μg/ml of aprotinin, leupeptin, and pepstatin) using a Teflon homogeniser. After tissue homogenisation, samples were centrifuged at 12 000 g for 30 min at 4 °C to sediment the cellular debris. The supernatant fraction was collected, and the protein concentration was determined by Bradford assay. Eighty μg of total protein from each tissue sample was gel electrophoresed on 7.5% polyacrylamide gels containing 1% SDS and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). Samples were analysed by Western blot as described previously (71) for detection of SRC-1. Briefly, SRC-1 from brain was probed for using a mouse monoclonal antibody generated against amino acids 477-947 of human SRC-1(1135-H4, 0.5 μg/ml, kindly provided by Dean Edwards, Bert O'Malley, Ming Tsai and Sergio Oñate, Baylor College of Medicine). Membranes were incubated in a horseradish peroxidase linked sheep-anti-mouse secondary antibody (1 : 6000, Amersham, Piscataway, NJ, USA) for 1 h. Immunoreactive bands were detected with an enhanced chemiluminescence kit (ECL; New England Biolabs, Ipswich, MA, USA) and membranes exposed to film (Blue Sensitive X-ray film, Laboratory Products Sales, Rochester, NY, USA). Membranes were stripped with stripping buffer (62.5 mM Tris, pH = 6.7, containing 100 mM 2-mercaptoethanol and 2% SDS) for 3 h, and then reprobed for CBP by incubation in a rabbit polyclonal antibody, PA1-847, generated against amino acids 162-176 of human CBP peptide (1 : 500, Affinity BioReagents, Golden, CO, USA). Membranes were incubated in sheep-anti-rabbit (1 : 5000, Amersham) and immunoreactive bands were visualised as described above.
Dual-label immunofluorescence for progestin receptors and nuclear receptor coactivators
A dual-label immunofluorescent technique was used to identify PR-containing neurones that express SRC-1 or CBP in behaviourally relevant brain regions. One week following ovariectomy, rats were injected subcutaneously with either 10 μg of 17β-oestradiol benzoate (EB, dissolved in 0.1 ml sesame oil) or vehicle at 48 and 24 h prior to being sacrificed. Ovariectomised rats treated with EB (n = 5) or vehicle (n = 4) were anaesthetised and perfused with 4% paraformaldehyde. Five thousand units of sodium heparin dissolved in 1 ml of saline were injected into the left ventricle. Saline (0.15 M, 25 ml) preceded the flow of 250 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.2) at a flow rate of 25 ml/min for 10 min. Brains were removed from the cranium, blocked and stored in 0.1 M sodium phosphate buffer (pH = 7.2) containing 20% sucrose at 4 °C overnight. Forty μm sections from the preoptic area through the midbrain region were cut on a freezing rotary microtome and stored in cryoprotectant at -20 °C until immunohistochemistry.
Brain sections were incubated in 0.05 M TBS containing 1% bovine serum albumin and 20% normal goat serum to reduce nonspecific binding. To detect PR and SRC-1, the sections were incubated in a cocktail containing a rabbit polyclonal antibody generated against the DNA binding domain of human PR (1 : 700, PR DAKO, Sweden) and the SRC-1 monoclonal antibody, 1135/H4 (1 μg/ml). Sections were incubated in a cocktail of fluorescently labelled secondary antisera containing CY3-labelled goat anti-rabbit serum (1.5 μg/ml, Jackson ImmunoResearch, West Grove, PA, USA) for visualisation of PR and fluorescein isothiocyanate (FITC)-labelled goat anti-mouse serum (10 μg/ml, Jackson ImmunoResearch) for detection of SRC-1.
To detect PR and CBP, MAB-462, a mouse monoclonal antibody generated against amino acids 922-933 of human PR (1 : 4000, Chemicon, Temecula, CA, USA) and the CBP polyclonal antibody PA1-847 (1 : 1000) were used. Sections were incubated in a cocktail of fluorescently labelled secondary antisera containing CY3-labelled goat anti-mouse serum (1 μg/ml, Jackson ImmunoResearch) for visualisation of PR and FITC-labelled goat anti-rabbit serum (3.5 μg/ml, Jackson ImmunoResearch) for detection of CBP. For immunolabelling of PR-containing neurones that express SRC-1 or CBP in the MCG, the same procedure described above was used with the following modifications of PR antibody concentrations to optimise visualisation of PR in the MCG: PR DAKO at 1 : 500 and PR MAB-462 at 1 : 500. Following the immunohistochemical procedure, the brain sections were mounted on glass slides, cover-slipped with Vectashield (Vector Laboratories, Burlingame, CA, USA) and stored at 4 °C.
Controls for this dual-label technique included the omission of the primary or secondary antibodies. In addition, primary antibodies were preadsorbed with CBP peptide (PEP-052, Affinity BioReagents) or the appropriate recombinant proteins. SRC-1 and human PR-A and PR-B recombinant proteins were obtained from whole cell extracts of Sf9 insect cells, infected with the appropriate recombinant transfer plasmids, from the Tissue Culture CORE Facility of the University of Colorado Cancer Center as described previously (45, 76).
Statistical analysis
The MPOA, VMN, ARC and MCG, which are rich in oestradiol-induced PR, were analysed with the experimenter blind to treatment groups. Images of immunofluorescence from the left side of one matched section per brain region for each rat (77) were captured at × 200 using an Olympus Fluoview FV300 confocal microscope. Images of PR-immunoreactive cells (PR-IR) were printed on transparencies and the corresponding images of coactivator (SRC-1 or CBP) immunoreactivity were printed on paper and all immunoreactive cells were counted manually. To determine if oestradiol influenced expression of coactivators, images of matched sections from oestradiol-treated and control animals were analysed for total cell count, mean optical density and area of immunoreactivity for SRC-1 and CBP using Image-Pro Plus 4.5.1 (Media Cybernetics, Silver Spring, MD, USA) as described previously (71). The differences in coactivator-IR or PR-IR between oestradiol and the control groups were compared using a two-tailed t-test (Sigma Stat 2.03, SPSS, San Rafael, CA, USA) and P < 0.05 was considered statistically significant.
Results
Western blot analysis of nuclear receptor coactivator expression in the brain
To confirm the specificity of the antibodies for SRC-1 and CBP in brain tissue, an analysis by Western blot was performed. Western blot analysis using the SRC-1 monoclonal antibody, 1135/H4, revealed that SRC-1 protein is expressed in the preoptic area/hypothalamus of female rats at its known molecular mass of 160 kDa (Fig. 1, lane 1). In addition, CBP was expressed at its expected molecular mass of 265 kDa in the rat preoptic area/hypothalamus (Fig. 1, lane 2).
Fig. 1.

Steroid receptor coactivator-1 (SRC-1) and CREB-binding protein (CBP) are expressed in rat brain. Western blot analysis shows that SRC-1 (lane 1) and CBP (lane 2) proteins are expressed in the female rat preoptic area/hypothalamus.
Expression of nuclear receptor coactivators in oestradiol-induced progestin receptor containing cells in the brain
Coexpression of SRC-1 and PR
Consistent with previous studies, very little to no PR-immunoreactive (PR-IR) cells were detected in vehicle-treated animals, oestradiol caused a dramatic increase in the expression of PR-containing cells in all the brain regions analysed (13, 14, 18-26). Consistent with our previous findings and those of others (50-55, 59), SRC-1-immunoreactive (SRC1-IR) cells were observed in many brain regions, including high levels of expression in the preoptic area, hypothalamus and midbrain. Specifically, we detected strong SRC-1 immunostaining in the VMN (Fig. 2B), MPOA (Fig. 3B), ARC and MCG. Taken together, these data indicate that SRC-1 protein is expressed in brain regions known to regulate female reproductive behaviour.
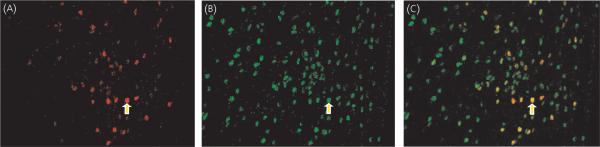
Fig. 2.
Coexpression of oestradiol-induced progesterone receptor (PR) and steroid receptor coactivator-1 (SRC-1) in cells of the ventromedial nucleus of the hypothalamus. The ventromedial nucleus of the hypothalamus of oestradiol benzoate-treated animals were immunostained simultaneously for (A) PR (red) and (B) SRC-1 (green). (C) Overlaid image show cells expressing both PR and SRC-1 (yellow). Red arrow points to cell containing PR immunoreactive cells (-IR) only, green arrow points to cell containing SRC1-IR only and yellow arrow points to one of many cells containing both PR-IR and SRC1-IR.
Fig. 3.
Cells in the medial preoptic area coexpress oestradiol-induced progesterone receptor (PR) and steroid receptor coactivator-1 (SRC-1). The medial preoptic area of oestradiol benzoate-treated animals were immunostained simultaneously for (A) PR (red) and (B) SRC-1 (green). (C) Overlaid image show cells expressing both PR and SRC-1 (yellow). Yellow arrow points to one of many cells containing both PR immunoreactive cells (-IR) and SRC1-IR.
We found that many of the cells containing oestradiol-induced PR also expressed SRC-1. The majority of the oestradiol-induced PR cells in the VMN (Fig. 2) and ARC expressed SRC-1 (Table 1). In addition, high levels of coexpression of PR and SRC-1 were observed in the MPOA (Fig. 3) and more than one-third of the PRIR cells in the MCG also expressed SRC-1 (Table 1). By contrast to the high proportion of PR cells that expressed SRC-1, the majority of SRC1-IR cells did not contain oestradiol-induced PR (Table 1). Although almost half of the SRC1-IR cells in the VMN expressed PR, most of the SRC1-IR cells in the MPOA, ARC and MCG did not contain E-induced PR (Table 1).
Table 1.
Cells Immunostained for Progesterone Receptor (PR), Steroid Receptor Coactivator-1 (SRC-1), or Both in the Ventral Medial Nucleus of the Hypothalamus (VMN), Medial Preoptic Area (MPOA), Arcuate Nucleus (ARC), and Midbrain Central Grey (MCG).
| PR and SRC-1 coexpression in oestradiol benzoate-treated females |
||||
|---|---|---|---|---|
| Brain region | Total PR cells | Total SRC-1 cells | % PR cells expressing SRC-1 | % SRC-1 cells expressing PR |
| VMN | 56 ± 10 | 128 ± 36 | 89.6 | 48.6 |
| MPOA | 42 ± 13 | 228 ± 80 | 63.0 | 13.0 |
| ARC | 71 ± 24 | 214 ± 75 | 82.6 | 28.8 |
| MCG | 10.6 ± 3.4 | 26.7 ± 5.8 | 35.6 | 17.0 |
Total number of cells are shown as the mean ± SEM.
Coexpression of CBP and PR
Because SRC-1 and CBP have been shown to function in concert to increase transcriptional activity of ER and PR in vitro (44) and in the brain (55), we investigated if PR-containing neurones coexpress CBP in these behaviourally relevant brain regions. As in the present study with SRC-1, little to no PR-IR cells were observed in the VMN, MPOA, ARC or MCG of vehicle-treated animals. By contrast, oestradiol treatment resulted in a large increase in PR-IR in these brain regions (Fig. 4A,D). It should be noted that the two primary antibodies used to detect PR, the rabbit polyclonal (DAKO) used in the SRC-1 studies and the mouse monoclonal (MAB-462) used in the CBP studies, recognise both PR-A and PR-B isoforms of the receptor. These antibodies were used at concentrations that yielded similar levels of oestradiol-induced PR-IR cells in these studies (compare total number of PR-IR cells in Tables 1 and 2).
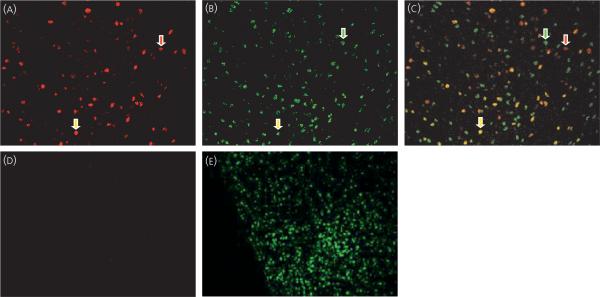
Fig. 4.
Coexpression of oestradiol-induced progesterone receptor (PR) and CREB-binding protein (CBP) in arcuate nucleus cells. Arcuate nucleus of animals treated with (A-C) oestradiol benzoate or (D-E) vehicle were immunostained simultaneously for (A and D) PR (red) and (B and E) CBP (green). (C) Overlaid image show cells expressing both PR and CBP (yellow). Red arrow points to cell containing PR immunoreactive cells (-IR) only, green arrow points to cell containing CBP-IR only and yellow arrow points to one of many cells containing both PR-IR and CBP-IR.
Table 2.
Cells Immunostained for Progesterone Receptor (PR), CREB-Binding Protein (CBP), or Both, in the Ventromedial Nucleus of the Hypothalamus (VMN), Medial Preoptic Area (MPOA), Arcuate Nucleus (ARC), and Midbrain Central Grey (MCG).
| PR and CBP coexpression in oestradiol benzoate-treated females |
||||
|---|---|---|---|---|
| Brain region | Total PR cells | Total CBP cells | % PR cells expressing CBP | % CBP cells expressing PR |
| VMN | 78 ± 21 | 109 ± 41 | 78.3 | 77.5 |
| MPOA | 41 ± 10 | 109 ± 28 | 83.1 | 34.6 |
| ARC | 48 ± 9 | 146 ± 27 | 83.6 | 28.0 |
| MCG | 4.5 ± 1.6 | 27.4 ± 6.2 | 59.8 | 25.4 |
Total number of cells are shown as the mean ± SEM.
CBP-immunoreactivity was expressed throughout the female rat brain, including the MPOA, VMN, ARC (Fig. 4B), and MCG, dorsal medial hypothalamus, and the paraventricular and supraoptic nuclei. By contrast, sparse CBP labelling was observed in the lateral hypothalamus. This neuroanatomical distribution of CBP expression is consistent with previous immunohistochemical studies (65).
Similar to the present findings with SRC-1, high levels of coexpression of oestrogen-induced PR and CBP in behaviourally relevant brain regions were observed. In the VMN, MPOA and ARC (Fig. 4), most of the oestradiol-induced PR containing cells also expressed CBP (Table 2). To a somewhat lesser extent, the majority of PR-IR cells in the MCG also expressed CBP (Table 2). Most of the CBP-IR cells in the VMN also expressed PR (Table 2). Although not a majority, many CBP expressing cells in the MPOA, ARC and MCG also expressed oestradiol-induced PR (Table 2).
Controls for dual-label immunohistochemistry
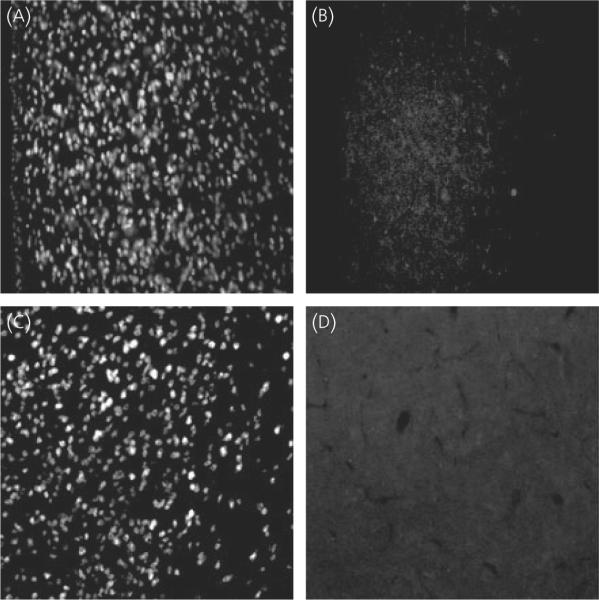
A variety of controls was performed to confirm the specificity of our dual-label immunofluorescent technique. Omission of the primary monoclonal antibody for SRC-1, 1135/H4, from the immunohistochemical procedure resulted in no detectable SRC-1 immunoreactive cells in any brain region. In addition, preadsorption of 1135/H4 with a 20-fold excess of human SRC-1 protein resulted in no detectable SRC-1 immunoreactive cells (Fig. 5A,B). Omission of the primary antibody for CBP (PA1-847) from the immunohistochemical procedure or preadsorption of PA1-847 with a 20-fold excess of CBP peptide resulted in no CBP-IR cells (Fig. 5C,D). Omission of either primary antibody for PR (MAB-462 or DAKO) from the immunohistochemical procedure or preadsorption of these antibodies with a 20-fold excess of human PR-A and PR-B protein resulted in no PR-IR cells. In further confirmation of the specificity of the double label immunofluorescent technique, intensely labelled PR-IR cells devoid of SRC-1 or CBP-IR were observed, as well as SRC1-IR or CBP-IR cells that lacked PR-IR.
Fig. 5.
Controls of steroid receptor coactivator-1 (SRC-1) and CREB-binding protein (CBP) immunostaining in rat brain. (A) Immunohistochemical staining of SRC-1 in the medial preoptic area (MPOA) using the monoclonal antibody, 1135/H4. (B) The MPOA stained with 1135/H4 preadsorbed with a 20-fold excess of human SRC-1 protein. (C) Immunostaining of CBP in the ventromedial nucleus (VMN) using PA1-847. (D) The VMN stained with PA1-847 preadsorbed with a 20-fold excess of CBP peptide.
Regulation of nuclear receptor coactivator expression by oestradiol
To determine if oestradiol benzoate alters the expression of SRC-1 or CBP expression in the VMN, MPOA, ARC or MCG, sections from EB- and vehicle-treated animals were compared. No differences were detected in the number of SRC1-IR cells between EB- and vehicle-treated animals in the VMN (EB: 128 ± 36 versus vehicle: 129 ± 30, P = 0.99), MPOA (228 ± 80 versus 176 ± 44, P = 0.61), ARC (214 ± 75 versus 170 ± 34, P = 0.29) or MCG (27 ± 6 versus 37 ± 4, P = 0.33). In addition, no differences were detected in the total area or mean optical density of SRC-1 immunoreactivity between EB- and vehicle-treated animals in these same brain regions. Similarly, no differences in the number of CBP-IR cells were detected between EB- and vehicle-treated animals in the VMN (EB: 109 ± 41 versus vehicle: 97 ± 7, P = 0.69), MPOA (109 ± 28 versus 90 ± 29, P = 0.68), ARC (146 ± 27 versus 140 ± 37, P 0.85) or 0.30). No differences were MCG (27 ± 6 versus 37 ± 6, P = 0.30). No differences were detected in total area or mean optical density of CBP immunoreactivity between EB- and vehicle-treated animals in these same brain regions. These data suggest that SRC-1 and CBP expression are not altered by EB treatment in the these brain regions as detected by immunofluorescence under the present experimental conditions.
Discussion
A variety of studies indicates that the nuclear receptor coactivators, SRC-1 and CBP, function together to regulate ER and PR function and activity in vitro (44, 45). Moreover, recent work from our laboratory, as well as others, indicates that SRC-1 and CBP function in the brain to modulate hormone-dependent gene expression, development and behaviour (54, 55, 66, 70, 71, 73). For example, SRC-1 and CBP function in the VMN to influence ER-mediated transactivation of the PR gene (55) and ER- and PR-dependent aspects of female sexual behaviour (71). For nuclear receptor coactivators to interact directly with ovarian steroid receptors in the brain, both the coactivator and receptor must be expressed within the same cells in the brain. Therefore, the present study investigated if oestradiol-induced PR-containing cells in behaviourally relevant brain regions also express the nuclear receptor coactivators, SRC-1 or CBP. Double-label immunofluorescence revealed that the majority of E-induced PR cells in the VMN (Fig. 2), MPOA (Fig. 3) and ARC, and many cells in the MCG, express SRC-1 (Table 1). In addition, most of the cells containing E-induced PR in these same brain regions also express CBP (Fig. 4 and Table 2). Given that a high percentage of E-induced PR-IR cells expresses either SRC-1 or CBP, it is likely that a significant proportion of these PR-containing cells express both SRC-1 and CBP. Other studies reveal that virtually all hypothalamic oestradiol-induced PR cells also express Egα (27, 28). Thus, the present studies indicate that a subpopulation of cells in behaviourally relevant brain regions coexpress ovarian steroid receptors (ERα and PR) and nuclear receptor coactivators (SRC-1 and CBP). Although it is important that future studies investigate the subnuclear distribution of these regulatory proteins (78-80), the present findings provide neuroanatomical evidence that nuclear receptor coactivators play a role in the action of ovarian hormones in behaviourally relevant brain regions. It is important to note that not all cells containing oestradiol-induced PR express SRC-1 or CBP (Tables 1 and 2). Although our immunohistochemical technique may not have been sensitive enough to detect very low levels of SRC-1 or CBP in all PR-containing cells, it may be that PR and ERα of some of these cells use other nuclear receptor coactivators (e.g. SRC-2 (70). Furthermore, many cells expressing SRC-1 or CBP do not express PR in the brain regions investigated. In these cells, SRC-1 and CBP may function with other nuclear receptors, such as ER or glucocorticoid receptors (71, 74).
The SRC-1 antibody used in the present experiments was generated against the mid-region of SRC-1 (81) and is likely to recognise both the SRC-1a and SRC-1e isoforms. These isoforms, which differ only at the extreme C-terminus (32, 82, 83), appear to have different functions (82, 84). Interestingly, the mRNAs of these SRC-1 isoforms are differentially expressed in some regions of the rat hypothalamus, including the VMN (53). In future studies, it will be important to investigate the differential expression of these SRC-1 isoforms by steroid receptor-containing cells.
In the present study, the possible regulation of SRC-1 or CBP expression by oestradiol was investigated. SRC-1 immunostaining in the VMN, MPOA, ARC and MCG was not altered by oestradiol treatment, suggesting that oestradiol does not influence SRC-1 expression in these brain regions under our experimental conditions. These findings are consistent with findings that SRC-1 mRNA expression is not influenced by oestradiol in the rat hypothalamus (50). However, other studies in rats have detected changes in SRC-1 protein over the oestrous cycle (58) and oestradiol regulation of SRC-1 mRNA in the hypothalamus (59). The lack of an effect of oestradiol on SRC-1 in the present study may be due to the use of ovariectomised, rather than intact (58), animals and a lower dose of oestradiol and/or investigating protein levels rather than mRNA (59). Furthermore, no changes in CBP-immunoreactivity following oestradiol treatment were detected in any of the brain regions analysed, which is consistent with data showing no effect of oestradiol on hypothalamic CBP protein (85) or mRNA levels of the closelyrelated p300 coactivator (50). However, it should be noted that it is possible that the present immunofluorescent technique was not sensitive enough to detect subtle changes in SRC-1 and/or CBP expression in these brain regions.
The present findings showing that many cells coexpress SRC-1 and PR are in contrast to a study in rat mammary tissue (75). Using a double-label immunohistochemical technique, SRC-1 was not detected in mammary epithelial cells that contained oestradiol-induced PR, suggesting that SRC-1 is not necessary for oestradiol-induction of PR in rat mammary gland (75). However, the present findings are supported by a variety of other studies. SRC-1 expression was significantly related to PR expression in hormone-responsive meningiomas (86). In addition, SRC-1 mRNA expression had a high correlation with PR mRNA in normal and malignant endometrium (87). Furthermore, a functional study in mice provides evidence that SRC-1 functions in uterine tissue with PR in a cell-type specific manner (88). These studies, taken together with the present findings, indicate that SRC-1 functions in a cell-type and tissue specific manner.
The heterogeneity of steroid responsiveness of individual neurones within a brain region is a fundamental issue of steroid hormone action in the brain. The presence or absence, as well as the overall ratio, of different coactivators within particular neurones may be one mechanism for fine-tuning steroid responsiveness within individual neurones. The neurones identified in the present study, which coexpress ovarian steroid receptors (PR and ERa) and coactivators (SRC-1 and CBP), are potential sites of functional interaction between receptors and coactivators with respect to hormone action in the brain. In addition, these neurones, located in the behaviourally relevant VMN and MPOA, ARC and MCG may be sites of nuclear receptor coactivator function in hormone-dependent female reproductive behaviour. The present studies provide strong neuroanatomical evidence for SRC-1 and CBP function with respect to ovarian hormone action in the brain. Future studies will need to continue to address the functional significance of these important regulatory proteins in hormone-dependent behaviour and physiology.
Acknowledgements
We thank Umar Imtiaz and Cheryl Jenks for their expert technical assistance. This research was supported by National Science Foundation IBN 0080818 and National Institutes of Health DK61935 to M.J.T.
References
- 1.Pfaff DW. Estrogens and Brain Function. Springer-Verlag; New York, NY: 1980. [Google Scholar]
- 2.Mani SK, O'Malley B. Mechanism of progesterone receptor action in the brain. In: Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, Pfaff DW, editors. Hormones, Brain and Behavior. Elsevier Science; Amseterdam: 2002. pp. 643–682. [Google Scholar]
- 3.Clemens LG, Weaver DR. The role of gonadal hormones in the activation of feminine sexual behavior. In: Adler NT, Pfaff DW, Goy RW, editors. Handbook of Behavioral Neurobiology. Plenum Press; New York, NY: 1985. pp. 183–227. [Google Scholar]
- 4.Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippo-campal dendritic spine shape and enhances synaptic protein immunore-activity and spatial memory in female mice. Proc Natl Acad Sci USA. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 6.Luine VN. Steroid hormone modulation of hippocampal dependent spatial memory. Stress. 1997;2:21–36. doi: 10.3109/10253899709014735. [DOI] [PubMed] [Google Scholar]
- 7.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 10.Pratt WB, Toft DO. Steroid receptor interaction with heat shock proteins and immunophilin chaperones. Endocrine Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 11.Blaustein JD, Feder HH. Cytoplasmic progestin receptors in guinea pig brain: characteristics and relationship to the induction of sexual behavior. Brain Res. 1979;169:481–497. doi: 10.1016/0006-8993(79)90398-6. [DOI] [PubMed] [Google Scholar]
- 12.MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- 13.Lauber AH, Romano GJ, Pfaff DW. Sex difference in estradiol regulation of progestin receptor messenger RNA in rat mediobasal hypothalamus as demonstrated by In situ hybridization. Neuroendocrinology. 1991;53:608. doi: 10.1159/000125781. [DOI] [PubMed] [Google Scholar]
- 14.Simerly RB. Distribution and regulation of steroid hormone receptor gene expression in the central nervous system. In: Seil FJ, editor. Advances in Neurology. Raven Press; New York, NY: 1993. pp. 207–226. [PubMed] [Google Scholar]
- 15.Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993;7:1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- 16.Kraus WL, Montano MM, Katzenellenbogen BS. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol. 1994;8:952–969. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- 17.Savouret JF, Bailly A, Misrahi M, Rauch C, Redeuilh G, Chauchereau A, Milgrom E. Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J. 1991;10:1875–1883. doi: 10.1002/j.1460-2075.1991.tb07713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–1812. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 19.Parsons B, MacLusky NJ, Krey L, Pfaff DW, McEwen BS. The temporal relationship between estrogen-inducible progestin receptors in the female rat brain and the time course of estrogen activation of mating behavior. Endocrinology. 1980;107:774–779. doi: 10.1210/endo-107-3-774. [DOI] [PubMed] [Google Scholar]
- 20.Blaustein JD, King JC, Toft DO, Turcotte J. Immunocytochemical localization of estrogen-induced progestin receptors in guinea pig brain. Brain Res. 1988;474:1–15. doi: 10.1016/0006-8993(88)90664-6. [DOI] [PubMed] [Google Scholar]
- 21.Turcotte JC, Blaustein JD. Immunocytochemical localization of midbrain estrogen receptor-containing and progestin receptor-containing cells in female guinea pigs. J Comp Neurol. 1993;328:76–87. doi: 10.1002/cne.903280106. [DOI] [PubMed] [Google Scholar]
- 22.Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene-disrupted mice. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung WC, Pak TR, Weiser MJ, Hinds LR, Andersen ME, Handa RJ. Progestin receptor expression in the developing rat brain depends upon activation of estrogen receptor alpha and not estrogen receptor beta. Brain Res. 2006;1082:50–60. doi: 10.1016/j.brainres.2006.01.109. [DOI] [PubMed] [Google Scholar]
- 24.Fenelon VS, Herbison AE. Progesterone regulation of GABA(A) receptor plasticity in adult rat supraoptic nucleus. Eur J Neurosci. 2000;12:1617–1623. doi: 10.1046/j.1460-9568.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 25.MacLusky NJ, McEwen BS. Progestin receptors in rat brain: distribution and properties of cytoplasmic progestin-binding sites. Endocrinology. 1980;106:192–202. doi: 10.1210/endo-106-1-192. [DOI] [PubMed] [Google Scholar]
- 26.Warembourg M. Radioautographic study of the rat brain, uterus and vagina after [3H]R-5020 injection. Mol Cell Endocrinol. 1978;12:67–79. doi: 10.1016/0303-7207(78)90102-8. [DOI] [PubMed] [Google Scholar]
- 27.Blaustein JD, Turcotte JC. Estradiol-induced progestin receptor immuno-reactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology. 1989;49:454–461. doi: 10.1159/000125152. [DOI] [PubMed] [Google Scholar]
- 28.Warembourg M, Jolivet A, Milgrom E. Immunohistochemical evidence of the presence of estrogen and progesterone receptors in the same neurons of the guinea pig hypothalamus and preoptic area. Brain Res. 1989;480:1–15. doi: 10.1016/0006-8993(89)91561-8. [DOI] [PubMed] [Google Scholar]
- 29.Edwards DP. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mamm Gland Biol Neoplasia. 2000;5:307–324. doi: 10.1023/a:1009503029176. [DOI] [PubMed] [Google Scholar]
- 30.Lonard DM, O'Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 32.Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 33.Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voegel JJ, Heine MJS, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 35.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 36.Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW. TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem. 1997;272:27629–27634. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwok RPS, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SGE, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–229. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 39.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 40.Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator, p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarti D, LaMorte VL, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 42.McKenna NJ, Nawaz Z, Tsai SY, Tsai MJ, O'Malley BW. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc Natl Acad Sci USA. 1998;95:11697–11702. doi: 10.1073/pnas.95.20.11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao TP, Ku G, Zhou N, Scully R, Livingston DM. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc Natl Acad Sci USA. 1996;93:10626–10631. doi: 10.1073/pnas.93.20.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith CL, Oñate SA, Tsai MJ, O'Malley BW. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Nat Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol Endocrinol. 1999;13:910–924. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- 46.Kim MY, Hsiao SJ, Kraus WL. A role for coactivators and histone acetylation in estrogen receptor alpha-mediated transcription initiation. EMBO J. 2001;20:6084–6094. doi: 10.1093/emboj/20.21.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Wong J, Tsai SY, Tsai MJ, O'Malley BW. Sequential recruitment of steroid receptor coactivator-1 (SRC-1) and p300 enhances progesterone receptor-dependent initiation and reinitiation of transcription from chromatin. Proc Natl Acad Sci USA. 2001;98:12426–12431. doi: 10.1073/pnas.231474798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Qiu Y, Demayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 49.Molenda HA, Kilts C, Allen RL, Tetel MJ. Nuclear receptor coactivator function in reproductive physiology and behavior. Biol Reprod. 2003;69:1449–1457. doi: 10.1095/biolreprod.103.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Misiti S, Schomburg L, Yen PM, Chin WW. Expression and hormonal regulation of coactivator and corepressor genes. Endocrinology. 1998;139:2493–2500. doi: 10.1210/endo.139.5.5971. [DOI] [PubMed] [Google Scholar]
- 51.Shearman LP, Zylka MJ, Reppert SM, Weaver DR. Expression of basic helix-loop-helix/PAS genes in the mouse suprachiasmatic nucleus. Neuroscience. 1999;89:387–397. doi: 10.1016/s0306-4522(98)00325-x. [DOI] [PubMed] [Google Scholar]
- 52.Martinez de Arrieta C, Koibuchi N, Chin WW. Coactivator and corepressor gene expression in rat cerebellum during postnatal development and the effect of altered thyroid status. Endocrinology. 2000;141:1693–1698. doi: 10.1210/endo.141.5.7467. [DOI] [PubMed] [Google Scholar]
- 53.Meijer OC, Steenbergen PJ, de Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- 54.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 mediates the development of sex specific brain morphology and behavior. Proc Nat Acad Sci USA. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 56.Charlier TD, Lakaye B, Ball GF, Balthazart J. Steroid receptor coactivator SRC-1 exhibits high expression in steroid-sensitive brain areas regulating reproductive behaviors in the quail brain. Neuroendocrinology. 2002;598:1–19. doi: 10.1159/000066624. [DOI] [PubMed] [Google Scholar]
- 57.Charlier TD, Balthazart J, Ball GF. Sex differences in the distribution of the steroid coactivator SRC-1 in the song control nuclei of male and female canaries. Brain Res. 2003;959:263–274. doi: 10.1016/s0006-8993(02)03758-7. [DOI] [PubMed] [Google Scholar]
- 58.Camacho-Arroyo I, Neri-Gomez T, Gonzalez-Arenas A, Guerra-Araiza C. Changes in the content of steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid hormone receptors in the rat brain during the estrous cycle. J Steroid Biochem Mol Biol. 2005;94:267–272. doi: 10.1016/j.jsbmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Mitev YA, Wolf SS, Almeida OF, Patchev VK. Developmental expression profiles and distinct regional estrogen responsiveness suggest a novel role for the steriod receptor coactivator SRC-1 as a discriminative amplifier of estrogen signaling in the rat brain. FASEB J. 2003;17:518–519. doi: 10.1096/fj.02-0513fje. [DOI] [PubMed] [Google Scholar]
- 60.Charlier TD, Ball GF, Balthazart J. Plasticity in the expression of the steroid receptor coactivator 1 in the japanese quail brain: effect of sex, testosterone, stress and time of the day. Neuroscience. 2006;140:1381–1394. doi: 10.1016/j.neuroscience.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Iannacone EA, Yan AW, Gauger KJ, Dowling ALS, Zoeller RT. Thyroid hormone exerts site-specific effects on SRC-1 and NCoR expression selectively in the neonatal rat brain. Mol Cell Endocrinol. 2002;186:49–59. doi: 10.1016/s0303-7207(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 62.Tetel MJ, Ungar TC, Hassan B, Bittman EL. Photoperiodic regulation of androgen receptor and steroid receptor coactivator-1 in Siberian hamster brain. Mol Brain Res. 2004;131:79–87. doi: 10.1016/j.molbrainres.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bousios S, Karandrea D, Kittas C, Kitraki E. Effects of gender and stress on the regulation of steroid receptor coactivator-1 expression in the rat brain and pituitary. J Steroid Biochem Mol Biol. 2001;78:401–407. doi: 10.1016/s0960-0760(01)00123-6. [DOI] [PubMed] [Google Scholar]
- 64.Meijer OCLS, Lachize S, Steenbergen PJ, de Kloet ER. Steroid receptor coregulator diversity: what can it mean for the stressed brain? Neuro-science. 2006;138:891–899. doi: 10.1016/j.neuroscience.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Stromberg H, Svensson SP, Hermanson O. Distribution of CREB-binding protein immunoreactivity in the adult rat brain. Brain Res. 1999;818:510–514. doi: 10.1016/s0006-8993(98)01219-0. [DOI] [PubMed] [Google Scholar]
- 66.Auger AP, Perrot-Sinai TS, Auger CJ, Ekas L, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auger CJ, Bentley GE, Auger AP, Ramamurthy M, Ball GF. Expression of cAMP response element binding protein-binding protein in the song control system and hypothalamus of adult European starlings (Sturnus vulgaris) J Neuroendocrinol. 2002;14:805–813. doi: 10.1046/j.1365-2826.2002.00842.x. [DOI] [PubMed] [Google Scholar]
- 68.Nishihara E, O'Malley BW, Xu J. Nuclear receptor coregulators are new players in nervous system development and function. Mol Neurobiol. 2004;30:307–325. doi: 10.1385/MN:30:3:307. [DOI] [PubMed] [Google Scholar]
- 69.Charlier TD, Balthazart J. Modulation of hormonal signaling in the brain by steroid receptor coactivators. Rev Neurosci. 2005;16:339–357. doi: 10.1515/revneuro.2005.16.4.339. [DOI] [PubMed] [Google Scholar]
- 70.Apostolakis EM, Ramamurphy M, Zhou D, Onate S, O'Malley B. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- 71.Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor-and progestin receptor-dependent aspects of sexual behavior in female rats. Horm Behav. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yousefi B, Jingu H, Ohta M, Umezu M, Koibuchi N. Postnatal changes of steroid receptor coactivator-1 immunoreactivity in rat cerebellar cortex. Thyroid. 2005;15:314–319. doi: 10.1089/thy.2005.15.314. [DOI] [PubMed] [Google Scholar]
- 73.Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neuorscience. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grenier J, Trousson A, Chauchereau A, Cartaud J, Schumacher M, Massaad C. Differential recruitment of p160 coactivators by glucocorticoid receptor between Schwann cells and astrocytes. Mol Endocrinol. 2005;20:254–267. doi: 10.1210/me.2005-0061. [DOI] [PubMed] [Google Scholar]
- 75.Shim WS, DiRenzo J, DeCaprio JA, Santen RJ, Brown M, Jeng MH. Segregation of steroid receptor coactivator-1 from steroid receptors in mam-mary epithelium. Proc Natl Acad Sci USA. 1999;96:208–213. doi: 10.1073/pnas.96.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tetel MJ, Jung S, Carbajo P, Ladtkow T, Skafar DF, Edwards DP. Hinge and amino-terminal sequences contribute to solution dimerization of human progesterone receptor. Mol Endocrinol. 1997;11:1114–1128. doi: 10.1210/mend.11.8.9963. [DOI] [PubMed] [Google Scholar]
- 77.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York, NY: 1998. [Google Scholar]
- 78.Arnett-Mansfield RL, Graham JD, Hanson AR, Mote PA, Gompel A, Scurr LL, Gava N, DeFazio A, Clarke CL. Focal subnuclear distribution of progesterone receptor is ligand-dependent and associated with transcriptional activity. Mol Endocrinol. 2006 Oct 4; doi: 10.1210/me.2006-0041. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 79.Baumann CT, Ma H, Wolford R, Reyes JC, Maruvada P, Lim C, Yen PM, Stallcup MR, Hager GL. The glucocorticoid receptor interacting protein 1 (GRIP1) localizes in discrete nuclear foci that associate with ND10 bodies and are enriched in components of the 26S proteasome. Mol Endocrinol. 2001;15:485–500. doi: 10.1210/mend.15.4.0618. [DOI] [PubMed] [Google Scholar]
- 80.Stenoien DL, Mancini MG, Patel K, Allegretto EA, Smith CL, Mancini MA. Subnuclear trafficking of estrogen receptor-a and steroid receptor coactivator-1. Mol Endocrinol. 2000;14:518–534. doi: 10.1210/mend.14.4.0436. [DOI] [PubMed] [Google Scholar]
- 81.Oñate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai MJ, Edwards DP, O'Malley BW. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- 82.Kalkhoven E, Valentine JE, Heery DM, Parker MG. Isoforms of steroid receptor co-activator 1 differ in their ability to potentiate transcription by the oestrogen receptor. EMBO J. 1998;17:232–243. doi: 10.1093/emboj/17.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeshita A, Yen PM, Misiti S, Cardona GR, Liu Y, Chin WW. Molecular cloning and properties of a full-length putative thyroid hormone receptor coactivator. Endocrinology. 1996;137:3594–3597. doi: 10.1210/endo.137.8.8754792. [DOI] [PubMed] [Google Scholar]
- 84.Meijer OC, van der Kalkhoven ELS, Steenbergen PJ, Houtman SH, Dijkmans TF, Pearce D, de Kloet ER. Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology. 2005;146:1438–1448. doi: 10.1210/en.2004-0411. [DOI] [PubMed] [Google Scholar]
- 85.Abizaid A, Mezei G, Thanarajasingam G, Horvath TL. Estrogen enhances light-induced activation of dorsal raphe serotonergic neurons. Eur J Neurosci. 2005;21:1536–1546. doi: 10.1111/j.1460-9568.2005.03964.x. [DOI] [PubMed] [Google Scholar]
- 86.Carroll RS, Brown M, Zhang J, DiRenzo J, De Mora JF, Black PM. Expression of a subset of steroid receptor cofactors is associated with progesterone receptor expression in meningiomas. Clin Cancer Res. 2000;6:3570–3575. [PubMed] [Google Scholar]
- 87.Kershah SM, Desouki MM, Koterba KL, Rowan BG. Expression of estrogen receptor coregulators in normal and malignant human endometrium. Gynecol Oncol. 2004;92:304–313. doi: 10.1016/j.ygyno.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Han SJ, Jeong J, Demayo FJ, Xu J, Tsai SY, Tsai MJ, O'Malley BW. Dynamic cell type specificity of SRC-1 coactivator in modulating uterine progesterone receptor function in mice. Mol Cell Biol. 2005;25:8150–8165. doi: 10.1128/MCB.25.18.8150-8165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]