Abstract
Vascular endothelial growth factor (VEGF) is a key angiogenic factor and has been used experimentally for induction of neovasculature in ischemic myocardium. However, blood vessels induced by VEGF are immature. Angiopoietin-1 (ang-1) has the ability to recruit and sustain periendothelial support cells and promote vascular maturation. Thus, co-expression of the two may yield a better result than expression of either one alone. Two adeno-associated viral vectors (AAV), CMVVEGF and CMVang-1 with the CMV promoter driving VEGF or ang-1 gene expression, respectively, were injected into ischemic mouse hearts individually or together in different ratios. The results show that co-injected groups had more capillaries than the CMVang-1 group and similar densities of capillaries and α-actin positive vessels as the CMVVEGF group. Neovasculature induced by CMVVEGF was leaky. In contrast, neovasculature in CMVang-1-injected or CMVVEGF and CMVang-1 co-injected hearts was less leaky than that in CMVVEGF-injected hearts. The group that received CMVang-1 and CMVVEGF in a 1:1 ratio had the smallest infarct size and best cardiac function and regional wall movement among all the groups. We conclude that ang-1 and VEGF can compensate for each others' shortcomings and yield a better therapeutic effect by acting together.
Keywords: Vascular endothelial growth factor, angiopoietin-1, adeno-associated viral vector, angiogenesis, ischemic heart
Introduction
Rapid and efficient over-expression of angiogenic growth factors in the peri-infarct zone is a promising approach for gene therapy of myocardial infarction. However, despite intensive studies in the last several decades, there is still no effective protocol for clinical use. Most studies have used a single gene as the therapeutic agent (1). The development of blood vessels in adult tissues is a complex, multi-step process in which different growth factors and cytokines act in concert. Although VEGF is a key component for angiogenesis, the blood vessels induced by VEGF are immature and leaky (2, 3). The angiopoietins comprise another class of angiogenic factors which are involved in the coordinated regulation of new vessel formation. Ang-1 has the ability of recruiting and sustaining periendothelial support cells, while angiopoietin-2 (ang-2) can disrupt blood vessel formation in the developing embryo by antagonizing the effects of ang-1 (4). When applied to the cornea micropocket, neither ang-1 nor ang-2 alone could promote neovascularization, while VEGF alone could induce neovasculature formation. Thus, neither VEGF nor angiopoietins alone is a good candidate for angiogenic therapy.
Experiments have shown that blood vessels in the skin of transgenic mice induced to over-express VEGF were leaky, whereas blood vessels in mice over-expressing ang-1 were not (5). Moreover, co-expression of ang-1 and VEGF in skin of transgenic mice models and in adult mice by adenoviral vector-mediated systemic delivery of these genes showed an additive effect on angiogenesis and resulted in leakage-resistant vessels typical of ang-1 alone (5-7). Thus, VEGF and ang-1 may provide a good combination for combined angiogenic gene therapy for myocardial infarction.
Although, we could induce neovascular formation and improve cardiac function with AAV-mediated VEGF gene transfer alone in our previous study (8), the functional improvement of treated hearts was still sub-optimal. In this study, we investigated whether using a combination of ang-1 and VEGF could result in further enhancement in cardiac function and show that AAV-mediated co-expression of ang-1 and VEGF results in less vascular leakage, smaller infarct size and better cardiac function.
Methods
AAV constructs and viral production
We used the same CMVVEGF and CMVLacZ vectors with CMV promoter driving VEGF or LacZ gene expression constructed for our previous experiments (8, 9). CMVang-1 was constructed by replacing VEGF cDNA in CMVVEGF vector with human ang-1 cDNA. We packaged these vectors in AAV serotype 2 capsid. Viral particles were prepared as described previously (8).
Gene expression in vitro
293 cells were seeded on 48 well plates, 3000 cells/well and infected with CMVang-1 and CMVVEGF individually or together in 2:1, 1:1 and 1:2 ratios (n=3) at MOI (multiplicity of infection) 2×106, 3×106, 4×106 for infection with individual vector or 6×106 (viral genomes) for co-infections of the two vectors. As negative control the cells were also infected with CMVLacZ at MOI 6×106. VEGF and ang-1 in supernatants were measured in duplicate by ELISA using Quantikin kit (R&D Systems, Minneapolis, MN).
Ischemic heart model and vector inoculation
Mice were housed in the animal care facility of the University of California, San Francisco (UCSF) and experiments were conducted according to the guidelines for rodent surgery with a protocol approved by the Institutional Animal Care and Use Committee of UCSF. CD1 mice (male and female) (Charles River, Wilmington, MA) were used. Mice were anesthetized using isoflurane inhalation. A tube was placed through the mouth into the trachea and connected to a Small Animal Volume Controlled Ventilator (Harvard Rodent Ventilator, Model 683). After respiration of the animal was controlled by the ventilator, a thoracotomy incision was made in the forth intercostal space and the heart was exposed by a surgical retractor. The LAD (left anterior descending coronary artery) was ligated permanently with a 7-0 nonabsorbable surgical suture. After the LAD ligation, the ischemic area became pale. Various doses of CMVVEGF and CMVang-1 in 50 μl HEPES buffer were injected individually or mixed together in different ratios at the border zone of the ischemic area immediately after LAD ligation. Mice injected with CMVLacZ vector or HEPES buffer served as controls. The thoracotomy incision was then be closed. The tracheal tube was gently retracted after the voluntary respiration has restored.
Western blots analyses
Proteins were separated on SDS-PAGE gel, and transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham Life Science, Buckinghamshire, England). The protein of VEGF, ang-1 and cyclin B1 were detected using specific antibodies obtained from Santa Cruz Biotechnology, Inc and Cell Signalling Technology (Boston, MA). The signals were detected by using Western blotting detecting reagent (Amersham), and visualized by exposure on X-ray films.
Echocardiogram
Transthoracic echocardiography was performed on conscious mice 4 weeks after LAD ligation and vector injection with a commercially available system (Acuson Sequoia c256) using a 15-MHz linear array transducer (15L8). Animals were habituated to the experimental environment prior to the echocardiogram by inserting them into a soft plastic cone for 10 minutes every day for three days. After the anterior chest was shaved, the mice were inserted into the plastic cone and laid naturally prone. Two-D long-axis images of the LV were obtained at the plane of the mitral and aortic valves at the largest LV cavity and adequate visualization of the LV apex, and a short-axis image was recorded at the level of the papillary muscles as described previously (10). A 2D guided M-mode echocardiogram was recorded through the anterior and posterior walls at a sweep speed of 200 mm/sec. Images were digitally acquired and stored on magnetooptical disk. All measurements were made from digital images captured on cine loops at the time of study with the use of a specialized software package (Acuson Sequoia). Since the LV cavity was distorted by the ligature and scar after LAD ligation, we measured the end-diastolic dimension (LVDd), end-systolic dimension (LVDs) at the junction of infarct and noninfarct myocardium. LV percentage of fractional shortening (%FS) was calculated as 100×(LVDd-LVDs)/LVDd.
Mile assay
Evans blue (0.5% in PBS, 30 mg/kg; EM Sciences, Cherry Hill, NJ) was injected into the femoral vein of anesthetized animals. Five minutes later, hearts were perfusion fixed through the aorta with a solution containing 1% paraformaldehyde in 50 mM citrate buffer, pH 3.5 for 1 minute and then removed. The LV including the interventricular septum was dissected out, blotted dry and weighed. Evans blue was extracted from the LV with formamide and measured on a spectrophotometer.
Histological staining
Serial sections were made from the apex of the heart to the site of the ligation and stained with Hematoxylin and Eosin (H&E) or trichrome to study morphology and myocardial fibrosis. The percentage of circumferential fibrous area of each heart was measured on trichrome stained serial sections. To reduce the variation of the measurement, 1 ml of 0.1 M of CdCl2 was injected into the right ventricle to stop the heart beat at diastolic stage. Anti-PECAM-1 (platelet endothelial cell adhesion molecule-1) (Santa Cruz Biotechnology, Inc., Santa Cruz), anti-smooth muscle α-actin (Sigma, St. Louis, MO) antibodies and Eriochrom blue-black R (LabChem Inc., Pittsburgh, PA) were used to stain endothelial cells, vascular smooth muscles and cardiac muscle respectively and anti-CD4, CD8 and CD45 antibodies (Santa Cruz) for inflammatory cells. Proliferating cells were identified by anti-Ki67 antibody (Vector Laboratory, Burlingame, CA). Nuclei were stained by To-Pro3 (Invitrogen, Carlsbad, CA).
Statistical analysis
One-way ANOVA with post hoc LSD (least significant difference) correction was used to compare the differences among groups, with statistical significance considered at P≤0.05. The data are presented as mean ± SD.
Results
VEGF and ang-1 expression
We analyzed the ratios of ang-1 and VEGF proteins expression mediated by co-infection of CMVang-1 and CMVVEGF by infecting 293 cells with these two vectors, individually or together, in 1:1, 2:1 and 1:2 ratios. The proteins in the supernatants were quantified by ELISA. As a negative control, we infected the cells with CMVLacZ. The measurements of the two proteins in the supernatants of CMVVEGF and CMVang-1-infected cells were subtracted by the measurements of the supernatant of CMVLacZ-infected cells. VEGF and ang-1 proteins in the supernatant of the cells infected with CMVVEGF and CMVang-1 in a 1:1 ratio is about equal (p=0.9). The expression of VEGF and ang-1 were correlated with viral doses, R2 = 0.9214 for ang-1 and R2 = 0.973 for VEGF, although the ratios of the two proteins in the supernatants didn't reflect the ratio of viruses when the cells were infected with CMVang-1 and CMVVEGF at 2:1 and 1:2 ratios (Table 1).
Table 1.
Ang-1 and VEGF proteins in the supernatants of infected 293 cells (μg/ml) (n=3)
| Groups | Ang-1 | VEGF |
|---|---|---|
| vegf (2×106 MOI) | 4±2.8 | 3805±293 |
| vegf (3×106 MOI) | -8.27±25 | 4747±184 |
| vegf (4×106 MOI) | 22±8 | 5674±743 |
| ang (2×106 MOI) | 4987±249 | 113±83 |
| ang (3×106 MOI) | 5105±1150 | -67±133 |
| ang (4×106 MOI) | 5674±743 | 202±150 |
| ang:vegf 2:1 | 5528±458 | 3608±57 |
| ang:vegf 1:1 | 4492±518 | 4452±207 |
| ang:vegf 1:2 | 4153±162 | 5979±359 |
| CMVLacZ | 49±34 | 226±195 |
ang: CMVang-1, vegf: CMVVEGF. Values are mean ± SD. The measurements of CMVVEGF and CMVang-1- infected samples were subtracted by the measurements of the CMVLacZ-infected sample.
In vivo gene expression was analyzed by Western blot analysis 4 weeks after the vector injection (n=3). More VEGF protein was detected in the groups injected with CMVVEGF alone or CMVVEGF/CMVang-1 in a 1:1 ratio than in the groups injected with CMVang-1 or HEPES. More ang-1 protein was detected in the groups injected with CMVang-1 alone or CMVVEGF/CMVang-1 (1:1) than in CMVVEGF or HEPES buffer groups (Figure 1). Since the antibodies we used react with VEGF and ang-1 of human and mouse, the lower levels of VEGF detected in CMVang-1 and HEPES buffer groups and the lower levels of ang-1 detected in CMVVEGF and HEPES buffer groups were probably due to cross-reaction with endogenous mouse VEGF and ang-1, respectively.
Figure 1.
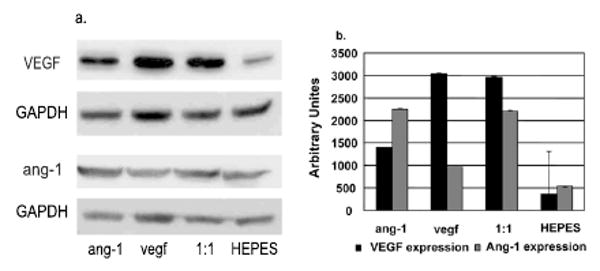
VEGF and ang-1 expression. a. Western blots. ang-1: CMVang-1, vegf: CMVVEGF, 1:1: CMVang-1 and CMVVEGF in a 1:1 ratio and HEPES: HEPES buffer. GAPDH was used as a loading control. b. Graph showing the quantification of VEGF and ang-1 expression (n=3). The expression of VEGF and ang-1 were normalized by the expression of GAPDH.
AAV-mediated co-expression of ang-1 and VEGF results in better cardiac function than expression of either protein alone
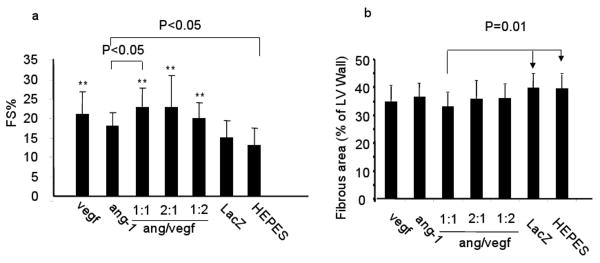
To compare the therapeutic effect of CMVVEGF, CMVang-1 and combinations of the 2 vectors in different ratios (1:1. 2:1 and 1:2) (Table 2), we analyzed cardiac function 4 weeks after treatment using echocardiogram (n=10). The FS% of all treated groups were larger than the two control groups (CMVLacZ and HEPES buffer-injected groups) except for the CMVang-1-injected group (ANOVA, p<0.003, post hoc LSD, p<0.05) (Figure 2a). The LVDd and LVDs of VEGF: ang-1, 1:1 ratio group (4.2±0.32 and 3.2±0.37) were significantly smaller than CMVLacZ (4.7±0.58 and 3.9±0.72) and HEPES buffer (4.9±0.60 and 4.3±0.70) groups (p<0.05). This group also had significantly smaller LVDd and LVDs and larger FS% than the CMVang-1 group (Figure 2a).
Table 2.
Viral doses
| Viral constructs | Ratios | Doses (genome copies) |
|---|---|---|
| CMVVEGF | 1×1011 | |
| CMVang-1 | 1×1011 | |
| CMVVEGF:CMVang-1 | 1:1 | 5×1010 each vector |
| CMVVEGF:CMVang-1 | 1:2 | 5×1010 CMVVEGF and 1×1011 CMVang-1 |
| CMVVEGF:CMVang-1 | 2:1 | 1×1011 CMVVEGF and 5×1010 CMVang-1 |
| CMVLacZ | 1×1011 |
Figure 2.
LV FS% and infarct size. In this and the subsequent figures, hearts were injected with CMVVEGF alone (vegf), CMVang-1 alone (ang-1), CMVang-1 and CMVVEGF at 1:1, 1:2 and 1:2 ratios and with CMVLacZ (LacZ) and HEPES as controls. a. LV FS%. ** indicates the differences between these groups and the 2 control groups (LacZ and HEPES) were significant (p<0.05). b. Infarct size.
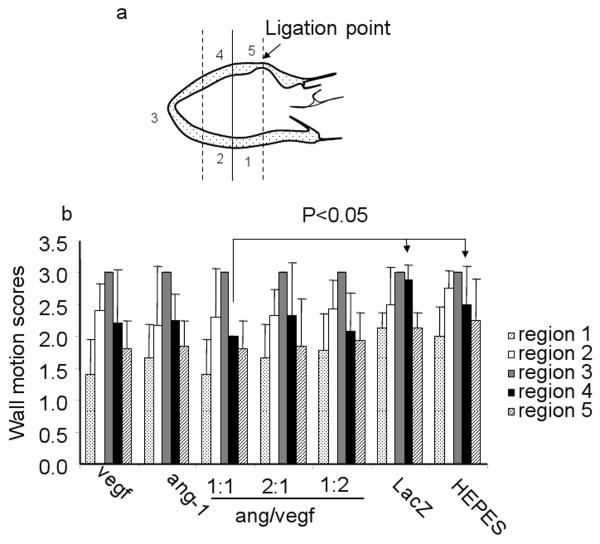
To measure the regional wall motion, we divided the LV wall into 5 regions (Figure 3a). The vectors were injected into the myocardium at region 4. The movement of each region was scored during echocardiography analysis as normokinesis (1), hypokinesis (1.5, 2 and 2.5) and akinesis (3). The apices (region 3, where the infarct was located) of all groups were akinetic and scored 3. Regions 1 and 5 were noninfarcted myocardium, while regions 2 and 4 embraced the junction between the infarcted and noninfarcted regions. There were no significant differences in wall motion scores of regions 1, 2, 3 and 5 among all the groups (Figure 3b). However, scores of region 4 of all treated groups were smaller, although not statistically significant, than those of the two control groups. VEGF:ang-1 1:1 was the only group that showed a statistically significant difference compared to the two control groups (p<0.05) (Figure 3b).
Figure 3.
Regional wall motion 4 weeks post treatment. a. A sketch showing the LV divided into 5 regions for analysis. Infarct scar was located at region 3. The regions 2 and 4 embraced the junction of infarct and noninfarct myocardium. The regions 1 and 5 are noninfarct myocardium. Vectors were injected into region 4. b. Wall motion scores.
We also measured the infarct size from the same mice after functional analyses using thichrome stained sections. Although all the treated groups have smaller infarct size than the control groups, the differences were not significant, except for the group that received co-injection of CMVang-1 and CMVVEGF in a 1:1 ratio (Figure 2b).
Co-expression of VEGF and ang-1 induces more capillaries than ang-1 alone
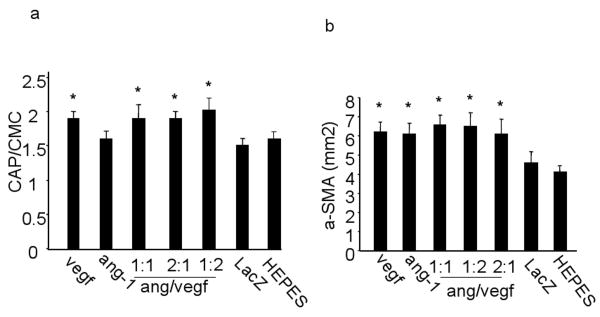
The capillaries and α-SMA (smooth muscle α-actin) positive vessels of above mice were quantified to analysis angiogenesis. Capillary density was expressed as the number of capillaries per cardiomyocyte. The density of α-SMA positive vessels was expressed as the number of α-SMA positive vessels per mm2. Injection of CMVVEGF significantly increased the densities of both capillaries (1.9±0.12) and α-SMA positive vessels (6.2±0.48), as compared to CMVLacZ (1.5±0.13 and 4.6±0.58) and HEPES buffer (1.6±0.05 and 4.1±0.43). However, injection of CMVang-1 increased the number of α-SMA positive vessels (6.1±0.61) only. The densities of the capillaries and α-SMA positive vessels in the 2 vector-co-injected hearts in ratios: 1:1 (1.9±0.16 and 6.6±0.49), 2:1 (1.9±0.15 and 6.5±0.68) and 1:2 (2.0±0.15 and 6.1±0.81) were similar to hearts injected with CMVVEGF alone (Figure 4a and b). Thus, over-expression of VEGF, with or without ang-1, in ischemic myocardium induced both arteriogenesis and capillary angiogenesis, while ang-1 only increased the number of arterioles.
Figure 4.
Densities of capillaries and α-SMA positive vessels. (a) Capillary (CAP) numbers per cardiomyocyte (CMC) and (b) densities of α-SMA positive vessels per mm2 in hearts collected 4 weeks after vector injection. * indicates the differences between these groups and control groups (HEPES and CMVLacZ groups) are statistically significantly.
AAV-mediated co-expression of ang-1 with VEGF reduced vessel leakage caused by VEGF over-expression
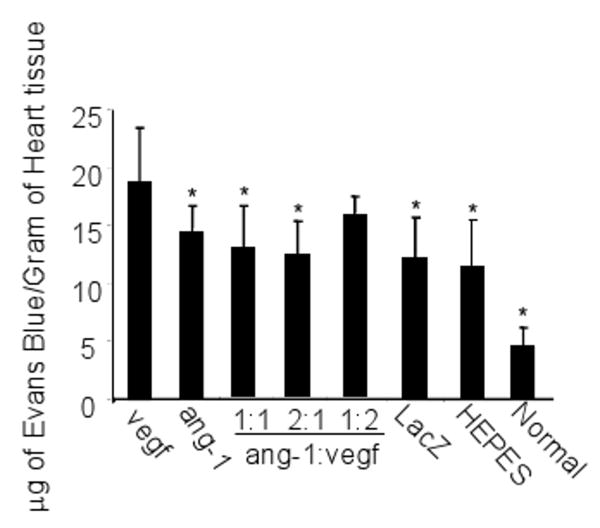
We studied whether over-expression of VEGF mediated by AAV vector caused leaky vessels in the heart and, if so, whether co-expression of ang-1 could prevent the leakiness. CMVVEGF and CMVang-1 were injected individually or together in different ratios (Table 2) into ischemic myocardium immediately after the LAD ligation (n=10). Normal unoperated hearts and ischemic hearts injected with HEPES buffer or the same dose of CMVLacZ were used as controls. Vessel leakage was analyzed 4 weeks after the surgery. Significantly more Evans blue was extracted from CMVVEGF-injected hearts (18.9±4.63 μg/g of myocardium) than from CMVLacZ (12.1±3.58 μg/g) and HEPES buffer (11.3±4.10 μg/g) injected hearts (Figure 5) (P<0.01), indicating that the vasculature in CMVVEGF-injected ischemic myocardium was more leaky. The Evans blue extracted form CMVang-1-injected hearts (10.6±2.35) was less than the VEGF-injected group (Figure 5). In addition, the amounts of Evans blue extracted from the hearts injected with CMVang-1 and CMVVEGF in 1:1 (12.9±3.64) and 2:1 (12.3±2.88) ratios were significantly less than the amount extracted from hearts injected with CMVVEGF alone (P<0.05), while the difference between the CMVVEGF and AAVang-1:CMVVEGF 1:2 groups (15.6±1.68) was not statistically significant (Figure 5). Thus, ang-1 reduced the vessel leakage caused by VEGF in a dose-dependent manner.
Figure 5.
Evans Blue dye leakage 4 weeks after LAD ligation and vector injection. Normal hearts from unoperated and uninjected mice were studied similarly as a control. * indicates that the differences between these groups and CMVVEGF (vegf) group are statistically significant.
Co-Injection of Ang-1 with VEGF Increased Cell Proliferation
Sections of the hearts collected of the animals after the echocardiogram analysis were stained with antibody against Ki67. Proliferating cells around the vector injection sites were counted under a microscope by a scientist who has no knowledge of the experimental groups. Except for the CMVang-1:CMVVEGF 1:2 group (74.0±8.7 cells/mm2), all the angiogenic factor-treated groups (CMVVEGF: 116.7±12.6 cells/mm2; CMVang-1: 115.3±43.8 cells/mm2; CMVang-1:CMVVEGF 1:1: 101.5±39.9 cells/mm2; 2:1: 130,5±55.6 cells/mm2;) had more proliferating cells than the HEPES buffer (34.8±16 cells/mm2) and CMVLacZ (44.2±25.3 cells/mm2) injected groups (P<0.01, ANOVA) (Figure 6a). The cell numbers were similar among groups injected with CMVVEGF, CMVang-1, CMVVEGF/CMVAng-1 in 1:1 and 2:1 ratios. Expression of Cyclin B1 was also upregulated in hearts injected with CMVVEGF and CMVang-1 alone or together in a 1:1 ratio (Figure 6b), indicating that there were more proliferating cells in these hearts than control hearts. Judging by the morphology and double immunostaining, the proliferating cells included cardiomyocytes, endothelial cells and vascular smooth muscles cells (Figure 6c). Few cells were found reactive to antibodies against CD4, CD8 or CD45. Thus, they were not inflammatory cells.
Figure 6.
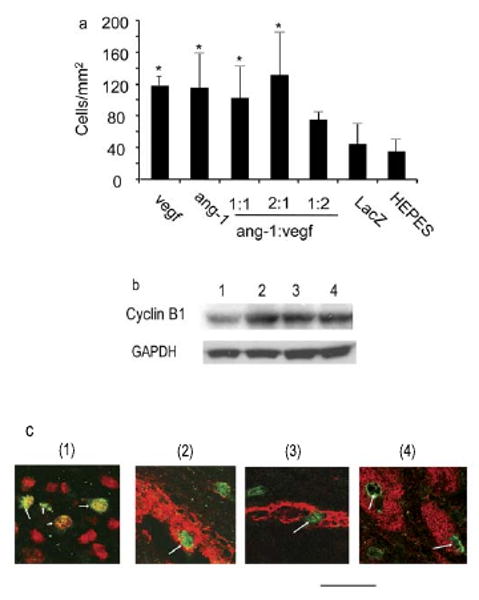
Proliferating cells. a. Graph showing the number of proliferating cells in the different groups quantified on sections stained by anti-Ki67 antibody. * indicates that the differences between these groups and the two control groups are statistically significant. b. Western blots show cyclin B1 expression. Proteins were extracted from hearts injected with CMVLacZ (lane 1), CMVang-1 (lane 2), CMVVEGF (lane 3), and CMVang-1 and CMVVEGF in a 1:1 ratio (lane 4). GAPDH was used as a loading control. c. Representative sections double-stained with antibodies specific for Ki67 (green and arrows) together with To-Pro3 (red) for nuclei (1), with antibody specific for SMA (red) to show proliferating vascular smooth muscle cells (2), with antibody specific for PECAM-1 (red) to show proliferating endothelial cells (3) or with Eriochrom blue-black R (red) to show proliferating cardiomyocytes (4). Scale bar=22 μm.
Discussion
In this study, we tested whether AAV-mediated co-expression of ang-1 and VEGF in ischemic myocardium could mediate stronger angiogenesis, less leaky vessels and better cardiac function than expression of either single gene alone. Although co-expression of VEGF and ang-1 in ischemic hindlimb and heart has been proven to induce leakage resistant vessels and resulted in some improvements in angiogenesis and therapy (11-21). Most of the experiments used plasmid as the vector, which only results in short-term gene expression. We used AAV, a vector that can mediate long-lasting transgene expression in tissues (22), to deliver ang-1 and VEGF genes into ischemic mouse myocardium. AAV vector has only been used to mediate co-expression of VEGF and ang-1 in hindlimb (14, 19). Although skeletal and cardiac muscles have many similarities, the results obtained from hindlimb may not apply to the heart directly. We expected that co-expression of VEGF and ang-1 mediated by AAV vector may yield different results from those mediated by plasmid and adenovirus. Cho et al. showed that short-term expression of ang-1 in adult mice induced transient vascular enlargement that spontaneously reversed within a month, while sustained over-expression of ang-1 produced prolonged vascular enlargement and increased blood flow (23). Similarly, long-term (4 weeks), sustained exposure to VEGF produced long-lasting, acquired vascular remodeling in liver, whereas short-term (2 weeks) exposure to VEGF produced reversible vascular remodeling (24).
In this study, we obtained better therapeutic effects by AAV-mediated co-expression of ang-1 and VEGF in mouse ischemic hearts than expression of either gene alone. The best therapeutic benefit was obtained by co-expression of VEGF and ang-1 in a 1:1 ratio. Co-expression of VEGF and ang-1 resulted in significantly larger FS% than expression of ang-1 alone. Although expressing VEGF alone could significantly improve cardiac function, improvement of regional wall motion was obtained only by co-expression of VEGF and ang-1 in a 1:1 ratio. The groups that were injected with CMVVEGF and CMVang-1 in 1:2 and 2:1 ratios received more total viral doses (1.5×1011) than those injected with single vectors (1×1011). However, the groups injected with CMVVEGF and CMVang-1 in a 1:1 ratio had the same viral dose as the CMVVEGF and CMVang-1 groups and had the best regional wall motion and smaller infarct size among all the groups. Thus, the better therapeutic effects observed in the co-expression group was not due to the differences in viral dosage but to the optimal ratio of VEGF and ang-1.
Although the mitogenic activity of ang-1 on endothelial cells has been proven (25), In our experiment, AAV-mediated ang-1 expression did not increase capillary density in ischemic hearts, while AAV-mediated VEGF expression increased the densities of both CAP and α-SMA positive vessels. Expression of ang-1 did increase the number of α-SMA positive vessels; however, co-expression of ang-1 with VEGF did not increase the vessel density above the levels of VEGF. This result is somewhat different from that of Siddiqui et al., where they transiently expressed VEGF and ang-1 in normal and ischemic mouse hearts by plasmid-mediated gene transfer (18). They showed that ang-1 alone could increase CAP density in both normal and ischemic mouse hearts. This discrepancy could be due to differences in time and duration of gene expression and the time points at which the vessel number were quantified. In their study, peak gene expression was detected at 24 to 48 hours after vector injection. We and others (26-28) have shown that AAV serotype 2 mediated gene expression in the myocardium had a slow onset and took 2-4 weeks to reach the peak. Plasmid-mediated gene expression is transient, whereas AAV-mediated gene express is long-term. Arsic et al. using AAV vectors to express VEGF and ang-1 in the normal perfused limb of rats obtained results similar to ours.
In summary, our results indicated that the precise balance between VEGF and ang-1 may be essential for inducing functional neovasculature in ischemic myocardium and a combination gene therapy with growth factors is a rational approach to create more stable vessels for functional improvement in ischemic tissue.
Acknowledgments
This work was supported by a National Institutes of Health Grant HL67969 (YWK), American Heart Association Scientist Development Grant, 0535018N (HS), and in part by a grant from the Wayne and Gladys Valley Foundation (WG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gyongyosi M, Khorsand A, Zamini S, et al. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005;112(9 Suppl):I157–65. doi: 10.1161/01.CIRCULATIONAHA.105.525782. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Collen D. Role of vascular endothelial growth factor and vascular endothelial growth factor receptors in vascular development. Curr Top Microbiol Immunol. 1999;237:133–58. doi: 10.1007/978-3-642-59953-8_7. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Chen D, Takahashi T, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83(3):233–40. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 5.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 6.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6(4):460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK, Munn LL. Leaky vessels? Call Ang1! [news] Nat Med. 2000;6(2):131–2. doi: 10.1038/72212. [DOI] [PubMed] [Google Scholar]
- 8.Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97(25):13801–6. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su H, Joho S, Huang Y, et al. Adeno-associated viral vector delivers cardiac-specific and hypoxia-inducible VEGF expression in ischemic mouse hearts. Proc Natl Acad Sci U S A. 2004;101(46):16280–5. doi: 10.1073/pnas.0407449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizaka S, Sievers RE, Zhu BQ, et al. New technique for measurement of left ventricular pressure in conscious mice. Am J Physiol Heart Circ Physiol. 2004;286(3):H1208–15. doi: 10.1152/ajpheart.00011.2003. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Chen Y, Zhang F, et al. Synergistically therapeutic effects of VEGF165 and angiopoietin-1 on ischemic rat myocardium. Scand Cardiovasc J. 2007;41(2):95–101. doi: 10.1080/14017430701197593. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Jiangl N, Gao W, et al. Augmentation of revascularization and prevention of plasma leakage by angiopoietin-1 and vascular endothelial growth factor co-transfection in rats with experimental limb ischaemia. Acta Cardiol. 2006;61(2):145–53. doi: 10.2143/AC.61.2.2014327. [DOI] [PubMed] [Google Scholar]
- 13.Chen F, Tan Z, Dong C, et al. Combination of VEGF(165)/Angiopoietin-1 gene and endothelial progenitor cells for therapeutic neovascularization. Eur J Pharmacol. 2007;568(13):222–30. doi: 10.1016/j.ejphar.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Tan Z, Dong CY, Chen X, Guo SF. Adeno-associated virus vectors simultaneously encoding VEGF and angiopoietin-1 enhances neovascularization in ischemic rabbit hind-limbs. Acta Pharmacol Sin. 2007;28(4):493–502. doi: 10.1111/j.1745-7254.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi A, Ito Y, Morikawa M, et al. Pre-administration of angiopoietin-1 followed by VEGF induces functional and mature vascular formation in a rabbit ischemic model. J Gene Med. 2003;5(11):994–1004. doi: 10.1002/jgm.439. [DOI] [PubMed] [Google Scholar]
- 16.Chae JK, Kim I, Lim ST, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20(12):2573–8. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 17.Shyu KG, Chang H, Isner JM. Synergistic effect of angiopoietin-1 and vascular endothelial growth factor on neoangiogenesis in hypercholesterolemic rabbit model with acute hindlimb ischemia. Life Sci. 2003;73(5):563–79. doi: 10.1016/s0024-3205(03)00318-7. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui AJ, Blomberg P, Wardell E, et al. Combination of angiopoietin-1 and vascular endothelial growth factor gene therapy enhances arteriogenesis in the ischemic myocardium. Biochem Biophys Res Commun. 2003;310(3):1002–9. doi: 10.1016/j.bbrc.2003.09.111. [DOI] [PubMed] [Google Scholar]
- 19.Arsic N, Zentilin L, Zacchigna S, et al. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol Ther. 2003;7(4):450–9. doi: 10.1016/s1525-0016(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Ma W, Yang Z, et al. VEGF165 and angiopoietin-1 decreased myocardium infarct size through phosphatidylinositol-3 kinase and Bcl-2 pathways. Gene Ther. 2005;12(3):196–202. doi: 10.1038/sj.gt.3302416. [DOI] [PubMed] [Google Scholar]
- 21.Ye L, Haider H, Jiang S, et al. Improved angiogenic response in pig heart following ischaemic injury using human skeletal myoblast simultaneously expressing VEGF165 and angiopoietin-1. Eur J Heart Fail. 2007;9(1):15–22. doi: 10.1016/j.ejheart.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Kessler PD, Podsakoff GM, Chen X, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci U S A. 1996;93(24):14082–7. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho CH, Kim KE, Byun J, et al. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res. 2005;97(1):86–94. doi: 10.1161/01.RES.0000174093.64855.a6. [DOI] [PubMed] [Google Scholar]
- 24.Dor Y, Djonov V, Abramovitch R, et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. Embo J. 2002;21(8):1939–47. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res. 2005;65(15):6820–7. doi: 10.1158/0008-5472.CAN-05-0522. [DOI] [PubMed] [Google Scholar]
- 26.Chu D, Sullivan CC, Weitzman MD, et al. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J Thorac Cardiovasc Surg. 2003;126(3):671–9. doi: 10.1016/s0022-5223(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 27.Su H, Huang Y, Takagawa J, et al. AAV Serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect. Gene Ther. 2006 doi: 10.1038/sj.gt.3302787. [DOI] [PubMed] [Google Scholar]
- 28.Vassalli G, Bueler H, Dudler J, von Segesser LK, Kappenberger L. Adeno-associated virus (AAV) vectors achieve prolonged transgene expression in mouse myocardium and arteries in vivo: a comparative study with adenovirus vectors. Int J Cardiol. 2003;90(23):229–38. doi: 10.1016/s0167-5273(02)00554-5. [DOI] [PubMed] [Google Scholar]