Abstract
Autism spectrum disorders (ASD) are complex neurodevelopmental disorders that manifest in childhood. Immune dysregulation and autoimmune reactivity may contribute to the etiology of ASD and are likely the result of both genetic and environmental susceptibilities. A common environmental contaminant, 2,2′,4,4′-tetrabrominated biphenyl (BDE-47), was tested for differential effects on the immune response of Peripheral blood mononuclear cells (PBMC) isolated from children with ASD (n=19) and age-matched typically developing controls (TD, n=18). PBMC were exposed in vitro to either 100 nM or 500 nM BDE-47, before challenge with bacterial lipopolysaccharide (LPS), an innate immune activator, with resultant cytokine production measured using the Luminex™ multiplex platform. The cytokine responses of LPS stimulated PBMC from ASD and TD subjects diverged in the presence of 100 nM BDE. For example, cells cultured from the TD group demonstrated significantly decreased levels of the cytokines IL-12p40, GM-CSF, IL-6, TNFα, and the chemokines MIP-1α and MIP-1β following LPS stimulation of PBMC pretreated with 100 nM BDE-47 compared with samples treated with vehicle control (p<0.05). In contrast, cells cultured from subjects with ASD demonstrated an increased IL-1β response to LPS (p=0.033) when pretreated with 100 nM BDE-47 compared with vehicle control. Preincubation with 500 nM BDE-47 significantly increased the stimulated release of the inflammatory chemokine IL-8 (p<0.04) in cells cultured from subjects with ASD but not in cells from TD controls. These data suggest that in vitro exposure of PBMC to BDE-47 affects cell cytokine production in a pediatric population. Moreover, PBMC from the ASD subjects were differentially affected when compared with the TD controls suggesting a biological basis for altered sensitivity to BDE-47 in the ASD population.
Keywords: ASD, autism, LPS, monocyte, innate immunity, BDE-47
Introduction
Autism Spectrum Disorders (ASD) are neurodevelopmental disorders characterized by impairments in social interaction, verbal and nonverbal communication, and stereotyped behaviors and interests (Lord C et al., 2000). The etiology of ASD is not well understood, but most likely involves a complex interplay between both genetic and environmental factors. Although susceptibility is highly heritable, genetic studies have not yet revealed a definitive marker common to all forms of autism. As ASD are typically diagnosed by 3 years of age, there has been much interest in prenatal or early postnatal infectious and environmental exposures that could contribute to the development of ASD. Heavy metal exposure, vaccinations and perinatal infection at critical periods of neurodevelopment have been hypothesized to influence the development of autism (Libbey et al., 2005; Mutter et al., 2005; Windham et al., 2006). However, the involvement of such factors is a controversial topic in the field and their effects remain widely debated. It is also likely that any environmental trigger could vary with a particular individual and influence neurodevelopment in specific ways dependent upon the genetic susceptibility of that individual.
A growing body of research has suggested that immune responses can influence neurodevelopment and that significant alterations in the immune system may play a key role in some individuals with autism (Ashwood et al., 2006). For example, several genetic studies link autism with genes that are associated with various immune functions such as HLA, Complement C4, PTEN, MET and REELIN (Burger et al., 1998; Campbell et al., 2006; Polleux et al., 2004; Skaar et al., 2005). Various immunological abnormalities have been described in subjects with ASD, specifically in the levels of inflammatory mediators and autoimmune responses (reviewed in Ashwood et al., 2006). In particular, increased production of cytokines from the innate immune system have been observed in the plasma and CNS of subjects with ASD, including interleukin-6 (IL-6) tumor necrosis factor alpha (TNFα) and macrophage chemotactic protein-1 (MCP-1) (Crooneberghs et al., 2002, and Jyonouchi et al., 2002, Vargas et al., 2005). Furthermore, evidence for an immune role in autism comes from recent animal models, which indicate that the maternal immune response to infection can influence fetal brain development via increased levels of circulating cytokines (Patterson, 2002). For example, infection of neonatal rats with Borna disease virus (BDV) leads to neuronal death in the hippocampus, cerebellum and neocortex, and a behavioral syndrome that has similarities to autism (Hornig et al., 2001). These abnormalities are correlated with major alterations of cytokine expression in various brain regions, indicating a likely role of cytokines as mediators of CNS injury in this model (Plata-Salaman et al., 1999; Sauder and de la Torre, 1999). Mouse models of maternal influenza virus infection at mid-gestation have similar results in neuropathological and behavioral abnormalities in the offspring, consistent with those seen in autism and are again suggestive of a strong immune component (Patterson, 2002; Shi et al., 2003).
Neonatal exposure to environmental toxicants, including organic mercury, polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) may interfere with normal immune and/or neurologic development (Lawler et al., 2004). PBDEs are used as flame-retardants in many industries including textile, building and the manufacture of electronic appliances such as computers and televisions (Birnbaum et al., 2006; WHO, 1994) and as such, are widely dispersed in the global environment. BDE-47 (2,2′,4,4′-tetra-BDE) is among the most prevalent congers found in human tissues and environmental samples (Wilford et al., 2005). The estimated half life of BDE-47 in human tissue is 1.8 years ((Geyer et al., 2004)) and levels detected in human tissues are rapidly increasing ((Meironyte et al., 1999); (Noren and Meironyte, 2000); (McDonald, 2002)). There are several possible routes of exposure for BDE compounds including the consumption of contaminated food sources (both plant and animal), inhalation of indoor or outdoor air, ingestion of dust, as well as direct dermal exposure. In addition, there are several studies that report BDEs in human milk ((Darnerud et al., 2001); (Fangstrom et al., 2005); (Schecter et al., 2003)) suggesting that there is a potential for neonatal exposure in breast fed infants. Indeed, levels of PBDE in breast milk have increased dramatically (60-fold) in the last 25 years, equivalent to a doubling of the concentration every 5 years ((Meironyte et al., 1999)). In key stages of cerebral development of rats or mice exposure to PBDEs, including BDE-47, results in irreversible damage to brain function, and disorders of voluntary behavior. Additionally, a decrease in learning and memory capabilities have been found which worsen with increased concentrations of PBDEs ((Eriksson et al., 1998); (Darnerud et al., 2001); (Viberg et al., 2002); (Branchi et al., 2002); (Kuriyama et al., 2005)). Early neonatal exposures of rodents to PBDEs result in permanent adverse neurodevelopmental consequences. These include alterations in spontaneous behaviors, deficits in learning and memory, and deficits in habituation to a novel situation ((Eriksson et al., 2001); (Branchi et al., 2002)). Behavioral deficits were only induced by early life exposures (day 3 and 10 for the mouse, but not day 19) demonstrated effects from PBDE compounds on the cholinergic nervous system, critical to numerous cognitive and behavioral functions ((Eriksson et al., 2002);(Viberg et al., 2002). Additionally, exposure to PBDE influences levels of three important signaling proteins that regulate neuronal survival, growth and synaptogenesis. Male mice exposed to PBDEs on postnatal day 3 altered the concentration of Brain Derived Neurotrophic Factor (BDNF), Growth Associated Protein 43 (GAP-43) and calcium/calmodulin-dependent protein kinase II (CaMKII) on postnatal day 10. CaMKII and GAP-43 increased significantly in the hippocampus, while BDNF decreased significantly. ((Viberg et al., 2008)). Little is known about possible interactions of BDE-47 with the immune system, particularly during early development. However, given the increasing exposure of children through flame-retardants and breast milk, the potential for the developing neural and immune systems to be in contact with this pervasive organic pollutant is increasingly likely and justify a preliminary study of autism and PBDEs.
This study was designed to help understand the cellular mechanisms by which specific environmental toxicants may interact with the immune system in a pediatric population, and in particular, those children with ASD. The experiments sought to examine innate cellular function in PBMCs following in vitro exposure to the specific environmental toxicant BDE-47, in children with ASD.
Materials and Methods
Subjects
Participants in the study were recruited through the CHARGE (Childhood Autism Risk from Genetics and Environment) study conducted at the UC Davis M.I.N.D. Institute (Hertz-Picciotto et al., 2006). Participants ranged from 2–5 years of age and included 18 children with a confirmed diagnosis of ASD (median age 3.42 years, range 2.42–5 years: 3 females) and 19 unrelated age- and gender-matched typically developing general population controls (median age 3.5 years, range 2.5–4.75 years: 1 females). Subjects who had been ill within 2 weeks of the blood draw, were taking antibiotics, or had recently taken (within 7 days) anti-inflammatory drugs were excluded from this study. This study was approved by the UC Davis institutional review board and complied with all requirements regarding human subjects. Informed consent was obtained from a legal guardian of each participant. To confirm diagnosis, all ASD participants completed the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS) which were administered at the M.I.N.D. Institute by qualified trained clinicians. The ADI-R is a comprehensive clinical interview administered to parents or caregivers of children suspected of having autism. The ADI-R assesses function in areas of language and communication, reciprocal social interaction and repetitive, restricted, stereotyped behaviors or interests. Results from the interview are interpreted using a diagnostic algorithm that correlates with the DSM-IV and ICD-10 definitions of autism. The ADOS is an observational assessment of the children suspected of having autism in various structured and unstructured situations that provide a standardized set of conditions to observe behavior in the areas of communication, play and other areas relevant to autism. Children from the general population were screened for autism traits using the Social Communication Questionnaire and no children in the control group scored above the cut-off.
Peripheral Blood Mononuclear Cell (PBMC) Isolation
Peripheral blood was collected in acid-citrate-dextrose Vacutainers (BD Biosciences; San Jose, CA), centrifuged at 2300 rpm for 10 min and plasma removed from sample. Blood cell suspension was adjusted to a volume of 20 mL with Hank’s Balanced Salt Solution (HBSS) (VWR; West Chester, PA), layered on room temperature Histopaque (Sigma; St. Louis, MO) and centrifuged at 1700 rpm for 30 min to isolate PBMC. PBMC were extracted and washed twice with HBSS and the number of viable PBMC was determined by Trypan Blue exclusion (Sigma, St. Louis, MO). Cells were >95% viable. No significant differences in PBMC cell numbers were noted between the study populations (data not shown).
PBMC Stimulation assays
PBMCs were adjusted to a concentration of 3.0 × 106 cells mL−1 with a solution of the culture supplement T-Stim (0.2%) (BD Biosciences, Franklin Lakes, NJ) in serum-free X-Vivo media, which is used to grow and maintain cultured human mononuclear cells (Cambrex, Walkersville, MD). For each subject, the PBMC suspension were placed into 3 separate polypropylene vials (Savillex Corp., Minnetonka, MN) for treatment with DMSO (vehicle control), 100 nM or 500 nM BDE 47, and incubated at 37°C, 5% C02/95% air for 4 hours in sterile borosilicate glass tubes. Glass tubes were used for the preincubation phase to prevent adsorption of the BDE 47 to the vessel during incubation as well as to avoid cell loss and activation due to monocyte adherence. Thus, no loss of cells due to adherence or a decrease in cell viability was observed during the pre-incubation period. We chose to use BDE 47 at 100 nM and 500 nM in vitro exposure levels for two reasons. First, in a study by Shecter et al, in a series of 39 individual analyses from two samples pools of 100 samples each, the US blood level range was from 4.6 to 365.5 ppb (Schecter et al., 2005). Further, a dose response study ranging from 5 to 1000 nM exposures, the most significant effect was noted between 100 and 500 nM of BDE 47. The cells were then centrifuged at 4000 rpm and washed twice with X-vivo 15 supplemented with 0.2% T-stim (BD, Franklin Lakes, NJ). 100 μL of PBMC suspension was plated in four individual wells of a 96-well tissue culture plate (Corning, Corning, NY). 100 μL of stimulant or media was added to each well for a final volume of 200 μL. The PBMCs were stimulated with 2.5 μg ml−1 lipopolysaccharide (LPS; Sigma, St. Louis, MO) or 100 μL X-Vivo alone. All antigen dilutions were made in X-Vivo. The cells were incubated at 37°C for 48 hrs at which time the plates were centrifuged and supernatants from each of the 6 test conditions were individually harvested by pipette and stored at -80°C until the date of the cytokine assay.
Cytokine Concentration Measurement
PBMC culture supernatants were analyzed by the Luminex multiplex platform using the Human Cytokine Antibody Bead Kit (Biosource, Camarillo, CA), Human Chemokine Antibody Bead Kit (Biosource) and IL-12p40 Antibody Bead Kit (Biosource). The assay kits allowed for the detection of the cytokine or chemokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p40, IFNγ, TNFα, GM-CSF, MIP-1α, MIP-1β, MCP-1, RANTES and Eotaxin. Analyte specific antibody conjugated beads were incubated with 50 μL of 1x biotinylated detection antibody solution and 50 μL of either standard, or 25 μL of sample in 25 μL of assay diluent for 3 hrs at room temperature, in the dark, on an orbital shaker (500–600 rpm). After wells were washed and aspirated by vacuum manifold aspiration, 100 μL of strepavidin-conjugated R-phycoerythrin was added to each well and incubated for 30 min at room temperature, in the dark, on an orbital shaker (500–600 rpm). R-phycoerythrin fluorescence was measured on a Bio-Plex 200 System (Bio-Rad Laboratories Inc., Hercules, CA) to determine concentration.
Statistical analysis
The cells were incubated in triplicate per treatment. The data was analyzed by Wilcoxon rank sign tests to take into account both the magnitude of the response, and how many responses went up or down within a subject population. SPSS software, version 15 (SPSS Inc., Chicago, IL.) was used to analyze the data. Values were considered significant for p < 0.05.
Results
Following LPS stimulation of PBMC cultures, cytokines and chemokines that are predominantly produced by cells of the monocytes/macrophage lineage could be detected using the Luminex bead based assay; these included IL-1β, IL-6, IL-8, IL-10, IL-12p40, TNFα, GM-CSF, MIP-1α and MIP-1β. In contrast, cytokines that are largely produced by lymphocytes, such as IL-2, IL-4, IL-5, IFNγ, MCP-1, RANTES and Eotaxin could not be reliably detected using this assay. This result was expected as LPS stimulates innate immune response and signals through the CD14-Toll Like receptor-4 (TLR4) complex present on monocytes.
Pretreatment of PBMC with BDE-47, at either the 100nM or 500nM, had no effect on cytokine production in unstimulated cell cultures from either ASD or TD subjects (Table 1 and 2). The production of the chemokine IL-8 was reduced in unstimulated cell cultures from ASD or TD subjects pretreated with BDE-47 100nM (p<0.022, Table 1) but not at the 500nM concentration. In contrast, the production of the chemokine, MIP-1α, was significantly increased at both 100nM and 500nM concentrations of BDE-47 in unstimulated cell cultures from ASD or TD children (p<0.023, Table 1 and Table 2). In addition, 500nM BDE-47 pretreatment increased the production of MIP-1β in unstimulated PBMC cultures from ASD but not TD children (p<0.008, Table 2).
Table 1.
Effect of 100nM or 500nM BDE-47 on LPS stimulated PBMC cultures from children with autism spectrum disorders (ASD) compared with typically developing (TD) controls.
| DMSO Mean (pg/ml) | SEM | 100nM Mean (pg/ml) | SEM | 500nM Mean (pg/ml) | SEM | |||
|---|---|---|---|---|---|---|---|---|
| GM-CSF | Media | ASD | 150.9 | 8.1 | 147.4 | 5.8 | 165.6 | 24.6 |
| TD | 177.3 | 18.6 | 159.4 | 10 | 149.3 | 6.8 | ||
| LPS | ASD | 737.5 | 52 | 742.2 | 40.6 | 676a | 45.3 | |
| TD | 790 | 57 | 695.2a | 41.9 | 694.2a | 44.5 | ||
|
| ||||||||
| IL-12p40 | Media | ASD | 15.5 | 0.5 | 15 | 0 | 169.2 | 153.1 |
| TD | 69.9 | 551.8 | 31.5 | 16.5 | 20.1 | 5.1 | ||
| LPS | ASD | 1220.8 | 216.1 | 1465.4 | 240.6 | 1261.5 | 196.9 | |
| TD | 1377.6 | 166.1 | 1148.5a | 154.4 | 1163.5a | 122.8 | ||
|
| ||||||||
| TNFα | Media | ASD | 18.4 | 2.8 | 15.2 | 1.4 | 115.7 | 100.2 |
| TD | 49 | 26.2 | 17.6 | 5.2 | 21.9 | 7.2 | ||
| LPS | ASD | 2358.7 | 502.6 | 2275.6 | 355.5 | 2026.6 | 359.9 | |
| TD | 2041.8 | 553.7 | 1202.7a | 216.9 | 1704.1 | 361.7 | ||
|
| ||||||||
| IL-6 | Media | ASD | 113.1 | 29 | 81.7 | 8.4 | 300.1 | 180.5 |
| TD | 467.5 | 244.3 | 260 | 154.2 | 175.4 | 73.1 | ||
| LPS | ASD | 19375.5 | 3081.8 | 12507a | 1507.1 | 11161.3a | 928.9 | |
| TD | 20497.5 | 3433.8 | 10973.3a | 1389.3 | 11709.5a | 1075.6 | ||
|
| ||||||||
| IL-1β | Media | ASD | 15 | 0 | 15 | 0 | 19.2 | 3 |
| TD | 16 | 0.6 | 15.1 | 0.1 | 15.2 | 0.8 | ||
| LPS | ASD | 204.8 | 29.8 | 247.5a | 29 | 229.5 | 32 | |
| TD | 187.1 | 36.4 | 188.8 | 35.8 | 191.8 | 26.8 | ||
|
| ||||||||
| IL-10 | Media | ASD | 5 | 0 | 5 | 0 | 9.2 | 4.2 |
| TD | 9.8 | 3.4 | 8.6 | 2.5 | 5.9 | 0.9 | ||
| LPS | ASD | 83.2 | 17.3 | 93.5 | 20.9 | 86.5 | 16.1 | |
| TD | 109.6 | 25.9 | 122.5 | 48.2 | 123.8 | 27.2 | ||
|
| ||||||||
| IL-8 | Media | ASD | 30830.2 | 5636.3 | 17999.6a | 2775.6 | 35605.7 | 4420.7 |
| TD | 42997.2 | 9615.6 | 26592.1a | 6494 | 46250.8 | 9517.6 | ||
| LPS | ASD | 167912.3 | 23548.1 | 187017.5 | 30164.2 | 308515.1a | 67522.6 | |
| TD | 183592.4 | 35491 | 160737.8 | 37100.9 | 198903.6 | 36218.7 | ||
|
| ||||||||
| MIP-1α | Media | ASD | 10 | 0 | 430.2a | 78 | 1858.3a | 1435.1 |
| TD | 453.1 | 443.1 | 621.6a | 251.3 | 962.7b | 375 | ||
| LPS | ASD | 14956.6 | 2207.4 | 13986.9 | 1943.3 | 14565 | 2667.2 | |
| TD | 17833.7 | 2373.9 | 12262.2a | 1675.6 | 17353.9 | 2552.5 | ||
|
| ||||||||
| MIP-1β | Media | ASD | 118.2 | 74.4 | 289.9 | 55.1 | 1166.6a | 797.6 |
| TD | 644.8 | 269.5 | 421 | 167.2 | 553.9 | 269.1 | ||
| LPS | ASD | 7066 | 1362.7 | 6429.3 | 1176.2 | 8009.8 | 1542.7 | |
| TD | 7430.8 | 1212.3 | 4615a | 446.4 | 7629.1 | 745.1 | ||
p<0.05 for cytokine release following100nM BDE-47 pretreatment compared with DMSO treated cell cultures
p<0.05 forcytokine release following500nM BDE-47 pretreatment compared with DMSO treated cell cultures
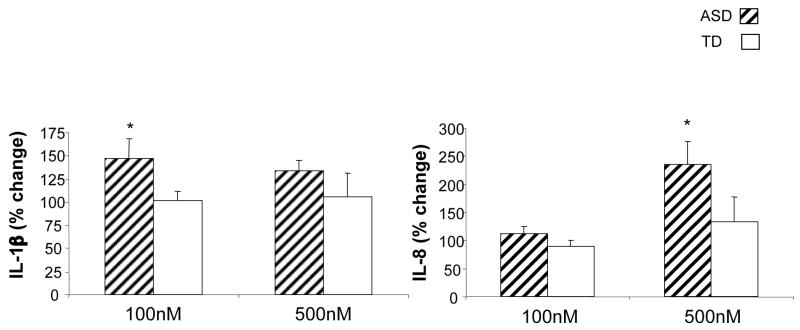
In BDE-47 pre-treated cell cultures that were stimulated with LPS, a divergent profile of cytokine and chemokine production was observed for cells cultured from ASD children compared with TD children. In PBMC cultures from TD controls that were pretreated with BDE-47 at 100nM concentration, significantly decreased production of GM-CSF, IL-12p40, TNFα, IL-6, MIP-1α and MIP-1β was detected following LPS-stimulation (p<0.05, Table 1). In contrast, in PBMC cultures from ASD children, only IL-6 production was significantly decreased under the same conditions (p=0.039, Table 1). Furthermore, IL-1β production was significantly increased in LPS-stimulated cell cultures pretreated with 100nM BDE-47 from ASD children (p=0.033, Table 1). Moreover, the percentage change in the LPS induced IL-1β response following pre-treatment with 100nM BDE-47 when compared with pre-treatment with DMSO, was significantly increased in cells cultured from ASD children (p=0.03) but not TD controls (Figure 1).
Figure 1.
Effect of polybrominated diphenyl ethers (PBDE47) on LPS stimulated cytokine response in children with autism spectrum disorders (ASD) compared with typically developing (TD) controls. Mean percent change in induced cytokine production of (A) IL-1β and (B) IL-8 from LPS-stimulated cell cultures with BDE-47 pre-treatment compared with DMSO pre-trement is shown.
At the 500nM BDE-47 concentration, a similar response was noted, with decreased cytokine production in cell cultures from TD subjects for GM-CSF, IL-12p40 and IL-6 (p<0.05, Table 2) following LPS-stimulation. There was also decreased cytokine production for GM-CSF and IL-6 in cell cultures from ASD children (p<0.02, Table 2). Of note, however, there was an increase in production of the chemokine IL-8 in LPS-stimulated cell cultures from ASD children that were pretreated with 500nM of BDE-47 (p=0.039, Table 2). Despite the similar baseline control levels of IL-8, the LPS induced IL-8 response following pre-treatment with 500nM BDE-47 when compared with pre-treatment with DMSO, was significantly increased in cells cultured from ASD children (p=0.02) but not TD controls (Figure 1).
Discussion
In the absence of BDE-47, cell cultures stimulated with LPS elicit an innate immune response with the production of cytokines primarily from the monocyte/macrophage cell lineage. Previous reports have shown innate immune responses are elevated in postmortem brain specimens and CSF samples from ASD subjects compared with controls (Vargas et al.,2005). In the current study, we demonstrate for the first time, that BDE-47 pretreatment of LPS-stimulated cell cultures results in divergent innate cytokine responses in ASD children compared with TD general population pediatric controls. In cell cultures from TD controls, LPS stimulated cytokine/chemokine production was significantly reduced in the presence of 100nM BDE-47 for a number of cytokines including, GM-CSF, IL-12p40, TNFα, IL-6 MIP-1α and MIP-1β whereas only IL-6 was decreased in cell cultures from ASD children. In contrast, we demonstrate that there is increased production of IL-1β in similar cell cultures from ASD subjects, compared with no detectable change in IL-1β in cell culture from TD controls. Similarly, pre-treatment with 500nM concentrations of BDE-47 induced increased production of IL-8 in LPS stimulated cell cultures from ASD children but not TD controls. These results suggest that innate immune cytokine response may be differentially affected by BDE-47 in subjects with ASD compared with TD controls.
The innate immune response (also called natural or native immunity) is considered the first line of defense and provides a powerful and rapid response to eliminate microbes from host tissue. Innate immunity relies primarily on pattern recognition of structures that are shared by various classes of microbes but not mammalian cells, such as LPS present in gram negative bacteria and double stranded RNA in viruses. Upon encountering invading pathogens, cells of the innate immune system are responsible for their phagocytosis, processing, and the subsequent presentation of antigens in combination with MHC molecules to cells of the adaptive immune system resulting in the propagation of the immune response. Monocyte and monocyte- derived cell lineages comprise the major cell types responsible for the phagocytosis and presentation of foreign antigenic molecules from pathogens to T cells, and are an important source of immunomodulatory cytokines and chemokines. Interference in this process can have profound effects on both the innate and acquired immune systems.
Previous studies indicate that there is an over-production of pro-inflammatory cytokines, in particular IL-1β and TNFα from peripheral mononuclear cells stimulated with bacterial LPS in children with autism, findings which strongly indicate an inappropriate monocyte driven innate immune response (Singh, 1996). However, these studies were all performed a subset of autism subjects that have gastrointestinal (GI) abnormalities. As the subjects studied herein were not evaluated for GI abnormalities by a pediatric gastroenterologist it may be that we were testing a different group of autistic individuals compared with these previous reports. In addition, the age range of our study were much younger than these previous reports and may account for the fact that although we found a slight elevation in TNFα and IL-1β levels in the ASD cases compared with controls, this did not reach statistical significance. Furthermore, our experimental conditions required that we use DMSO as a control. Although in previous experiments we found that LPS stimulated cytokine responses are the same with or without DMSO, we cannot directly compare our experiments with the previous published reports.
The presence of specific monocyte phenotypes that produce enhanced levels of cytokines has also been associated with other immune-mediated disorders such as multiple sclerosis (TNFα) and Type 1 diabetes (IFNα) (Bergh et al., 2004). In the CNS, antigen presenting cells from the monocyte cell lineage have been found in healthy meninges, the choroid plexus, and cerebrospinal fluid (Pashenkov et al., 2003). During inflammation in the CNS there is a recruitment of these cells where they are thought to play equal roles in the defense against infections, but may also contribute to the break-down of tolerance to CNS autoantigens (Pashenkov et al., 2003).
Microglial cells share many similarities with cells of the monocyte lineage and are considered part of the CNS innate immune system. The CNS is largely populated by astroglia and microglia that not only respond to cytokines, but also produce cytokines upon activation. Cell culture studies have shown that neuropoietic cytokines such as IL-1 and IL-8 can have direct effects on neurons and glial cells, including changes in proliferation, survival, death, neurite outgrowth and gene expression (Mehler et al., 98; Gadient et al., 1999). Microglial cells participate in many reactive processes in the CNS and have been implicated in the exacerbation of neurological conditions such as multiple sclerosis, Alzheimer’s disease, AIDS, and viral encephalitis (Nelson et al., 2002). Vargas et al., recently showed the presence of innate immune activation in both brain specimens and cerebrospinal fluid (CSF) from subjects with autism (Vargas et al., 2005), with active neuroinflammation present in the cerebral cortex and cerebellum. This inflammatory process was characterized by a marked cellular activation of microglial and astroglial cells and the presence of an altered cytokine pattern, including enhanced proinflammatory cytokine levels in the CSF. These results suggest that abnormal innate immune responses in the neuroglia of individuals with autism may influence neural function and neural development.
Organochlorine (OC) contaminants, notably polychlorinated biphenyls (PCBs), are ubiquitous in all ecosystems and found in the tissues of humans and wildlife. We have previously demonstrated this ubiquitous environmental contaminant to be associated with various adverse conditions, such as impaired immunological function in marine wildlife (Neale et al., 2002). Although the immunotoxicity of coplanar, dioxin-like PCBs is well documented, the adverse effects exerted by non-coplanar, non-dioxinlike PCBs have received little attention. A direct causal relationship between PCB exposure and the observed detrimental effects on the immune system observed in marine mammals has yet to be fully established in humans. Levin et al (2005) demonstrated a suppression of phagocytosis by non-coplanar PCBs, suggesting a previously unrecognized aryl hydrocarbon receptor (AhR)-independent pathway. Similarly, brominated flame retardants are a novel class of environmental contaminants; within this group, the polybrominated diphenyl ethers are currently used in large quantities, disperse similarly to PCBs and DDT, and bioaccumulate and biomagnify (Herzke et al., 2005; Meerts et al., 2001). Little work has been done on the effects of PBDEs on immune function in humans. In one study on harbor porpoises, thymic atrophy and splenic depletion were significantly correlated with an increase in both PCB and PBDE levels (Beineke et al., 2005). Moreover, the structural similarities between PBDEs and immunotoxic halogenated aromatic compounds suggest that the PBDEs may exert an affect on the immune system (Fernlof et al., 1997). However, the choice of which cogener to use may be critical to realize any effect. There is in vitro evidence that BDE-47 and its major metabolites inhibit aromatase activity (CYP19) and can be cytotoxic (Canton et al., 2005). In addition, many of the biochemical effects of PBDEs, with BDE-47 being the most potent, on protein kinase activity and calcium homeostatis in rat neuronal cultures have been shown to be similar to those seen following exposure to structurally related PCBs (Kodavanti et al., 2005).
Little is known about the effects of BDE-47 and immune function. However, we demonstrate herein that BDE-47 can affect innate immune responses previously shown to be elevated in subjects with ASD. This may be due to the lipophilic nature of the BDEs, which would allow more efficient incorporation into the lipid bilayer of (Rahman, et al 2001). We further hypothesize that patients with autism may have an altered sensitivity to the immunomodulatory effects of BDEs. It may be this increased susceptibility that is responsible for some of the immune anomalies that we have previously noted (Ashwood et al., 2006). The precise mechanism by which PDE effects PBMC function in the pediatric populations described herein is under further investigation.
Acknowledgments
This research was supported by Award Number P01ES011269 from the National Institute of Environmental Health Sciences and Award Numbers R833292 and R829388 from the Environmental Protection Agency. The content is solely the responsibility of the investigators and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health or the Environmental Protection Agency.. In addition, funding was received from Cure Autism Now, Autism Speaks, The Peter Emch Foundation and a generous gift from the Johnson Family. We would like to thank the staff of both the UC Davis M.I.N.D Institute and the CHARGE study for their technical support. The commitment of the families who took part in these studies, at both the M.I.N.D Institute and as part of the CHARGE study, is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004;24(6):664–73. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van De Water JA. The immune response in autism: a new frontier for autism research. J Leukocyte Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Beineke A, Siebert U, McLachlan M, Bruhn R, Thron K, Failing K, Muller G, Baumgartner W. Investigations of the potential influence of environmental contaminants on the thymus and spleen of harbor porpoises (Phocoena phocoena) Environ Sci Technol. 2005;39:3933–8. doi: 10.1021/es048709j. [DOI] [PubMed] [Google Scholar]
- Bergh FT, Dayyani F, Ziegler-Heitbrock L. Impact of type-I-interferon on monocyte subsets and their differentiation to dendritic cells. An in vivo and ex vivo study in multiple sclerosis patients treated with interferon-beta. J Neuroimmunol. 2004;146:176–188. doi: 10.1016/j.jneuroim.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Cohen Hubal EA. Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect. 2006;114(11):1770–5. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicology. 2002;23:375–384. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Burger RA, Warren RP. Possible immunogenetic basis for autism. Ment Retard Dev Disabil Res Rev. 1998;4:137–141. [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103(45):16834–9. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton RF, Sanderson T, Letcher RJ, Bergman A, van den Berg M. Inhibition andInduction of Aromatase (CYP19) Activity by Brominated Flame Retardants in H295R Human Adrenocortical Carcinoma Cells. Toxicol Sci. 2005;88(2):447–55. doi: 10.1093/toxsci/kfi325. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiol. 2002;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson C, Winblad B, Schultzberg M. Kainic acid induced expression of interleukin-1 receptor antagonist mRNA in the rat brain. Brain Res Mol Brain Res. 1998;58:195–208. doi: 10.1016/s0169-328x(98)00125-9. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Ankarberg E, Viberg H, Fredriksson A. The developing cholinergic system as target for environmental toxicants, nicotine and polychlorinated biphenyls (PCBs): implications for neurotoxicological processes in mice. Neurotox Res. 2001;3:37–51. doi: 10.1007/BF03033229. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Viberg H, Jakobsson E, Orn U, Fredriksson A. A brominated flame retardant, 2,2′,4,4′,5-pentabromodiphenyl ether: uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol Sci. 2002;67:98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- Fangstrom B, Strid A, Grandjean P, Weihe P, Bergman A. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ Health. 2005;4:12. doi: 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernlof G, Gadhasson I, Podra K, Darnerud PO, Thuvander A. Lack of effects of some individual polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) congeners on human lymphocyte functions in vitro. Toxicol Lett. 1997;90:189–97. doi: 10.1016/s0378-4274(96)03848-9. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, Schramm K, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann G, Lenoir D, Schmid P, McDonald TA. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 2004;66:3867–3871. [Google Scholar]
- Gadient RA, Patterson PH. Leukemia inhibitory factor, Interleukin 6, and other cytokines using the GP130 transducing receptor: roles in inflammation and injury. Stem Cells. 1999;17:127–137. doi: 10.1002/stem.170127. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, Van de Water JA, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Env Health Persp. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzke D, Berger U, Kallenborn R, Nygard T, Vetter W. Brominated flame retardants and other organobromines in Norwegian predatory bird eggs. Chemosphere. 2005;61:441–9. doi: 10.1016/j.chemosphere.2005.01.066. [DOI] [PubMed] [Google Scholar]
- Hornig M, Solbrig M, Horscroft N, Weissenbock H, Lipkin WI. Borna disease virus infection of adult and neonatal rats: models for neuropsychiatric disease. Curr Top Microbiol Immunol. 2001;253:157–77. doi: 10.1007/978-3-662-10356-2_8. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46:76–84. doi: 10.1159/000065416. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol Sci. 2005;88:181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low-dose PBDE 99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect. 2005;113:149–154. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler CP, Croen LA, Grether JK, Van de Water JA. Identifying environmental contributions to autism: Provocative clues and false leads. Ment Retard Dev Disabil Res Rev. 2004;10:292–302. doi: 10.1002/mrdd.20043. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. J Neurovirol. 2005;11:1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- McDonald TA. A perspective on the potential health risks of PBDEs. Chemosphere. 2002;46:745–755. doi: 10.1016/s0045-6535(01)00239-9. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, andpolybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA. Cytokines in brain development and function. Adv Protein Chem. 1998;52:223–251. doi: 10.1016/s0065-3233(08)60437-4. [DOI] [PubMed] [Google Scholar]
- Meironyte D, Noren K, Bergman A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J Toxicol Environ Health A. 1999;58:329–341. doi: 10.1080/009841099157197. [DOI] [PubMed] [Google Scholar]
- Mutter J, Naumann J, Schneider R, Walach H, Haley B. Mercury and autism: accelerating evidence? Neuroendocrinol. Lett. 2005;26:439–446. [PubMed] [Google Scholar]
- Neale JC, van de Water JA, Harvey JT, Tjeerdema RS, Gershwin ME. Proliferative responses of harbor seal (Phoca vitulina) T lymphocytes to model marine pollutants. Dev Immunol. 2002;9:215–21. doi: 10.1080/10446670310001593523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Ann Med. 2002;34:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- Noren K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40:1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Teleshova N, Link H. Inflammation in the central nervous system: the role for dendritic cells. Brain Pathol. 2003;13:23–33. doi: 10.1111/j.1750-3639.2003.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12:115–8. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- Peng R, Li Y, Brezner K, Litherland S, Clare-Salzler MJ. Abnormal peripheral blood dendritic cell populations in type 1 diabetes. Ann N Y Acad Sci. 2003;1005:222–225. doi: 10.1196/annals.1288.031. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C, Turrin N. Cytokine interactions and cytokine balance in the brain: relevance to neurology and psychiatry. Mol Psychiatry. 1999a;4(4):302–6. [PubMed] [Google Scholar]
- Plata-Salamán CR, Ilyin SE, Gayle D, Romanovitch A, Carbone KM. Persistent Borna disease virus infection of neonatal rats causes brain regional changes of mRNAs for cytokines, cytokine receptor components and neuropeptides. Brain Res Bull. 1999b;49(6):441–51. doi: 10.1016/s0361-9230(99)00081-7. [DOI] [PubMed] [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Sauder C, de la Torre JC. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J Neuroimmunol. 1999;96(1):29–45. doi: 10.1016/s0165-5728(98)00272-0. [DOI] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U. S. mothers’ milk. Environ. Health Perspect. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Shi LM, Fatemi H, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66:143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, DeLong GR, Moore JH, McCauley JL, Sutcliffe JS, Ashley-Koch AE, Cuccaro ML, Folstein SE, Gilbert JR, Pericak-Vance MA. Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry. 2005;10:563–571. doi: 10.1038/sj.mp.4001614. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Viberg H, Mundy W, Eriksson P. Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth, and synaptogenesis. Neurotoxicology. 2008;29:152–159. doi: 10.1016/j.neuro.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Viberg H, Mundy W, Eriksson P. Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth, and synaptogenesis. Neurotoxicology. 2008;29:152–159. doi: 10.1016/j.neuro.2007.10.007. [DOI] [PubMed] [Google Scholar]
- WHO. WHO IPCS Environmental Health Criteria Document. WHO; Geneva: 1994. Brominated Diphenyl Ethers; p. 162. [Google Scholar]
- Wilford BH, Shoeib M, Harner T, Zhu J, Jones KC. Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: implications for sources and exposure. Environ Sci Technol. 2005;39:7027–35. doi: 10.1021/es050759g. [DOI] [PubMed] [Google Scholar]
- Windham G, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay Area. Environ Health Perpect. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]