Abstract
Background
Fatty liver is increasingly common in obese adolescents. We determined its association with glucose dysregulation in 118 (37M/81F) obese adolescents of similar age and % total fat.
Methods and Findings
Fast-MRI and simple MRI were used to quantify hepatic fat content and abdominal fat distribution. All subjects had a standard OGTT. Insulin sensitivity was estimated by the Matsuda Index and HOMA-IR. Baseline total and HMW-adiponectin and IL-6 levels were measured. The cohort was stratified according to tertiles of Hepatic Fat Content. While age and %fat were comparable across tertiles, ethnicity differed in that fewer Blacks and more Whites and Hispanics were in the Moderate and High category of HFF. Visceral and the visceral to subcutaneous fat ratio increased and insulin sensitivity decreased across tertiles. Two hr plasma glucose rose with increasing hepatic steatosis (p<0.008). 73.7% of the subjects in the High HFF had the Metabolic Syndrome compared to 19.5% and 30.6% respectively in the Low and Moderate category. Both total and HMW-adiponectin decreased, and IL-6 increased with increasing hepatic steatosis.
Conclusion
In obese adolescents, independent of total fat, increasing severity of fatty liver is associated with glucose dysregulation, the Metabolic Syndrome and with a pro-inflammatory milieu.
Introduction
Ectopic fat deposition in insulin sensitive tissues such as liver and muscle strongly correlates with insulin resistance (1). Previously, we reported that increased intramyocellular fat in obese adolescents was associated with impaired glucose intolerance and insulin resistance (2). Furthermore, moderate elevation in ALT levels, a poor surrogate of fatty liver, was found to be associated with high-normal glucose levels (3), whereas abnormal ALT levels were reported in youngsters with type 2 diabetes (T2DM) (4), raising the question of a potential role of fatty liver in the onset of T2DM in obese youth. Fatty liver in obese adolescents is becoming increasingly common (5-7). Nevertheless, its role in the dysregulation of glucose metabolism is unclear. We hypothesize that, independent of overall obesity, the severity of hepatic steatosis will strongly affect the presence of prediabetes and diabetes in obese adolescents. Furthermore, we determined if the balance between anti-inflammatory markers such as total and High Molecular Weight (HMW) adiponectin and pro-inflammatory markers like IL-6 would vary as a function of the degree of hepatic steatosis. Using Fast-MRI we quantified intrahepatic fat content in a large multiethnic group of obese adolescents matched for age and overall adiposity.
Methods
The Cohort
118 obese adolescents, 13 to 16 years old with a BMI-z score ranging from 2.35 to 2.5 participated in this study. All subjects were recruited from our Pediatric Obesity Clinic. Sixty percent of the subjects were reported previously (3, 8, 9). To be eligible for this study, subjects could not be on medications known to affect liver function or alter glucose or lipid metabolism. Information related to alcohol consumption was obtained in all subjects using a questionnaire. Autoimmune hepatitis, Wilson disease, α-1-antitrypsin deficiency, hepatitis B and C and iron overload, were excluded with appropriate tests in subjects with persistent elevation in ALT (>6months). The Yale University School of Medicine Human Investigation Committee approved the study, and written informed consent and assent were obtained.
Oral Glucose Tolerance Test (OGTT)
All subjects were invited to the Yale Center for Clinical Investigation (YCCI) at 8 a.m. after an overnight fast (3, 8). Following placement of an indwelling venous line, baseline samples were obtained for glucose, insulin, c-peptide, lipid profile, liver enzymes, total and HMW-adiponectin, leptin and IL-6 levels. Thereafter, a standard 3-hr OGTT was performed (3, 8). Impaired fasting glucose (IFG), impaired glucose tolerance (IGT) or combined (IFG+IGT) was defined in accordance with the American Diabetes Association guidelines (10). Insulin sensitivity was measured with whole body insulin sensitivity index (WBISI) using the Matsuda Index (11, 12) and the HOMA-IR (13). The insulinogenic index (IGI), was calculated as the ratio of the increment in plasma insulin level to that in plasma glucose level during the first 30 min after glucose ingestion (14). The Disposition Index (DI) represents the insulin response in the context of the insulin resistant milieu. DI was calculated as the product of WBISI and the IGI (15,16). Definition of the Metabolic Syndrome was based on the pediatric criteria per Ford et al (17).
Fast-MRI: Liver Fat Content
Measurement of liver fat content was performed by MRI using the 2-point Dixon (2PD) method as modified by Fishbein et al. (18) based on phase-shift imaging where Hepatic Fat Fraction (HFF) is calculated from the signal difference between the vectors resulting from in-phase and out-of-phase signals. Using the MRIcro software program, five regions of interest were drawn on each image, and the mean pixel signal intensity level was recorded. The HFF was calculated in duplicate from the mean pixel signal intensity data using the formula: [(Sin-Sout)/(2×Sin)]×100. The imaging parameters were: matrix size = 128×256, flip langle (α) = 30°, TR = 18 ms, TEs = 2.38/4.76 ms out-of-phase (OP) and in-phase (IP), respectively), bandwidth = 420 Hz/pixel, 6 averages, slice thickness = 10 mm, 1 slice, 2.3 sec/slice (for 2-points), scan time = 14 sec on a single breath-hold (19). Recent studies indicated that T1 and T2 can introduce errors in the caluculation of fat fraction (20-22). Without T1 correction low fat fractions can be overestimated (22) while higher fat fractions remain relatively unaffected. It should be noted that in our methodology we used a small flip angle (30 degrees) to minimize T1 effects. It should also be noted that a simpler explanation for some of the negative fat fractions arises in low SNR regions where in-phase/out-of-phase difference is within the noise, or where the tissue is composed of either mostly all fat or mostly all water such that the in-phase/out-of-phase differences are also around zero. The T2* correction is very important in cases where iron overload might lead to T2* changes (21), but none of the patients in our study had iron overload problems and thus this affect can be eliminated as a concern that might influence our results. Furthermore, while some studies recently have been published employing fat fractions that have been corrected for T1 and T2* this is currently an area of active research and the correction schemes are not necessarily as straight forward. For example, fat has multiple spectral components and this single measurement of the T2* decay curve is not adequate (such a measurement yields oscillating signal behavior (22)). For these reasons we did not correct for T1 or T2* effects but do not feel such corrections would significantly alter the results presented in this strictly defined subject group, and could in fact introduce additional errors in the data.
Validation of Fast-MRI
We validated the modified 2PD method against 1H-NMR in 34 lean and obese adolescents (26 of these subjects are included in the present study) and found a very strong correlation between the two methods (r=0.954, p<0.0001)(19). To assess its repeatability, measurements were obtained (within the same day) on 12 subjects. The within-subject standard deviation for HFF was 1.9%. This degree of reproducibility is well within the boundaries of that necessary to make this a viable method to assess the relation between HFF and metabolic outcomes. Kim et al. (19) demonstrated that a 2PD HFF cut-off of 3.6%, provided good sensitivity (80%) and specificity (87%) compared to a 1H-NMR reference.(19) Comparisons between the 2PD method and histologic determination of fatty liver have been made, albeit only in adults. Fishbein et al. (23) found in 38 patients undergoing biopsy for a variety of liver diseases a highly significant correlation between liver histology and MRI determination of HFF, particularly with macrovesicular steatosis (r=0.920, p<0.001). Fast MRI has also been found to be able to track longitudinal changes in liver fat content in adults during pioglitazone treatment (24) and in a case report involving an obese adolescent with NAFLD undergoing gradual weight loss (25).
Abdominal MRI and Total Body Composition (DEXA)
Abdominal MRI studies were performed on a Siemens Sonata 1.5 Tesla system (2,9). Total body composition was measured by dual-energy X-ray absorptiometry with a Hologic scanner (Boston, MA).
Biochemical Analysis
Plasma glucose levels were measured using the YSI 2700 STAT Analyzer (Yellow Springs Instruments) and lipid levels using an Autoanalyzer (model 747-200, Roche-Hitachi). Plasma insulin, proinsulin, leptin and total adiponectin levels were measured using double antibody radioimmunoassays from Millipore; HMW-adiponectin using ELISA kits from Millipore, C-peptide using double antibody radioimmunoassays from Diagnostic Products Corporation and IL-6 using ELISA high sensitivity kits from R&D Systems. Liver enzymes were measured using standard automated kinetic enzymatic assays.
Statistics
To evaluate a potential relation between metabolic parameters and intrahepatic fat accumulation, subjects were stratified according to tertiles of Hepatic Fat Content or (%HFF). Tertile 1 is labeled as Low HFF (median %HFF 0.7, Range - 4.6 to 1.6) , Tertile 2, Moderate HFF (median 4.5%, Range 1.8 to 9.1), and Tertile 3, High HFF, (median 28.8, Range 9.9 to 44.9). Analysis of covariance and logistic regression, with adjustment for age, gender and race/ethnicity, were used to compare means and proportions respectively across HFF tertiles. Where appropriate, log transformations were used to normalize variables with positively skewed distributions. For the prevalence of impaired glucose regulation, a test for trend was conducted in logistic regression by including the continuous HFF in the model. Unless otherwise stated, data are represented as mean±SEM or mean±95% Confidence Intervals (CI). Two logistic regression models were utilized to define significant predictors of high HFF tertile. The independent variables used in model 1 were gender, race/ethnicity, age, ALT, visceral fat and total adiponectin. Model 2 replaced total adiponectin with HMW-adiponectin. The Odds Ratio (OR) and 95% confidence intervals (CIs) for the unadjusted and adjusted HFF risk factors were then calculated via the logistic regression model. Multiple linear regressions were also used to evaluate the role of HFF in determining 2h-G during the OGTT. The independent variables entered were gender, race/ethnicity, age, ALT, adiponectin (or HMW-adiponectin alternatively), visceral fat and HFF. For all analyses, a p-value of <0.05 was considered statistically significant. All analyses were performed using SPSS 15.0 for Windows (SPSS Inc, Chicago, IL).
Results
Anthropometric Phenotypes According to Liver Fat Content (Table 1)
Table 1.
Demographic, anthropometric and clinical characteristics of the study cohort across tertile of Liver Fat Content (HFF %), adjusted for age, gender, race/ethnicity.
| Characteristic | Low Liver Fat Content (n=42) | Moderate Liver Fat Content (n=40) | High Liver Fat Content (n=36) | P-value Adjusted |
|---|---|---|---|---|
| Age (y) | 14.0 (2.9) | 15.3 (2.6) | 14.7 (2.7) | |
| Gender-no. (%) | ||||
| Male (n=37) | 7 (17%) | 12 (30%) | 18 (50%) | |
| Female (n=81) | 35 (83%) | 28 (70%) | 18 (50%) | |
| Race/Ethnicity-no. (%) | ||||
| White (n=49) | 7 (17%) | 21 (52%) | 21 (58%) | |
| African-American (n=39) | 24 (57%) | 10 (25%) | 5 (14%) | |
| Hispanic (n=30) | 11 (26%) | 9 (23%) | 10 (28%) | |
| Weight (Kg) | 101 (95.2, 106) | 101 (95.4, 106) | 102 (96.7, 108) | 0.91 |
| BMI (Kg/m2) | 36.4 (34.7, 38.1) | 37.1 (35.5, 38.7) | 38.3 (36.6, 39.9) | 0.32 |
| BMIz-score | 2.43 (2.35, 2.51) | 2.43 (2.36, 2.51) | 2.49 (2.42, 2.57) | 0.42 |
| Waist | 110 (106, 114) | 107 (104, 111) | 113 (109, 117)!! | 0.09 |
| DEXA | ||||
| %fat | 43.6 (41.2, 46.0) | 45.9 (43.7, 48.1) | 46.0 (43.6, 48.4) | 0.31 |
| Tissue Lipid Content | ||||
| Liver | ||||
| Hepatic Fat Fraction (HFF%)* | 0.7 (-1.6, 1.1) | 4.5 (2.8, 5.9) | 26.2 (15.2, 35.9) | - |
| Abdominal Region | ||||
| VAT (cm2) | 58.1 (49.3,67.0)‡ | 66.4 (58.0, 74.9)!! | 92.3 (83.2, 101) | <0.001 |
| SAT (cm2) | 582 (528, 637) | 573 (521, 625) | 610 (554, 665) | 0.61 |
| VAT/SAT | 0.10 (0.09, 0.12) | 0.12 (0.11, 0.13)!! | 0.16 (0.14, 0.17)‡ | <0.001 |
| DeepSubQ (cm2) | 201 (180, 223) | 208 (188, 229) | 220 (198, 241) | 0.47 |
| SupSubQ (cm2) | 156 (137, 175) | 153 (135, 171) | 145 (126, 164) | 0.74 |
| Deep/SupSubQ | 1.36 (1.19, 1.54)‡ | 1.47 (1.30, 1.64) | 1.75 (1.58, 1.91) | 0.007 |
Data are presented as means (95% C.I.).
Data are presented as median (IQR).
Data in bold indicate significance.
P<0.05 for difference between low and moderate
P<0.05 for difference between low and high
P<0.05 for difference between moderate and high
The cohort was stratified according to tertiles of hepatic fat content (%HFF). Subjects in tertile 1 had the lowest %HFF, and thus this group is referred to as Low Hepatic Fat Content. Subjects in the other 2 tertiles were labeled as Moderate and High to denote different degrees of Hepatic Fat Content.. As shown in Table 1, males were more represented in the Moderate (30%) and High HFF (50%) category compared to the Low HFF category (17% p=0.01). While age, BMI, BMI-z score, waist and % fat were comparable across categories, there was a clear shift in the racial/ethnic distribution, in that fewer Blacks were in the Moderate (25%) and High (14%) category of fatty liver (p<0.0001).
Despite similar % total fat, abdominal visceral fat increased, whereas total subcutaneous fat was similar across categories. The visceral to subcutaneous ratio increased significantly across tertiles (p<0.001). When the posterior subcutaneous fat was further divided into deep and superficial subcutaneous fat, we found a similar content in deep superficial subcutaneous fat distribution across tertiles, and an increase in the deep to superficial subcutaneous ratio, especially in the High HFF category.
Metabolic Phenotypes According to Liver Fat Content (Table 2)
Table 2.
Metabolic characteristics of the study cohort across tertile of Liver Fat Content (HFF %),
| Characteristic | Low Liver Fat Content | Moderate Liver Fat Content | High Liver Fat Content | P-value Adjusted |
|---|---|---|---|---|
| Fasting Plasma Glucose (mmol/l)* | 5.25 (5.08, 5.43) | 5.30 (5.14, 5.47) | 5.35 (5.18, 5.52) | 0.77 |
| 2h-Glucose (mmol/l)* | 6.49 (6.05, 6.94)‡ | 7.13 (6.70, 7.55) | 7.26 (6.82, 7.70) | 0.04 |
| Fasting Plasma Insulin (μU/ml)ˆ | 27.4 (23.3, 32.1)† | 35.8 (30.6, 42.1)!! | 47.0 (40.0, 55.1)‡ | <0.001 |
| 2h-Plasma Insulin (μU/ml)ˆ | 95.9 (72.1, 128)† | 167 (126, 220) | 225 (170, 300)‡ | <0.001 |
| Fasting C-Peptide (pmol/l)ˆ | 988 (877, 1113)† | 1202 (1071, 1351)!! | 1487 (1316, 1680)‡ | <0.001 |
| 2h C-Peptide (pmol/l)ˆ | 2741 (2375, 3164)† | 3629 (3158, 4169) | 4155 (3598, 4801)‡ | <0.001 |
| Proinsulin (pM)ˆ | 18.5 (15.0, 22.6)† | 24.8 (20.2, 30.4)!! | 33.1 (27.0, 40.7)‡ | <0.001 |
| Insulin Sensitivity and Secretion Indexes | ||||
| HOMA-IRˆ | 6.81 (5.71, 8.12) | 8.39 (7.11, 9.90)!! | 11.3 (9.51, 13.4)‡ | <0.001 |
| WBISI(Matsuda Index) ˆ 10-4dl/min per microunits/ml | 1.70 (1.30, 2.21) | 1.19 (0.93, 1.51) | 0.82 (0.74, 1.17)‡ | 0.007 |
| Insulinogenic Index (IGI)ˆ | 4.45 (3.19, 5.72) | 5.21 (4.01, 6.40) | 6.40 (518, 7.63)‡ | 0.05 |
| Disposition Index (DI)ˆ | 6.02 (4.88, 7.43) | 5.20 (4.24, 6.37) | 4.64 (3.76, 5.74)‡ | 0.05 |
| Lipid Levels | ||||
| HDL (mmol/l)* | 1.21 (1.13, 1.29) | 1.13 (1.06, 1.21) | 1.12 (1.03, 1.20) | 0.24 |
| TG (mmol/l)ˆ | 0.87 (0.73, 1.02) | 0.88 (0.75, 1.04)!! | 1.36 (1.15, 1.60)‡ | <0.001 |
| Liver Enzymes | ||||
| ALT (UI/L)ˆ | 16.7 (13.5, 20.7) | 15.1 (12.3, 18.6)!! | 38.1 (30.6, 47.6)‡ | <0.001 |
| AST (UI/L)ˆ | 21.4 (19.3, 24.4) | 21.1 (18.9, 23.7)!! | 30.8 (27.3, 34.8)‡ | <0.001 |
| GGT (UI/L)ˆ | 18.6 (15.8, 21.8) | 19.3 (16.4, 22.7)!! | 32.8 (27.8, 38.8)‡ | <0.001 |
| Total Adiponectin | 10.2 (8.9, 11.4) † | 8.1 (6.9, 9.3)!! | 6.0 (4.7, 7.3) ‡ | <0.001 |
| HMW Adiponectin | 2.3 (1.8, 2.8) | 1.8 (1.4, 2.3) !! | 1.2 (0.7, 1.62) ‡ | 0.006 |
| IL-6 | 1.5 (1.1, 1.9) | 2.0 (1.6, 2.4) !! | 2.6 (2.2, 3.0) ‡ | 0.001 |
Data are presented as *means (95% C.I.), ˆ geometric means (95% C.I.). Data in bold indicate significance.
P<0.05 for difference between low and moderate
P<0.05 for difference between low and high
P<0.05 for difference between moderate and high
While there were no differences in fasting glucose, 2h-glucose increased across tertiles, with the high tertile being significantly greater than the low tertile. Both fasting and post-OGTT insulin and C-peptide levels increased significantly across categories. Fasting proinsulin also increased across tertiles. It should be noted that for the above described metabolic parameters, significant differences were already present between the Low and Moderate tertiles of HFF. The WBISI decreased and HOMA-IR increased significantly across categories, with significant differences in the high tertile compared to both moderate and low tertiles. The IGI tended to be higher and the DI tended to be lower in the high tertile compared to the low tertile. Plasma triglycerides significantly increased across categories. HDL-cholesterol levels, although lower in the high tertile, lost significance after adjusting for age, gender and race. Of note, ALT, AST and GGT levels were significantly higher in the High tertile compared to both Moderate and Low.
Impaired Glucose Regulation and Metabolic Syndrome Prevalence According to Hepatic Fat Content (HFF) (Figure 1)
Figure 1.
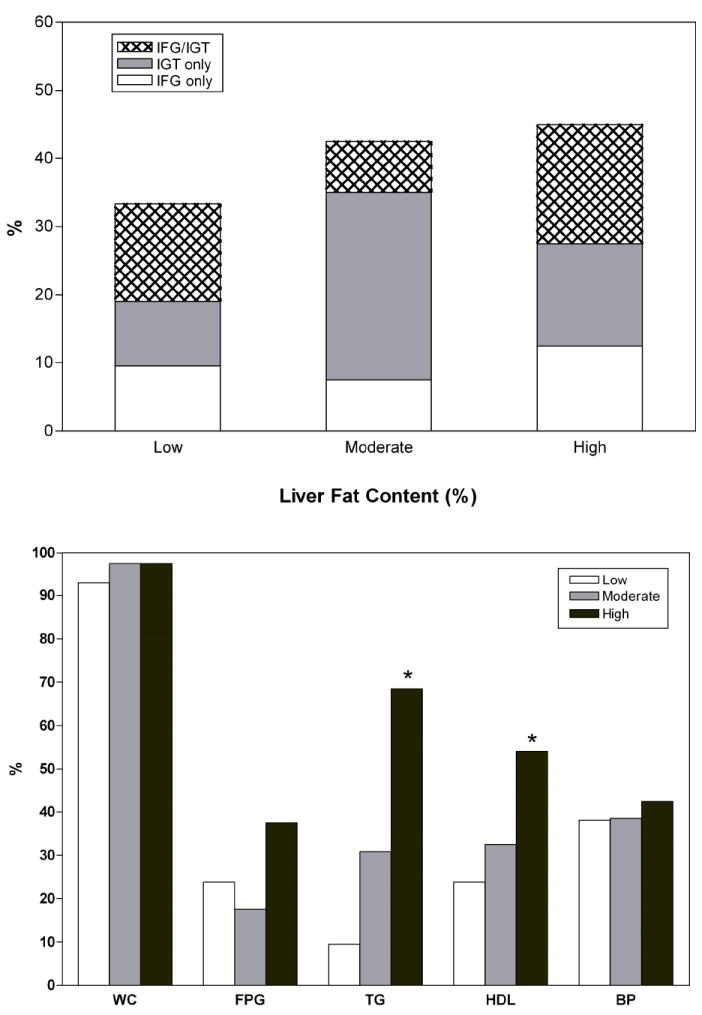
Panel A. Prevalence of prediabetes in obese adolescents according to the degree of Liver Fat Content (%) measured by the fast-MRI. The prevalence rates of impaired glucose regulation (IFG, IGT, IFG/IGT) tended to rise across tertiles (p for trend = 0.07).
Panel B. Prevalence rate of each component of the Metabolic Syndrome (Ford’s criteria) according to the degree of Liver Fat Content (%) measured by the fast-MRI. There were no differences in waist circumference (WC), Fasting Plasma Glucose (FPG) and Blood pressure (BP) across categories. The prevalence rates for Triglycerides (TG) and HDL (L-HDL) levels were significant differences between Low Liver Fat Content and the remaining groups (P=0.000). P values were adjusted for age, gender and race/ethnicity.
White box= Low Liver Fat content
Light Gray= Moderate Liver Fat Content
Dark Gray= High Liver Fat Content
While there were no pairwise differences across tertiles the prevalence rates of impaired glucose regulation (IFG, IGT, IFG/IGT) tended to rise across tertiles (p for trend = 0.07).
Paralleling the severity of fatty liver, there was a significant increase in the prevalence of the metabolic syndrome (p<0.001). In particular, 73.7% of the subjects in the High Fatty liver category met the criteria for the metabolic syndrome compared to 19.5% and 30.6% respectively in the Low and Moderate category. Shown in Figure 1B are the prevalence rates for each single component of the Metabolic Syndrome across categories of Liver Fat Content. Waist circumference, fasting plasma glucose and blood pressure prevalence rates were not significantly different across categories of Liver Fat Content, whereas the prevalence rates for elevated triglycerides and low HDL-cholesterol levels were (p<0.001).
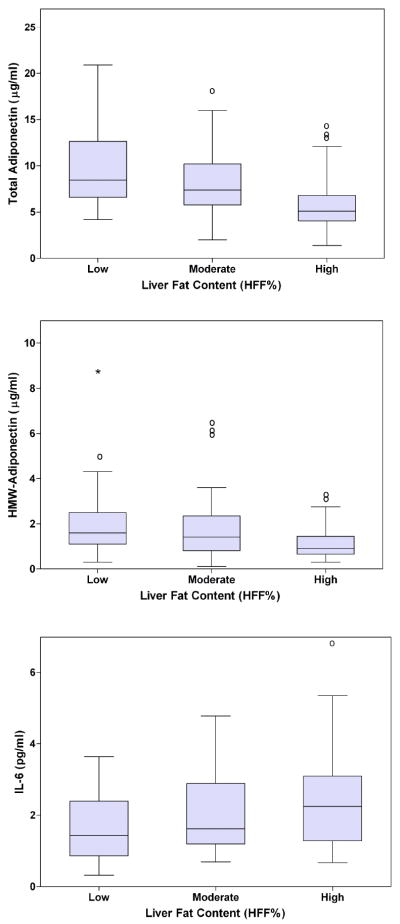
Adipokines According to the Liver Fat Content (Figure 2)
Figure 2.
Panel A. Box-plot for Total Adiponectin according to the degree of Liver Fat Content (%) measured by fast-MRI. P = 0.001, adjusted for age, gender and race/ethnicity.
Panel B. Box-plot for HMW-Adiponectin according to the degree of Liver Fat Content (%) measured by fast-MRI. P = 0.001, adjusted for age, gender and race/ethnicity.
Panel C. Box-plot for IL-6 according to the degree of Liver Fat Content (%) measured by fast-MRI. P = 0.008, adjusted for age, gender and race/ethnicity.
Total and HMW-adiponectin levels decreased significantly across tertiles of liver fat content (p<0.001). Leptin levels across category did not significantly differ by tertile (data not shown). In contrast, a difference emerged across categories for IL-6 (p= 0.009) (Fig.2).
Relationship
In the whole cohort, HFF was significantly correlated with visceral fat (ρ=.55, p<0.001), ALT (ρ=0.56, p<0.001), WBISI (ρ =-0.36, p<0.001), total adiponectin (ρ =-0.40, p<0.001), HMW-adiponectin (ρ=-0.34, p<0.001). Significant predictors of high HFF tertile were evaluated in two logistic regression models differing for the use of total adiponectin (model 1) or HMW-adiponectin (model 2). ALT (adjusted OR 1.06, 95% C.I. 1.02-1.11, p=0.007) and visceral fat deposition (adjusted OR 1.04, C.I. 1.01-1.08, p=0.02) were significantly associated with high HFF. Total adiponectin was significantly associated with HFF (OR 0.69, C.I. 0.47-0.97, p=0.04), but HMW-adiponectin was not significantly associated with HFF. In particular we found that for each 10 unit increase in ALT or visceral fat there was a 59% and 40% increase, respectively, in the odds of having high HFF. To further analyze the role of HFF in determining Impaired Glucose Regulation, we used multiple linear regression analyses. HFF was significantly associated with 2h-glucose (2h-G) suggesting that for each 10 unit increase in HFF there is an increase of 0.35 mmol/l (6.2 mg/dl) of the 2h-G ( p=0.02).
Discussion
In a multiethnic group of obese adolescents with a narrow range of BMIs and total body fat, we determined the impact of the degree of hepatic steatosis, measured by fast gradient MRI, on the prevalence of prediabetic and diabetic phenotypes. The novel finding is that, independent of obesity the severity of fatty liver is associated with the presence of pre-diabetes (IGT and IFG/IGT). Of note, hepatic steatosis independently predicted prediabetes in obese adolescents. Paralleling the severity of hepatic steatosis, there was a significant decrease in insulin sensitivity and impairment in beta-cell function as indicated by the fall in the disposition index. Moreover, increasing degree of fatty liver was associated with an imbalance between anti- and pro-inflammatory markers. These important findings highlight, for the first time, the close relation between the amount of liver fat content, measured by a reliable technique, Fast-MRI, and glucose dysregulation in a large group of adolescents with similar degree of obesity and maturational age.
A high prevalence of fatty liver in association with T2DM has been reported in adults (5, 26, 27). However, it remains unclear whether hepatic steatosis is a consequence or a cause of the metabolic derangements in insulin sensitivity (27, 28). Furthermore, hepatic steatosis was found to predict the metabolic syndrome, independent of overall obesity. Thus, fatty liver disease may be the hepatic component of the metabolic syndrome (30,31), as demonstrated by Schwimmer et al. (30) in obese adolescent with biopsy proven fatty liver (32). In the present study, the prevalence of the metabolic syndrome was 44.4% and 74.2% in the Moderate and High category respectively, compared to 24.2% in the Low category. Given that the liver plays a key role in glucose metabolism, we believe, based on the current findings that it is reasonable to assume that hepatic steatosis may play a role in the development of T2DM in obese youth. Although not directly measured here, a steatotic liver is usually very insulin resistant and consequently overproduces glucose (33). Our study would suggest that those youngsters diagnosed with fatty liver need to have further evaluation to rule out diabetes or impaired glucose tolerance. Furthermore, in light of the fact that in the adult population diabetes is a very important prognostic factor indicating the presence of more advanced fibrosis (34) and a risk factor for more rapid progression of fibrosis, a similar scenario may be also present in obese youngsters with fatty liver. Although our study cannot prove causality it does suggest that the presence of fatty liver may predate the onset of full blown diabetes, because of the presence of both IGT and IFG/IGT conditions, which are the precursors of diabetes.
Fatty liver is known to be associated with central obesity (26). The current study further confirms this association in obese adolescents, regardless of the degree of obesity. Moreover, in a logistic regression, VAT significantly predicted, together with elevated level of ALT, liver fat content. Previously we reported that intrahepatic fat content correlated positively with ALT, and that elevation of ALT levels, even within the normal range, was associated with insulin resistance (3). Metabolic alterations were further exaggerated when ALT levels rose outside the normal range.(3) In the present study, subjects with Moderate liver fat content had totally normal ALT levels. It is only in the High category of fatty liver that the ALT levels were abnormally elevated. Thus, ALT is not a sensitive marker for fatty liver since a normal ALT may not exclude it.
Consistent with our previous report (3, 9) and with that by Louthan et al. (35) hepatic steatosis was inversely correlated with total adiponectin. For the first time, this study reports data on the active form of adiponectin, the HMW form, which decreased as the HFF increased across categories. This further supports the hypothesis that hypoadiponectinemia is related to hepatic steatosis (36, 37). In the logistic regression analyses, however, neither total adiponectin nor HMW-adiponectin were significant predictors of fatty liver. Hypoadiponectinemia and severe insulin resistance appear to be more consistent with the presence of steatohepatitis than with only steatosis and may be one of the pathogenetic links between central obesity and the development of inflammatory forms of Non Alcoholic Fatty Liver Disease (NAFDL) (36). Consistent with this hypothesis is the fact that in the three subjects with NASH (biopsy-proven), the HMW was extremely low (data not shown).
While total and HMW-adiponectin decreased, IL-6 levels were significantly higher in the adolescents with High liver fat content. Thus, mild to moderate hepatic steatosis is associated with an imbalance between anti and proinflammatory markers that together may ignite inflammation in the liver.
Although the Fast-MRI is an accepted technique for measuring hepatic fat content, there are no studies that have validated it with liver histology in the pediatric population. These studies are greatly needed. Despite these limitations, we showed in our group that this technique stands strong against the 1H-NMR technique, which is considered the reference method for quantifying fat in the liver.
In conclusion, we found that fatty liver is associated with prediabetic phenotypes and thus may be considered a strong risk factor for T2DM in youth, independent of overall obesity. The risk for prediabetes and metabolic syndrome is proportional to the degree of hepatic steatosis. Furthermore, as the hepatic fat content increases, the balance between anti- and pro-inflammatory markers changes in favor of a pro-inflammatory milieu.
Acknowledgments
We are grateful to all of the adolescents who participated in the study, to the research nurses for the excellent care given to our subjects, and to Aida Groszmann, Andrea Belous and Corduta Todeasa for their superb technical assistance.
This study was supported by grants from the National Institutes of Health (NIH) (R01-HD40787, R01-HD28016, and K24-HD01464 to Dr. Caprio, and by CTSA Grant Number UL1 RR0249139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); and R01-EB006494 (Bioimage Suite). This publication was supported and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Contributors AMG Cali did all the metabolic studies. AM De Oliveira and R Kursawe helped doing the metabolic studies. H Kim, R T Constable and S Taksali did the Fast-MRI analyses.. S Escalera and M Reyes-Mugica are expert pediatric hepatologists. J Dziura and Shu Chen did the statistical analyses. M Shaw, M Savoye and B Pierpont helped in the clinical care of the participants and data management. AMG Cali and S Caprio took part in the study design. All investigators contributed to the writing and the review of the report.
Reference List
- 1.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000 Jul;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003 Sep 20;362(9388):951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006 Nov;91(11):4287–4294. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 4.Nadeau KJ, Klingensmith G, Zeitler P. Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. J Pediatr Gastroenterol Nutr. 2005 Jul 1;41:94–98. doi: 10.1097/01.mpg.0000164698.03164.e5. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 6.Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006 Oct;434:413–427. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006 Oct;118(4):1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 8.Cali AM, Zern TL, Taksali SE, de Oliveira AM, Dufour S, Otvos JD, et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care. 2007 Dec;30(12):3093–3098. doi: 10.2337/dc07-1088. [DOI] [PubMed] [Google Scholar]
- 9.Taksali SE, Caprio S, Dziura J, Dufour S, Cali AM, Goodman TR et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008 Feb;57(2):367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association: Diagnosis and classification of diabetes mellitus (Position Statement) Diabetes Care. 2006;29(Suppl.1):S43–S48. [PubMed] [Google Scholar]
- 11.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999 Sep;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 12.Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004 Mar;89(3):1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 13.Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002 Jul;19(7):527–534. doi: 10.1046/j.1464-5491.2002.00745.x. [DOI] [PubMed] [Google Scholar]
- 14.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994 Apr;113:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 15.Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006 Mar;49(3):571–579. doi: 10.1007/s00125-005-0109-z. [DOI] [PubMed] [Google Scholar]
- 16.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993 Nov;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005 Apr;28(4):878–881. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- 18.Fishbein MH, Gardner KG, Potter CJ, Schmalbrock P, Smith MA. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15(3):287–293. doi: 10.1016/s0730-725x(96)00224-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF et al. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med. 2008 Mar;59(3):521–527. doi: 10.1002/mrm.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwenzer NF, Machann J, Petros M, Stefan N, Schraml C, Fritsche A, Claussen CD, Schick F. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest Radiol. 2008;43(5):330–337. doi: 10.1097/RLI.0b013e31816a88c6. [DOI] [PubMed] [Google Scholar]
- 21.Westphalen ACA, Qayyum A, Yeh BM, Merriman RB, Lee JA, Lamba A, Lu Y, Coakley FV. Liver fat: effect of hepatic iron deposition on evaluation with opposed-phase MR imaging. Radiology. 2007;242(2):450–455. doi: 10.1148/radiol.2422052024. [DOI] [PubMed] [Google Scholar]
- 22.Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, Lavine JE, Sirlin CB. Relaxation effects in the quantification of fat using gradient echo imaging. Magnetic Resonance Imaging. 2008;26:347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005 Aug;39(7):619–625. doi: 10.1097/00004836-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004 Jan;39(1):188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 25.Fishbein MH, Stevens WR. Rapid MRI using a modified Dixon technique: a non-invasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatr Radiol. 2001 Nov;31(11):806–809. doi: 10.1007/s002470100547. [DOI] [PubMed] [Google Scholar]
- 26.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E906–E916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 27.Kotronen A, Juurinen L, Hakkarainen A, Westerbacka J, Corner A, Bergholm R, et al. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care. 2008 Jan;31(1):165–169. doi: 10.2337/dc07-1463. [DOI] [PubMed] [Google Scholar]
- 28.Iozzo P, Turpeinen AK, Takala T, Oikonen V, Bergman J, Gronroos T, et al. Defective liver disposal of free fatty acids in patients with impaired glucose tolerance. J Clin Endocrinol Metab. 2004 Jul;89(7):3496–3502. doi: 10.1210/jc.2003-031142. [DOI] [PubMed] [Google Scholar]
- 29.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002 Jul;87(7):3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 30.Musso G, Gambino R, Bo S, Uberti B, Biroli G, Pagano G, et al. Should nonalcoholic fatty liver disease be included in the definition of metabolic syndrome? A cross-sectional comparison with Adult Treatment Panel III criteria in nonobese nondiabetic subjects. Diabetes Care. 2008 Mar;31(3):562–568. doi: 10.2337/dc07-1526. [DOI] [PubMed] [Google Scholar]
- 31.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008 Jan;28(1):27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 32.Schwimmer J, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the Metabolic Syndrome in Pediatric Nonalcoholic Fatty liver disease. Circulation. 2008;118:227–283. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007 Aug;133(2):496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 34.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999 Dec;30(6):1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 35.Louthan MV, Barve S, McClain CJ, Joshi-Barve S. Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease. J Pediatr. 2005 Dec;147(6):835–838. doi: 10.1016/j.jpeds.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004 Jul;40(1):46. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 37.Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, et al. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005 Jan;54(1):117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]