Abstract
Context
Prolonged calorie restriction (CR) increases lifespan in rodents. Whether prolonged CR affects biomarkers of longevity, markers of oxidative stress, and reduces metabolic rate, beyond that expected from reduced metabolic mass, has not previously been tested in humans.
Objectives
To examine the effects of 6 months of calorie restriction, with or without exercise in nonobese (25≤BMI<30) humans.
Design, Setting, and Participants
Healthy, sedentary men and women (n=48) were randomized to one of four groups for 6-mo; Control=100% of energy requirements; CR=25% diet restriction; CREX=12.5%CR+12.5% increase in energy expenditure; LCD=low calorie diet until 15% weight reduction followed by weight maintenance.
Main Outcome Measures
Body composition, dehydroepiandrosterone sulfate (DHEAS), glucose, insulin, protein carbonyls, DNA damage, 24h energy expenditure (24h-EE, metabolic chamber) and core body temperature.
Results
Weight change at M6 was -1.0(1.1)% (Control), -10.4(0.9)% (CR), -10.0(0.8)% (CREX), -13.9(0.7)% (LCD). At M6, fasting insulin was reduced from baseline in CR, CREX and LCD groups (all, p<0.01), whereas DHEAS and glucose were unchanged. Core temperature was reduced in CR by 0.2(0.05)°C and by 0.3(0.08)°C in CREX (both, p<0.05). After adjustment for changes in body composition, sedentary 24h-EE was unchanged in controls (-18(52) kcal/d; p>0.05), but decreased in CR (-135(42)kcal/d), CREX (-117(52)kcal/d) and LCD (-125(35)kcal/d, (all, p<0.008). These “metabolic adaptations” (~6% more than expected based on loss of metabolic mass) were statistically different from controls (p<0.05). DNA damage was also reduced from baseline in CR, CREX and LCD groups at M6 (p≤ 0.002).
Conclusion
These results show that two previously reported biomarkers of longevity (fasting insulin and body temperature) are reduced by prolonged CR in humans and support the theory that metabolic rate is reduced beyond the level expected for reduced metabolic body size. Studies of longer duration are now required to determine if CR attenuates the aging process in humans.
Keywords: aging, metabolic rate, oxidative damage, calorie restriction, core temperature, insulin
Introduction
Prolonged calorie restriction (CR) increases lifespan in rodents and other shorter-lived species1. Whether this occurs in longer-lived species is unknown, although the effect of prolonged CR in non-human primates is under investigation. One hypothesis to explain the anti-aging effects of CR is reduced energy expenditure (EE) with the consequent reduction in reactive oxygen species (ROS) production 2, 3. However, other metabolic effects associated with CR, including altered insulin sensitivity and signaling, altered neuroendocrine function, altered stress response, or a combination of these may retard aging 4.
Total EE is made up of resting EE (50-80% of energy), the thermic effect of feeding (~10%), and non-resting EE (10-40%) 5. Whether energy expenditure is reduced beyond the level expected for a given reduction in the size of the metabolizing mass following CR is debated. Leibel et al. 6 showed that 10% weight loss reduced sedentary 24h energy intake for weight maintenance between 15-20% in obese subjects, suggesting that metabolic adaptation is occurring in humans. However, weight loss was achieved quickly with liquid diets and except for a few normal weight subjects in that study, the effects of prolonged CR on energy expenditure in non-obese humans have not been tested. In rhesus monkeys, resting EE adjusted for fat-free mass and fat mass was lower after 11 years of CR 7. Similarly, total EE was lower in CR monkeys following 10 years of weight clamping 8. Studies in rodents have proven more controversial with reports of decreased, no change, or increased adjusted EE between CR and ad libitum fed animals 9-13.
One of the most widely accepted theories of aging is the oxidative stress theory, which states that oxidative damage produced by ROS accumulate over time leading to the development of disease such as cancer, aging, and ultimately death. ROS are byproducts of energy metabolism, with 0.2-2.0% of oxygen consumption resulting in ROS formation 15, 16. ROS attack lipids, proteins, and DNA generating a number of products that affect normal cell functioning 17. Studies in rodents demonstrate a 30% decrease in 8-oxo7,8-dihidro2’deoxyguanosine (8oxodG) in brain, skeletal muscle and heart, similar reductions in carbonyl content in brain and muscle18- 22 and exhibit transcriptional patterns that suggest decreased oxidative stress in response to CR23. However, rhesus monkeys subjected to calorie restriction exhibit divergent responses in the expression of genes involved in oxidative stress 24.
Core temperature, dehydroepiandrosterone sulfate (DHEAS) and insulin are proposed biomarkers of CR and longevity in rodents and monkeys 25. Data from the Baltimore Longitudinal Study of Aging support the association between longevity and temperature, insulin, and DHEAS with men with plasma insulin concentration or oral temperature below median and DHEAS above median living longer 26. Furthermore, in a cross-sectional study comparing individuals on self-imposed nutritionally adequate CR for 6 years to normal weight controls, Fontana et al 27. reported that CR subjects had lower serum glucose, insulin, and markers of atherosclerosis.
The aims of this study were to establish whether prolonged CR by diet alone or in conjunction with exercise can be successfully implemented in non-obese subjects and to determine the effects of the interventions on established biomarkers of CR, sedentary energy expenditure, and oxidative damage to DNA and proteins.
Methods
The Comprehensive Assessment of the Long Term Effects of Reducing Intake of Energy (CALERIE) study is a randomized clinical trial funded by the National Institute on Aging conducted at the Pennington Biomedical Research Center, Louisiana. The Internal Review Board at Pennington and an independent data safety monitoring board approved the protocol and subjects provided written informed consent. The study was conducted between August 2002 and July 2004.
Subjects
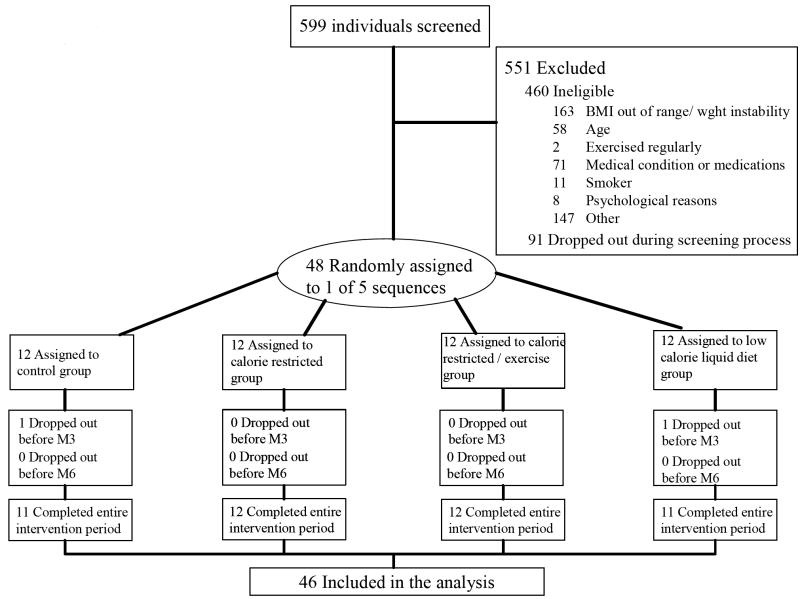
Potential participants completed 3 screening visits during which many parameters were recorded to ensure subjects were physically and psychologically healthy, including height, weight, blood pressure, electrocardiogram, chemistry 15 panel, and complete blood counts. Five hundred ninety nine individuals were screened; 551 were excluded, 460 of these were ineligible and 91 withdrew during screening (Figure 1). Race and ethnicity were self reported.
Figure 1.
Participant Flow in the Trial.
Baseline
Total energy expenditure was measured twice (two-week periods) at baseline using doubly labeled water, once while participants followed their usual diet at home (B1) and another time while being provided with a weight maintenance diet (B2). Briefly, subjects provided 2 urine samples before being dosed (2.0 g of 10% enriched H218O and 0.12 g of 99.9% enriched 2H2O per kg of estimated total body water) and additional timed samples were taken at 4.5 and 6h and 7 and 14 days after dosing. Carbon dioxide production and energy expenditure were calculated as previously described 28, 29. After B2, subjects attended a 5-day inpatient stay (M0) where numerous metabolic tests were conducted. Subjects repeated the inpatient stay at M3 and M6.
Intervention
After stratification by sex and BMI, subjects (n=48) were sequentially randomized into one of four groups for 6-months: Control (weight maintenance diet), CR = 25% calorie restriction of baseline energy requirements, CREX = 12.5% CR + 12.5% increase in energy expenditure by structured exercise, LCD = very low calorie diet until 15% reduction in body weight followed by weight maintenance diets. Except for the intervention team all personnel involved in data collection were blinded to subject information including treatment assignment.
Diets
Energy requirements at baseline were individually calculated from measured EE. Menus were then prescribed for each subject within 100 kcals of daily target intake. Menus were designed using Moore’s Extended Nutrient Database (MENu 2000, PBRC, Baton Rouge, LA) and ProNutra 3.0 (Viocare, Princeton, NJ). Participants were provided with all their food at baseline (B2) and for the first 12 weeks after randomization. Participants ate 2 meals at the centre each week-day with 1 meal plus snacks packaged for take-out. From weeks 13-22, participants self selected their diet based on individual calorie targets. From weeks 22-24 they returned to the in-feeding protocol. All diets (except LCD) were based on American Heart Association recommendations (≤ 30% fat). LCD participants were placed on 890 kcal/d (HealthOne, Health and Nutrition Technology, Carmel, CA) given as 5 shakes containing 75g protein, 110g carbohydrate, 5g of fat plus a 10g bolus of fat per day. Once target weight loss (-15%) was achieved, participants were slowly re-fed to an energy level that maintained body weight. Generally, target weight was achieved by week 8 in men and by week 11 in women.
Behavioral and Exercise Strategies
Subjects attended weekly group meetings and initiated a mid-week phone call to report energy intake so that any problems adhering to the protocol were quickly addressed. Cognitive-behavioral techniques were utilized to foster adherence to diet and exercise prescriptions including self-monitoring and stimulus control. The Health Management Resources Calorie System (HMR™, Boston, MA) was used to train participants to estimate the caloric content of food.
CREX participants increased energy expenditure by 12.5% above resting by undergoing structured exercise (walking, running, cycling) five days per week. The target energy cost was 403(63) kcal per session for women and 569(118) kcal per session for men. Individual exercise prescriptions were calculated by measuring the oxygen cost (V-Max29 Series, SensorMedics, Yorba Linda, CA) at three levels of the prescribed activity and an equation for estimating energy expenditure was generated. Mean exercise duration per session was 53(11) min in women and 45(14) min in men. Participants were required to conduct 3 sessions per week under supervision and wore portable heart rate monitors (Polar S-610, Polar Beat, Port Washington, NY) to assess compliance during unsupervised sessions.
Biochemical Analysis
Fasting serum insulin, DHEAS, thyroxine (T4) and tri-iodothyronine (T3) were measured using immunoassays (DPC 2000, Diagnostic Product Corporation, Los Angeles, CA). Glucose was analyzed using a glucose oxidase electrode (Syncron CX7, Beckman, Brea, CA). The carbonyl content in proteins was determined using a modified 2,4-dinitrophenylhydrazine (DNPH) assay according to the method of Mates et al 30.
Metabolic Tests
Weight was measured weekly in a hospital gown following a 12-h fast, after participants had voided. All other tests were conducted while participants were inpatient at M0, M3 and M6. Fasting blood samples were taken. Body composition was measured by DEXA (Hologics, QDA 4500A Bedford, MA). Sedentary EE (24h-EE) was measured over 23-hours in a whole room indirect calorimeter as previously described 31. Three meals and one snack were provided at scheduled intervals and participants were instructed to eat all their food within 30 minutes. EE was calculated from VO2, VCO2 and 24h urinary nitrogen excretion 32 and extrapolated to 24h. Sleeping EE was calculated between 02:00-05:00 am, when the motion detectors were reading zero activity. At baseline, energy intake was matched to measured energy expenditure. In keeping with the assigned protocols, controls were fed the same calories on their return visits, whereas CR subjects were fed 25% less and CREX subjects 12.5% less than measured baseline 24h-EE at M3 and M6. At M3, LCD participants were fed so energy intake was matched to measured energy expenditure. In conjunction with the metabolic chamber at baseline and M6, core temperature was measured every minute by telemetry pills (CorTemp™, HQ inc, Palmetto, FL) 33. Mean 24-h, day (8am –10:30pm) and night (2am – 5am) temperatures were computed. Due to malfunctions with the monitor or subjects passing the pill, complete data was only obtained in 7/11 controls, 11/12 CR, 8/12 CREX and 9/11 LCD subjects.
DNA Fragmentation measured by Single Cell Gel Electrophoresis (Comet assay)
The comet assay was conducted according to Deutsch et al 34. Briefly, whole blood cells were suspended in low melting point agarose on commercially available slides (Trevigen, Gaithersburg, MD). The slides were viewed under a UV microscope (Nikon Microphot FXA, Hamamatsu high resolution 512 lines, Image I AT software, FITC 3 filter). The extent of DNA damage was determined by calculating the comet tail moment, which is the integrated density in the comet tail multiplied by the distance from the center of the nucleus to the center of mass of the tail of 25 cells using freely available software (Herbert M Geller; http://www2.umdnj.edu/~geller/lab/comet-Scoring-Macro.txt). In 20 individuals measured on two consecutive days, the intraclass correlation coefficient of the method was 0.95.
Statistical Analysis
Data in text and tables are provided as means (SEM). SAS Version 9.1 was used for analysis. The change in variables from baseline at M3 and M6 were analyzed by repeated measures with treatment and time interactions and baseline values included as covariates. Linear regression at baseline (n=48) was used to generate equations for predicting EE and the predicted values were generated using the equation with measured FFM. Differences between predicted and measured EE were calculated and analyzed by ANOVA as described above. To reinforce our conclusion, a similar approach was used to generate predictive equations from a reference population of 865 non diabetic individuals measured in a similar metabolic chamber 35. A normalizing and variance-stabilizing logarithmic transformation was applied to the calculated tail moments for the comet assay. The target samples size of 48 provided the necessary power to detect a 15% reduction in the primary endpoint, sedentary energy expenditure.
Results
Two individuals withdrew prior to completion of the study, one from the control group at week 4 (personal reasons) and one from the LCD group at week 5 (lost to follow up). Subjects were provided monetary compensation during and upon completion of the study. This, along with frequent contact with the interventionist facilitated the excellent retention rate.
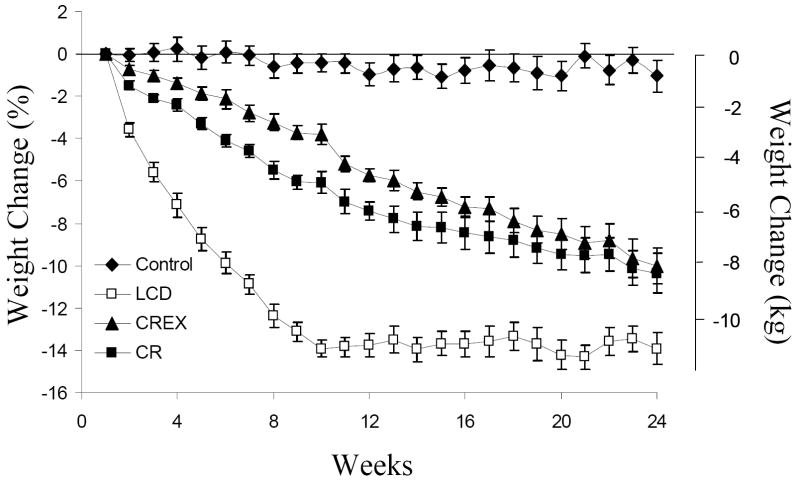
Baseline characteristics of subjects by sex are described in Table 1. Weight loss at M6 by group was −1.0(1.1) % (C), −10.4(0.9)% (CR), -10.0(0.8)% (CREX), -13.9(0.7)% (LCD) of initial body weight (Figure 1). Fat mass (FM) was significantly reduced in all 3 intervention groups as compared to baseline and the controls at M3 and M6 (M6; CR - 24(3)%, CREX -25(3)%, LCD -32(3)%, p<0.001). FFM was significantly reduced in CR - 5(1)%, CREX -3(1)% and LCD -6(1)% groups as compared to baseline and controls at M6 (all, p<0.001).
Table 1.
Screening characteristics of subjects completing the study (n = 46).
| Male (n = 20) | Female (n = 26) | |
|---|---|---|
| Race (C/AA/O) | 14 / 5 / 1 | 16 / 10 / 0 |
| Age (y) | 37 (2) [26 – 48] | 37 (1) [26 –44] |
| Weight (kg) | 88.6 (1.3) [77.2 – 103.0] | 75.0 (1.0) [58.1 – 90.5] |
| BMI (kg./m2) | 27.8 (0.2) [25.7 – 29.8] | 27.2 (0.2) [25.0 – 29.9] |
| Body Fat (%) | 24.7 (0.7) [16.5 – 31.0] | 37.2 (0.8) [29.1 – 46.2] |
Values are given as Means (SEM) [Range].
C= Caucasian, AA = African-American, O = Other
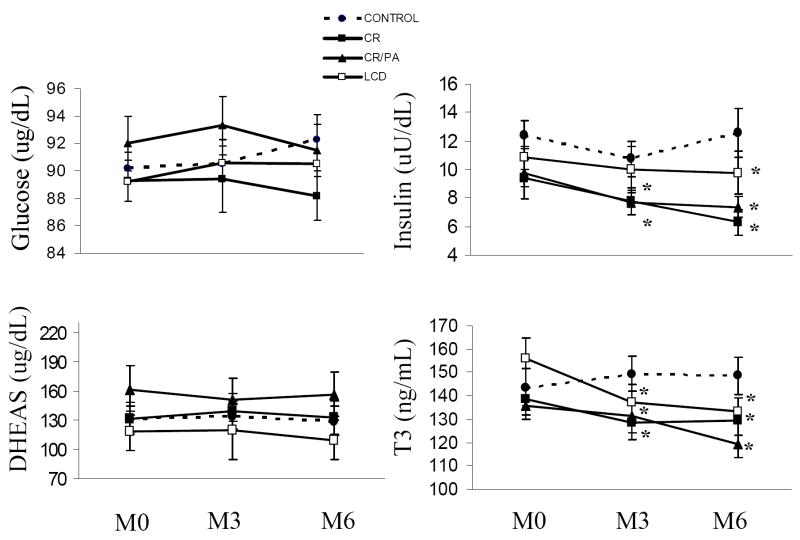
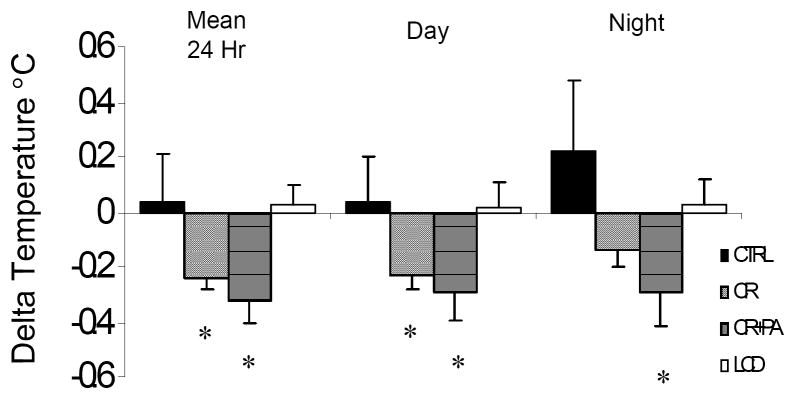
Fasting insulin was significantly reduced from baseline at M3 and M6 in CR and CREX groups (both, p<0.01; Figure 2) and at M6 in all groups (all, p<0.01, Figure 2). Fasting glucose and DHEAS were not changed in any group. Subjects randomized to CR (- 0.2(0.05)°C) and CREX (-0.3(0.08) °C) had reduced mean 24-h core body temperature (both, p<0.05, Figure 3). There was no change in core temperature in control or LCD groups.
Figure 2.
Percentage weight loss by group. Initial weight was recorded as the mean of 5 weights measured weekly during the baseline phase. The change in weight over time was significantly different between the control group and the three intervention groups (p<0.001) and between LCD and CR, CREX groups (p<0.001), but percent weight loss at Week 24 was not significantly different between LCD, CR and CREX groups.
Footnote: CR = calorie restriction, CREX = calorie restriction plus exercise, LCD = liquid calorie diet.
Figure 3.
Fasting plasma glucose, insulin, dehydroepiandrosterone sulphate (DHEAS), and triiodo-thyronine (T3) at baseline, Month 3 and Month 6.
Footnote: CR = calorie restriction, CREX = calorie restriction plus exercise, LCD = liquid calorie diet.
SI conversion factors: to convert glucose to mmol/L, multiply by 0.0555: insulin to pmol/L, multiply by 6.945; DHEAS to nmol/L, multply by 3.47; and T3 to nmol/L, multiply by 0.0154.
* Statistically different from baseline p<0.05.
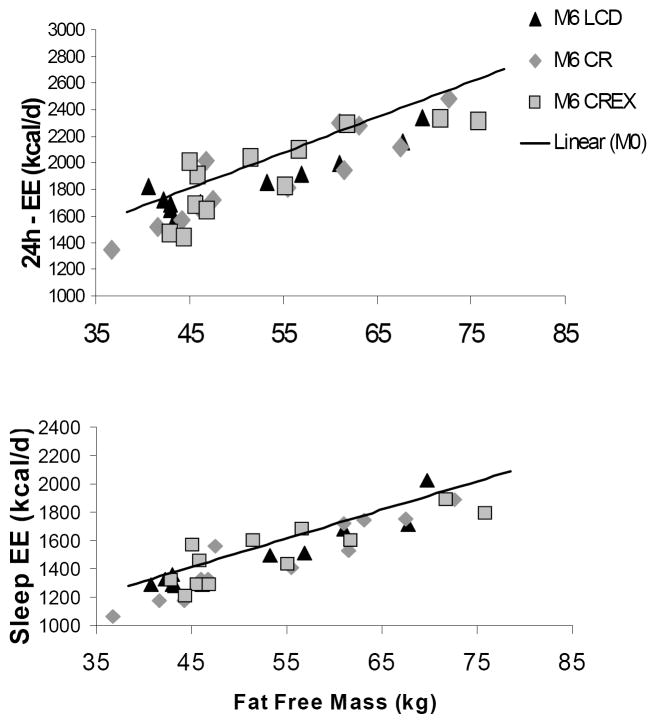
Absolute 24-h EE and sleeping EE were significantly reduced from baseline in CR, CREX and LCD groups (all, p<0.001, Table 2). At baseline, FFM accounted for 86% of the variance in sedentary 24h-EE [24h-EE (kcal/d) = 596 + 26.8 * FFM, r2 = 0.86, p<0.001], whereas FM, age and sex did not statistically account for any additional variance. Compared to predicted values from this equation, measured daily 24h-EE at M3 and M6 were unchanged in controls and reduced in CR, CREX, LCD (Table 2). Individual data points at M6 and the baseline regression line for 24h-EE vs. FFM are presented in Figure 4. When subjects from the three intervention groups were pooled, adjusted 24-h EE values were statistically lower than controls at M3 and M6 (p < 0.05). Since the above equations were generated in only 48 subjects, we also compared the 24h-EE data from each group to 865 individuals measured in a similar metabolic chamber at NIDDK in Phoenix 35. Importantly, 24h-EE was not different between the reference population and the CR, CREX or LCD groups at baseline or at any time point in the controls. However, adjusted 24h-EE was significantly lower at M3 and M6 in CR, CREX and LCD (all, p<0.01). Similar to 24h-EE, measured sleeping EE was lower than predicted at M3 and M6 in CR and CREX groups (Table 2 and Figure 4). There were no significant changes from baseline in the level of spontaneous physical activity and in the thermic effect of food expressed as percentage of energy intake.
Table 2.
Absolute energy expenditures (24-h sedentary and sleeping) measured in a metabolic chamber at M0, M3 and M6. The measured – predicted values for 24h-EE and Sleep-EE are calculated as the difference between the measured and the predicted values*.
| Actual 24h-EE | Predicted 24h-EE* | P | Sleep-EE | Predicted sleep-EE** | P | |
|---|---|---|---|---|---|---|
| Control | ||||||
| - M0 | 2129 (102) | 2110 (80) | - | 1654 (69) | 1642 (60) | - |
| - M3 | 2119 (109) | 2118 (84) | >0.05 | 1642 (92) | 1698 (63) | >0.05 |
| - M6 | 2092 (97) | 2110 (84) | >0.05 | 1513 (37) | 1642 (63) | >0.05 |
| CR | ||||||
| - M0 | 2079 (102) | 2100 (95) | - | 1600 (88) | 1635 (72) | - |
| - M3 | 1900 (101) | 2048 (91) | 0.001 | 1472 (75) | 1595 (69) | 0.0001 |
| - M6 | 1899 (101) | 2034 (88) | 0.002 | 1473 (77) | 1585 (66) | 0.001 |
| CREX | ||||||
| - M0 | 2106 (102) | 2085 (93) | - | 1615 (78) | 1623 (70) | - |
| - M3 | 1972 (101) | 2057 (89) | 0.04 | 1524 (76) | 1602 (67) | 0.02 |
| - M6 | 1917 (91) | 2034 (86) | 0.008 | 1511 (62) | 1585 (65) | 0.03 |
| LCD | ||||||
| - M0 | 2085 (90) | 2055 (92) | - | 1658 (78) | 1600 (69) | - |
| - M3 | 1842 (60) | 1965 (82) | 0.007 | 1489 (54) | 1533 (62) | >0.05 |
| - M6 | 1852 (71) | 1977 (87) | 0.006 | 1479 (73) | 1542 (66) | >0.05 |
Means (SEM). Predicted EE were calculated as *24h-EE = 596 + 26.8*FFM (r2 = 0.86, p<0.001) and **Sleep-EE = 501 + 20.2 * FFM (r2 = 0.76, p<0.001) generated from the 48 subjects measured at baseline.
Figure 4.
Change in core body temperature from baseline to M6 measured over 23h inside a metabolic chamber set to 22.2 (0.2)°C.
Footnote: Values are in 7/11 controls, 11/12 CR subjects, 8/12 CREX subjects and 9/11 LCD subjects. Mean total temperature, mean day temperature (8am – 10:30pm), and night temperature (2am – 5am). * Statistically different from baseline p< 0.05.
Plasma T3 was reduced from baseline in the CR (-10.2 ng/dL (0.15 nmol/L)) and LCD (-18.9ng/dL (0.29nmol/L)) groups at M3 (both, p<0.01) and in CR (-8.9ng/dL (0.13nmol/L)), CREX (-4.52ng/dL (0.07nmol/L)) and LCD (-23.24ng/dL (0.36nmol/L)) groups at M6 (all, p<0.02). A significant treatment effect for plasma T3 (p=0.001; Figure 2) with only a tendency for a time effect (p=0.07) was observed. Similar results were found for change in plasma T4 in response to treatment (p<0.05). When the subjects in the three treatment groups were combined, we observed significant linear relationships between the change in plasma thyroid hormones and deviations in measured 24h-EE from predicted values at M3 only (T3; r=0.40, p=0.006 and T4; r=0.29, p=0.05).
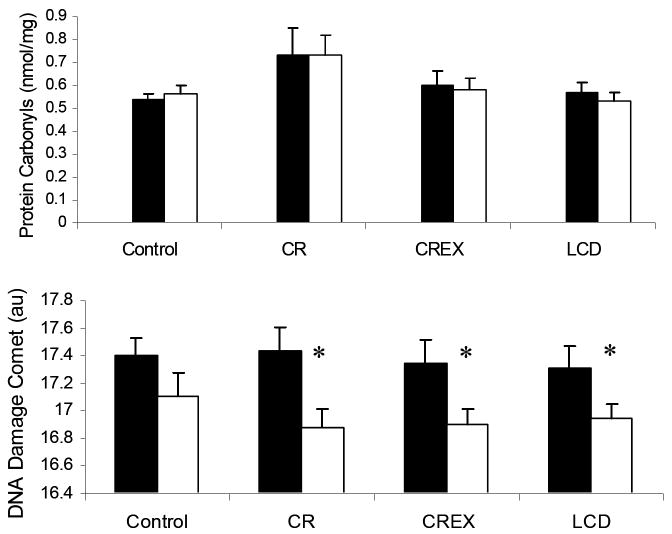
Serum protein carbonyl concentrations were not changed from baseline to M6 in any group (Figure 5). DNA damage was reduced from baseline in CR (-0.56 (0.11)au), CREX (-0.45 (0.12)au) and LCD (-0.35 (0.12)au) groups at M6 (all, p<0.005), but not in the controls (Figure 5). This decrease was not statistically different to controls when the three treatment groups were combined. We found no significant relationships between the changes in DNA damage and the changes in adjusted EE, FM, or body weight.
Figure 5.
Correlation between: Top panel: measured 24h-EE and fat-free mass. [24h-EE (kcal/d) = 596 + 26.8 * FFM, r2 = 0.86, p<0.001]; Bottom panel: measured sleep-EE and fat-free mass. EE [sleeping EE = 501 + 20.2 * FFM, r2 = 0.76, p<0.001]. Fat Free mass was the major determinant of sleep-EE.
Footnote: EE = energy expenditure, CR = calorie restriction, CREX = calorie restriction plus exercise, LCD = liquid calorie diet. Regression lines are drawn at baseline in all subjects (n=48) with individual’s values at M6 in CR, CREX and LCD groups.
Discussion
Since the pioneering experiments by McCay et al. 36, it has been known that calorie restriction (CR) extends lifespan in rodents and other lower species. However, little is known about the long-term effects of CR in humans. In the present study, we examined the effects of 6-months CR on biomarkers of CR, energy expenditure, and oxidative stress in humans. Our results indicate that prolonged CR caused: 1) a reversal of two out of three previously reported robust biomarkers of longevity (fasting insulin and core body temperature); 2) a metabolic adaptation (decrease in EE larger than expected on the basis of loss of metabolic mass) associated with lower thyroid hormone concentrations and 3) a reduction in DNA fragmentation as a result of less damage to DNA.
Numerous biomarkers of CR have been identified in rodents including temperature, DHEAS, glucose, and insulin. Roth et al. 26 recently observed that body temperature, insulin, and DHEAS were also altered in CR monkeys, validating their usefulness as biomarkers in longer lived species. Importantly, they also showed that these parameters were altered in longer-lived men. These findings support the role of these factors as biomarkers of longevity in humans. Similar to the primate model, we observed significantly reduced fasting insulin and body core temperature in CR and CREX groups. However, DHEAS and fasting glucose were unchanged by intervention. Most likely, this study was of insufficient duration to detect changes in DHEAS, which have been calculated to fall 2-4% per year in humans. Fasting glucose is not consistently altered by prolonged CR in primates and thus we question whether fasting glucose is useful as a biomarker in longer-lived species. On the other hand, Fontana et al. 27 observed that fasting glucose and insulin were substantially reduced in CR subjects who had been following self-prescribed nutritionally adequate CR diets for 6 years.
Previous studies are inconclusive regarding whether metabolic rate is reduced following prolonged CR. In rodents, adjusted resting EE was not different from controls after restricting energy intake for six months 11 or for the entire life span 12. In monkeys, adjusted resting EE was reduced by 60kcal/d after 11 years of CR 7, but previously these authors had reported no metabolic adaptation after 42 months 38. Indeed, there are numerous reports in the literature showing either reduced or unchanged adjusted EE after prolonged CR in monkeys 8, 25. In humans, the effects of prolonged, nutrient dense, calorie restricted diets on non-obese subjects have not been formally investigated. In the Keys starvation study 39, adjusted resting EE was decreased and this coincided with a reduction in temperature indicating a real metabolic adaptation 40. Adjusted 24h-EE was also lower in 5 subjects after 2-years CR in the Biosphere 2 experiment as compared to 152 control subjects 41. However, resting-EE was not altered in overweight women following liquid calorie diet to normal body weight and 10 days of weight stabilization 42. In this study, we observed a metabolic adaptation over 24-hour in sedentary conditions and during sleep following 6-months of CR. The metabolic adaptation in the CREX group was similar to that observed in CR group, suggesting that energy deficit rather than CR itself is driving the decrease in energy expenditure. Importantly, the metabolic adaptations were closely paralleled by a drop in thyroid hormone plasma concentrations confirming the importance of the thyroid pathway as a determinant of energy metabolism43. Of significance, the metabolic adaptation occurred in the first 3-months after intervention with no further adaptation at 6 months, even though weight loss continued in CR and CREX groups. Metabolic adaptation was also observed over 24 hours but not during sleep in LCD subjects who were weight stable when measured at M3 and M6. Possible explanations for the lack of significant adaptation during sleep in this group include a smaller sample size and that two men were regaining weight at M6. Interestingly, core temperature and fasting insulin at M3 were also not changed in this group, despite the largest amount of weight loss. Whether metabolic adaptation persists during weight loss maintenance remains to be determined in humans. Spontaneous physical activity and the thermic effect of food were not changed from baseline. However, even if these two factors can account for some of the metabolic adaptation, it should be remembered that the thermic effect of food account for only 10% of daily energy expenditure 44 and the cost of activity is already accounted for by a decrease in body weight. Therefore, these 2 factors can only account for a minor part of the metabolic adaptation.
The inverse relationship between increased free radical production, oxidative damage to DNA and maximum lifespan has been demonstrated in numerous studies 45, 46. Caloric restriction in mice down regulates genes involved in oxidative stress and reduces oxidative damage (8oxodG), lipid peroxidation and protein carbonyls 18, 20, 21, 23. In non-human primates, genes involved in protection against oxidative stress are not altered by CR, although protein carbonylation is reduced 22. In obese humans, protein carbonylation is also reduced after 4 weeks of CR 47. Whilst we observed no change in protein carbonylation, we are the first to report a significant decline in DNA damage following six months of CR in nonobese humans. Contrary to our hypothesis, the reduction in DNA damage was not associated with reduced total or adjusted O2 consumption in the metabolic chamber. Considering the lack of correlation between these parameters and the lack of response in protein carbonylation in response to CR, we are hesitant to conclude that CR reduces oxidative stress overall. Clearly, more studies investigating different measures of oxidative stress, such as 24-h urinary samples of 8oxodG are required. Furthermore, other factors (such as mitochondrial function) may play an important role in the accumulation of oxidative stress. Indeed, the importance of uncoupling proteins in protection against ROS production, independent of changes in proton kinetics and mitochondrial respiration has recently been demonstrated48.
The results of this study show that prolonged CR by diet or by a combination of diet and exercise was successfully implemented as evidenced by reduced weight, fat mass, fasting serum insulin and body core temperature. This study is unique in that individual energy requirements were carefully measured at baseline allowing us to feed and prescribe individual diet goals for each subject. Furthermore, we observed that “metabolic adaptation” develops in response to energy deficit in non-obese humans at 3 and 6 months leading to reduced oxygen consumption per unit of fat-free mass, even after weight stability is achieved. Finally, this study confirms previous findings that calorie restriction results in a decline in DNA damage. However, longer studies are required to determine if these effects are sustained and impact the aging process.
Figure 6.
Fasting plasma protein carbonyls and DNA Damage measured by the Comet assay. *Statistically different from baseline p<0.005.
Acknowledgments
The authors want to thank the remaining members of Pennington CALERIE Research Team including: Steven Anton, Emily York-Crowe, Catherine Champagne, Paula Geiselman, Michael Lefevre, Jennifer Howard, Jana Ihrig, Brenda Dahmer, Anthony Alfonso, Darlene Marquis, Connie Murla, Aimee Stewart, Amanda Broussard and Vanessa Tarver. Our gratitude is extended to the excellent staffs of the Outpatient Clinic, Inpatient Clinic, Metabolic Kitchen and Clinical Chemistry Laboratory. We also want to thank Claudia Van Skiver for developing the behavioral treatment manual and training the staff on how to use the HMR™ energy counting system.
Our thanks also go to Health and Nutrition Technology, Carmel, CA for providing us with the HealthOne formula used in the study.
Finally, our profound gratitude goes to all the volunteers who spent so much time in participating in this very demanding research study.
This work was supported by grant U01 AG20478
Abbreviations
- CR
calorie restriction
- FFM
fat-free mass
- DHEAS
dehydroepiandrosterone sulfate
- EE
energy expenditure
Literature Cited
- 1.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986 Apr;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 2.Roth GS, Ingram DK, Black A, Lane MA. Effects of reduced energy intake on the biology of aging: the primate model. Eur J Clin Nutr. 2000 Jun;54(Suppl 3):S15–20. doi: 10.1038/sj.ejcn.1601020. [DOI] [PubMed] [Google Scholar]
- 3.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004 Sep 3;305(5689):1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 4.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003 Sep;78(3):361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 5.Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989 May;49(5 Suppl):968–975. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- 6.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995 Mar 9;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 7.Blanc S, Schoeller D, Kemnitz J, et al. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003 Jan;88(1):16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- 8.DeLany JP, Hansen BC, Bodkin NL, Hannah J, Bray GA. Long-term calorie restriction reduces energy expenditure in aging monkeys. J Gerontol A Biol Sci Med Sci. 1999 Jan;54(1):B5–11. doi: 10.1093/gerona/54.1.b5. discussion B12-13. [DOI] [PubMed] [Google Scholar]
- 9.Ballor DL. Effect of dietary restriction and/or exercise on 23-h metabolic rate and body composition in female rats. J Appl Physiol. 1991 Sep;71(3):801–806. doi: 10.1152/jappl.1991.71.3.801. [DOI] [PubMed] [Google Scholar]
- 10.Dulloo AG, Girardier L. 24 hour energy expenditure several months after weight loss in the underfed rat: evidence for a chronic increase in whole-body metabolic efficiency. Int J Obes Relat Metab Disord. 1993 Feb;17(2):115–123. [PubMed] [Google Scholar]
- 11.McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol. 1985 Apr;248(4 Pt 1):E488–490. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- 12.McCarter RJ, Palmer J. Energy metabolism and aging: a lifelong study of Fischer 344 rats. Am J Physiol. 1992 Sep;263(3 Pt 1):E448–452. doi: 10.1152/ajpendo.1992.263.3.E448. [DOI] [PubMed] [Google Scholar]
- 13.Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech Ageing Dev. 2005 Jun-Jul;126(67):783–793. doi: 10.1016/j.mad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Harman D. Aging: A theory based on free radical radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 16.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002 Nov 22;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell BaJMCG. Free Radicals in Biology and Medicine. New York: Oxford University Press; 1999. [Google Scholar]
- 18.de Oliveira SL, Diniz DB, Amaya-Farfan J. Carbohydrate-energy restriction may protect the rat brain against oxidative damage and improve physical performance. Br J Nutr. 2003 Jan;89(1):89–96. doi: 10.1079/BJN2002749. [DOI] [PubMed] [Google Scholar]
- 19.Drew B, Phaneuf S, Dirks A, et al. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003 Feb;284(2):R474–480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- 20.Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996 Sep 1;333(1):189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- 21.Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994 Oct 20;76(23):215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 22.Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. Faseb J. 2000 Sep;14(12):1825–1836. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]
- 23.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999 Aug 27;285(5432):1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 24.Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5093–5098. doi: 10.1073/pnas.081061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane MA, Baer DJ, Tilmont EM, et al. Energy balance in rhesus monkeys (Macaca mulatta) subjected to long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 1995 Sep;50(5):B295–302. doi: 10.1093/gerona/50a.5.b295. [DOI] [PubMed] [Google Scholar]
- 26.Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002 Aug 2;297(5582):811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 27.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA. Field use of D2 18O to measure energy expenditure of soldiers at different energy intakes. J Appl Physiol. 1989 Nov;67(5):1922–1929. doi: 10.1152/jappl.1989.67.5.1922. [DOI] [PubMed] [Google Scholar]
- 29.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988 Nov;118(11):1278–1289. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 30.Mates JM, Perez-Gomez C, Olalla L, Segura JM, Blanca M. Allergy to drugs: antioxidant enzymic activities, lipid peroxidation and protein oxidative damage in human blood. Cell Biochem Funct. 2000 Jun;18(2):77–84. doi: 10.1002/(SICI)1099-0844(200006)18:2<77::AID-CBF851>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput. 2003 Sep;41(5):572–578. doi: 10.1007/BF02345320. [DOI] [PubMed] [Google Scholar]
- 32.Acheson KJ, Schutz Y, Bessard T, Flatt JP, Jequier E. Carbohydrate metabolism and de novo lipogenesis in human obesity. Am J Clin Nutr. 1987 Jan;45(1):78–85. doi: 10.1093/ajcn/45.1.78. [DOI] [PubMed] [Google Scholar]
- 33.Rising R, Fontvieille AM, Larson DE, Spraul M, Bogardus C, Ravussin E. Racial difference in body core temperature between Pima Indian and Caucasian men. Int J Obes Relat Metab Disord. 1995 Jan;19(1):1–5. [PubMed] [Google Scholar]
- 34.Deutsch WA, Kukreja A, Shane B, Hegde V. Phenobarbital, oxazepam and Wyeth 14,643 cause DNA damage as measured by the Comet assay. Mutagenesis. 2001 Sep;16(5):439–442. doi: 10.1093/mutage/16.5.439. [DOI] [PubMed] [Google Scholar]
- 35.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes Relat Metab Disord. 1999 Jul;23(7):715–722. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 36.McCay CCM, Maynard LA. The effect of retarded growth upon the length of the lifespan and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 37.Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001 Oct;281(4):E757–765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 38.Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med. 2000 Nov 15;29(10):946–968. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 39.Keys A, Brozek J, Henschel A, Michelson O, Taylor H. The Biology of Human Starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 40.Rising R, Keys A, Ravussin E, Bogardus C. Concomitant interindividual variation in body temperature and metabolic rate. Am J Physiol. 1992 Oct;263(4 Pt 1):E730–734. doi: 10.1152/ajpendo.1992.263.4.E730. [DOI] [PubMed] [Google Scholar]
- 41.Weyer C, Walford RL, Harper IT, et al. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr. 2000 Oct;72(4):946–953. doi: 10.1093/ajcn/72.4.946. [DOI] [PubMed] [Google Scholar]
- 42.Weinsier RL, Hunter GR, Zuckerman PA, et al. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr. 2000 May;71(5):1138–1146. doi: 10.1093/ajcn/71.5.1138. [DOI] [PubMed] [Google Scholar]
- 43.Toubro S, Sorensen TI, Ronn B, Christensen NJ, Astrup A. Twenty-four-hour energy expenditure: the role of body composition, thyroid status, sympathetic activity, and family membership. J Clin Endocrinol Metab. 1996 Jul;81(7):2670–2674. doi: 10.1210/jcem.81.7.8675595. [DOI] [PubMed] [Google Scholar]
- 44.Tataranni PA, Larson DE, Snitker S, Ravussin E. Thermic effect of food in humans: methods and results from use of a respiratory chamber. Am J Clin Nutr. 1995 May;61(5):1013–1019. doi: 10.1093/ajcn/61.4.1013. [DOI] [PubMed] [Google Scholar]
- 45.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996 Jul 5;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sohal RS, Svensson I, Brunk UT. Hydrogen peroxide production by liver mitochondria in different species. Mech Ageing Dev. 1990 Apr 30;53(3):209–215. doi: 10.1016/0047-6374(90)90039-i. [DOI] [PubMed] [Google Scholar]
- 47.Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001 Jan;86(1):355–362. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 48.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005 Sep;289(3):E429–438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]