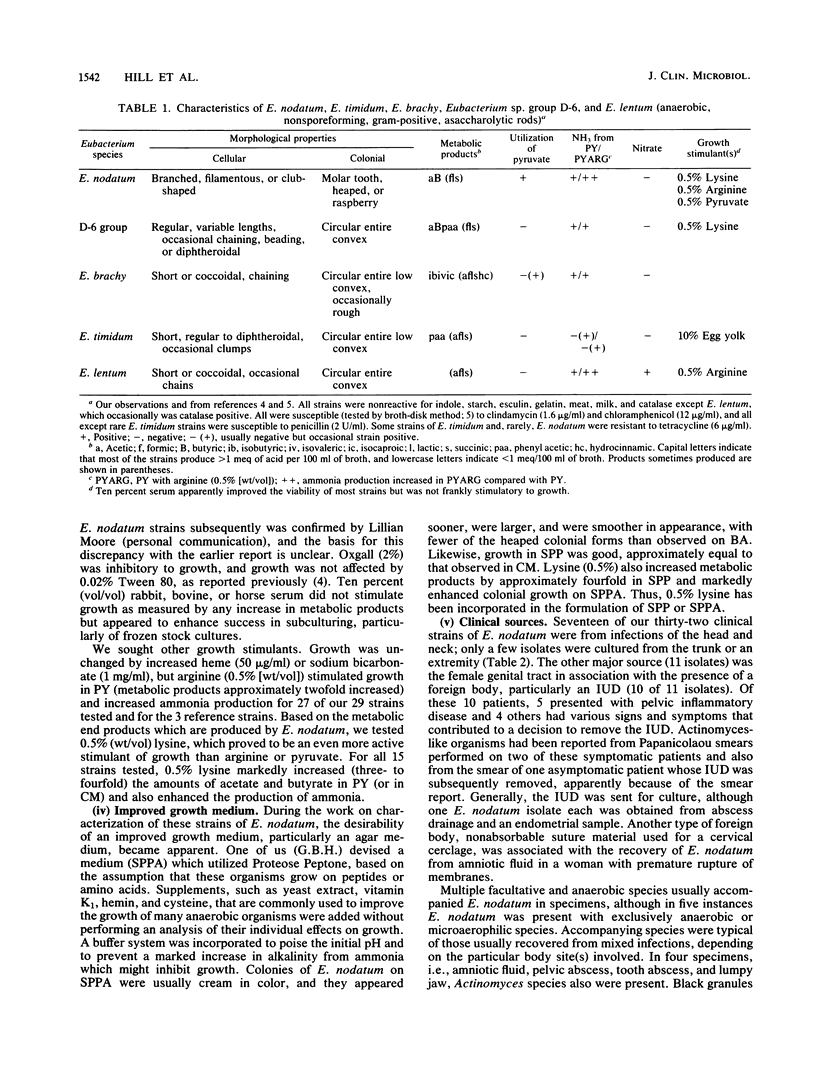
Abstract
Three new species, Eubacterium nodatum, Eubacterium timidum, and Eubacterium brachy, were described, primarily from subgingival samples taken from patients with moderate and severe adult periodontitis. Except for the isolation of E. brachy from a pleuropulmonary infection, these species have not been reported from other infected body sites. We report on the isolation of these species and an undescribed group (D-6) of asaccharolytic eubacteria also found in periodontal disease from numerous different sites of infection, mostly the head and neck. A similarity in cellular morphological properties of E. nodatum and Actinomyces sp. was noted previously. Additional similarities, particularly to Actinomyces israelii, that we found are the formation of molar tooth colonies and the isolation from cases of lumpy jaw and from the genital tract of women in association with the use of an intrauterine contraceptive device. E. timidum and E. brachy did not occur more often from any particular site outside of the head, neck, and respiratory tract. The group D-6 strains came from a variety of sites in the trunk and pelvis. These species are all obligately anaerobic, asaccharolytic, and generally nonreactive, and they grow poorly and slowly on media commonly used to isolate anaerobic bacteria. L-Lysine (0.5%) markedly stimulated the growth of E. nodatum and, to a lesser extent, another acetate- and butyrate-producing group, Eubacterium sp. group D-6, but we did not find comparable stimulants for the other species. We found the production of phenyl acetate to be a helpful marker in the identification of E. timidum and Eubacterium sp. group D-6. Although the isolation and identification of most of these species remain somewhat difficult, the evidence from dental infections and the present report suggests that these species are potential pathogens that are likely to be overlooked in infected clinical material without special attention to more prolonged incubation and use of enriched isolation media.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gupta P. K., Hollander D. H., Frost J. K. Actinomycetes in cervico-vaginal smears: an association with IUD usage. Acta Cytol. 1976 Jul-Aug;20(4):295–297. [PubMed] [Google Scholar]
- Hill G. B., Eschenbach D. A., Holmes K. K. Bacteriology of the vagina. Scand J Urol Nephrol Suppl. 1984;86:23–39. [PubMed] [Google Scholar]
- Jones M. C., Buschmann B. O., Dowling E. A., Pollock H. M. The prevalence of actinomycetes-like organisms found in cervicovaginal smears of 300 IUD wearers. Acta Cytol. 1979 Jul-Aug;23(4):282–286. [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Smibert R. M., Burmeister J. A., Palcanis K. G., Ranney R. R. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985 May;48(2):507–519. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson E., Holmberg K. A longitudinal study of Actinomyces israelii in the female genital tract. Acta Obstet Gynecol Scand. 1984;63(3):207–216. doi: 10.3109/00016348409155498. [DOI] [PubMed] [Google Scholar]
- Pine L., Malcolm G. B., Curtis E. M., Brown J. M. Demonstration of Actinomyces and Arachnia species in cervicovaginal smears by direct staining with species-specific fluorescent-antibody conjugate. J Clin Microbiol. 1981 Jan;13(1):15–21. doi: 10.1128/jcm.13.1.15-21.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford J. C. Pleuropulmonary infection associated with Eubacterium brachy, a new species of Eubacterium. J Clin Microbiol. 1980 Nov;12(5):722–723. doi: 10.1128/jcm.12.5.722-723.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry J. F., Wilkins T. D. Arginine, a growth-limiting factor for Eubacterium lentum. J Bacteriol. 1976 Aug;127(2):780–784. doi: 10.1128/jb.127.2.780-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann W. R., Hill G. B. Modified extraction procedure for gas-liquid chromatography applied to the identification of anaerobic bacteria. J Clin Microbiol. 1986 Feb;23(2):392–394. doi: 10.1128/jcm.23.2.392-394.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


