Abstract
The DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) is a cardinal defense against the mutagenic and carcinogenic effects of alkylating agents. We have reported evidence that absence of detectable MGMT activity (MGMT− phenotype) in human brain is a predisposing factor for primary brain tumors that affects ca. 12% of individuals [J.R. Silber, et. al. Proc. Natl. Acad. Sci. USA 93 (1996) 6941–6946]. We report here that MGMT− phenotype in the brain of children and adults, and the apparent increase in risk of neurocarcinogenesis, may arise during gestation. We found that MGMT activity in 71 brain specimens at 6 to 19 weeks post-conception was positively correlated with gestational age (P ≤ 0.0015). Moreover, the proportion of specimens exhibiting MGMT− phenotype (MGMT content < 0.42 fmol/106 cells or 255 molecules/cell) declined progressively from 76% (16/21) at 6 to 8 weeks to 13% (1/8) at 15 to 19 weeks. All liver specimens that accompanied MGMT− brain (15/15) had measurable MGMT activity, demonstrating that the phenotype was not systemic in these cases. In contrast to MGMT, apurinic endonuclease, DNA polymerase ϐ and lactate dehydrogenase activities were found in every brain extract assayed, and showed no significant relationship with gestational age. The observed gestational pattern has at least two implications for neurocarcinogenesis. (1) Early in development, brain tissue that has MGMT− phenotype and is rapidly proliferating may be especially vulnerable to alkylation-induced mutations, including mutations that lead to brain tumors. (2) Persistence of prenatal MGMT deficiency into postnatal life in a sub-population of individuals may increase brain tumor risk. Our findings provide possible mechanistic insight into epidemiologic data associating maternal alkylating agent exposure with brain tumor incidence.
1. Introduction
Alkylating agents, particularly N-nitroso compounds, are potent neurocarcinogens in certain rodent strains and primate species [1–3]. Transplacental exposure can be especially damaging. For example, a single dose of ethylnitrosourea to pregnant rats, representing ca. 25% of the LD50 for adults, resulted in a 98–100% incidence of neurogenic tumors in offspring [reviewed in 1,2]. Central nervous system tumors were also frequent in the offspring of patas and rhesus monkeys exposed to ethylnitrosourea early in gestation [3]. Methyl- and ethylnitrosourea-induced tumors of rodent brain are associated with persistent O6-alkylguanine adducts in DNA [4–6], and administration of N-nitrosodimethylamine to pregnant patas monkeys generates O6-methylguanine in fetal brain DNA [7]. O6-alkylguanine adducts are highly genotoxic. These lesions are mutagenic, producing predominantly GC to AT transitions [8,9]. They are also clastogenic and recombinogenic, promoting sister chromatid exchange, chromosome deletions and rearrangements [reviewed in 9]. Moreover, specific O6-alkylguanine adducts in particular genes are believed to be causally related to induction of cancer in animal models [10,11].
The brain of rodents [12] has low levels of O6-methylguanine-DNA methyltransferase (MGMT), a DNA repair protein that removes alkyl adducts from the O6 atom of guanine [8]. MGMT repairs DNA alkylation damage by transferring a single alkyl group from O6-methylguanine to an internal cysteine in a “suicide” reaction that irreversibly inactivates the protein. Methyl groups are the preferred substrate, though larger alkyl groups are removed at slower rates. MGMT is a crucial defense against mutagenesis, as evidenced by bacteria, yeast, and mammalian cells that are deficient in MGMT and display increased rates of spontaneous and alkylating agent-induced mutation [8–11,13]. MGMT also protects against alkylation-induced carcinogenesis, as demonstrated in transgenic mice. Expression of exogenous MGMT in mouse tissues decreases tumor formation induced by alkylators [10,11], and conversely, deletion of the MGMT gene is accompanied by increased methylnitrosourea-induced tumor formation [10,11,14,15].
Emerging evidence indicates that suppression of MGMT expression is associated with human carcinogenesis [14,15]. In the case of brain, MGMT content is relatively low [15], and we have observed that a substantial fraction of specimens contains no measurable activity [15]. We have also observed that MGMT− phenotype in brain is associated with gliomas [16], the most common primary brain tumor. We found that histologically normal brain adjacent to tumors displayed MGMT− phenotype in over half of cases (64/117 or 55%), whereas brain tissue from control individuals without brain tumors exhibited MGMT− phenotype in a minority of cases (5/43 or 12%). This 4.6-fold difference in frequency was highly significant (P ≤ 0.001), indicating that MGMT− phenotype may be a risk factor for human neurocarcinogenesis. The incidence of MGMT− phenotype in normal brain was independent of tumor grade and histology [17], suggesting that inability to remove O6-alkylguanine adducts promotes the genesis of glial tumors of all major diagnostic types. Few exogenous risk factors for gliomas have been identified [18,19], suggesting that endogenous factors such as MGMT− phenotype may be especially important.
We report here that 50% of human embryonic (< 9 weeks post-fertilization [20]) and early fetal brain specimens we examined exhibited MGMT− phenotype. We also report that the proportion of brain specimens with MGMT− phenotype declined progressively during the initial half of gestation, reaching the fraction observed in postnatal brain [16]. This developmental pattern suggests that MGMT− phenotype in the brain of children and adults, together with a concomitant increase in brain tumor risk [16], may originate in utero. The deficiency of MGMT activity during gestation has potential significance for prevention via reduction of maternal alkylating agent exposure [19], and for the time of occurrence of mutations that culminate in brain tumors.
2. Materials and Methods
2.1 Tissue
Fresh tissue obtained in accord with an IRB-approved protocol was provided by the Central Laboratory for Human Embryology, Department of Pediatrics, University of Washington. Brain and liver were transported to our laboratory in cold DMEM/F12 supplemented with 10% fetal bovine serum. Specimens were washed with PBS, and flash-frozen in multiple aliquots. To determine cell number, a small piece of fresh tissue was suspended in PBS and completely disrupted by serial passage through 18, 20 and 22 gauge needles. The cell suspension was stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, and total cell number determined by counting using a hemacytometer. Preparation of extracts (i.e., high speed supernatants of whole tissue sonicates) was carried out as previously described [16].
2.2 MGMT
MGMT activity was measured in a standard biochemical assay that quantitates transfer of radioactivity from a DNA substrate containing [methyl3H]O6-methylguanine to protein, as we have previously described in detail [16,17,21]. We have used this procedure to quantitate MGMT in extracts of human brain tumors [17,21,22] and normal brain [16,17,21], and to document the association between brain tumors and MGMT− phenotype in adjacent, histologically normal brain [17,21,22]. All activities reported here represent the mean of at least 5 determinations that generally differed by no more than 20%, and are validated by numerous controls [17,21]. As in previous reports [16,17,21], every MGMT+ specimen displayed linearity of activity with added extract.
MGMT− phenotype is a functional term that refers to the limit of detection in the particular procedure employed, and varies among procedures and laboratories. We define MGMT− phenotype in this study as MGMT activity < 0.42 fmol/106 cells (< ca. 0.16 fmol/mg protein or < 255 molecules/cell). The limit of detection in our assay is comparable to that of oligonucleotide-based assays (ca. 200 molecules/cell [23]) and Western assay (ca. 135 molecules/cell when 4.6 × 106 cell equivalents are analyzed [24]) and is lower than that of histocytochemical procedures (7000–30,000 molecules/cell [25,26]). Every MGMT− extract, when mixed 8:1 (v/v) with MGMT+ extracts, yielded activity expected for the MGMT+ extracts, indicating that MGMT− extracts did not contain a diffusible inhibitor of MGMT activity.
2.3 Apurinic endonuclease
Activity was assayed as described previously [27,28]. Briefly, acid depurinated, supercoiled plasmid DNA was incubated with extracts, after which nicked, relaxed and supercoiled DNA was resolved by agarose gel electrophoresis. Photographs of ethidium stained gels were scanned by using Adobe Workshop 2.5, and DNA band density was quantitated by using NIH Image version 1.55. Activity (fmol abasic sites incised/min/cell) was calculated from the fraction of nicked molecules, and was a linear function of added extract in all cases. E. coli endonuclease, III a reference enzyme, also yielded linearity with added enzyme.
2.4 DNA polymerase ϐ
DNA polymerase ϐ (Pol ϐ) activity was quantitated, as in earlier work [16], by using an activity gel assay [29] with recombinant rat pol ϐ as a reference. Briefly, extracts were subjected to electrophoresis in an SDS-polyacrylamide gel containing gapped DNA in the matrix; following renaturation, Pol ϐ was visualized by incorporation of radioactivity from α-32P-TTP into a 39 kDa band. Activity, determined by phosphor imaging analysis of band intensity, was a linear function of the amount of Pol ϐ standard (0.01–1.0 pg), and of added extract, in all cases.
2.5 Lactate dehydrogenase
Activity was quantitated by the Cabaud-Wroblewski procedure [30] with a kit from Sigma, and was a linear function of added extract.
2.6 Statistical analysis
Linear regression analysis was carried out with Microsoft Excel (Microsoft Corp., Redmond, WA). For purposes of calculation, MGMT− extracts were assigned an MGMT activity of 0.21 fmol/106 cells, i.e., one-half of the lower limit of detection.
3. Results
The purpose of this work was assess to the idea that the frequent MGMT− phenotype in human brain, and an accompanying risk of neurocarcinogenesis, may arise during gestation. The hypothesis we investigated derived from an earlier study [16] in which we observed an age dependence of MGMT− phenotype in the histologically normal brain of brain tumor patients. In that study, we found that the frequency of MGMT− phenotype in brain adjacent to primary brain tumors was correlated with age, increasing from 22% in children to fully 75% in adults over 50. In contrast, the frequency of MGMT− phenotype in the brain of individuals without brain tumors (12%) was unrelated to age. These observations suggested the following hypothesis: MGMT− phenotype in brain is a prenatal characteristic that persists into postnatal life in a subpopulation of individuals -- 12% in our sample -- who are thereby predisposed to the age-dependent occurrence of brain tumors.
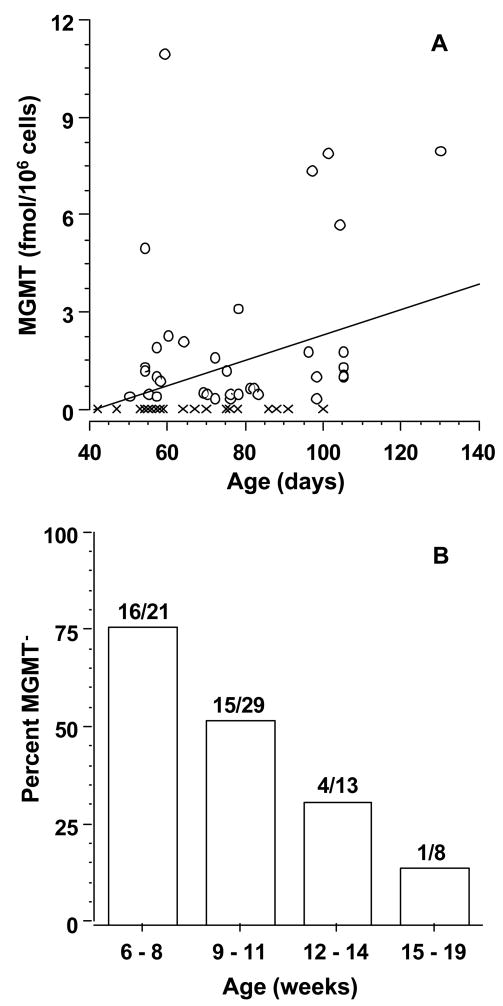
To investigate this hypothesis, we investigated the developmental pattern of MGMT activity in brain. We quantitated MGMT activity in extracts of 71 brains ranging in age from approximately 42 days (6 weeks) to 130 days (19 weeks) post-fertilization. We found that half of extracts (36/71) contained no detectable MGMT activity (MGMT− phenotype, < 0.42 fmol/106 cells (< ca. 0.16 fmol/mg protein or < 255 molecules/cell). MGMT activity in the remaining 35 extracts varied from 0.49 ± 0.08 to 11 ± 1.1 fmol/106 cells (295 to 6,625 molecules/cell), a range consistent with that previously observed in adult [16] and pediatric [22] brain (240 to 18,700 molecules/cell). As shown in Fig. 1A, MGMT activity in developing brain was strongly correlated with gestational age (r= 0.371, P ≤ 0.0015). As illustrated in Fig. 1B, this correlation reflected a decline in the proportion of MGMT− extracts from 76% (16/21) at 6 to 8 weeks of age to 13% (1/8) at 15 weeks and older. Liver, in addition to brain, was obtained in 36 cases. All liver extracts were MGMT+, including 15 paired with MGMT− brain, and had activity that varied from 0.43 ± 0.10 to 255 ± 30 fmol/106 cells (260 to 154,000 molecules/cell).
Fig. 1.
MGMT activity in developing brain. (A) MGMT activity in high speed supernatants of tissue extracts is shown as a function of days post-fertilization. The overlapping crosses at 53–60 days on the X-axis represent 18 specimens without detectable activity (MGMT− phenotype). The line was derived by linear regression analysis. X, MGMT− phenotype; O, MGMT+ phenotype. (B) The percent of specimens with MGMT− phenotype is shown as a function of weeks post-fertilization. The number of specimens in each age group was 21, 29, 13 and 8, respectively, in order of increasing age.
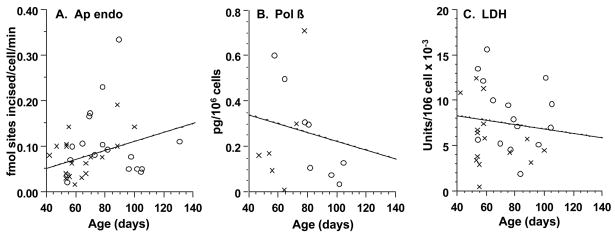
To determine whether the gestational pattern of MGMT activity in brain extended to other activities that repair alkylated DNA, we assayed apurinic endonuclease (Ap endo) activity and Pol ϐ, key base excision repair activities [31]. Ap endo activity was found in all 36 extracts examined, 18 of which had no measurable MGMT (Fig. 2A). The mean activity (0.10 ± 0.091 fmol abasic sites incised/cell/min) was similar to that previously observed in adult [27] and pediatric [28] brain (0.13 ± 0.053 fmol abasic sites incised/cell/min). Pol ϐ activity was likewise found in all 14 extracts examined, 6 of which had no measurable MGMT (Fig. 2B). The mean activity (0.24 ± 0.22 pg/106 cells) was also similar to that observed [16] in the brain of adults (0.16 ± 0.15 pg/106 cells). Importantly, neither Ap endo nor Pol ϐ activities showed a statistically significant relationship with age, as determined by linear regression analysis (r = 0.16; P > 0.40 and r = −0.25; P > 0.35 respectively) and illustrated by the mean activities during development (Fig. 3B,C). These observations are consistent with the essentiality of Ap endo activity [32] and pol ϐ for early mouse development [33]. We also assayed lactate dehydrogenase, an enzyme with no known DNA repair function. All 30 extracts examined had activity, and, again, the activity had no statistically significant relationship with age, as determined by linear regression analysis (r = −0.16; P ≤ 0.35) (Fig. 2C) and illustrated by the mean activities during development (Fig. 3D). In striking contrast, the age dependence of mean MGMT activity for the entire sample population (Fig. 3A), was manifest in each of the three subsets of extracts assayed for Ap endo (r = 0.64; P ≤ 0.0001), Pol ϐ (r = 0.62; P ≤ 0.020) and lactate dehydrogenase (r = 0.39; P ≤ 0.04) activities.
Fig. 2.
Apurinic endonuclease (A), DNA polymerase ϐ (B), and lactate dehydrogenase (C) in developing brain. Activities in high-speed supernatants of tissue extracts are shown as a function of days post-fertilization, together with the lines derived by linear regression analysis. X, MGMT−; O, MGMT+.
Fig. 3.
Dependence of MGMT (A), apurinic endonuclease (B), DNA polymerase ϐ (C), and lactate dehydrogenase (D) activites on gestational age in developing brain. Mean activities and standard errors of the mean are shown as a function of weeks post-fertilization. The number of extracts in each age group is indicated. For purposes of calculation, a value of 0.21 fmol/106 cells (half the limit of detection) was assigned to MGMT− extracts.
To summarize, the data we present here show that: (1) Half of embryonic and early fetal brain specimens we examined contained no detectable MGMT activity (MGMT− phenotype, <255 molecules/cell); (2) the proportion of brain specimens that exhibited MGMT− phenotype decreased from 76% to 13% between 6 to 19 weeks post-fertilization; (3) the proportion of specimens with MGMT− phenotype in brain at 19 weeks of gestation reached the proportion observed [16] in adult brain (12%)1. Taken together, the results provide evidence of developmental regulation of MGMT activity in human brain, and indicate that MGMT− phenotype in the brain of children and adults may arise as a “developmental holdover”.
Discussion
Primary malignant brain tumors are among the most rapidly lethal human cancers, with two year survival rates for adult gliomas being less than 20% [34]. The incidence is strongly age dependent, peaking in the sixth and seventh decades of life [18]. However, 10% of new cases are children under the age of fifteen, and brain tumors are the leading cause of death from childhood cancer [35]. Despite intensive investigation, the etiology of primary brain tumors remains largely unknown. Heritable syndromes that predispose to neurocarcinogenesis, and high dose ionizing radiation, a strongly associated exogenous risk factor, account for relatively few cases [18,19]. Our evidence [16] indicates that lack of detectable MGMT activity (MGMT− phenotype) in brain is a susceptibility factor that affects a sizable minority of individuals (12% in our sample population). Apparently, failure to express this important DNA repair activity may be among the earliest events in progenitor tissue that predispose to tumor formation.
Based on earlier findings [16], we postulated that MGMT− phenotype in brain is a gestational trait that is carried into postnatal life by a subgroup of individuals who thereby incur increased risk for brain tumors. In support of this premise, we found that MGMT activity was strongly correlated with gestational age, and the proportion of specimens displaying MGMT− phenotype gradually fell to that found in adults. The data are consistent with a transition from MGMT− to MGMT+ phenotype that may involve uniform up-regulation of MGMT activity in all cells, or an increase in the proportion of cells that express MGMT activity. The putative transition is not highly stage-specific, and would occur during a period when major morphological features of the brain are still forming [20]. As noted above, the frequency of MGMT− phenotype that we observed at a gestational age of 15 to 19 weeks (13%) was essentially the same as we found in adult brain (12%). Overall, the results indicate that MGMT activity in human brain is developmentally regulated and suggest that the MGMT− phenotype in the brain of children and adults may arise in utero. Our data do not address the possibility that MGMT− phenotype in the brain of children and adults might also arise by loss of MGMT expression in MGMT+ tissue, either late in gestation or post-gestationally.
The observed pattern of MGMT activity in developing brain has potential implications for the occurrence of alkylation-induced genomic damage that contributes to brain tumors. The mammalian blood-brain barrier, which is an important defense against exposure to endogenous and exogenous alkylators, is not completely formed until after birth [36]. One implication is that brain may be especially susceptible to the mutagenic and clastogenic effects of O6-alkylguanine adducts early in gestation when brain cells have MGMT− phenotype and are dividing rapidly. Even if such cells eventually become MGMT+, previously suffered point mutations and chromosomal aberrations would be fixed. In accord with this idea, gliomas frequently exhibit genetic alterations that may be attributable to unrepaired O6-alkylguanine. For example, GC to AT transitions (the characteristic point mutation caused by O6-methylguanine [8,9]) in p53 [37], RB [38] and PTEN [39], together with loss of the second allele, underlie the abrogation of programmed cell death and cell cycle regulation found in human gliomas [40]. Human embryonic brain may thus resemble embryonic rodent brain, which is deficient in MGMT and particularly vulnerable to nitrosourea-induced tumors [1–3]. These considerations suggest that alkylation damage incurred during embryogenesis may contribute disproportionately (relative to damage incurred at other periods of life) to the overall incidence of human brain tumors, and raise the issue of maternal exposure (19). Another implication of the observed gestational pattern is that the MGMT− phenotype that prevails in embryonic brain may persist into postnatal life in a subpopulation of individuals, thus increasing risk for alkylation-related neurocarcinogenesis.
Human exposure to alkylating agents is believed to be continuous and life-long, and to originate from diverse, endogenous and exogenous sources. Possible endogenous alkylators include S-adenosylmethionine, lipid peroxidation products and nitrosated bile acids, peptides and amino acids [41]. Notably, azaserine and nitrosated glycine react with DNA in vitro to produce O6-methylguanine [41,42]. Based on the neurocarcinogenicity of N-nitroso compounds and certain precursors in experimental animals [1–3], it has been hypothesized that exogenous agents of particular relevance may include N-nitrosamides and nitrosamide precursors, e.g. sodium nitrite [19]. Although epidemiologic evaluation has been complicated by manifold problems, and the data have not been consistent [reviewed in reference 19], several recent studies offer some support for this hypothesis. Notably, the majority of studies to date of maternal diet and childhood brain tumors suggests that exposure during gestation to endogenously formed nitroso compounds may be associated with tumor occurrence [19]. Our present observations imply that maternal exposure during the first trimester could have particular causal relevance. Other recent studies suggest that exposure to dietary sources of nitroso compounds is associated with increased risk of adult gliomas, and, conversely, that ingestion of agents that inhibit endogenous formation of these compounds is associated with reduced risk [e.g., 43,44]. Stronger epidemiologic data might be obtainable for adult tumors if it were possible to identify individuals with MGMT− phenotype in brain, since this subgroup may have heightened susceptibility to neurocarcinogenesis by nitroso compounds. Unfortunately, there is no readily assayable biomarker for this subpopulation, such as MGMT in lymphocytes [16].
The mechanisms controlling MGMT expression in normal mammalian tissue are not yet completely defined, although epigenetic regulation is mediated by CpG methylation in MGMT− cell lines and human tumors [45]. DNA methylation is involved in early development [46] and could be especially important in determining tissue-specific MGMT expression in human brain. Polymorphisms have been documented at the human MGMT locus [47,48], some of which might be relevant as well. Thus, individuals with MGMT− phenotype in brain could carry an MGMT allele(s) that is particularly subject to down-regulation. Alternatively, MGMT− phenotype in brain may be independent of genotype at the MGMT locus and represent an ontologic variation determined entirely by other factors.
Acknowledgments
This work was supported by grants from the NIH (CA70790, CA82622), the American Cancer Society (RPG-97-019-01) and the American Federation for Aging Research.
Footnotes
In the work on adult brain [16], MGMT− phenotype was defined as <0.34 fmol MGMT/106 cells or < 204 molecules/cell. The 20% difference relative to the definition used here (204 vs. 255 molecules/cell) reflects the small amounts of embryonic tissue available and does not alter the conclusions or implications of the present study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kleihues P, Lantos PL, Magee PN. Chemical carcinogenesis in the nervous system. Int Rev Exp Pathol. 1976;15:153–232. [PubMed] [Google Scholar]
- 2.Schlegel J, Stumm G, Mennel HD. Chemical carcinogenesis in the nervous system: past and future. Exp Toxic Pathol. 1994;45:455–466. doi: 10.1016/S0940-2993(11)80504-X. [DOI] [PubMed] [Google Scholar]
- 3.Rice JM, Rehm S, Donovan PJ, Perantoni AO. Comparative transplacental carcinogenesis by directly acting and metabolism-dependent alkylating agents in rodents and nonhuman primates. In: Napalkov NP, Rice JM, Yamasaki H, editors. Perinatal and Multigenerational Carcinogenesis. IARC Scientific Publications: Lyon; 1989. [PubMed] [Google Scholar]
- 4.Goth R, Rajewsky MF. Persistence of O6-ethylguanine in rat brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci USA. 1974;71:639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleihues P, Buchler J. Long-term persistence of O6-methylguanine in rat brain DNA. Nature. 1977;269:625–626. doi: 10.1038/269625a0. [DOI] [PubMed] [Google Scholar]
- 6.Singer B, Bodell WJ, Cleaver JE, Thomas GH, Rajewsky MF, Thon W. Oxygens in DNA are the main targets for ethylnitrosourea in normal and xeroderma pigmentosum fibroblasts and rat brain cells. Science. 1978;276:85–88. doi: 10.1038/276085a0. [DOI] [PubMed] [Google Scholar]
- 7.Chabra SK, Souliotis VL, Harbaugh JW, Krasnow SW, Jones AB, Anderson LM, Kyrtopoulos SA. O6-methylguanine adduct formation and modulation by ethanol in placenta and fetal tissues after exposure of pregnant Patas monkeys to N-nitrosodiethylamine. Cancer Res. 1995;55:6010–6020. [PubMed] [Google Scholar]
- 8.Margison GP, Santibanez Koref MF, Povey AC. Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis. 2002;17:483–487. doi: 10.1093/mutage/17.6.483. [DOI] [PubMed] [Google Scholar]
- 9.Kaina B. Mechanisms and consequences of methylating agent-induced SCEs and chromosomal aberrations: a long road traveled and still a far way to go. Cytogenet Genome Res. 2004;104:77–86. doi: 10.1159/000077469. [DOI] [PubMed] [Google Scholar]
- 10.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 11.Margison GP, Santibanez-Koref MF. O6-alkylguanine-DNA alkyltransferase: role in carcinogenesis and chemotherapy. Bioessays. 2002;24:255–266. doi: 10.1002/bies.10063. [DOI] [PubMed] [Google Scholar]
- 12.Washington WJ, Foote RS, Dunn WC, Generoso WM, Mitra S. Age-dependent modulation of tissue-specific repair activity for 3-methyladenine and O6-methylguanine in DNA in inbred mice. Mech Aging Dev. 1989;48:43–52. doi: 10.1016/0047-6374(89)90024-9. [DOI] [PubMed] [Google Scholar]
- 13.Xiao W, Samson L. The Saccharomyces cerevisiae MGT1 DNA repair methyltransferase gene: its promoter and entire coding sequence, regulation and in vivo biological functions. Nucleic Acids Res. 1992;20:3599–3606. doi: 10.1093/nar/20.14.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esteller M, Herman JG. Generating mutations but providing chemosensitivity: the role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene. 2004;8:1–8. doi: 10.1038/sj.onc.1207316. [DOI] [PubMed] [Google Scholar]
- 15.Margison GP, Povey AC, Kaina B, Santibanez Koref MF. Variability and regulation of O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 2003;24:625–635. doi: 10.1093/carcin/bgg005. [DOI] [PubMed] [Google Scholar]
- 16.Silber JR, Blank A, Bobola MS, Mueller BA, Kolstoe DD, Ojemann GA, Berger MS. Lack of the DNA repair protein O6-methylguanine-DNA methyltransferase in histologically normal brain adjacent to primary brain tumors. Proc Natl Acad Sci USA. 1996;93:6941–6946. doi: 10.1073/pnas.93.14.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silber JR, Bobola MS, Ghatan S, Blank A, Kolstoe DD, Berger MS. O6-methylguanine-DNA methyltransferase activity in adult gliomas: relation to patient and tumor characteristics. Cancer Res. 1998;58:1068–1073. [PubMed] [Google Scholar]
- 18.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncol. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietrich M, Block G, Pogoda JM, Buffler P, Hecht S, Preston-Martin S. A review: dietary and endogenously formed N-nitroso compounds and risk of childhood brain tumors. Cancer Causes Control. 2005;16:619–635. doi: 10.1007/s10552-005-0168-y. [DOI] [PubMed] [Google Scholar]
- 20.England MA. Anatomical development of the central nervous system. In: Levene MI, Lilford RJ, Bennett MJ, Punt J, editors. Fetal and Neonatal Neurology and Neurosurgery. Churchill Livingstone: New York; 1995. [Google Scholar]
- 21.Silber JR, Blank A, Bobola MS, Ghatan S, Kolstoe DD, Berger MS. O6-methylguanine-DNA methyltransferase-deficient phenotype in human gliomas: frequency and time to tumor progression after alkylating agent-based chemotherapy. Clin Cancer Res. 1999;5:807–814. [PubMed] [Google Scholar]
- 22.Bobola MS, Berger MS, Ellenbogen RG, Roberts TS, Geyer JR, Silber JR. O6-methylguanine-DNA methyltransferase in pediatric primary brain tumors: relation to patient and tumor characteristics. Clin Cancer Res. 2001;7:613–619. [PubMed] [Google Scholar]
- 23.Wu RS, Hurst-Calderone S, Kohn K. Measurement of O6-alkylguanine-DNA alkyltransferase activity in human cells and tumor tissues by restriction endonuclease inhibition. Cancer Res. 1987;47:6229–6235. [PubMed] [Google Scholar]
- 24.Ostrowski LE, Pegram CN, von Wronski MA, Humphrey PA, He X, Shiota S, Mitra S, Brent TP, Bigner DD. Production and characterization of antipeptide antibodies against human O6-methylguanine-DNA methyltransferase. Cancer Res. 1991;51:3339–3344. [PubMed] [Google Scholar]
- 25.Brent TP, von Wronski MA, Edwards CC, Bromley M, Margison GP, Rafferty JA, Pegram CN, Bigner DD. Identification of nitrosourea-resistant human rhabdomyosarcomas by in situ immunostaining of O6-methylguanine-DNA methyltransferase. Oncol Res. 1993;5:83–86. [PubMed] [Google Scholar]
- 26.Belanich M, Pastor M, Randall T, Guerra D, Kibitel J, Alas L, Li B, Citron M, Wasserman P, White A, Eyre H, Jaeckle K, Schulman S, Rector D, Prados M, Coons S, Shapiro W, Yarosh D. Retrospective study of the correlation between the DNA repair protein alkyltransferase and survival of brain tumor patients treated with carmustine. Cancer Res. 1996;56:783–788. [PubMed] [Google Scholar]
- 27.Bobola MS, Blank A, Berger MS, Stevens BA, Silber JR. Apurinic/apyrimidinic endonuclease activity is elevated in human adult gliomas. Clin Cancer Res. 2001;7:3510–3518. [PubMed] [Google Scholar]
- 28.Bobola MS, Finn LS, Ellenbogen RG, Geyer JR, Berger MS, Braga JM, Meade EH, Gross ME, Silber JR. Apurinic/apyrimidinic endonuclease activity is associated with response to radiation and chemotherapy in medulloblastoma and primitive neuroectodermal tumors. Clin Cancer Res. 2005;15:7405–7414. doi: 10.1158/1078-0432.CCR-05-1068. [DOI] [PubMed] [Google Scholar]
- 29.Blank A, Silber JR, Thelen MP, Dekker CA. Detection of enzymatic activities in sodium dodecyl-sulfate-polyacrylamide gels: DNA polymerases as model enzymes. Anal Biochem. 1983;135:423–430. doi: 10.1016/0003-2697(83)90705-4. [DOI] [PubMed] [Google Scholar]
- 30.Cabaud PG, Wroblewski F. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am J Clin Path. 1958;30:234–246. doi: 10.1093/ajcp/30.3.234. [DOI] [PubMed] [Google Scholar]
- 31.Dianov GL, Sleeth KM, Dianova II, Allinson SL. Repair of abasic sites in DNA. Mutat Res. 2003;531:157–163. doi: 10.1016/j.mrfmmm.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Xanthoudakis S, Smeyne RJ, Wallace JD, Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc Natl Acad Sci USA. 1996;93:8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Betz UA, Vosshenrich CA, Rajewsky K, Muller W. Bypass of lethality with mosaic mice generated by Cre-loxP-mediated recombination. Curr Biol. 1996;6:1307–1316. doi: 10.1016/s0960-9822(02)70717-3. [DOI] [PubMed] [Google Scholar]
- 34.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 35.Bleyer WA. Epidemiologic impact of children with brain tumors. Childs Nerv Syst. 1999;15:758–63. doi: 10.1007/s003810050467. [DOI] [PubMed] [Google Scholar]
- 36.Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- 37.Ohgaki H. Genetic pathways to glioblastomas. Neuropathology. 2005;25:1–7. doi: 10.1111/j.1440-1789.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 38.Davies MP, Gibbs FE, Halliwell N, Joyce KA, Roebuck MM, Rossi ML, Salisbury J, Sibson DR, Tacconi L, Walker C. Mutation in the PTEN/MMAC1 gene in archival low grade and high grade gliomas. Br J Cancer. 1999;79:1542–1548. doi: 10.1038/sj.bjc.6690246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krex D, Mohr B, Appelt H, Schackert HK, Schackert G. Genetic analysis of a multifocal glioblastoma multiforme: a suitable tool to gain new aspects in glioma development. Neurosurgery. 2003;53:1377–1384. doi: 10.1227/01.neu.0000093426.29236.86. [DOI] [PubMed] [Google Scholar]
- 40.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 41.Sedgwick B. Nitrosated peptides and polyamines as endogenous mutagens in O6-alkylguanine-DNA alkyltransferase deficient cells. Carcinogenesis. 1997;18:1561–1567. doi: 10.1093/carcin/18.8.1561. [DOI] [PubMed] [Google Scholar]
- 42.Shuker DEG, Margison GP. Nitrosated glycine derivatives as a potential source of O6-methylguanine in DNA. Cancer Res. 1997;57:366–369. [PubMed] [Google Scholar]
- 43.Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for onset glioma in the San Francisco Bay area. Cancer Causes Control. 1997;8:13–24. doi: 10.1023/a:1018470802969. [DOI] [PubMed] [Google Scholar]
- 44.Huncharek M, Kupelnick B, Wheeler L. Dietary cured meat and the risk of adult glioma: a meta-analysis of nine observational studies. J Environ Pathol Toxicol Oncol. 2003;22:129–137. doi: 10.1615/jenvpathtoxoncol.v22.i2.60. [DOI] [PubMed] [Google Scholar]
- 45.Esteller M, Herman JG. Generating mutations but providing chemosensitivity: the role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene. 2004;23:1–8. doi: 10.1038/sj.onc.1207316. [DOI] [PubMed] [Google Scholar]
- 46.Razin A, Shemer R. DNA methylation in early development. Human Mol Gen. 1995;4:1751–1755. doi: 10.1093/hmg/4.suppl_1.1751. [DOI] [PubMed] [Google Scholar]
- 47.National Center for Biotechnology Information. http://www.ncbi.nlm.nih.gov/projects/SNP/
- 48.NIEHS SNPs Program. http://egp.gs.washington.edu/