Abstract
Failure and high systemic toxicity of conventional cancer therapies have accelerated the focus on the search for newer agents, which could prevent and/or slow-down cancer growth and have more human acceptability by being less or non-toxic. Silymarin is one such agent, which has been extensively used since ages for the treatment of liver conditions, and thus has possibly the greatest patient acceptability. In recent years, increasing body of evidence has underscored the cancer preventive efficacy of silymarin in both in vitro and in vivo animal models of various epithelial cancers. Apart from chemopreventive effects, other noteworthy aspects of silymarin and its active constituent silibinin in cancer treatment include their capability to potentiate the efficacy of known chemotherapeutic drugs, as an inhibitor of multidrug resistance associated proteins, and as an adjunct to the cancer therapeutic drugs due to their organ-protective efficacy specifically liver, and immunostimulatory effects. Widespread use of silymarin for liver health in humans and commercial availability of its formulations with increased bioavailability, further underscore the necessity of carrying out controlled clinical trials with these agents in cancer patients. In this review, we will briefly discuss the outcomes of clinical trials being conducted by us and others in cancer patients to provide insight into the clinical relevance of the observed chemopreventive effects of these agents in various epithelial cancer models.
Keywords: Silymarin, Silibinin, anticancer, chemoprevention, epithelial cancers
Introduction
Modernization along with rapid industrialization of the present world was much desired proposition, however, with passing time, it is being increasingly recognized that this growth and prosperity are not without associated harmful consequences. Modernization has affected all walks of human life, making it much more comfortable; however, it has led to drastic changes in the day to day life style resulting in adverse health effects. Another important adverse consequence of industrialization is that human population at large is now exposed to much diverse range of harmful chemicals, mostly as pollutants generated through rapid industrialization. Overall, marked increase in the environmental and occupational hazards together with altered life style has resulted in the increased incidence of various diseases, such as cardiovascular disorders, diabetes, obesity and cancer. Out of these, most concerning is the cancer, which not only is a disease with great economic burden, but also has tremendous social and psychological implications. Even though, there has been a decline in deaths due to cancer in United States during past decade or so, yet overall incidence of new cancer remains unchanged. However, in global context, deaths due to cancer are actually projected to increase in coming years (WHO Report). As a result, over the years, increased efforts have been devoted to determine the risk factors for the development of cancer, where numerous epidemiological studies have concluded that geographical variation in the incidence of cancer throughout the world is associated mostly with life style, especially dietary habits and environment. Closer examination of the trends of cancer incidence in different geographical locations revealed that incidence varies with a variation in dietary habits. In recent years, tremendous interest has been generated in studying the relationship between dietary habits and cancer incidence.
Now, it is recognized that diets rich in fruits and vegetables have an association with lower risk of cancer, suggesting that cancer risk could be modified/prevented by making appropriate dietary manipulations (Steinmetz et al., 1991; Block et al., 1992; Kelemen et al., 2006; Pavia et al., 2006). Additionally, long duration of development of most types of cancers once initiating event has taken place, provides an excellent time frame window to intervene or prevent them from becoming full blown clinically defined cancer. Taking cue from these observations, there is a strong interest in isolating and characterizing the nutritive and non-nutritive components of fruits and vegetables, often referred to as phytochemicals as potential chemopreventive agents (Surh, 2003). In this review article, we will provide detailed insight into the chemopreventive efficacy of silymarin based on the evidence generated from various studies conducted using cell culture or animal models of epithelial cancers. Though numerous studies are available reporting clinical trials with silymarin/silibinin, most of them have been carried out in patients with adverse liver conditions. However, we will briefly discuss the clinical trials being carried out with silibinin, an active constituent of silymarin, in cancer conditions, especially in prostate cancer patients by our group.
Milk Thistle Extract, Silymarin and Silibinin: Pharmacology and Biological Activities
With the increased acceptance of “anything natural” and recognition of the therapeutic value of the variety of plants/plant products used traditionally for the treatment of various ailments by indigenous healers and herbalists, extensive research has been conducted in recent years to identify potential phytochemicals as effective cancer chemopreventive agents. In this regard, Milk Thistle Extract has been the most extensively studied plant product for its overall health beneficial effects. Milk Thistle (Silybum Marianum, Family Asteraceae) is one of the earliest known herbal medicines with hepato-protective effects, and has been documented in ancient literature as a plant with excellent capacity in carrying off the bile, or one with the ability to remove obstructions of the liver and spleen (Foster, 1999; Pliny the Elder, 77 A.D; Culpeper, 1650). Chemically, active constituent of milk thistle extract is basically a flavolignan, silymarin, which in itself represents the mixture of four isomeric flavonoids such as silibinin, isosilibinin, silydianin and silychristin. Fig. 1 represents the chemical structure of silibinin, which is a major active as well as most studied constituent of silymarin. Recently, using combination of liquid chromatography and electrospray ionization mass spectrometry, six main active constituents of silymarin have been separated, and include silydianin, silychristin, silybin A, silybin B, isosilybin A and isosilybin B (Lee et al., 2006). The chemical structures of these isomers have been shown by us previously (Davis-Searles et al., 2005). Fruit and seeds of this plant are major source of silymarin, though it can also be found in trace amounts in other parts of the plant (Luper, 1998). Currently, milk thistle extract is marketed around the world including the United States as silymarin and silibinin encapsulated formulation/tablet with an enhanced bioavailability under the trade names, such as Siliphos, Silipide and Legalon.
Fig. 1.
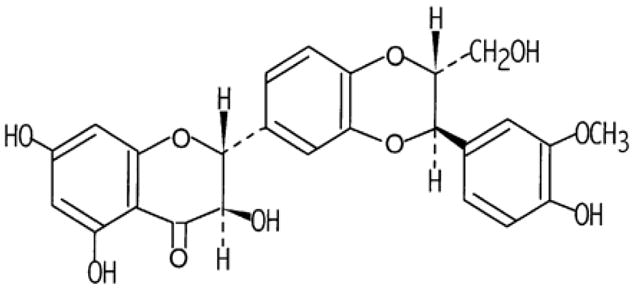
Chemical structure of Silibinin.
Silymarin is being used to treat liver conditions since ages, and thus is considered safe for human use. So far there is no report showing the toxicity due to silymarin, though few clinical studies have reported minor adverse effects. The reported adverse effects include headaches, gastrointestinal and dermatological symptoms (Wellington and Jarvis, 2001). Due to its low solubility, bioavailability of silymarin has been the major concern especially during its clinical trials. We conducted the bioavailability studies of silibinin, which is the major active constituent of Silymarin in SENCAR mice. We observed that within 30 minutes of 50mg/Kg oral dose of silibinin to mice, peak levels of free silibinin could be detected in liver, lung, stomach and pancreas. In other organs, such as prostate and skin, peak levels were observed at one hour of administration. Our results clearly demonstrated the bioavailability of systemically administered silibinin (Zhao and Agarwal, 1999). Additionally, recent literature is abundant with the studies reporting different formulations with tested increased bioavailability using different animal models (Abrol et al., 2005; El-samaligy et al., 2006). A detailed study on the bioavailability and clinical efficacy in humans has been conducted with Silibinin Phytosome called as Siliphos (Kidd and Head, 2005). In this study, the authors found greater bioavailability of silibinin in the form of siliphos over the non-complexed silibinin when administered to rats/humans, and levels of free silibinin were detectable in the liver which was the target organ of the study. Thus, from the above cited literature, it is clear that bioavailability of silymarin/silibinin is not a major issue for its potential use in clinical settings.
Silymarin has been extensively used for its hepatoprotective effects since ancient times, though other health beneficial effects are being recognized in recent years. Most of these effects have been attributed to direct and/or indirect anti-oxidant capacity of silymarin, such as being scavenger of reactive oxygen species, scavenger of phenylglyoxylic ketyl radicals, chain breaking antioxidant (Luper, 1998). In recent years, increased efforts have been devoted to understand the mechanism of hepatoprotective effect of silymarin, utilizing experimental models of various liver disorders, as well as in human patients with liver diseases. Findings of these studies have in part enlightened certain aspects of hepatoprotective mechanism of silymarin. In case of alcoholic liver diseases (ALD), wherein oxidative stress and inflammation are key players in pathogenesis, silymarin was found to exert hepatoprotective effects by attenuating the tumor necrosis factor (TNF) production along with decreasing the serum alanine aminotransferase (ALT) activity, inhibiting lipid peroxidation, and increasing the intracellular reduced glutathione content in mouse model of ALD (Song et al., 2006). Silibinin, an active constituent of silymarin also affords protection against T cell-dependent hepatitis through the modulation of immune response (Schumann et al., 2003). Silymarin is not only hepatoprotectant in ALD, it also acts as a fetoprotectant in ethanol-induced behavioral deficits (Busby et al., 2002). Similarly, silymarin was found to retard collagen accumulation associated with both early and advanced biliary fibrosis in experimental model of complete bile duct occlusion induced by Ethibloc in rats (Luper, 1998). In experimental model of choleostasis induced by 17α-ethynylestradiol (EE) in rats, silymarin exerted protective effects by normalizing the EE-induced decrease in bile salt pool size and HCO3−, and by counteracting cholestatic effect of its glucuronidated metabolite (Crocenzi et al., 2001). In yet another study conducted by the same group, hepatoprotective effects of silymarin were observed in monohydroxylated bile salt (BS) taurolithocholate (TLC)-induced choleostatsis in rats by preventing the impairment of both BS-dependent and -independent fractions of the bile flow (Crocenzi et al., 2003). Preventive effects of silymarin against diet induced hypercholesterolemia were observed in rats, and the effects were comparable to that observed with hypocholesterolemic drug, Probucol (Krecman et al., 1998). Hepatoprotective effects of silymarin were also observed in carbon tetrachloride (CCl4) and acetaminophen (AAP) induced liver toxicity, partly due to its antioxidant capacity (Luper, 1998). Even silibinin exerts hepatoprotective effects partly through inhibition of leukotriene formation by Kupffer cells (Dehmlow et al., 1996), and as an in vivo immune response modifier (Schumann et al., 2003). Silymarin was also found to exert protective effect against post-ischemic mitochondrial injury in rat liver occurring during liver transplantation and/or surgery by preventing the alterations in mitochondrial respiration, decrease in membrane potential and increased susceptibility to mitochondrial permeability transition (MPT) induction (Rolo et al., 2003).
In addition to hepatoprotective effects, silymarin has shown protective effects against cisplatin induced renal toxicity (Karimi et al., 2005), and causes recovery of pancreatic function in experimental model of diabetes mellitus syndrome induced with alloxan in rats (Soto et al., 2004). Protective effects against cisplatin- or ifosfamide-induced nephrotoxicity without significant effect on the anti-tumor efficacy of these drugs have been reported in rat model of cisplatin-induced nephropathy. Results of these studies suggest the potential use of silymarin as protective agent against cisplatin-induced nephrotoxicity in cancer patients (Bokemeyer et al., 1996). Additionally, silymarin and its constituents have shown protective effects on rat cardiomyocytes exposed to doxorubicin due to its anti-oxidant, iron chelating and cell membrane stabilizing capacity (Chlopcikova et al., 2004).
Anti-atherosclerotic effects of silymarin have been demonstrated using in vitro system, wherein silymarin inhibits the expression of adhesion molecules in HUVEC cells (Kang et al., 2003). Silymarin has also been reported to possess neurotrophic and neuroprotective effects as it promotes differentiation and survival of neural cells (Kittur et al., 2002). Immuno-stimulatory effects of silymarin have also been reported as it increases the secretion of interferon-γ (IFN-γ), interleukin-4 (IL-4), and interleukin-10 (IL-10) cytokines in stimulated lymphocytes (Wilarusmee et al., 2002). Protective effects of silymarin were observed against acetaminophen induced brain damage in rats, which were ascribed to its antioxidative potential in preventing lipid peroxidation, and additionally, due to its capacity to replenish intracellular reduced glutathione pool (Nencini et al., 2006).
One of the major impediments to successful chemotherapeutics is multidrug resistance, which often involves the overexpression of P-glycoprotein (Pgp) or multidrug resistance-associated protein 1 (MRP1). Silymarin was found to be an inhibitor of P-glycoprotein and MRP1-mediated drug transport. Silymarin increased daunomycin accumulation in Pgp-positive cells as against to Pgp-negative cells, which could be utilized in increasing the absorption and bioavailability of drugs that are Pgp substrates. Similarly, silymarin increased the accumulation of daunomycin and vinblastine in Panc-1 cells and human breast cancer cells, by inhibiting breast cancer resistance protein (BRCP) (Morris and Zhang, 2006; Chung et al., 2005). On another front, maintenance of genomic integrity is most critical, and cells are well equipped with variety of DNA repair enzymes. Silymarin has been found to upregulate the activity/expression of one such enzyme, O6-Methylguanine-DNA methyltransferase (MGMT) in various cancer cell lines (Niture et al., 2006).
Clinical Studies
Several clinical studies have been conducted with silymarin studying the hepatoprotective efficacy under various pathological conditions of liver and other organs. These studies are worth mentioning here, as findings of these studies are highly suggestive of carrying out the controlled clinical trials with silymarin without any major concerns about the toxicity and bioavailability of this compound in humans. In a 12 month open controlled study with two well matched groups of insulin-treated diabetic patients with alcoholic cirrhosis, administration of 600mg silymarin per day in addition to standard therapy, resulted in significantly decreased fasting blood glucose levels, mean daily blood glucose levels, glycosuria and HbA1c levels after 4 months of treatment as compared to control group receiving standard therapy alone. Findings of this study led to a conclusion that silymarin administration leads to reduced lipid peroxidation and insulin resistance with simultaneous decrease in endogenous insulin overproduction and the need for exogenous insulin administration (Velussi et al., 1997). In another double blind multicenter trial of silymarin in alcoholic patients with histologically and laparoscopically proven liver cirrhosis, 450 mg of silymarin (150mg/three times per day) as compared to placebo for two years showed no effect of silymarin on the survival and the clinical course of the disease. However, no relevant side effects were observed in any group during the study period of two years (Pares et al., 1998). In yet another study, workers with a history of exposure to toluene and xylene vapors for 5–20 years and clinically proven with abnormal liver function tests and/or abnormal hematological values were given Legalon three times per day (t.i.d) orally for 30 days. Clinical outcome of this study revealed that these workers presented themselves with improved liver function tests and platelet counts as compared to non-treated workers with similar medical history (Szilard et al., 1988). However, this study had several flaws, such as lack of proper control groups, and small size of sample population as well as lot of heterogeneity in the sample population, as was the case with another study done by Ferenci et al., in patients with cirrhosis of liver (Ferenci et al., 1998). More recently, a preliminary pilot study was conducted with RealSIL-IBI from Lorenzi Pharmaceutical, Italy, which basically is a complex of silibinin with vitamin E and phospholipids in patients with non alcoholic fatty liver disease with or without the presence of hepatitis C virus (HCV)-related chronic hepatitis. Outcome of this study found the complex to be active in vivo, with some therapeutic value, such as improvement in insulin resistance and markers of fibrosis in plasma of the patients (Trappoliere et al., 2005).
Anticancer and Cancer Preventive Efficacy of Silymarin
Though initial impetus of research was on understanding the hepatoprotective effects of silymarin, over the years, many other biological effects of silymarin as discussed above have been recognized. One of them not discussed earlier in this review is the pioneer work done by our group over the years in recognizing the anti-cancer efficacy of silymarin. We were the first one to demonstrate the anti-tumor promoting activity of silymarin against 12- O -tetradecanoylphorbol-13-acetate (TPA)-induced tumor promotion in mouse epidermis, which was attributed to its ability to inhibit the activity and expression of epidermal ornithine decarboxylase (Agarwal et al., 1994). Anticancer activity of silymarin or silibinin has been observed against breast cancer (Zi et al., 1998, Tyagi et al., 2004), skin cancer (Ahmad et al., 1998, Lahiri-Chatterjee et al., 1999), androgen-dependent and-independent prostate cancer (Zi and Agarwal, 1999; Zi et al.,2000; Bhatia et al., 1999; Deep et al., 2006; Thelen et al., 2004) cervical cancer (Bhatia et al., 1999), bladder cancer (Tyagi et al., 2004), hepatocellular carcinoma (Varghese et al., 2005), colon cancer (Agarwal et al., 2003), ovarian cancer (Scambia et al., 1996) and lung cancer (Sharma et al., 2003, Chu et al., 2004). Findings of these in vitro studies in various cancer cell culture systems have provided the platform for further research to explore their efficacy under in vivo conditions, and finally clinical application of this non-toxic agent as potential chemopreventive in cancer patients. Apart from anticancer efficacy, another important effect of silymarin with its implication in cancer control/prevention is its anti-angiogenic potential through inhibition of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2) secretion (Jiang et al., 2000; Yang et al., 2003). From all above mentioned studies, there are sufficient evidences that silymarin/silibinin possesses anticancer efficacy, and additionally, as discussed further, preventive efficacy in various in vivo models of epithelial cancers summarized in Table 1. Collectively, all the findings advocate the necessity of developing and testing silymarin/silibinin for its potential use in clinics in cancer patients.
Table 1.
Summary of the outcome of in vivo chemopreventive studies conducted with silymarin and silibinin against cancers of different organs/sites
| Cancer site | Outcome with Silymarin | Outcome with Silibinin |
|---|---|---|
| Skin Cancer (NMSC) | Positive | Positive |
| Prostate Cancer | Positive | Positive |
| Breast Cancer | Negative | ND |
| Colon Cancer | Positive | ND |
| Lung Cancer | ND | Positive/No Effect |
| Bladder | Positive | ND |
| Ovarian Cancer | ND | Positive |
| Hepatocellular Carcinoma | Positive | ND |
| Pancreatic Cancer | ND | ND |
ND: Not Determined.
Cancer Preventive Efficacy of Silymarin/Silibinin and Associated Mechanisms
Skin Cancer
As mentioned earlier, we were the first one to recognize the anti-tumor promoting activity of silymarin in two stage skin carcinogenesis model, and further extension of this work by our group revealed that silymarin possesses novel capacity to reduce the expression of endogenous tumor promoter TNFα in 7,12-dimethylbenz(a)anthracene (DMBA) initiated and TPA/Okadiac Acid (OA) promoted skin tumors in SENCAR mice (Zi et al., 1997). Silymarin was also found to possess in vivo therapeutic effects when given through diet against DMBA-initiated and TPA-promoted skin papilloma in SENCAR mice in yet another study conducted by us. In this study, we observed that silymarin was not only able to inhibit the growth of tumors, but importantly, was also able to cause regression of established tumors with no sign of toxicity to the animals. Additionally, detectable levels of silibinin were achieved in plasma and other tissues such as liver, lung, and skin tumors of the animals. Mechanistically, silymarin exerted its effect through the modulation of mitogen activated protein kinases and induction of apoptotic pathway. This observation has important implications for human skin cancer intervention at the benign stage, clinically defined as actinic keratosis (Singh et al., 2002).
UV exposure is the major etiological factor for non-melanoma skin cancer (NMSC), and our group revealed that silymarin exerts protective effects against UVB radiation induced skin tumor initiation, tumor promotion as well as complete carcinogenesis in SKH-1 hairless mice (Katiyar et al., 1997), wherein, silymarin caused significant inhibition in UVB-caused sunburn and apoptotic cell formation, skin edema, depletion of catalase activity and induction of cyclooxygenase (COX) and ornithine decarboxylase (ODC) expression and activity. Even silibinin exerted antiproliferative effects when applied topically pre- or post-UVB exposure, as well as when given in diet to SKH-1 hairless mice (Mallikarjuna et al., 2004). In additional mechanistic studies, our group found that silibinin affords protection against UV induced skin damage in SKH-1 hairless mice via the upregulation of p53-p21 cascade, a sensor and guardian of DNA from damage (Dhanalakshmi et al., 2004). Dietary feeding of silibinin prevented early biomarkers of UVB radiation induced skin carcinogenesis, such as reduced number of UVB-induced thymidine dimer-positive cells, proliferating cell nuclear antigen, apoptotic sunburn cells (Mallikarjuna et al., 2005). Using mouse epithelial JB6 cells, our group got further insight into the mechanism of efficacy of silibinin against UVB-induced skin tumorigenesis. It was observed that silibinin activates DNA-PK-p53 pathway for apoptotic response to UVB-induced DNA damage in JB6 cells (Dhanalakshmi et al., 2005). Topical application or dietary administration of silibinin also inhibited all the members of mitogen-activated protein kinases (MAPKs) family (ERK1/2, JNK and p38) and Akt activation induced by either acute or chronic UVB exposure of SKH-1 mouse skin (Mallikarjuna et al., 2005). Findings of all these studies provide a strong notion that silymarin/silibinin is an effective preventive agent against photocarcinogenesis.
Prostate Cancer
Prostate cancer is the second leading cause of cancer related deaths in US men (American Cancer Society, 2005). Failure of conventional treatment modalities, especially of hormone refractory PCA has lead to increased efforts in identifying new approaches to improve the outcome of this disease. In this direction, use of various chemopreventive agents, especially of natural origin has gained importance as these agents usually are the part of normal diet or taken as dietary supplements, and thus have lower or no systemic toxicity. In this regard, silymarin/silibinin showed potential anti-cancer activity against both hormone dependent- and independent- prostate cancer cell lines as mentioned earlier.
Studies aiming at testing in vivo efficacy of silymarin/silibinin conducted by our group revealed that dietary feeding of silibinin to athymic nude mice bearing DU145 prostate tumor xenograft significantly inhibits tumor growth and increases secreted levels of insulin-like growth factor binding protein 3 (IGFBP-3) in plasma without any toxicity symptoms in the animals fed with the agent (Singh et al., 2002). These findings were in concurrence with our in vitro studies with PC-3 cell lines, where we observed that silibinin upregulates the expression of IGFBP-3, and thus causes increased levels of secreted IGFBP-3 in conditioned medium (Zi et al., 2000). These observations have strong implications, as mitogenic and cell survival signaling mediated by IGFBP-insulin like growth factor-1 (IGF-1)/IGF-1R (insulin like growth factor-1 receptor) are often constitutively upregulated in human prostate carcinoma cell lines and are often implicated in advanced and androgen independent stage of prostate cancer (Stattin et al., 2000). In yet another study conducted by Kohno et al., dietary administration of silymarin at 100 or 500 ppm dose level for 40 weeks significantly reduced the incidence of prostatic adenocarcinoma in 3,2-dimethyl-4-aminobiphenyl (DMAB)-induced prostate carcinogenesis in male F344 rats (Kohno et al., 2005). Importantly, authors of this study also report that no toxicity symptoms were observed in the animals fed with silymarin supplemented diet for 40 weeks. Together, these observations convincingly support conducting clinical trials with silibinin in prostate cancer patients.
Hepatocellular Carcinoma
There is no dearth of evidence for hepatoprotective efficacy of silymarin/silibinin against liver conditions; however, there is only one study reporting the in vivo chemopreventive efficacy of silymarin in N-nitrosodiethylamine induced hepatocarcinogenesis in Wistar albino male rats (Ramakrishna et al., 2006). More efforts are needed in this direction in near future.
Breast Cancer
Studies conducted by us and others revealed that silibinin exerts synergistic anti-cancer effects with chemotherapeutic drugs such as doxorubicin, cisplatin and carboplatin in human breast carcinoma MCF-7 and MDA-MB468 cells (Scambia et al., 1996; Tyagi et al., 2004); however, there is only one study where authors studied the in vivo cancer preventive efficacy in rat model of mammary carcinogenesis. Contrary to anticancer efficacy of silymarin against breast cancer cell lines, in this model, dietary supplementation of silymarin modestly increased the number of mammary tumors in 1-methyl-1-nitrosourea (MNU) treated animals. In this study, increased incidence and multiplicity of mammary tumors were also observed by silymarin administration in neu-transgenic mice (Malewicz et al., 2006). More studies possibly are needed to further identify and characterize the mammary tumorigenesis enhancing effect of silymarin, which would help establishing a caution for its invariable use as anti-cancer and cancer preventive agent.
Bladder Cancer
We found that silibinin inhibits the growth and proliferation of human bladder transitional cell carcinoma (TCC) cells by causing cell cycle arrest and induction of apoptosis (Tyagi et al., 2004). Under in vivo conditions, there is only one report available, wherein, dietary administration of silymarin at the initiation or post initiation phase of N-butyl-N-(4-hydroxybutyl)nitrosamine (OH-BBN)-induced urinary bladder carcinogenesis in male ICR mice significantly decreased the incidence of bladder neoplasms and preneoplastic lesions. Immunohistochemical analysis revealed that silymarin significantly reduced the labeling index for BrdU and the cyclin D1-positive cell ratio in various bladder lesions (Vinh et al., 2002). These findings suggest that silymarin may be potential chemopreventive agent effective against bladder cancer.
Lung Cancer
Chemopreventive efficacy of silibinin against lung cancer was extensively explored by our group after the initial observation of anticancer effects of silibinin against lung carcinoma cell lines in culture (Sharma et al., 2003). Initial studies done by our group revealed that silibinin suppresses the growth of human non-small-cell lung carcinoma A549 xenograft in athymic BALB/c nu/nu mice. Silibinin also enhanced the therapeutic potential of traditional chemotherapeutic drug doxorubicin via an inhibition of doxorubicin-induced chemoresistance by modulating NF-κB mediated signaling pathway in this model. In addition, silibinin also improved the doxorubicin associated adverse health effects (Singh et al., 2004).
Further studies were carried out to assess the chemopreventive efficacy of silibinin in urethane-induced lung tumors in A/J mice. We observed that lung tumors in silibinin fed mice had significantly lower multiplicity and marked reduction in the size, when given for either 18 weeks or 29 weeks post urethane injection. Tumors in animals fed with silibinin had lesser positive cells for markers of proliferation such as PCNA and cyclinD1, and reduced microvessel density and expression of VEGF. Overall, the results of this study revealed the antiangiogenic potential of silibinin in animal model of lung tumorigenesis (Singh et al., 2006). However, in yet another chemical induced lung tumorigenesis model, no protective effect of silibinin was found when given prior to benzo(a)pyrene induced pulmonary adenoma formation and growth in A/J mice (Yan et al., 2005). Firstly, it is possible that silibinin exerts its effects primarily at post-initiation stage of lung tumorigenesis, and additionally, the dose levels of silibinin used in this study were much lower, and might not have achieved physiologically relevant levels in the animals. Secondly, in most of the in vitro lung cancer models, silibinin has been shown to inhibit the invasion of these cell lines (Chen et al., 2005; Chu et al., 2004). This might explain the absence of any protective effect against lung tumors in this particular model.
Colon Cancer
Though anticancer efficacy of silymarin/silibinin has been observed against colon carcinoma cell lines in number of studies, yet only two studies are available in literature evaluating the chemopreventive efficacy of silymarin using in vivo model of colon cancer. In the first study, authors report that rats fed with silymarin supplemented diet led to significant decrease in number of aberrant crypt foci (represent precursor lesions for colonic adenomas) in an azoxymethane (AOM)-induced rat colon cancer model (Volate et al., 2005). In another similar but more detailed study, it was observed that silymarin administration through diet for 4 weeks either during or after carcinogen (Azoxymethane) exposure led to a significant reduction in the frequency of aberrant crypt foci formation. In long term experiment, silymarin significantly reduced the incidence and multiplicity of colonic adenocarcinomas when fed during initiation or post initiation phase of AOM-induced colon carcinogenesis (Kohno et al., 2002). Silymarin exerted these beneficial effects via the reduction of proliferating cell nuclear antigen (PCNA) positive cells and increase in the number of apoptotic cells. Beneficial effects of silymarin also included decrease in the levels of prostaglandin E2 (PGE2), β-glucuronidase and polyamine content of colonic mucosa. In yet another model of 1,2-dimethylhydrazine induced colon carcinogenesis in SD rats, the frequency of adenocarcinoma of the colon, both total and occurrence at proximal or distal regions was significantly reduced with dietary administration of silymarin (Gershbein et al., 1994).
Ovarian Cancer
In case of ovarian cancer, studies have been conducted with either silibinin or IdB 1016, also marketed as Silipide, which represents the complex of silibinin with phosphotidylcholine to improve its bioavailability. In the completed studies, the authors observed that administration of IdB 1016 by oral gavage to nude mice bearing tumor xenograft of human ovarian cancer cell line A2780 produces significant tumor weight inhibition (TWI%) of 78% and a log10cell kill (LCK) of 1.1. In the same study, they were also able to measure the free levels of silibinin both in plasma and tumor tissue. However, no significant difference was observed in the VEGF levels in IdB1016 treated and untreated xenografts, though downregulation of VEGF receptor3 and upregulation of angiopeoitin-2 was observed upon gene array analysis (Gallo et al., 2003). In another study by same group, the authors reported that Silipide was able to potentiate the cytotoxicity of anticancer drug, cisplatin (CDDP) under in vitro conditions. Even under in vivo conditions, silipide was able to significantly enhance the anti-tumor activity of CDDP measured in terms of TWI % and log10cell kill(LCK) values. Importantly, mice receiving the combination therapy recovered from weight loss earlier to the ones receiving CCDP alone (Giacomelli et al., 2002).
Clinical Trials with Silibinin in Cancer Patients
There is one isolated report wherein, silibinin formulation, Silipide was given orally to patients with confirmed colorectal adenocarcinoma at doses ranging from 360 to 1440 mg of silibinin per day for seven weeks. Though intervention with silibinin was found to be ineffective in modulating the circulating levels of IGFBP-3, IGF-1 and pyrimidopurinone adduct of dexoguanosine, a marker for oxidative DNA damage; however it is worthy to note that high levels of silibinin were achieved in colorectal mucosa of the patients, which further supports the need of conducting more elaborate clinical trials for evaluating its efficacy as potential chemopreventive agent (Hoh et al., 2006). Our group has also recently completed a Phase-I clinical trial with silibinin in prostate cancer patients (Flaig et al., 2006). This trial was designed to assess the toxicity of high dose silybin-phytosome, a commercial preparation, and recommend a dose for phase II trial. Briefly, Silybin-phytosome was administered orally to prostate cancer patient at a daily dose of 2.5 – 20 grams, in three divided doses; each course was 4 weeks in duration. From the results obtained in the study, we concluded that thirteen grams of oral silybin-phytosome daily, in 3 divided doses, appears to be well tolerated in patients with advanced prostate cancer and is the recommended phase II dose. Further, an asymptomatic liver toxicity is the most commonly seen adverse event. Another important observation in this study was that we were able to achieve 100 μM plasma levels of free silibinin in patients, though this level was not sustained. Currently, we are in the process of starting a Phase II clinical trial to assess the effect of silibinin administration on prostate cancer progression using surrogate biomarkers as end points.
Conclusion
From the findings of all the studies summarized above briefly, it is clear that silymarin and its active constituent silibinin could have potential beneficial effects on the outcome of various epithelial malignancies. Accordingly, the future need is to conduct controlled clinical trials with these agents against various cancers, specifically employing commercially available standardized silymarin and silibinin preparations with improved bioavailability as well as proven efficacy against other pathological conditions in humans. In this direction, the results of the open preliminary pilot study with pharmaceutical complex of silibinin, RealSIL-IBI in patients with non alcoholic fatty liver disease with and without the presence of hepatitis-c virus-related chronic hepatitis are quite promising. In this study, no adverse effects of this complex were reported in the patients, and there was improvement in the insulin resistance and markers of fibrosis in plasma (Trappoliere et al., 2005). Additionally, in number of clinical trials, mostly in patients with liver diseases, silibinin/silymarin is well tolerated (Gordon et al., 2006; Strickland et al., 2005). These observations have significant relevance for translating the basic research to clinical settings, as two major hurdles in this transition i.e. bioavailability and toxicity, have been somewhat defined for silymarin and silibinin. Even the proven hepatoprotective effects of silymarin and silibinin confer added advantage of using them in adjuvant therapy, not limiting only to their cancer chemopreventive efficacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrol S, Trehan A, Katare OP. Comparative study of different silymarin formulations: formulation, characterisation and in vitro/in vivo evaluation. Curr Drug Deliv. 2005;2:45–51. doi: 10.2174/1567201052772870. [DOI] [PubMed] [Google Scholar]
- Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- Agarwal R, Katiyar SK, Lundgren DW, Mukhtar H. Inhibitory effect of silymarin, an anti-hepatotoxic flavonoid, on 12-O-tetradecanoylphorbol-13-acetate-induced epidermal ornithine decarboxylase activity and mRNA in SENCAR mice. Carcinogenesis. 1994;15:1099–1103. doi: 10.1093/carcin/15.6.1099. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Gali H, Javed S, Agarwal R. Skin cancer chemopreventive effects of a flavonoid antioxidant silymarin are mediated via impairment of receptor tyrosine kinase signaling and perturbation in cell cycle progression. Biochem Biophys Res Commun. 1998;247:294–301. doi: 10.1006/bbrc.1998.8748. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts and Figures. 2005:1–60. [Google Scholar]
- Bhatia N, Agarwal R. Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. Prostate. 2001;46:98–107. doi: 10.1002/1097-0045(20010201)46:2<98::aid-pros1013>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. Review. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Fels LM, Dunn T, Voigt W, Gaedeke J, Schmoll HJ, Stolte H, Lentzen H. Silibinin protects against cisplatin-induced nephrotoxicity without compromising cisplatin or ifosfamide anti-tumour activity. Br J Cancer. 1996;74:2036–2041. doi: 10.1038/bjc.1996.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby A, La Grange L, Edwards J, King J. The use of a silymarin/phospholipid compound as a fetoprotectant from ethanol-induced behavioral deficits. J Herb Pharmacother. 2002;2:39–47. [PubMed] [Google Scholar]
- Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact. 2005;156:141–150. doi: 10.1016/j.cbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Chlopcikova S, Psotova J, Miketova P, Simanek V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part I Silymarin and its flavonolignans. Phytother Res. 2004;18:107–110. doi: 10.1002/ptr.1415. [DOI] [PubMed] [Google Scholar]
- Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40:143–149. doi: 10.1002/mc.20018. [DOI] [PubMed] [Google Scholar]
- Chung SY, Sung MK, Kim NH, Jang JO, Go EJ, Lee HJ. Inhibition of P-glycoprotein by natural products in human breast cancer cells. Arch Pharm Res. 2005;28:823–828. doi: 10.1007/BF02977349. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Sanchez Pozzi EJ, Pellegrino JM, Favre CO, Rodriguez Garay EA, Mottino AD, Coleman R, Roma MG. Beneficial effects of silymarin on estrogen-induced cholestasis in the rat: a study in vivo and in isolated hepatocyte couplets. Hepatology. 2001;34:329–339. doi: 10.1053/jhep.2001.26520. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Sanchez Pozzi EJ, Pellegrino JM, Rodriguez Garay EA, Mottino AD, Roma MG. Preventive effect of silymarin against taurolithocholate-induced cholestasis in the rat. Biochem Pharmacol. 2003;66:355–364. doi: 10.1016/s0006-2952(03)00253-3. [DOI] [PubMed] [Google Scholar]
- Culpeper N. A Physical Directory: or a Translation of the Dispensatory made by the College of Physicians of London. London, England: Peter Cole; 1650. [Google Scholar]
- Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–754. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis. 2004;25:99–106. doi: 10.1093/carcin/bgg188. [DOI] [PubMed] [Google Scholar]
- Dhanalakshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005;280:20375–20383. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- El-Samaligy MS, Afifi NN, Mahmoud EA. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int J Pharm. 2006;319:121–129. doi: 10.1016/j.ijpharm.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Flaig TW, Gustafson DL, Su L-J, Zirrolli J, Harrison G, Pierson AS, Agarwal R, Glodé LM. A Phase I and Pharmacokinetic Study of Silybin-Phytosome in prostate cancer patients. Investigational New Drugs, Accepted. 2006 doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- Foster S. Milk Thistle: Silybum marianum. Austin, Tex: American Botanical Council.; 1999. [Google Scholar]
- Gallo D, Giacomelli S, Ferlini C, Raspaglio G, Apollonio P, Prislei S, Riva A, Morazzoni P, Bombardelli E, Scambia G. Antitumour activity of the silybin-phosphatidylcholine complex, IdB 1016, against human ovarian cancer. Eur J Cancer. 2003;39:2403–2410. doi: 10.1016/s0959-8049(03)00624-5. [DOI] [PubMed] [Google Scholar]
- Gershbein LL. Action of dietary trypsin, pressed coffee oil, silymarin and iron salt on 1,2-dimethylhydrazine tumorigenesis by gavage. Anticancer Res. 1994;14:1113–1116. [PubMed] [Google Scholar]
- Giacomelli S, Gallo D, Apollonio P, Ferlini C, Distefano M, Morazzoni P, Riva A, Bombardelli E, Mancuso S, Scambia G. Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin. Life Sci. 2002;70:1447–1459. doi: 10.1016/s0024-3205(01)01511-9. [DOI] [PubMed] [Google Scholar]
- Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, Francis AJ, Roberts SK. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:275–280. doi: 10.1111/j.1440-1746.2006.04138.x. [DOI] [PubMed] [Google Scholar]
- Gu M, Dhanalakshmi S, Mohan S, Singh RP, Agarwal R. Silibinin inhibits ultraviolet B radiation-induced mitogenic and survival signaling, and associated biological responses in SKH-1 mouse skin. Carcinogenesis. 2005;26:1404–1413. doi: 10.1093/carcin/bgi096. [DOI] [PubMed] [Google Scholar]
- Hoh C, Boocock D, Marczylo T, Singh R, Berry DP, Dennison AR, Hemingway D, Miller A, West K, Euden S, Garcea G, Farmer PB, Steward WP, Gescher AJ. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res. 2006;12:2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- Jiang C, Agarwal R, Lu J. Anti-angiogenic potential of a cancer chemopreventive flavonoid antioxidant, silymarin: inhibition of key attributes of vascular endothelial cells and angiogenic cytokine secretion by cancer epithelial cells. Biochem Biophys Res Commun. 2000;276:371–378. doi: 10.1006/bbrc.2000.3474. [DOI] [PubMed] [Google Scholar]
- Kang JS, Park SK, Yang KH, Kim HM. Silymarin inhibits TNF-alpha-induced expression of adhesion molecules in human umbilical vein endothelial cells. FEBS Lett. 2003;550:89–93. doi: 10.1016/s0014-5793(03)00827-5. [DOI] [PubMed] [Google Scholar]
- Karimi G, Ramezani M, Tahoonian Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid Based Complement Alternat Med. 2005;2:383–386. doi: 10.1093/ecam/neh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- Kelemen LE, Cerhan JR, Lim U, Davis S, Cozen W, Schenk M, Colt J, Hartge P, Ward MH. Vegetables, fruit, and antioxidant-related nutrients and risk of non-Hodgkin lymphoma: a National Cancer Institute-Surveillance, Epidemiology, and End Results population-based case-control study. Am J Clin Nutr. 2006;83:1401–1410. doi: 10.1093/ajcn/83.6.1401. [DOI] [PubMed] [Google Scholar]
- Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos) Altern Med Rev. 2005;10:193–203. [PubMed] [Google Scholar]
- Kittur S, Wilasrusmee S, Pedersen WA, Mattson MP, Straube-West K, Wilasrusmee C, Lubelt B, Kittur DS. Neurotrophic and neuroprotective effects of milk thistle (Silybum marianum) on neurons in culture. J Mol Neurosci. 2002;18:265–269. doi: 10.1385/jmn:18:3:265. [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Sugie S, Tsuda H, Tanaka T. Dietary supplementation with silymarin inhibits 3,2′-dimethyl-4-aminobiphenyl-induced prostate carcinogenesis in male F344 rats. Clin Cancer Res. 2005;11:4962–4967. doi: 10.1158/1078-0432.CCR-05-0137. [DOI] [PubMed] [Google Scholar]
- Kohno H, Tanaka T, Kawabata K, Hirose Y, Sugie S, Tsuda H, Mori H. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int J Cancer. 2002;101:461–468. doi: 10.1002/ijc.10625. [DOI] [PubMed] [Google Scholar]
- Krecman V, Skottova N, Walterova D, Ulrichova J, Simanek V. Silymarin inhibits the development of diet-induced hypercholesterolemia in rats. Planta Med. 1998;64:138–142. doi: 10.1055/s-2006-957391. [DOI] [PubMed] [Google Scholar]
- Lahiri-Chatterjee M, Katiyar SK, Mohan RR, Agarwal R. A flavonoid antioxidant, silymarin, affords exceptionally high protection against tumor promotion in the SENCAR mouse skin tumorigenesis model. Cancer Res. 1999;59:622–632. [PubMed] [Google Scholar]
- Lee JI, Narayan M, Barrett JS. Analysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Aug 28; doi: 10.1016/j.jchromb.2006.07.063. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Luper S. A review of plants used in the treatment of liver disease: part 1. Altern Med Rev. 1998;3:410–421. [PubMed] [Google Scholar]
- Malewicz B, Wang Z, Jiang C, Guo J, Cleary MP, Grande JP, Lu J. Enhancement of mammary carcinogenesis in two rodent models by silymarin dietary supplements. Carcinogenesis. 2006;27:1739–1747. doi: 10.1093/carcin/bgl032. [DOI] [PubMed] [Google Scholar]
- Mallikarjuna G, Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64:6349–6356. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–2130. doi: 10.1016/j.lfs.2005.12.003. Review. [DOI] [PubMed] [Google Scholar]
- Nencini C, Giorgi G, Micheli L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine. 2006 Apr 22; doi: 10.1016/j.phymed.2006.02.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Niture SK, Velu CS, Smith QR, Bhat GJ, Srivenugopal KS. Increased expression of the MGMT repair protein mediated by cysteine prodrugs and chemopreventative natural products in human lymphocytes and tumor cell lines. Carcinogenesis. 2006 Aug 31; doi: 10.1093/carcin/bgl155. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pares A, Planas R, Torres M, Caballeria J, Viver JM, Acero D, Panes J, Rigau J, Santos J, Rodes J. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83:1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan G, Raghavendran HR, Vinodhkumar R, Devaki T. Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem Biol Interact. 2006;161:104–114. doi: 10.1016/j.cbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Protection against post-ischemic mitochondrial injury in rat liver by silymarin or TUDC. Hepatol Res. 2003;26:217–224. doi: 10.1016/s1386-6346(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Scambia G, De Vincenzo R, Ranelletti FO, Panici PB, Ferrandina G, D’Agostino G, Fattorossi A, Bombardelli E, Mancuso S. Antiproliferative effect of silybin on gynaecological malignancies: synergism with cisplatin and doxorubicin. Eur J Cancer. 1996;32A:877–882. doi: 10.1016/0959-8049(96)00011-1. [DOI] [PubMed] [Google Scholar]
- Schumann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. Silibinin protects mice from T cell-dependent liver injury. J Hepatol. 2003;39:333–340. doi: 10.1016/s0168-8278(03)00239-3. [DOI] [PubMed] [Google Scholar]
- Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23:2649–2655. [PubMed] [Google Scholar]
- Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23:499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62:3063–3069. [PubMed] [Google Scholar]
- Singh RP, Mallikarjuna GU, Sharma G, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin Cancer Res. 2004;10:8641–8647. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, Agarwal R. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, McClain C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006;30:407–413. doi: 10.1111/j.1530-0277.2006.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. Silibinin protects mice from T cell-dependent liver injury. J Hepatol. 2003;39:333–340. doi: 10.1016/s0168-8278(03)00239-3. [DOI] [PubMed] [Google Scholar]
- Soto C, Mena R, Luna J, Cerbon M, Larrieta E, Vital P, Uria E, Sanchez M, Recoba R, Barron H, Favari L, Lara A. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004;75:2167–2180. doi: 10.1016/j.lfs.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, Kaaks R. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92:1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I Epidemiology. Cancer Causes Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- Strickland GT, Tanamly MD, Tadros F, Labeeb S, Makld H, Nessim D, Mikhail N, Magder LS, Afdhal NH, Medhat A, Abdel-Hamid M. Two-year results of a randomised double-blinded trial evaluating silymarin for chronic hepatitis C. Dig Liver Dis. 2005;37:542–543. doi: 10.1016/j.dld.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. Review. [DOI] [PubMed] [Google Scholar]
- Szilard S, Szentgyorgyi D, Demeter I. Protective effect of Legalon in workers exposed to organic solvents. Acta Med Hung. 1988;45:249–256. [PubMed] [Google Scholar]
- Thelen P, Jarry H, Ringert RH, Wuttke W. Silibinin down-regulates prostate epithelium-derived Ets transcription factor in LNCaP prostate cancer cells. Planta Med. 2004;70:397–400. doi: 10.1055/s-2004-818965. [DOI] [PubMed] [Google Scholar]
- Trappoliere M, Federico A, Tuccillo C, de Sio I, Di Leva A, Niosi M, D’Auria M, Loguercio C Real Sud Group 2. Effects of a new pharmacological complex (silybin + vitamin-E + phospholipids) on some markers of the metabolic syndrome and of liver fibrosis in patients with hepatic steatosis. Minerva Gastroenterol Dietol. 2005;51:193–199. Preliminary study. [PubMed] [Google Scholar]
- Trappoliere M, Federico A, Tuccillo C, de Sio I, Di Leva A, Niosi M, D’Auria M, Loguercio C Real Sud Group 2. Effects of a new pharmacological complex (silybin + vitamin-E + phospholipids) on some markers of the metabolic syndrome and of liver fibrosis in patients with hepatic steatosis. Minerva Gastroenterol Dietol. 2005;51:193–199. Italian - Preliminary study. [PubMed] [Google Scholar]
- Tyagi AK, Agarwal C, Chan DC, Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol Rep. 2004;11:493–499. [PubMed] [Google Scholar]
- Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis. 2004;25:1711–1720. doi: 10.1093/carcin/bgh180. [DOI] [PubMed] [Google Scholar]
- Varghese L, Agarwal C, Tyagi A, Singh RP, Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res. 2005;11:8441–8448. doi: 10.1158/1078-0432.CCR-05-1646. [DOI] [PubMed] [Google Scholar]
- Velussi M, Cernigoi AM, De Monte A, Dapas F, Caffau C, Zilli M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26:871–879. doi: 10.1016/s0168-8278(97)80255-3. [DOI] [PubMed] [Google Scholar]
- Vinh PQ, Sugie S, Tanaka T, Hara A, Yamada Y, Katayama M, Deguchi T, Mori H. Chemopreventive effects of a flavonoid antioxidant silymarin on N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis in male ICR mice. Jpn J Cancer Res. 2002;93:42–49. doi: 10.1111/j.1349-7006.2002.tb01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26:1450–1456. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- Ward MH. Vegetables, fruit, and antioxidant-related nutrients and risk of non-Hodgkin Pliny the Elder. Historia Naturalis. 2006 doi: 10.1093/ajcn/83.6.1401. 77 A.D. [DOI] [PubMed] [Google Scholar]
- Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–489. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- Wilasrusmee C, Kittur S, Shah G, Siddiqui J, Bruch D, Wilasrusmee S, Kittur DS. Immunostimulatory effect of Silybum Marianum (milk thistle) extract. Med Sci Monit. 2002;8:BR439–443. www.who.int/mediacentre/factsheets/fs297/en/index.html. [PubMed]
- Yan Y, Wang Y, Tan Q, Lubet RA, You M. Efficacy of deguelin and silibinin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2005;7:1053–1057. doi: 10.1593/neo.05532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Lin JK, Chen WS, Chiu JH. Anti-angiogenic effect of silymarin on colon cancer LoVo cell line. J Surg Res. 2003;113:133–138. doi: 10.1016/s0022-4804(03)00229-4. [DOI] [PubMed] [Google Scholar]
- Zhao J, Agarwal R. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention. Carcinogenesis. 1999;20:2101–2108. doi: 10.1093/carcin/20.11.2101. [DOI] [PubMed] [Google Scholar]
- Zi X, Mukhtar H, Agarwal R. Novel cancer chemopreventive effects of a flavonoid antioxidant silymarin: inhibition of mRNA expression of an endogenous tumor promoter TNF alpha. Biochem Biophys Res Commun. 1997;239:334–339. doi: 10.1006/bbrc.1997.7375. [DOI] [PubMed] [Google Scholar]
- Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin Cancer Res. 1998;4:1055–1064. [PubMed] [Google Scholar]
- Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci U S A. 1999;96:7490–7495. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi X, Zhang J, Agarwal R, Pollak M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res. 2000;60:5617–5620. [PubMed] [Google Scholar]


