Abstract
On the basis of the accelerated age-related effects in type II muscle, we hypothesized that with aging the semimembranosus (type II) muscle would accumulate a greater amount of oxidized proteins compared to proteins in the soleus (type I) muscle. In this study, 3-nitrotyrosine (3-NT) was used as a stable marker of protein oxidative damage. The presence of 3-NT was evaluated in muscles from young adult, old, and very old Fischer 344 rats to provide an indication of the time course of muscle protein oxidative damage. A significant age-associated increase in nitrotyrosine-modified proteins was observed. The modified proteins identified by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry include the sarcoplasmic reticulum Ca+2-ATPase, aconitase, β-enolase, triosephosphate isomerase, and carbonic anhydrase III. These proteins, involved in metabolism and calcium homeostasis, exhibited an age-dependent increase in 3-NT content in both muscles. However, significant levels of 3-NT modification were present at an earlier age in the semimembranosus muscle.
Oxidative damage to cellular macromolecules has long been implicated in the aging process. The “Free Radical Hypothesis of Aging,” first introduced by Denham Harman in 1956, has been modified to the “Oxidative Stress Hypothesis of Aging.” Oxidative stress is defined as an imbalance of pro-oxidants and antioxidants that results in the accumulation of oxidative damage to a variety of macromolecules (1). The Oxidative Stress Hypothesis of Aging postulates that the cumulative oxidative damage that occurs during aging results in a progressive loss in function of various cellular processes (1).
Skeletal muscle is particularly vulnerable to oxidative stress. This is due, in part, to the rapid and coordinated changes in energy supply and oxygen flux that occur during contraction, resulting in increased electron flux and leakage from the mitochondrial electron transport chain (2). Skeletal muscle also contains a high concentration of myoglobin, a heme-containing protein known to confer greater sensitivity to free radical–induced damage to surrounding macromolecules by converting hydrogen peroxide to other more highly reactive oxygen species (ROS) (3). The greater concentration of myoglobin in type I fibers (slow-twitch) compared to type II fibers (fast-twitch) suggests the potential for fiber type–specific differences in susceptibility to oxidative stress.
Other fundamental differences between type I and type II fibers may confer differing degrees of susceptibility to oxidative stress. For example, the major energy pathway used in type I fibers occurs through oxidative metabolism, whereas the glycolytic pathway is the primary means for generating energy in type II fibers. Thus, type I fibers, compared with type II fibers, likely produce greater ROS via mitochondrial oxidative phosphorylation. To counter the effects of ROS, type I fibers have higher antioxidant capacities that prevent or attenuate oxidative damage (4–6). Although type II fibers may generate lower levels of ROS during metabolism than do type I fibers, type II fibers may be more susceptible to oxidative stress because their antioxidant defenses are less robust. These fiber-type differences in susceptibility to oxidative stress may be mechanistically related to the aging phenotype, where type II fibers suffer the greatest age-related atrophy and loss in function (6). In the present study, we hypothesized that proteins in the rat semimembranosus muscle will accumulate oxidative damage at an earlier age compared to proteins in the soleus muscle.
In this study, we compared the protein oxidative damage of skeletal muscle proteins in two muscles, the soleus and semimembranosus, each composed of different skeletal muscle fiber types. Specifically, the soleus muscle is composed of >90% type I fibers, whereas the semimembranosus is composed of. >90% type IIB fibers (7). We used 3-nitrotyrosine (3-NT) as a stable marker of protein oxidative damage. This posttranslational modification can alter protein function and is associated with acute and chronic disease states (8). When tyrosine is nitrated by peroxynitrite, a highly reactive molecule generated by the reaction of nitric oxide with superoxide, 3-NT is formed (9). Muscle cells are exposed to periodic fluxes of nitric oxide and superoxide, thus providing favorable conditions for the formation of peroxynitrite. The presence of 3-NT was evaluated in muscles from young adult, old, and very old Fischer 344 (F344) rats to provide an indication of the time course of protein oxidative damage. The purpose of the experiments was to ask whether there are muscle-specific differences in oxidative damage that could partially account for the loss in soleus and semimembranosus function with age.
Materials and Methods
Materials
Eighteen male F344 rats were purchased from the Minneapolis Veterans Administration Aged Rodent Colony, maintained by the University of Minnesota. A monoclonal antibody recognizing 3-NT was purchased from Upstate Biotechnology (Lake Placid, NY). Monoclonal antibodies recognizing sarcoplasmic reticulum (SR) Ca+2-ATPase 2a (SERCA2a) and SR Ca+2-ATPase 1 (SERCA1) were purchased from Affinity Bioreagents (Golden, CO). Goat anti-mouse biotinylated secondary antibody was purchased from Rockland Immunochemical, Inc. (Gilbertsville, PA). Alkaline phosphatase–conjugated anti-mouse secondary antibody, Alkaline Phosphatase Conjugated Substrate Kit containing 5-bromo-4-chloro-3′-indolylphospate ρ-toluidine and nitro-blue tetrazolium chloride (BCIP-NBT), streptavidin, and all agents for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) were supplied by BioRad (Hercules, CA). A full-range molecular weight marker was purchased from Amersham-Pharmacia-Hoefer (Piscataway, NJ). Immobilon-P polyvinylidene difluoride (PVDF) membrane (0.45 μm) for Western immunoblotting was purchased from Millipore (Bedford, MA). The bicinchoninic acid (BCA) protein assay kit was obtained from Pierce (Rockford, IL).
Preparation of Skeletal Muscle Homogenates
Ages of rats included in this study were 7–11 months (young adult), 22–25 months (old), and 27–30 months (very old) (10). Six rats per age group were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)–approved animal facility at the University of Minnesota, which is maintained at 20°C with a 12-hour light/dark cycle. Standard rat chow and water were provided ad libitum. An animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Minnesota and followed the guidelines established by the National Institutes of Health.
Prior to skeletal muscle dissection, rats were killed with a lethal dose of pentobarbital sodium (100 mg/kg body weight). The soleus and semimembranosus muscles were removed, weighed, and immediately frozen in liquid nitrogen and stored at −80°C until homogenization. For the homogenization procedure, a small piece of each muscle was cut, cooled in liquid nitrogen, and ground using a mortar and pestle. The ground tissue was then suspended in a buffer containing 50 mM Tris (pH 7.4) and 10% sucrose, and was stored at −80°C. Protein concentrations were determined using the BCA protein assay reagents (Pierce). Bovine serum albumin was used as a protein standard.
One-Dimensional Gel Electrophoresis
Prior to Western immunoblotting to localize proteins containing 3-NT, skeletal muscle proteins were electrophoretically separated by SDS–PAGE using a Mighty Small SE 250 system (Amersham-Pharmacia-Hoefer) and a 10% resolving gel with 3% stacking gel (11). Protein loads for preparations from skeletal muscle were 20 μg per lane. To increase the resolution of the bands, the use of a large format gel (16 cm × 18 cm) for SDS–PAGE (Amersham-Pharmacia-Hoefer) was necessary. For each sample, two gels were run in parallel. One gel was silver stained using the mass spectrometry(MS)-compatible Silver Stain Plus Kit (BioRad). Thealternate gel was used for Western immunoblotting. Images were captured using a Fluor-S MultiImaging System (BioRad).
Western Immunoblotting
Proteins were electrophoretically separated by SDS–PAGE using 5%, 10%, or 13% resolving gels with a 3% or 4% stacking gel; they were then transferred to PVDF membranes (11). Membranes were incubated with a 3-NT (1:2000) monoclonal antibody. Goat anti-mouse alkaline phosphatase–conjugated secondary antibody (1:3000) and the substrate BCIP-NBT were used to visualize the immunoreaction. Membranes were imaged using the Fluor-S Multi-Imaging System (BioRad) and quantified by densitometric analysis using Sigma Scan Pro (Systat, Point Richmond, CA).
Selection and Preparation of Proteins for MS
Western immunoblotting of one-dimensional gels were used to identify proteins modified by 3-NT. To align protein bands exhibiting an immunoreaction with bands on silver-stained gels, images of Western immunoblots and their corresponding gels were printed on transparencies and overlaid on a lightbox. Selected bands were excised and proteins digested in gel overnight at 37°C with trypsin as described (12). Prior to protease digestion, the cysteine residues were reduced with dithiothreitol and alkylated using iodoacetamide. Peptides were extracted from gels by repeated swelling and shrinking of the gel using 25 mM ammonium hydrogen carbonate and acetonitrile 1:1 (vol/vol), followed by 5% formic acid and acetonitrile 1:1 (vol/vol) (13). Extracted peptides were evaporated to near dryness in a Speed Vac (GMI, Inc., Ramsey, MN) and stored at −80°C prior to mass spectral analysis.
Matrix-Assisted Laser Desorption Ionization-Time of Flight MS
Prior to matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) MS analysis, a portion of the peptide mixture was desalted using Millipore C18 ZipTips using the protocol of the manufacturer. Full scan mass spectra from 500 to 3500 m/z of the tryptic peptide mixtures were collected in positive mode by averaging;50–200 spectra. Data were acquired on a QSTAR XL quadrupole-TOF Applied Biosystems (ABI; Foster City, CA) mass spectrometer. The peptide data were collected in the reflectron mode, with an accelerating potential of 19 kV using α-cyano-4-hydroxycinnamic acid (Agilent Technologies, Palo Alto, CA) diluted 1:1 (vol/vol) with a 50:50 acetonitrile/Nanopure water mixture and 0.1% trifluoroacetic acid. External calibration was performed using human angiotensin II (monoisotropic [MH+] of m/z 1046.5417; Sigma), angiotensin I (monoisotropic [MH+] of m/z 1296.6853; Sigma), and adrenocorticotropin hormone fragment 18–39 (monoisotropic [MH+] of m/z 2465.1989; Sigma). ABI’s Bayesian Peptide Reconstruct Tool, which is very robust (because multiple peaks in an isotope series are used during the Bayesian calculation), was used to generate a peak list for sample peptides.
Measured peptide mass to charge ratios (m/z) were used to search the National Center for Biotechnology Information (NCBI) and Swiss-Prot sequence databases for protein identifications using Mascot (www.matrixscience.com). BioAnalyst (ABI) software and Mascot were used to obtain tandem mass spectrometry (MS/MS) sequence information from the product ion spectra. All searches were performed with a mass tolerance between 50 and 100 ppm and with a fragment mass tolerance of 100 amu for MS/MS analysis. Positive identification required a minimum of three peptide matches and a probability score that indicates high concordance between the masses of experimentally derived peptides with theoretical masses of peptides from the matched protein. For most proteins, a positive identification from at least one product ion spectrum was also obtained.
Western Immunoblotting for Sarcoplasmic Reticulum Ca2+-ATPase, SERCA1, and SERCA2a
To determine the oxidative damage status of SERCA1 and SERCA2a, proteins were electrophoretically separated by SDS–PAGE with a 5% gel and then transferred to PVDF membranes. Subsequently, the membranes were incubated with a 3-NT (1:2000) monoclonal antibody, a monoclonal antibody recognizing SERCA2a (1:500) antibody, or a monoclonal antibody recognizing SERCA1 (1:5000) antibody. Biotinylated goat anti-mouse secondary antibody (1:3000), biotinylated alkaline phosphatase (1:3000) with streptavidin (1:3000), and the substrate BCIP-NBT were used to visualize the immunoreaction.
Statistical Analysis
Muscle-specific immune reactions were compared between age groups by using a one-way analysis of variance (ANOVA), with the level of significance set at p ≤ .05. When appropriate, post hoc analysis was performed using the Tukey–Kramer test.
Results
To discern muscle- and age-specific differences in tyrosine nitration, the soleus and semimembranosus muscles were isolated from rats at ages corresponding with young adulthood (98% survival rate), early senescence (50% survival rate), and late senescence (15% survival rate) (10). Proteins from homogenates of the semimembranosus and soleus muscle were separated by 5%, 10%, and 13% SDS–PAGE to preferentially resolve higher, middle, and lower molecular mass proteins, respectively. For each experiment, two matched gels were run in parallel. One gel was stained with silver to verify identical protein loads for each sample and to provide peptides for MS analysis. For the second gel of the matched set, proteins were electrophoretically transferred to a PVDF membrane and probed for 3-NT using an antibody that specifically recognizes this posttranslational modification. Figure 1 shows representative silver-stained gels and Western immunoblots of proteins from the soleus and semimembranosus muscles from the three age groups examined. In both muscles, the 3-NT antibody reacted with proteins of similar molecular weights, suggesting that the protein targets of peroxynitrite-induced oxidation were consistent, irrespective of the muscle (Figure 1).
Figure 1.
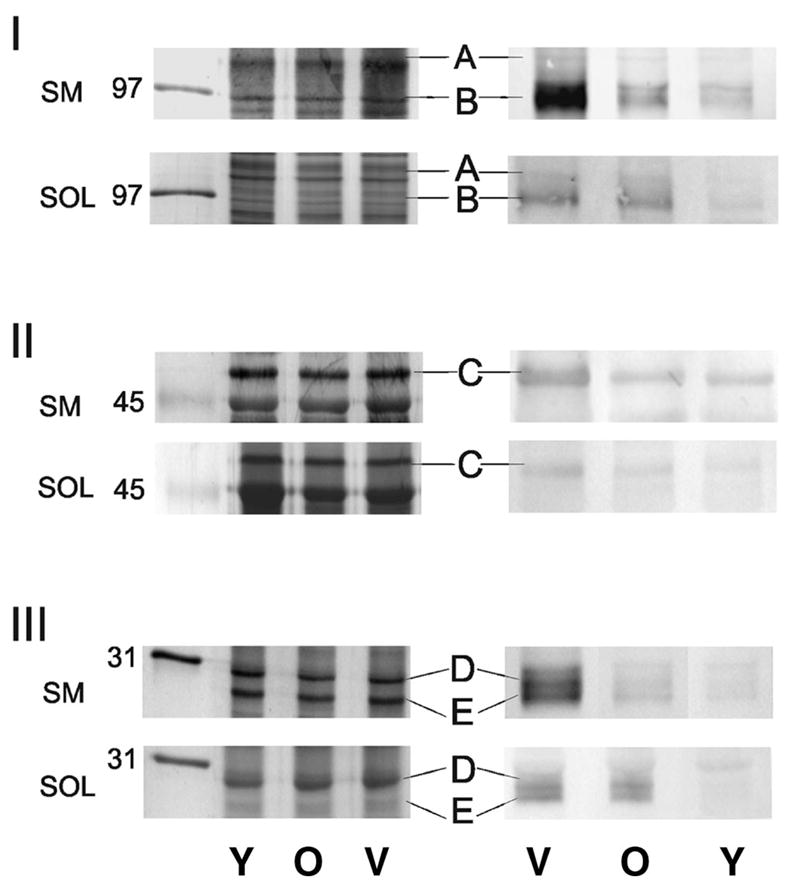
Representative silver-stained gels and Western blots for semimembranosus (SM) and soleus (SOL) muscle proteins. Panels I, II, and III show 5%, 10%, and 13% gels (left) and Western immunoblots (right) for the reaction of an antibody that recognizes 3-nitrotyrosine. Protein loads were 20 μg. Letters A–E indicate proteins demonstrating significant age-related changes in immune reactions that were identified by mass spectrometry. Mobilities of molecular weight markers are indicated in kilodaltons. V = very old; O = old; Y = young.
Densitometric analysis was performed on immune reactions corresponding to individual protein bands to evaluate relative 3-NT content between age groups within each muscle. Although silver-stained gels confirm similar protein loads for each sample (Figure 1, left column), the Western blot clearly demonstrates an age-dependent increase in nitrotyrosine content within select proteins in each muscle (Figure 1, right column). Although many protein bands showed a positive immune reaction with the 3-NT antibody, we performed in-gel trypsin digestion and MS analysis on only the protein bands that demonstrated an age-dependent statistically significant (i.e., >2-fold) difference in immune reaction in at least one muscle (summarized in Figure 2).
Figure 2.
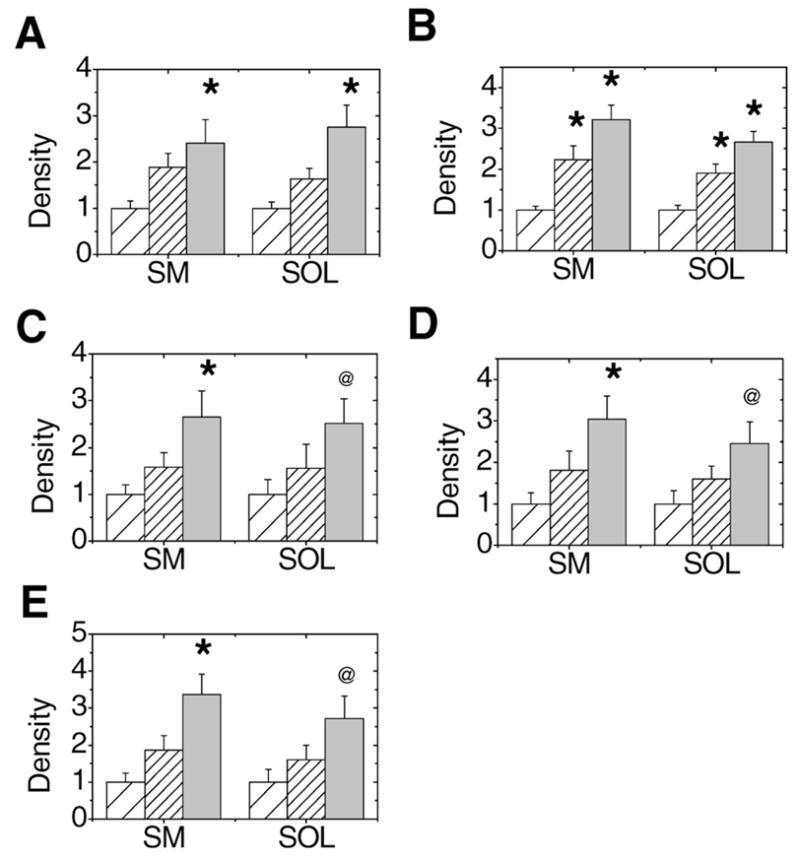
Densitometric analysis of immune reactions for nitrated proteins in semimembranosus (SM) and soleus (SOL) muscle homogenates. Young adult, old, and very old age groups are represented with sparse hatch, dense hatch, and light gray bars, respectively. A–E, Plots summarize the mean (± standard error) of densitometric values from each band that were normalized to the immuno-reaction in a standard sample from young adult muscle homogenate. Blots represent sarcoplasmic reticulum Ca2+-ATPase (A), aconitase (B), β-enolase (C), carbonic anhydrase III (D), and triosephosphate isomerase (E). *p ≤ .05, significantly different between young adult and old or very old rats; @p ≤ .10, comparing young adult and very old rats.
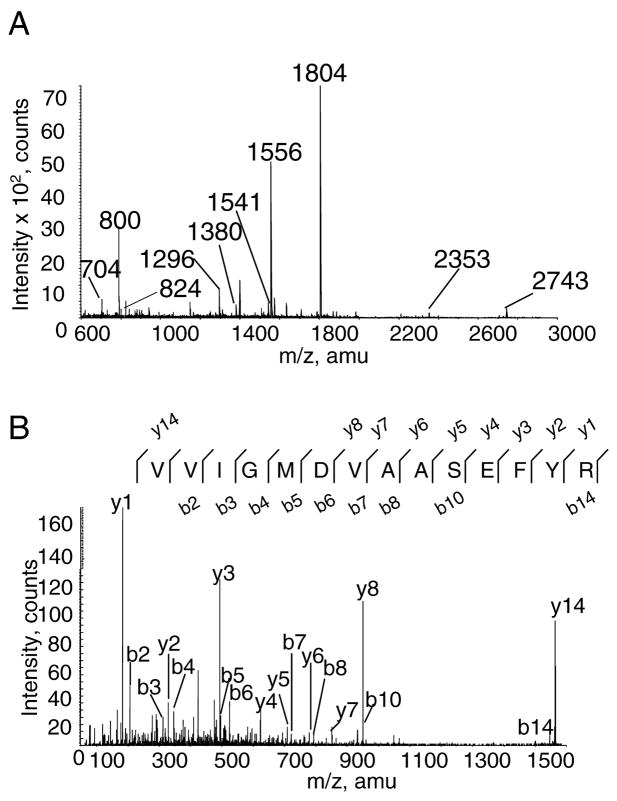
For the five 3-NT–containing proteins that showed a significant age-associated increase in immune reaction, protein identities were obtained using MALDI-TOF MS. Peptide fingerprints from full scans were used to obtain an initial identification. In most instances, tandem MS sequencing of one or more peptides confirmed the protein’s identity. Figure 3A shows a representative MALDI-TOF mass spectrum of peptides generated from an in-gel trypsin digest of a protein band that was identified as β-enolase. Thirteen peptides representing 35% of the primary sequence for β-enolase were identified from the peptide mass fingerprint (Table 1). In this example, two peptides were sequenced by MS/MS analysis, thus providing unambiguous identification of the protein. The tandem MS spectrum for peptide 1556 m/z is shown in Figure 3B. The fragmentation of this peptide produced a product ion spectrum containing 9 of 14 b ions and 9 of 14 y ions that matched the amino acid sequence for residues 239–252 of β-enolase.
Figure 3.
Mass spectrometric (MS) analysis of tryptic peptides from β-enolase. A, Full scan of a matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) MS peptide mass fingerprint from band C. The spectrum shows that 10 of the 13 peaks matched the theoretical m/z values for residues in the sequence from β-enolase. B, Product ion spectrum of the 1556 m/z of the VVIGMDVAASEFYR peptide formed by MALDI ionization. The amino acid sequence is displayed above the spectrum, and the y- and b-type ions found experimentally are written above the corresponding peak in the spectrum. amu = atomic mass unit.
Table 1.
MALDI-TOF MS Identification of 3-Nitrotyrosine-Immunopositive Bands
| Protein | Accession No.* | Mass (Da) | MALDI-TOF MS |
||
|---|---|---|---|---|---|
| Score† | % Coverage | Peptides | |||
| SR Ca2+ATPase‡,§ | gi 17157987 | 110,707 | 165 | 24 | 17 |
| Aconitase | gi 18079339 | 86,164 | 79 | 21 | 11 |
| β-enolase‡ | gi 54035288 | 47,142 | 108 | 35 | 13 |
| Carbonic anhydrase III‡ | gi 31377484 | 29,567 | 141 | 62 | 11 |
| Triosephosphate isomerase | gi 6678413 | 27,044 | 64 | 43 | 6 |
Notes:
Acquired from the National Center for Biotechnology Information (NCBI) database.
MOWSE scores obtained from Mascot (www.matrixscience.com), score >54 indicates a significant match.
Protein confirmed by sequencing one or more peptides using tandem MS.
SERCA1 was identified by MALDI-TOF MS, whereas the immunoblots for 3-nitrotyrosine, SERCA1, and SERCA2a demonstrate that SERCA2a is the nitrated protein.
MALDI-TOF MS = matrix-assisted laser desorption ionization-time of flight mass spectrometry; SR = sarcoplasmic reticulum; SR Ca2+ATPase or SERCA = sarcoplasmic reticulum Ca+2-ATPase.
Table 1 summarizes the proteins identified by MS analysis that demonstrated a significant age-associated increase in 3-NT content. For all of these proteins, there was a general trend of increasing 3-NT content with aging. In several instances, however, significance was achieved in only the semimembranosus muscle at p ≤ .05. For example, β-enolase, triosephosphate isomerase (TPI), and carbonic anhydrase III contained significant levels of 3-NT in the semimembranosus muscle of very old rats compared with young rats. In the soleus muscle, 3-NT accumulated more in these proteins in very old rats compared with young rats, but the significance level was p ≤ .10.
For aconitase and the SR Ca-ATPase, significant elevations in 3-NT content were observed in both muscles. Of note, the significant protein nitration occurred at an earlier age for aconitase than it did for other proteins. For the SR Ca-ATPase, two fiber type–specific isoforms are found in skeletal muscle. These isoforms, SERCA1 and SERCA2a, are present in fast-twitch and slow-twitch fibers, respectively. In our initial screening, quantitation of Western immunoblots and protein identification of the 3-NT–positive band migrating at;100,000 daltons were performed using 10% SDS–PAGE gels. MS analysis identified the SERCA1 isoform as a component of the immune reactive protein band. However, it is likely that both SERCA1 and SERCA2a are comigrating in this band due to the similarity in mass between these two isoforms, thus preventing separation of the two proteins on a 10% gel. Because previous research had shown that modification of the SR Ca-ATPase was limited to the SERCA2a isoform, we performed additional experiments using 5% SDS–PAGE to clearly resolve SERCA1 and SERCA2a (Figure 4). Probing membranes with the antibody that recognizes 3-NT clearly shows that the immune reaction is limited to the SERCA2a protein.
Figure 4.
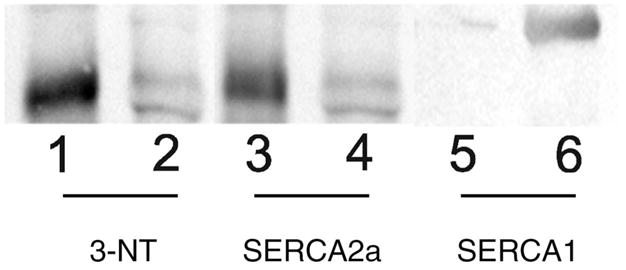
Representative 5% Western blot indicating nitration of SERCA2a (sarcoplasmic reticulum Ca+2-ATPase) in soleus (lanes 1, 3, and 5) and semimembranosus muscles (lanes 2, 4, and 6). Lanes 1 and 2, Reaction of an antibody that recognizes 3-nitrotyrosine. Lanes 3 and 4, Reaction to antibody that recognizes SERCA2a. Lanes 5 and 6, Reaction of an antibody that recognizes SERCA1.
Discussion
The free radical theory of aging and subsequent contemporary free radical damage theories provide a mechanistic explanation for the age-related decline in physiological function. The basis of these hypotheses is that oxidative damage accumulates in cells and tissues over time, and contributes to the decline in physiologic function with age. It has been shown that oxidative damage in cells occurs as a result of the reaction of reactive oxygen and nitrogen species with biological macromolecules. Our previous work demonstrated that, in addition to a significant decline in cell size (atrophy), the force-generating capacity and the structure of myosin in type IIB skeletal muscle fibers (semimembranosus muscle) are altered with aging when comparing young, old, and, very old animals (14–18). In contrast, the age-associated atrophy and significant functional declines of the type I fibers (soleus muscle) occur later (well into senescence) (19). These studies suggest that both fiber types are affected, but the time course of change is fiber type–dependent.
Age-Dependent Changes in Muscle
In the present study, the hypothesis we tested was that oxidative damage to muscle proteins is a mechanism that contributes to the age-related loss in function in muscles expressing predominantly type II fibers. To discern muscle-specific (fast-twitch, type II vs slow-twitch, type I) accumulation of oxidative damage to proteins with age, we investigated the accumulation of 3-NT in skeletal muscle proteins at three points across the life span of the F344 rat. The young adult age group (98% survival) represents an animal with no apparent functional limitations, i.e., the force-generating capacity of single type I and type II fibers is not compromised (6,19,20). In contrast, type I fibers from old animals (50% survival) show an 18% decline in function, and type II fibers exhibit a 30% reduction in function (15,19). At 25% survival (very old age), significant functional declines have been documented in both fiber types (15,19). These results suggest the time course of functional decline is fiber type–specific.
The soleus and the semimembranosus muscles have distinct biochemical differences in energy utilization and antioxidant defense. The soleus muscle has large concentrations of myoglobin, the energy pathway is through oxidative metabolism, and there is a highly developed antioxidant defense system. In contrast, the semimembranosus muscle has low concentrations of myoglobin, the energy pathway is through glycolysis, and the antioxidant defense system is not as well developed as that of the soleus muscle. These fiber-type differences in susceptibility to oxidative stress may be mechanistically related to the aging phenotype, where type II fibers suffer the greatest age-related atrophy and loss in function. In other words, oxidative damage accumulates differently in muscles with distinct biochemical composition and may contribute to the observed aging phenotype. On the basis of the fiber type-specific 30% decrease in function (noted above) in old rats, we would have expected to observe an accumulation of 3-NT in the semimembranosus earlier, by old age (50% survival). However, our data do not show a statistical increase in 3-NT in muscles from the old rats. Thus, accumulation of 3-NT in the proteins identified in this study does not explain muscle-specific phenotype changes in function with age.
Nitration and Protein Function
Skeletal muscles produce reactive oxygen and nitrogen species including superoxide and nitric oxide at low levels under resting conditions and at higher levels during contractile activity (4,5,21–23). The reaction of superoxide and nitric oxide form peroxynitrite, a highly reactive molecule that can covalently modify cysteine and tyrosine residues (9,24). In this study, we focused on 3-NT, a stable product of peroxynitrite-induced tyrosine nitration that we monitored using an antibody that recognized this posttranslation modification.
Tyrosine nitration can inhibit protein function, for example, by altering a protein’s conformation, imposing steric restrictions to the catalytic site, and preventing tyrosine phosphorylation (25). Thus, the functional significance of tyrosine nitration depends on both the site of modification and the extent of the protein population containing functionally significant modifications. It has been suggested that to have biological significance, a loss-of-function modification requires a large fraction of the protein to become nitrated at specific critical tyrosines. A notable example of enzyme activity inhibition linked to nitration in vivo is the mitochondrial enzyme, manganese superoxide dismutase. This protein is nitrated by peroxynitrite at Tyr-34 in the active site by a manganese-catabolized process, which leads to enzyme inactivation. Nitrated and inactivated manganese superoxide dismutase is found in acute and chronic inflammatory processes both in animal models and human diseases (26). An alternative scenario is a gain-of-function modification, in which case a small fraction of nitrated protein can elicit a substantive biological change. This scenario has been demonstrated in a few proteins, such as cytochrome c, which acquires a strong peroxidase activity after nitration (25). The functional consequences for tyrosine nitration of the SR Ca-ATPase have been demonstrated using peroxynitrite following in vitro chemical modification of the protein. Viner and colleagues (27) showed that peroxynitrite induced an increase in nitrotyrosine content that correlated with a significant loss in Ca-ATPase activity. Furthermore, the modification of tyrosine was specific to the SERCA2a isoform, even in fast-twitch muscle containing mainly the SERCA1 Ca-ATPase isoform.
The impact of protein tyrosine nitration on muscle performance has been demonstrated in in vitro studies (28–31). In isolated skeletal muscle preparations, peroxynitrite potentially impaired both energetics and contractility (23,28,30). Although these in vitro studies did not identify specific protein targets, the extent of functional decline is consistent with age-induced changes in single fiber contractile properties, and suggests that protein nitration may contribute to underlying mechanisms in age-related functional decrement (19).
Aging and Protein Nitration
The cellular content of oxidized proteins reflects the balance between the generation of reactive oxygen and nitrogen species and the removal of either the oxidants (by the antioxidant system) or the oxidant-damaged proteins (by the proteasome). With aging, it has been shown that under basal conditions, oxidant production is increased in old age and the redox state of muscle from aged animals shifts to a more oxidative environment (4,21). Although some anti-oxidants are increased in aging muscle, the extent of increase is muscle-specific and not global to all enzymes (32). Thus, the burden of defending against the increased load of free radicals may be greater than the compensatory change in antioxidants. If the antioxidant system is inadequate and proteins are modified, the proteasome must remove damaged proteins. Our laboratory and others have shown that proteasome function in muscle declines with aging (33–35). Thus, the fundamental changes in cell redox status and the ability to remove free radical–damaged proteins likely contribute to the age-related increase in oxidized proteins observed in this study.
MALDI-TOF MS analysis identified five proteins that exhibited an age-related increase in nitrotyrosine immune reactivity. The function of these proteins include energy production (TPI, β-enolase, aconitase, carbonic anhydrase III), and calcium homeostasis (SR Ca-ATPase). Mitochondrial aconitase is one of the major intracellular targets of nitric oxide, and the decrease in aconitase activity has been attributed to the direct reactions of nitric oxide with the iron–sulfur cluster (36). Earlier results show oxidative damage of this protein during biological aging in cardiac tissue (37). To date, carbonic anhydrase III in skeletal muscle has not been identified as a nitrated protein with age. However, previous studies have demonstrated oxidative modifications of carbonic anhydrase III in vivo with a concomitant decrease in catalytic activities in liver tissue (38,39). There is increasing evidence that links β-enolase and TPI as targets for nitration in Alzheimer’s disease, and in aging cardiac and skeletal muscle, providing a possible rationale for the observed disturbance in energy metabolism of aging tissue (37,40–43).
The accumulation of 3-NT in the skeletal muscle SR Ca-ATPase from the very old rats is consistent with previous findings. Viner and colleagues (27) demonstrated a progressive age-related accumulation of nitrotyrosine that was selectively limited to the SERCA2a isoform in preparations from both fast- and slow-twitch rat muscle. MS analysis of tryptic peptides from aged rats identified the sites of nitration at tyrosine residues 294, 295, and 753. The functional impact of modification of these tyrosine residues is suggested by the significant age-dependent loss in Ca2+-ATPase function in slow-twitch muscle preparations containing predominantly SERCA2a. From our work, accumulation of 3-NT in SERCA2a is observed in the soleus (slow-twitch) as well as in the semimembranosus (fast-twitch) muscle.
Recently, protein nitration has been reported in skeletal and cardiac muscle from rats of different ages (37,42). Although these studies have identified a subset of proteins that were nitrated with age (TPI and β-enolase), there were a number of constraints imposed by the research design that limit the application of these results to specific questions of muscle aging. First, these studies investigated a limited number of animals, that is, two or three per age group, precluding statistical comparisons between age groups. Second, the intensity of the 3-NT immune reaction was not compared, so the degree of change was not apparent. This information is important when comparing results among other studies or even between proteins within the same study, thus allowing one to determine if specific proteins are more susceptible to modification with aging. Third, comparisons between skeletal muscles of different fiber types were not possible because the hind-limb muscles were homogenized as one unit. Thus, fiber type–specific changes with age can not be distinguished. Finally, in some studies, the designation of “old” was not appropriate for the species. For example, in the investigation of cardiac muscle, the old rats were only 26 months old, which for F344BN F1 hybrid rats represents an age group that has not reached the 50% survival point (10,44). Generally, the minimum age for old animals should be at 50% survival or less on the survival curve (44).
Summary
We hypothesized that, with aging, the semimembranosus (type II) muscle would accumulate a greater amount of oxidized proteins (3-NT) than would the soleus (type I) muscle. The five modified proteins identified by MALDI-TOF MS and Western immunoblotting (SR Ca+2-ATPase [SERCA2a], aconitase, β-enolase, TPI, and carbonic anhydrase III) exhibited an age-dependent increase in 3-NT content in both type I and type II muscles. However, significant levels of 3-NT modification were present at an earlier age in the semimembranosus muscle.
Acknowledgments
This work was supported by National Institute on Aging Grants AG-17768 and AG-21626 (to L. V. Thompson).
We thank Janice Shoeman, Aimee Husom, and Sheng Zhong for technical assistance.
References
- 1.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haycock JW, Jones P, Harris JB, Mantle D. Differential susceptibility of human skeletal muscle proteins to free radical induced oxidative damage: a histochemical, immunocytochemical and electron microscopical study in vitro. Acta Neuropathol (Berl) 1996;92:331–340. doi: 10.1007/s004010050527. [DOI] [PubMed] [Google Scholar]
- 3.Ostdal H, Skibsted LH, Andersen HJ. Formation of long-lived protein radicals in the reaction between H2O2-activated metmyoglobin and other proteins. Free Radic Biol Med. 1997;23:754–761. doi: 10.1016/s0891-5849(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 4.Reid MB, Durham WJ. Generation of reactive oxygen and nitrogen species in contracting skeletal muscle: potential impact on aging. Ann N Y Acad Sci. 2002;959:108–116. doi: 10.1111/j.1749-6632.2002.tb02087.x. [DOI] [PubMed] [Google Scholar]
- 5.Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LV. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther. 2002;32:44–57. doi: 10.2519/jospt.2002.32.2.44. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 10.Lipman R, Chrisp C, Hazzard D, Bronson R. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol Biol Sci. 1996;51A:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Shevchenko A, Jensen ON, Podtelejnikov AV, et al. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci U S A. 1996;93:14440–144445. doi: 10.1073/pnas.93.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen O, Wilm M, Shevchenko A, Mann M. Sample preparation methods for mass spectrometric peptide mapping directly from 2-DE gels. Methods Mol Biol. 1999;112:513–530. doi: 10.1385/1-59259-584-7:513. [DOI] [PubMed] [Google Scholar]
- 14.Lowe D, Husom A, Ferrington D, Thompson L. Myofibrillar myosin ATPase activity in hindlimb muscles from young and aged rats. Mech Ageing Dev. 2004;125:619–627. doi: 10.1016/j.mad.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowe D, Surek J, Thomas D, Thompson L. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–C547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- 16.Lowe D, Thomas D, Thompson L. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am J Physiol Cell Physiol. 2002;283:C187–192. doi: 10.1152/ajpcell.00008.2002. [DOI] [PubMed] [Google Scholar]
- 17.Lowe D, Warren G, Snow L, Thompson L, Thomas D. Muscle activity and aging affect myosin structural distribution and force generation in rat fibers. J Appl Physiol. 2004;96:498–506. doi: 10.1152/japplphysiol.00842.2003. [DOI] [PubMed] [Google Scholar]
- 18.Prochniewicz E, Thomas D, Thompson L. Age-related decline in actomyosin function. J Gerontol Biol Sci Med Sci. 2005;60A:425–431. doi: 10.1093/gerona/60.4.425. [DOI] [PubMed] [Google Scholar]
- 19.Thompson LV, Brown MB. Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol. 1999;86:881–886. doi: 10.1152/jappl.1999.86.3.881. [DOI] [PubMed] [Google Scholar]
- 20.Thompson LV. Contractile properties and protein isoforms of single skeletal muscle fibers from 12- and 30-month-old Fischer 344 brown Norway F1 hybrid rats. Aging (Milano) 1999;11:109–118. [PubMed] [Google Scholar]
- 21.Bejma J, Ramires P, Ji LL. Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand. 2000;169:343–351. doi: 10.1046/j.1365-201x.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 22.Best TM, Fiebig R, Corr DT, Brickson S, Ji L. Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J Appl Physiol. 1999;87:74–82. doi: 10.1152/jappl.1999.87.1.74. [DOI] [PubMed] [Google Scholar]
- 23.Reid MB, Kobzik L, Bredt DS, Stamler JS. Nitric oxide modulates excitation-contraction coupling in the diaphragm. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:211–218. doi: 10.1016/s1095-6433(97)00417-0. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassina AM, Hodara R, Souza JM, et al. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 26.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schoneich C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J. 1999;340:657–669. [PMC free article] [PubMed] [Google Scholar]
- 28.Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol. 2001;90:45–54. doi: 10.1152/jappl.2001.90.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Mihm MJ, Bauer JA. Peroxynitrite-induced inhibition and nitration of cardiac myofibrillar creatine kinase. Biochimie. 2002;84:1013–1019. doi: 10.1016/s0300-9084(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 30.Mihm MJ, Yu F, Reiser PJ, Bauer JA. Effects of peroxynitrite on isolated cardiac trabeculae: selective impact on myofibrillar energetic controllers. Biochimie. 2003;85:587–596. doi: 10.1016/s0300-9084(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 31.Mihm MJ, Yu F, Weinstein DM, Reiser PJ, Bauer JA. Intracellular distribution of peroxynitrite during doxorubicin cardiomyopathy: evidence for selective impairment of myofibrillar creatine kinase. Br J Pharmacol. 2002;135:581–588. doi: 10.1038/sj.bjp.0704495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 33.Husom A, Peters E, Kolling E, Fugere N, Thompson L, Ferrington D. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Ferrington D, Husom A, Thompson L. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- 35.Radak Z, Takahashi R, Kumiyama A, et al. Effect of aging and late onset dietary restriction on antioxidant enzymes and proteasome activities, and protein carbonylation of rat skeletal muscle and tendon. Exp Gerontol. 2002;37:1423–1430. doi: 10.1016/s0531-5565(02)00116-x. [DOI] [PubMed] [Google Scholar]
- 36.Patel H, Li X, Karan HI. Amperometric glucose sensors based on ferrocene containing polymeric electron transfer systems-a preliminary report. Biosens Bioelectron. 2003;18:1073–1076. doi: 10.1016/s0956-5663(02)00226-9. [DOI] [PubMed] [Google Scholar]
- 37.Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol. 2005;288:H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 38.Cabiscol E, Levine RL. Carbonic anhydrase III. Oxidative modification in vivo and loss of phosphatase activity during aging. J Biol Chem. 1995;270:14742–14747. doi: 10.1074/jbc.270.24.14742. [DOI] [PubMed] [Google Scholar]
- 39.Cabiscol E, Tamarit J, Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol. 2000;3:3–8. [PubMed] [Google Scholar]
- 40.Castegna A, Aksenov M, Thongboonkerd V, et al. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 41.Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem. 2003;85:1394–1401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 42.Kanski J, Alterman MA, Schoneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radic Biol Med. 2003;35:1229–1239. doi: 10.1016/s0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- 43.Kanski J, Hong S, Schoneich C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. J Biol Chem. 2005;280:24261–24266. doi: 10.1074/jbc.M501773200. [DOI] [PubMed] [Google Scholar]
- 44.Burek J, Holander C. The Laboratory Rat: Vol. II. Research Applications. New York: Academic Press; 1980. [Google Scholar]