Abstract
Nucleotides and nucleosides within the airway surface liquid regulate mucociliary clearance (MCC) activities, the primary innate defense mechanism that removes foreign particles and pathogens from airway surfaces. Nucleotide and nucleoside actions in the airways are mediated mainly by two purinergic receptor subtypes, the Gq-coupled ATP/UTP-sensing P2Y2 receptor and the Gs-coupled A2b adenosine receptor. Activation of the A2b receptor results in cyclic AMP-dependent activation of the cystic fibrosis transmebrane regulator (CFTR) Cl- channel and stimulation of ciliary beat frequency. Agonist occupation of the P2Y2 receptor promotes inhibition of Na+ absorption as well as CFTR-dependent and CFTR-independent Cl-secretion, ciliary beating, and mucin secretion.
Introduction
Almost two decades have elapsed since the initial observation that extracellular nucleotides promote Cl- ion secretion from airway epithelial cells [1]. Extensive research has subsequently verified that a subset of purinergic receptors accounts for the physiological actions of ATP as well as other nucleotides and nucleosides within the airway surface liquid (ASL).
Extracellular nucleotide actions are mediated by two classes of broadly distributed cell surface P2-purinergic receptors: the ligand-gated ion channel P2X receptors comprise seven species activated by ATP; and, the P2Y family of G protein-coupled receptors composed of eight species, activated by adenine and uridine nucleotides and nucleotide-sugars [2]. In addition, adenosine, the final product of ATP hydrolysis, activates a separate family of G protein-coupled receptors, the A1, A2a, A2b, and A3 adenosine receptors [2]. The agonist selectivity and signaling properties of purinergic receptors are summarized in Table 1.
Table 1. Purinergic receptors, their agonists and signaling properties.
Nineteen purinergic receptor species have been identified at the molecular level. The A2b, P2Y2, P2Y6, and P2X4 receptors are present in airway epithelial cells [1;3-8]. Abbreviations: PLC, phospholipase C; PKC, protein kinase C; AC, adenylyl cyclase; cAMP, cyclic AMP; ↓, inhibition.
| Agonist | Signaling | |
|---|---|---|
| P2X receptors | ||
| P2X1-P2X7 | ATP | ATP-gated cation channel |
| P2Y receptors | ||
| P2Y1 | ADP | Gq/PLCβ → Ca2+/PKC |
| P2Y2 | ATP = UTP | Gq/PLCβ → Ca2+/PKC |
| P2Y4 | UTP | Gq/PLCβ → Ca2+/PKC |
| P2Y6 | UDP | Gq/PLCβ → Ca2+/PKC |
| P2Y11 | ATP | Gq/PLCβ → Ca2+/PKC and Gs → AC/cAMP |
| P2Y12 | ADP | Gi → ↓AC/↓cAMP |
| P2Y13 | ADP | Gi → ↓AC/↓cAMP |
| P2Y14 | UDP-glucose | Gi → ↓AC/↓cAMP |
| Adenosine receptors | ||
| A1, A3 | Gi → ↓AC/↓cAMP | |
| A2a, A2b | Gs → AC/cAMP |
The Gq/phospholipase C-coupled P2Y2 and P2Y6 receptors and the Gs/adenylyl cyclase-coupled A2b receptor are expressed on the apical surface of human airway epithelial cells [1;3-7] as well as P2X receptors [8], suggesting that adenosine and adenine and uridine nucleotides are endogenous modulators of airway functions. Indeed, ATP, UTP, UDP, and adenosine are present in physiologically relevant concentrations in ASL both in vivo and in vitro [9-11]. Functional and biochemical evidence indicate that release of nucleotides into ASL represents a major mechanism of autocrine/paracrine signaling to regulate MCC activities [7;10;12-14].
This review discusses recent advances in the understanding of how purinergic receptors modulate MCC activities.
ATP release provides a mechanism for MCC regulation
The MCC system ‘consists of three major components, all of which are regulated by extracellular nucleosides and nucleotides [9;14-16]: (i) ion transport elements in the epithelium, which produce an aqueous environment on the airway surface (i.e., ASL production); (ii) mucins, secreted by goblet cells or from submucosal glands, which mature into mucus, and (iii) cilia, which propel the mucus toward the mouth. Component failures may lead to airway inflammatory diseases. For example cystic fibrosis (CF) results from a failure in epithelial Cl- and fluid secretion, primary ciliary dyskinesia results from structural failures in the ciliary axoneme, which negatively affect ciliary activity, and chronic bronchitis and asthma result, in part, from mucin hypersecretion [17-20].
The recognition that airway epithelial cells release ATP constitutively [10;11] suggests a mechanism for the control of basal MCC activities. In vitro studies demonstrated that resting airway epithelia release ATP at a rate of 300-500 fmol/min cm2 [9;21]. Due to the action of ecto-ATPases, steady-state ATP concentrations on resting cells are in the 5-20 nM range, well below the EC50 value for P2Y2 receptor stimulation [9;10;21]. However, ATP metabolism provides a source of adenosine, which reaches steady state concentrations capable of promoting A2b receptor stimulation [9;22]. Cyclic AMP measurements in the presence or absence of adenosine deaminase verified that the A2b receptor on resting airway epithelial cells is tonically stimulated by endogenous adenosine [9]. In addition to constitutive release, enhanced ATP release from airway epithelial cells is associated with mechanical stress that mimics physiological stimulus, e.g., shear stress provided by tidal breathing. Therefore, in vivo ASL ATP may reach concentrations capable of promoting P2Y2 receptor activation (reviewed in [13;23]). Indeed, functional data demonstrated that ATP mediates acute MCC responses via P2Y2 receptor stimulation [15;16]. In sum, adenosine and ATP are physiological relevant stimuli that impart cyclic AMP-regulated and phospholipase C-dependent MCC activities, respectively, to the airways.
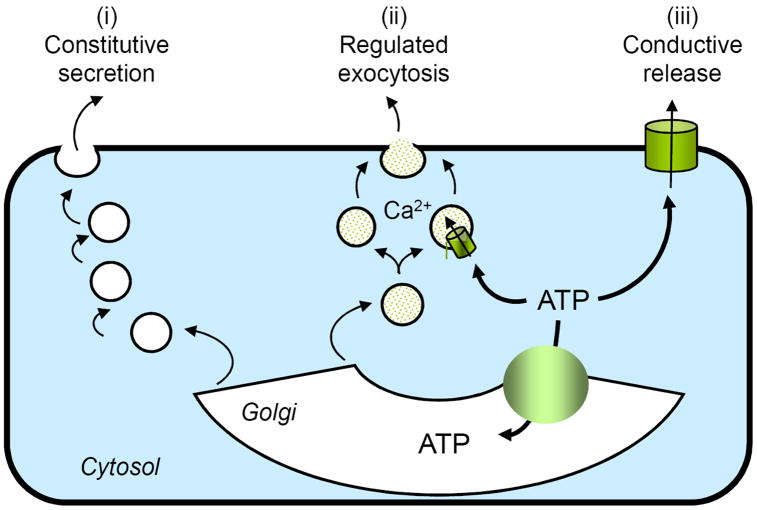
Lung epithelia exhibit a complex cellular composition, and thus, several mechanisms and pathways likely are involved in the release of nucleotides into the airways. Circumstantial evidence supports the involvement of both the secretory pathway and plasma membrane channels in the cellular release of nucleotides from non-excitatory tissues (Fig. 1). However, unambiguous evidence for either vesicular or conductive mechanisms in airway epithelia and in most peripheral tissues is lacking. Moreover, the regulatory processes involved in ATP release are largely unknown [23]. The fact that airway epithelial cells release UDP-sugars constitutively, in addition to ATP [24], suggests that nucleotides involved in glycosylation reactions within the secretory pathway are released as cargo molecules during the export of glycoconjugates, i.e., via the constitutive secretory pathway (Fig. 1). Moreover, recent studies with goblet-like airway epithelial cells indicated that ATP and UDP-sugars are released concomitantly with MUC5AC, a secretory mucin, during Ca2+-regulated exocytosis of mucin granules. This observation suggest that nucleotides are stored within and released from mucin granules in goblet cells [25] (Fig. 1). An important corollary derived from this observation is that nucleotide release is a mechanism by which mucin-secreting goblet cells produce paracrine signals for mucin hydration within the ASL. Less clear is how nucleotides are released from non-secretory (ciliated) cells in response to shear forces. Evidence for the involvement of either the secretory pathway or plasma membrane channels in the release of ATP from mechanically stimulated ciliated cells remains circumstantial.
Figure 1. Pathways for ATP release.
Several scenarios possibly account for the basal and stimulated release of nucleotides from airway epithelial cells. (i) ATP entering the Golgi lumen via specific transporters may be released as a residual cargo product of the constitutive secretory pathway. (ii) Secretory granules (e.g., mucin granules) containing ATP may be competent for Ca2+-regulated exocytosis. (iii) A not yet identified ATP conductance effluxes cytosolic ATP out of the cells.
Adenosine promotes CFTR Cl- channel activity
A key component of MCC function involves the regulation of ASL volume by electrolyte transport. ASL volume production reflects a balance between Cl- secretion and Na+ absorption. CFTR is a major element mediating liquid balance on normal airway surfaces. CFTR regulates Na+ absorption and acts as a Cl- channel [26]. Defective CFTR activity causes the syndrome of CF, characterized by enhanced Na+ absorption and failure to secrete Cl-, leading to ASL volume depletion and defective MCC [26].
CFTR is a cyclic AMP-regulated Cl- channel [27]. Electrophysiological and biochemical evidence suggests that CFTR activity in normal airways is controlled, primarily, by the A2b receptor (Fig. 2). Patch clamp studies with Calu-3 cells (an airway epithelial cell line that expresses large amounts of functional CFTR) revealed that basal CFTR Cl- channel activity is abolished by adenosine receptor antagonists [12]. Inclusion of the 5’ nucleotidase inhibitor AMPCP in the patch pipette also greatly reduced CFTR activity, indicating that adenosine was generated by hydrolysis of endogenous AMP, a product of ATP release and metabolism [12]. Subsequent studies indicated that the adenosine metabolizing enzyme adenosine deaminase (ADA) decreased cyclic AMP levels in resting cells [9]. Two major implications emanate from these observations: (i) in resting epithelial cells, adenosine accumulates in ASL at levels capable of promoting A2b receptor activation; and, (ii) adenosine levels are maintained by the tonic release of ATP and its subsequent metabolism within ASL. These assumptions were corroborated by measurements of adenosine levels and ATP release and hydrolysis rates on resting airway epithelia. In studies combining chemical derivatization of adenyl purines with HPLC analysis of the resulting fluorescent ethenylated species, adenosine levels in ASL in the 180-350 nM range were found [9;22].
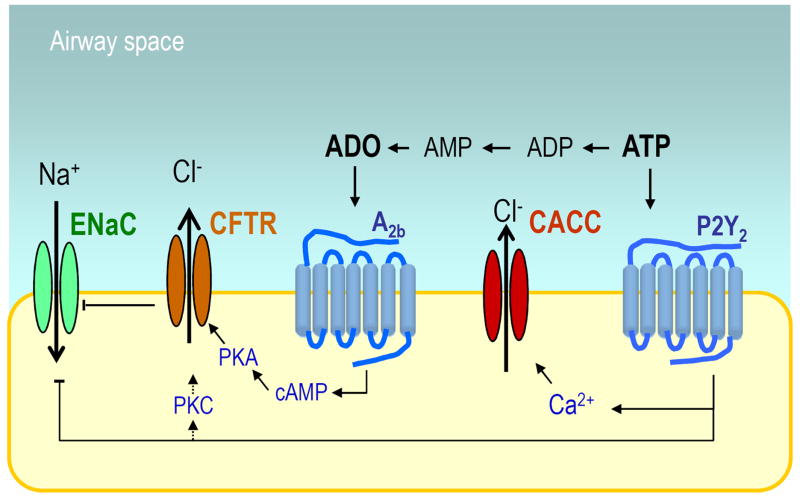
Figure 2. Purinergic regulation of ion transport.
ASL ATP promotes P2Y2 receptor-mediated Ca2+-activated Cl- channel (CaCC) activity, and inhibition of the epithelial Na+ channel ENaC (via PIP2 depletion, not shown). Potentially, the P2Y2 receptor promotes PKC-mediated CFTR activation. ATP hydrolysis results in adenosine accumulation (ADO), which in turns activates the A2b receptor, leading to cyclic AMP (cAMP) and protein kinase A (PKA)-mediated CFTR activation. CFTR inhibits ENaC by mechanisms that are not well understood.
These observations led to the hypothesis that, by promoting cyclic AMP-regulated CFTR activation, the A2b receptor regulates ASL volume production. Experimental validation of this hypothesis came from confocal microscopy studies in which ASL volume production was assessed in normal and CF airway epithelia in the absence or presence of A2b receptor antagonists or ADA. In normal cultures, the height of ASL at steady state is ~7 μm, the height of the extended cilia. Consistent with the notion that CFTR regulates ASL volume, ASL height in CF cultures (i.e., in the absence of functional CFTR) is reduced to < 4 μm. Addition of ADA or A2b receptor antagonists to normal cultures caused a depletion of ASL volume, which displayed a height of ~4 μm at steady state [9]. Thus, in terms of ASL volume production, normal cultures behaved as CF cultures when activation of the A2b receptor by endogenous adenosine was prevented.
An important element in the cascade that leads from A2b receptor occupation to CFTR activation was revealed during early studies of protein kinase A (PKA)-mediated CFTR activation. Exposure of excised CFTR-containing membrane patches to cyclic AMP analogues increased CFTR open probability, indicating that PKA remained associated with excised membrane patches, i.e., physically close to CFTR. These studies also revealed that CFTR is selectively regulated by the membrane-associated isozyme of PKA, PKA-II, and that PKAII is anchored at specific subcellular sites by A kinase anchoring proteins (AKAPs) [28]. Altogether, these observations suggest that CFTR activity is controlled by adenosine generated locally, i.e., near the A2b receptor site, and that A2b-receptor-mediated cyclic AMP production efficiently activates PKA in close proximity to CFTR.
In addition to PKA-promoted CFTR phosphorylation and activation, activation of protein kinase C (PKC) results in enhanced CFTR-mediated Cl- secretion. Two potential scenarios by which PKC promotes CFTR Cl- channel activity include: (i) phosphorylation of CFTR by PKC, which facilitates subsequent PKA-mediated activation [29]; and (ii) enhanced cell surface expression of CFTR via inhibition of endocytosis [30]. These observations suggest a mechanism of activation of CFTR by the P2Y2 receptor (Fig. 2).
Ca2+-activated Cl- channel
An alternative (i.e., CFTR-independent) Cl- channel (named CaCC) is also present on the mucosal surface of the airway epithelium and is activated by elevation of cytosolic Ca2+ levels [31] (Fig. 2). Under normal, resting conditions, cytosolic Ca2+ levels are low, and therefore, CaCC activity is negligible. However, robust CaCC activation is associated with conditions promoting Ca2+ mobilization from intracellular stores or the influx of extracellular Ca2+. In human airways, the P2Y2 and P2Y6 receptors and, likely, the P2X4 receptor promote Ca2+- dependent CaCC responses [4;7;8]. The identity of the CaCC had remained elusive for many years, but three independent groups have recently reported that members of the anoctamin (ANO)/TMEM16 family of membrane proteins are components of the CaCC [32-34]. The term anoctamin derives from the fact that these channels are anion selective and have eight (octa) transmembrane domains. P2Y2 receptor activation promoted Ca2+-mobilization and (ANO)/TMEM16-associated Cl- current [32-34].
Regulation of Na+ absorption
The epithelial Na+ channel (ENaC) is expressed at the apical membrane of airway epithelial cells and is the major regulator of Na+ absorption in the lung (Fig. 2). Airway epithelial ENaC is constitutively activated by proteolytic cleavage. The identities of the endogenous proteases that cleave ENaC on the airway epithelial cell surface have not been fully elucidated, but prostasin and other members of the channel-activating protein (CAP) family likely are involved [35-38]. A site in the extracellular loop of the γ-subunit of ENaC (RKRK186) has been recently identified as a prostasisn-dependent cleavage site involved in ENaC activation [36;37].
CF airway epithelia absorb Na+ at two to three times the normal rate. In normal airway epithelia, stimuli that raise intracellular cyclic AMP have no effect on the rate of Na+ absorption, but in CF, airway epithelial cyclic AMP further stimulates the already elevated rate of Na+ absorption. Little is known about how CFTR controls Na+ absorption. It has been hypothesized that CFTR expression restrains the activity of ENaC (Fig. 2), but the molecular basis for a functional connection between CFTR and ENaC remains elusive. One speculation is that reduced or absent functional CFTR results in decreased expression of a signaling pathway that inhibits constitutively activated ENaC or, conversely, permits the expression of a stimulatory pathway that is normally repressed in normal airway epithelial cells [39].
It has been known for a while that activation of the airway epithelial P2Y2 receptor results in inhibition of Na2+ absorption (Fig. 2). While early studies excluded the role of intracellular Ca2+ or protein kinase C, recent evidence suggests that inhibition of ENaC by P2Y2 receptor activation requires phospholipase C-catalyzed hydrolysis of phosphatidylinositol 4, 5-bisphosphate (PIP2). For example, Kunzelmann et al. illustrated that ATP-promoted inhibition of Na+ absorption in tracheal epithelial cells was suppressed by neomycin, which binds to PIP2, and by inhibitors of PIP kinase. These authors also showed that PIP2 co-immunoprecipitated the β subunit of ENaC and that co-expression of P2Y2 receptor and α,β,γ-ENaC in Xenopus oocytes resulted in ATP-promoted ENaC inhibition [40]. A series of studies with renal epithelia provided further support for the notion that the P2Y2 receptor regulates ENaC activity through PIP2 depletion [41]. Eaton and co-workers illustrated that PIP2 binding to the NH3-terminal region of the β subunit of ENaC increased the open probability of the channel [42-44]. Pochynyuk et al. demonstrated that conserved positive-charged regions at the NH3-terminal of β and γ ENaC are important for inhibition of ENaC activity in response to P2Y2 receptor-promoted PIP2 depletion [45;46].
P2Y2 receptor regulation of mucin secretion
Mucins are released from goblet cells via regulated exocytosis. In contrast to submucosal glands, which are innervated richly and regulated primarily by acetylcholine and possibly other neurotransmitters, goblet cells of the superficial epithelium are sparsely innervated and are regulated primarily by local mediators in the airway lumen, acting on their apical surfaces. The current body of data indicates that the P2Y2 receptor on apical membranes of surface goblet cells constitutes the major regulatory system for mucin secretion. The P2Y2 receptor promotes mucin secretion via complex Ca2+ and dyacylglycerol (DAG)-regulated mechanisms. Excellent reviews have been recently published on the regulation of mucin secretion in goblet cells [14;47;48] and, therefore, we will not expand on this topic.
Regulation of ciliary beat by P2Y and A2b receptors
Airway epithelial cells contain hundreds of motile cilia per cell that beat together to propel the mucus over the epithelial surface. Thus, in normally hydrated airways, the rate of MCC is determined by ciliary beat frequency (CBF). Coordinated ciliary beat is achieved by the co-orientation of ciliary structure and direction of beating, both within each cell and between individual ciliated cells, and is essential to the physiological functions of the epithelia (reviewed in [49]). Extracellular nucleotides regulate ciliary beating in the airways by mechanisms that involve Ca2+ mobilization via P2Y2 (and to a lesser extent P2Y6, [7]) receptor activation. P2Y receptor-regulated Ca2+-dependent CBF involves two components: (i) a transient Ca2+ signal associated with Ca2+ release from intracellular stores; and, (ii) a more sustained Ca2+ influx via plasma membrane Ca2+channels. In a recent study, Lorenzo et al. have elegantly shown that at non-saturating concentrations, ATP generates oscillatory Ca2+ signals in ciliated cells, which in turn increases CBF. At maximally effective concentrations, ATP elicited a robust CBF response, which was associated with a sustained Ca2+ influx via receptor operated Ca2+ entry mechanisms [50]. It has been proposed that an oscillatory Ca2+ signal increases CBF by acting directly on a ciliary target, most likely a calcium-binding protein [51;52]. An indirect mechanism for Ca2+-promoted CBF increase, e.g., phosphorylation of axonemal proteins, may be involved in response to sustained Ca2+ elevation [52].
In addition, a Ca2+-independent mechanism of CBF regulation operates in response to adenosine, i.e., via A2b receptor-elicited cyclic AMP formation and PKA activation [53]. The putative substrate for PKA phosphorylation that regulates CBF in human airway epithelial cells has not been identified. However, there is good evidence that cyclic AMP regulates CBF in mammalian cells via PKA-promoted phosphorylation of dynein light chain, which in turn possibly mediates a switch from the slow to the fast dynein-duty cycle and thus increases CBF (reviewed in [51]).
Conclusions and prospective
The above studies highlight the importance of purinergic signaling in the airways. While it is now evident that adenosine and ATP are crucial regulators of MCC activities in normal airways, the P2Y2 receptor has promising perspectives as a therapeutic target to promote CaCC activity and ENaC inhibition in CF lungs, improving the otherwise poor ASL volume production associated with defective CFTR. Therapeutic use of a P2Y2 receptor agonist to promote lung hydratation in CF is in late phases of clinical testing. Of note, the potential role of purinergic agonists in the pathophysiology of inflammatory airway diseases, e.g., chronic obstructed/inflammed lungs, has been incompletely unexplored. For example, adenosine receptors, P2X receptors, and the P2Y2, P2Y11, and P2Y14 receptors are expressed in inflammatory cells such as macrophages, lymphocytes, and granulocytes. Rrecent measurements of nucleotides in bronchoalveolar secretions from patients with lung inflammation indicated levels of adenosine, ATP, and UDP-sugars in range of promoting robust activation of their cognate receptor ([54] and data not shown). An interrelated area of active research focuses on the mechanisms of nucleotide release, in particular, the identification of biochemical signals that transduce mechanical forces into ATP release.
Acknowledgments
We thank Lisa Brown for editorial assistance of the manuscript. This work was supported by the National Institute of Health (NIH), National Heart, Lung, and Blood Institute, P01-HL034322.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 3.Lazarowski ER, Mason SJ, Clarke L, Harden TK, Boucher RC. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br J Pharmacol. 1992;106:774–782. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y(2) receptor. Proc Natl Acad Sci U S A. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobb BR, Fan LJ, Kovacs TE, Sorscher EJ, Clancy JP. Adenosine Receptors and Phosphodiesterase Inhibitors Stimulate Cl- Secretion in Calu-3 Cells. Am J Respir Cell Mol Biol. 2003;29:410–418. doi: 10.1165/rcmb.2002-0247OC. [DOI] [PubMed] [Google Scholar]
- 6.Szkotak AJ, Ng AM, Man SF, Baldwin SA, Cass CE, Young JD, Duszyk M. Coupling of CFTR-mediated anion secretion to nucleoside transporters and adenosine homeostasis in Calu-3 cells. J Membr Biol. 2003;192:169–179. doi: 10.1007/s00232-002-1073-x. [DOI] [PubMed] [Google Scholar]
- 7.Morse DM, Smullen JL, Davis CW. Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am J Physiol Cell Physiol. 2001;280:C1485–C1497. doi: 10.1152/ajpcell.2001.280.6.C1485. [DOI] [PubMed] [Google Scholar]
- 8.Zsembery A, Fortenberry JA, Liang L, Bebok Z, Tucker TA, Boyce AT, Braunstein GM, Welty E, Bell PD, Sorscher EJ, Clancy JP, Schwiebert EM. Extracellular zinc and ATP restore chloride secretion across cystic fibrosis airway epithelia by triggering calcium entry. J Biol Chem. 2004;279:10720–10729. doi: 10.1074/jbc.M313391200. [DOI] [PubMed] [Google Scholar]
- 9.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med. 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 12.Huang PB, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci U S A. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Button B, Boucher RC. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163:189–201. doi: 10.1016/j.resp.2008.04.020. * In human airway epithelia, cyclic compressive stress (CCS)-induced ATP release was sufficient to induce purinergic receptor-mediated increases in ASL height and MCC, via inhibition ENaC and stimulation of CFTR and CaCC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 15.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredburg JJ, Boucher RC. Normal and cystic fbrosis airway surface liquid homeostasis: The effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salathe M, O’Riordan TG, Wanner A. Treatment of mucociliary dysfunction. Chest. 1996;110:1048–1057. doi: 10.1378/chest.110.4.1048. [DOI] [PubMed] [Google Scholar]
- 18.Wanner A, Salathe M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 19.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucher RC. Relationship of airway epithelial ion transport to chronic bronchitis. Proc Am Thorac Soc. 2004;1:66–70. doi: 10.1513/pats.2306018. [DOI] [PubMed] [Google Scholar]
- 21.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127:591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 24.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of Cellular UDP-Glucose as a Potential Extracellular Signaling Molecule. Mol Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- 25.Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 27.Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl-channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- 28.Huang P, Trotter K, Boucher RC, Milgram SL, Stutts MJ. PKA holoenzyme is functionally coupled to CFTR by AKAPs. Am J Physiol Cell Physiol. 2000;278:C417–C422. doi: 10.1152/ajpcell.2000.278.2.C417. [DOI] [PubMed] [Google Scholar]
- 29.Jia Y, Mathews CJ, Hanrahan JW. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J Biol Chem. 1997;272:4978–4984. doi: 10.1074/jbc.272.8.4978. [DOI] [PubMed] [Google Scholar]
- 30.Chappe F, Loewen ME, Hanrahan JW, Chappe V. Vasoactive intestinal peptide increases cystic fibrosis transmembrane conductance regulator levels in the apical membrane of Calu-3 cells through a protein kinase C-dependent mechanism. J Pharmacol Exp Ther. 2008;327:226–238. doi: 10.1124/jpet.108.141143. [DOI] [PubMed] [Google Scholar]
- 31.Gabriel SE. Calciun-activated Cl- conductance in the airway epithelium. In: Fuller CM, editor. Calcim-Activated Chloride Channels. San Diego: Academic Press; 2002. pp. 193–207. [Google Scholar]
- 32.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. ** Co-transfection of murine anoctamin (ANO1) and P2Y2 or other Ca2+ mobilizing receptors in HEK 293T cells resulted in agonist-promoted Cl- currents. ANO1-associated currents were activated by Ca2+, with an EC50 value of 2.6 μM[32] [DOI] [PubMed] [Google Scholar]
- 33.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. ** TMEM16 siRNA reduced UTP- as well as ionomycin-promoted anion transport in polarized cultures of human bronchial epithelial cells. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. ** Using a novel expression cloning system, the axolotl oocyte xANO was identified as s an ortholog of the human and mouse ANO1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- 36.Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem. 2008;283:25290–25295. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- 38.Tong Z, Illek B, Bhagwandin VJ, Verghese GM, Caughey GH. Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2004;287:L928–L935. doi: 10.1152/ajplung.00160.2004. [DOI] [PubMed] [Google Scholar]
- 39.Huang P, Gilmore E, Kultgen P, Barnes P, Milgram S, Stutts MJ. Local regulation of cystic fibrosis transmembrane regulator and epithelial sodium channel in airway epithelium. Proc Am Thorac Soc. 2004;1:33–37. doi: 10.1513/pats.2306012. [DOI] [PubMed] [Google Scholar]
- 40.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- 41.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J Biol Chem. 2002;277:7641–7644. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 42.Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 43.Becchetti A, Malik B, Yue G, Duchatelle P, Al Khalili O, Kleyman TR, Eaton DC. Phosphatase inhibitors increase the open probability of ENaC in A6 cells. Am J Physiol Renal Physiol. 2002;283:F1030–F1045. doi: 10.1152/ajprenal.00011.2002. [DOI] [PubMed] [Google Scholar]
- 44.Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol. 2005;16:3182–3187. doi: 10.1681/ASN.2005040434. [DOI] [PubMed] [Google Scholar]
- 45.Pochynyuk O, Tong Q, Staruschenko A, Stockand JD. Binding and direct activation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. J Physiol. 2007;580:365–372. doi: 10.1113/jphysiol.2006.127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol. 2008;294:F38–F46. doi: 10.1152/ajprenal.00403.2007. * Purinergic signaling rapidly decreases ENaC open probability and apical membrane PIP2 levels with similar time courses. Inhibiting either purinergic receptors or PLC in resting cells increased ENaC activity, which was associated with elevation of apical membrane PIP2 levels. [DOI] [PubMed] [Google Scholar]
- 47.Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: From production to secretion. Am J Respir Cell Mol Biol. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care. 2007;52:1134–1146. [PubMed] [Google Scholar]
- 49.Vladar EK, Axelrod JD. Dishevelled links basal body docking and orientation in ciliated epithelial cells. Trends Cell Biol. 2008;18:517–520. doi: 10.1016/j.tcb.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci U S A. 2008;105:12611–12616. doi: 10.1073/pnas.0803970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Sanderson MJ. Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP. J Physiol. 2003;546:733–749. doi: 10.1113/jphysiol.2002.028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winters SL, Davis CW, Boucher RC. Mechanosensitivity of mouse tracheal ciliary beat frequency: roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am J Physiol Lung Cell Mol Physiol. 2007;292:L614–L624. doi: 10.1152/ajplung.00288.2005. [DOI] [PubMed] [Google Scholar]
- 54.Esther CR, Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Pedrosa Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular Purines are Biomarkers of Neutrophilic Airway Inflammation. Eur Respir J. 2008 doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]