Abstract
Symptomatic infection with Neisseria gonorrhoeae (Gc) results in a potent polymorphonuclear leukocyte (PMN)-driven inflammatory response, but the mechanisms by which Gc withstands PMN attack are poorly defined. Here we report that Gc can suppress the PMN oxidative burst, a central component of the PMN antimicrobial arsenal. Primary human PMNs remained viable after exposure to liquid-grown, exponential-phase, opacity-associated protein (Opa)-negative Gc of strains FA1090 and MS11 but did not generate reactive oxygen species (ROS), even after bacterial opsonization. Liquid-grown FA1090 Gc expressing OpaB, an Opa protein previously correlated with PMN ROS production, elicited a minor PMN oxidative burst. PMN ROS production in response to Opa− and OpaB+ Gc was markedly enhanced if bacteria were agar-grown or if liquid-grown bacteria were heat killed. Liquid-grown Opa- Gc inhibited the PMN oxidative burst elicited by isogenic dead bacteria, formylated peptides or Staphylococcus aureus but did not inhibit PMN ROS production by OpaB+ Gc or phorbol esters. Suppression of the oxidative burst required Gc-PMN contact and bacterial protein synthesis but not phagocytosis. These results suggest that viable Gc directly inhibits PMN signaling pathways required for induction of the oxidative burst, which may contribute to gonococcal pathogenesis during inflammatory stages of gonorrheal disease.
Keywords: Neisseria gonorrhoeae, neutrophil, reactive oxygen species, oxidative burst
Introduction
The obligate human pathogen Neisseria gonorrhoeae (the gonococcus; Gc) is the causative agent of the sexually transmitted infection gonorrhea, which affects over 62 million individuals worldwide every year (WHO, 2001). Acute gonococcal infection elicits a potent inflammatory response characterized primarily by the influx of polymorphonuclear leukocytes (neutrophils or PMNs) to the lower urogenital tract (Harkness, 1948). The resulting exudate consisting of PMNs with attached and internalized Gc is the clinical hallmark of gonorrheal disease (Shafer and Rest, 1989). Although PMNs possess a broad range of antimicrobial activities, Gc can be cultured from gonorrheal exudates, demonstrating that the bacteria can survive the host inflammatory response (Hook and Holmes, 1985). Gc defense against PMN killing undoubtedly contributes to the continued persistence of gonorrhea within the human population.
Indirect evidence suggests that Gc survive exposure to PMNs by defending against phagocyte-derived reactive oxygen species (ROS). In activated PMNs, the NADPH oxidase enzyme converts molecular oxygen into superoxide (reviewed in Roos et al., 2003). Superoxide serves as the precursor for other ROS including hydrogen peroxide and hypochlorous acid or bleach, which is generated by the PMN granule enzyme myeloperoxidase (Hampton et al., 1998). ROS have potent antimicrobial activity in vitro and additionally facilitate the release of PMN granule enzymes and peptides that are toxic for many microorganisms (Fang, 2004; Segal, 2005). The Gc genome encodes an extensive array of antioxidant gene products, many of which have been shown to protect the organism from ROS-mediated killing. Some of these gene products directly detoxify ROS (e.g., catalase, superoxide dismutase, cytochrome c peroxidase, and a manganese-dependent ROS quenching system), while others protect Gc from ROS-mediated damage to biomolecules (e.g., peptide methionine sulfoxide reductase and RecN) (reviewed in Seib et al., 2006). Our laboratory has recently shown that expression of many of these gene products is upregulated in Gc exposed to sublethal concentrations of hydrogen peroxide, and a subset of ROS-induced genes protect the bacteria from ROS and PMN-mediated killing (Stohl et al., 2005). The retention of this array of gene products in the Gc genome and the observation that their expression is responsive to oxidative stress imply that Gc encounter ROS as part of their life cycle and are adept at correcting the resulting damage.
Other evidence suggests that PMNs use means that are independent of ROS production to combat Gc infection. Seib et al. found that inactivation of Gc antioxidant genes, singly or in combination, had no effect on bacterial survival after exposure to PMNs, and a Gc catalase mutant was not compromised for survival in the murine genital tract (Seib et al., 2005; Soler-Garcia and Jerse, 2007). Furthermore, PMNs maintained in anoxic conditions or from patients with chronic granulomatous disease (CGD), who are genetically deficient in NADPH oxidase, retain some antigonococcal activity (Rest et al., 1982; Frangipane and Rest, 1992). PMNs may therefore combat Gc infection with antimicrobial factors that function independently of ROS. Non-oxidative PMN antimicrobial factors that reduce Gc viability in vitro include cathepsin G and the cationic antimicrobial peptide LL-37 (Rest and Pretzer, 1981; Shafer et al., 1986; Qu et al., 1996; Shafer et al., 1998), which are resisted in part by the activity of the Gc Mtr efflux pump (Shafer et al., 1998). The Mtr system facilitates Gc survival in the murine genital tract (Jerse et al., 2003; Warner et al., 2007), but whether Mtr directly protects Gc from PMN killing is not known. Thus, the non-oxidative factors directed against Gc in vivo and the Gc gene products that confer protection from these factors still remain to be defined.
Central to the question of whether PMNs direct oxidative or non-oxidative antimicrobial mechanisms against Gc is the degree to which PMNs generate ROS during gonorrheal infection. The reported ability of PMNs to mount an oxidative burst in response to Gc varies widely, with some of the variation due to differences in bacterial outer membrane surface components. ROS production in PMNs is stimulated by Gc that express selected members of the opacity-associated (Opa) protein family, which engage carcinoembryonic antigen-cell adhesion molecule (CEACAM) receptors on the phagocyte surface and facilitate bacterial internalization (Dehio et al., 1998). Opa expression alone is sufficient to promote ROS production in PMNs, as shown using E. coli heterologously expressing certain CEACAM-binding Opa proteins (Belland et al., 1992; Chen and Gotschlich, 1996). However, the Opa-mediated PMN oxidative burst is less potent than stimuli such as opsonized zymosan (Simons et al., 2005). In the absence of Opa expression, Gc fail to stimulate a sizable PMN oxidative burst, but the strains tested in these studies also lacked adhesins (e.g., type IV pili) that could potentially facilitate Opa-independent interactions with PMNs (Rest et al., 1982; Virji and Heckels, 1986; Fischer and Rest, 1988; Elkins and Rest, 1990). In reexamining the data in these reports, Opa- Gc do appear to trigger some degree of ROS production from PMNs, albeit less than their Opa+ counterparts (Rest et al., 1982; Virji and Heckels, 1986). Given these discrepancies in the literature, it is apparent that key questions remain regarding PMN responsiveness to Gc infection.
Here we have directly monitored the oxidative burst in primary human PMNs exposed to different strains of liquid-grown, exponential-phase Gc. Strikingly, piliated, Opa- Gc not only failed to elicit ROS production in PMNs but also suppressed the oxidative burst in response to a subset of factors known to stimulate NADPH oxidase activity. Suppression required live bacteria and occurred without affecting PMN viability, suggesting that Gc uses an active process to prevent PMN ROS production. These results identify a previously unappreciated mechanism by which Gc subvert PMN antimicrobial activities, which may assist the organism in establishing and maintaining infection in its obligate human host.
Results
PMNs do not produce a significant oxidative burst in response to live, exponentially-growing Gc
The ability of primary human PMNs to generate reactive oxygen species (ROS) following infection with live Gc was monitored using luminol-dependent chemiluminescence (LDCL) (Dahlgren and Karlsson, 1999). PMNs were isolated from venous blood of healthy human donors and incubated with 20 µM luminol in a defined glucose-rich medium that supports survival of PMNs and growth of Gc (Morse and Bartenstein, 1980). ROS production was measured as counts per sec of luminescence at 5 min intervals over 1 h. Unstimulated PMNs did not produce detectable ROS, whereas PMN exposure to the phorbol ester phorbol myristate acetate (PMA) potently stimulated ROS production within 15 min (Fig. 1A). PMA-elicited LDCL was completely abolished in the presence of the NADPH oxidase inhibitor diphenylidene iodonium hydrochloride, showing that PMN ROS production in this system required NADPH oxidase activity (data not shown). The magnitude of the oxidative burst of primary PMNs to PMA was found to vary among experiments, with variation found between donors, as well as from each individual donor on different days. Thus, comparisons between strains or treatment conditions were made within a single experiment conducted on one day with one donor's cells, and all results shown are representative of several experiments conducted with PMNs from at least three different donors.
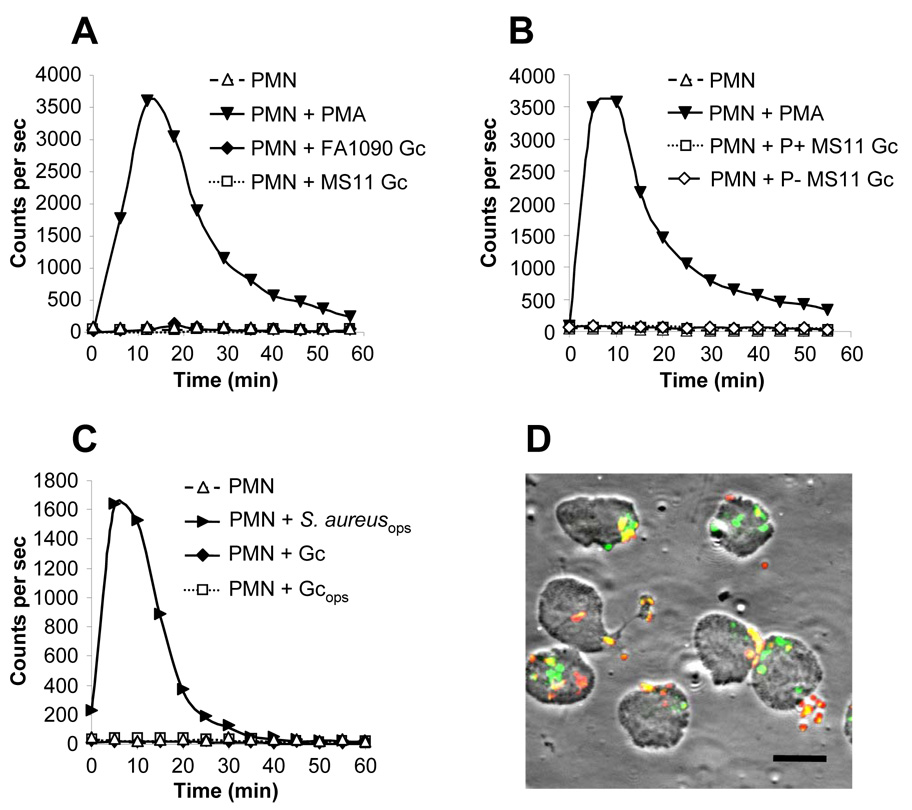
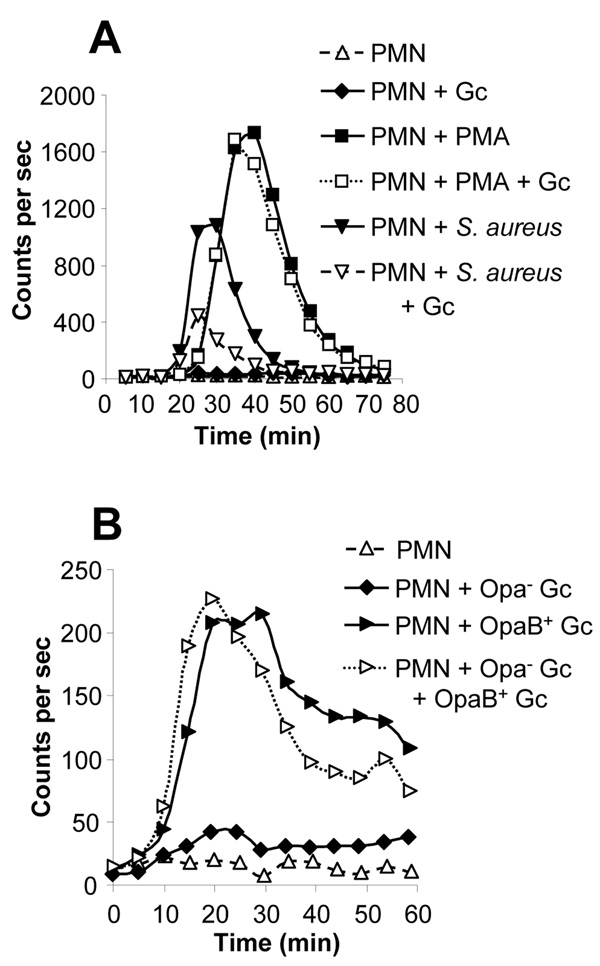
Fig. 1. Viable, exponentially-growing Opa- Gc do not induce the oxidative burst in PMNs.
A. Absence of the oxidative burst in Gc-infected PMNs. Primary human PMNs were left uninfected, stimulated with PMA, or exposed to piliated, Opa- Gc of strains FA1090 (MOI 90) or MS11 (MOI 80). ROS production was measured as counts per sec of LDCL. B. Neither piliated nor nonpiliated Gc stimulate PMN oxidative metabolism. ROS production was measured in PMNs infected with MS11 Gc that were piliated (MOI 100) or constitutively nonpiliated due to deletion of the pilE pilin structural gene (MOI 94). C. Neither opsonized nor unopsonized Gc stimulate PMN oxidative metabolism. ROS production was measured in PMNs infected with unopsonized MS11 Gc (MOI 200) or bacteria opsonized in 10% autologous serum, either MS11 Gc (Gcops; MOI 130) or S. aureus (S. aureusops; MOI 40). D. PMNs bind and phagocytose unopsonized Gc. PMNs were infected with unopsonized FA1090 Gc at a MOI of 10 for 30 min. Intracellular and extracellular bacteria were discriminated from one another based on accessibility to a Gc-specific antibody before and after PMN permeabilization. Extracellular bacteria appear red/yellow; intracellular bacteria appear green. Scale bar = 10 µm.
LDCL was then monitored in PMNs infected with Gc of strains FA1090 and MS11. For these experiments, piliated, Opa- Gc were grown in liquid culture for multiple rounds of replication; this protocol enriches for live, actively growing bacteria and has been previously used in our laboratory to demonstrate that the majority of Gc survive 1 h exposure to PMNs, which is aided by the bacterial gene products RecN and Ngo1686 (Stohl et al., 2005). PMNs exposed to FA1090 and MS11 Gc at multiplicities of infection (MOI) of ~100 colony-forming units (CFU) per PMN generated only 4% and 2% of the LDCL elicited by PMA stimulation, respectively (Fig. 1A). No oxidative burst was detected in PMNs exposed to MOIs of Gc ranging from 25 to 500 CFU per PMN or when infection was continued for > 2 h (data not shown). The absence of a notable oxidative burst was not attributable to the use of piliated Gc, since no ROS production was observed in PMNs exposed to isogenic non-piliated bacteria (Fig. 1B). Neither unopsonized Gc nor bacteria opsonized with autologous human serum elicited the oxidative burst in PMNs (Fig. 1C). The normal human serum retained opsonizing activity, since Staphylococcus aureus incubated with serum under the same conditions promoted strong ROS production from PMNs (Fig. 1C), whereas unopsonized S. aureus did not (data not shown). Gc-infected PMNs also did not generate detectable ROS by lucigenin- or isoluminol- enhanced chemiluminescence (data not shown). Therefore, PMNs fail to mount a respiratory burst when presented with either of two unrelated strains of viable, exponentially-growing Gc.
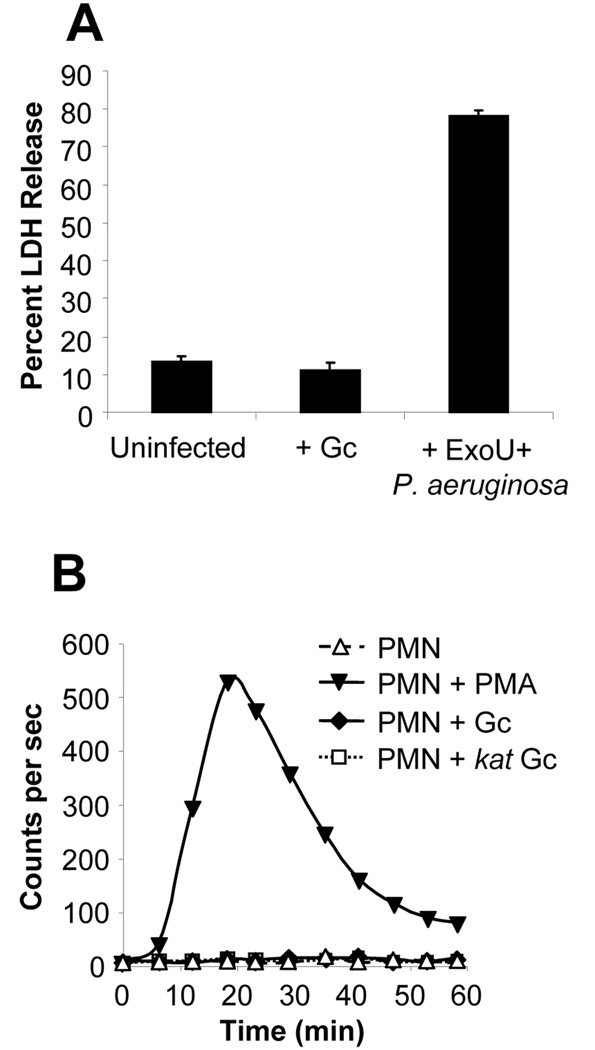
We tested and discounted several possibilities that could explain the absence of a sizable oxidative burst in PMNs exposed to Gc. The lack of ROS production was not due to inefficient bacteria-host cell contact, because no oxidative burst was detected even after Gc were centrifuged onto PMN monolayers (data not shown), which enhanced bacterial attachment and phagocytosis (Fig. 1D). Nor was it due to increased PMN lysis, as the amount of lactate dehydrogenase (LDH) activity released into PMN supernatants was not affected by Gc infection; in contrast, Pseudomonas aeruginosa secreting the cytotoxin ExoU stimulated significantly greater release of LDH from PMNs (Fig. 2A). Moreover, neither uninfected PMNs nor PMNs exposed to liquid-grown Gc exhibited morphological changes characteristic of apoptosis during the time course of the assay, indicating that Gc infection did not promote either necrotic or apoptotic PMN death (data not shown). Finally, Gc catalase activity was not detoxifying PMN-derived ROS such that it could not be detected by LDCL, because catalase-deficient Gc also failed to promote ROS production in PMNs (Fig. 2B). These results show that PMNs phagocytose Gc and that interaction with Gc does not compromise PMN viability, suggesting that Gc may be directly preventing PMNs from mounting the oxidative burst.
Fig. 2. The absence of an oxidative burst in Gc-infected PMNs is not due to reduced PMN viability or catalase-mediated degradation of PMN-derived ROS.
A. PMNs remain viable after infection with Gc at high MOIs. PMNs were left uninfected or were infected with MS11 Gc (MOI 390) for 1 h. Lactate dehydrogenase (LDH) release into the supernatant is expressed as a percentage of total cellular LDH activity. PMNs infected with ExoU-expressing P. aeruginosa for 3 h served as a positive control for PMN lysis. B. No detectable PMN oxidative burst in PMNs infected with catalase-deficient Gc. PMNs were left uninfected, stimulated with PMA, or infected with FA1090 Gc (MOI 80) or an isogenic kat mutant (MOI 50). ROS production was measured as in Fig. 1A.
Previous reports have indicated that PMNs mount a strong oxidative burst in response to Gc Opa proteins that engage granulocyte CEACAM receptors, such as OpaB in strain FA1090 (Elkins and Rest, 1990; Naids and Rest, 1991; Dehio et al., 1998). In contrast, we observed that live, exponentially-growing, piliated FA1090 Gc that predominantly expressed OpaB (OpaB+) elicited minimal ROS production from PMNs, only slightly greater than the isogenic Opa- parent (Fig. 3A). While Opa- Gc elicited essentially no oxidative burst in PMNs (total LDCL was < 1% of that stimulated by PMA), LDCL in response to OpaB+ Gc was 5% of the PMA-induced burst. Similar results were obtained with a FA1090 Gc parent carrying deletions of all 11 opa genes and an isogenic strain in which opaB was reintroduced at a second location in the Gc chromosome (Fulcher, 2004; data not shown). The effect of OpaB+ Gc on the PMN oxidative burst was independent of bacterial piliation state, because OpaB+ Gc carrying a deletion in pilE elicited similar amounts of ROS from PMNs as their isogenic piliated counterparts (data not shown). The OpaB+ -stimulated oxidative burst required bacterial phagocytosis, as ROS production was abolished in PMNs treated with cytochalasin D (data not shown). These results show the respiratory burst elicited from PMNs by liquid-grown OpaB+ Gc is minimal compared to the effect of other soluble (PMA) or particulate (S. aureus) stimuli.
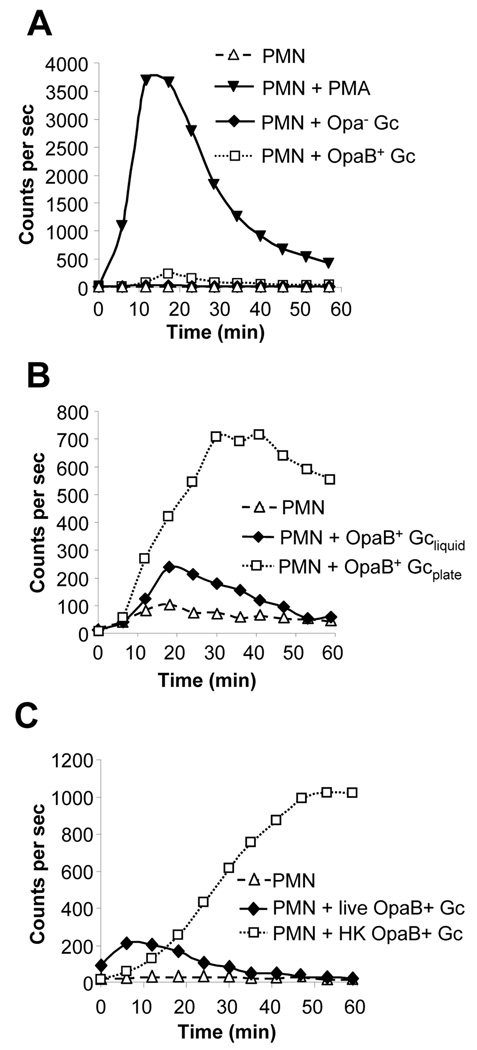
Fig. 3. Live, Opa-expressing Gc elicit a minimal oxidative burst in PMNs.
A. Minor oxidative burst in PMNs infected with live, exponentially-growing OpaB+ FA1090 Gc. PMNs were left uninfected, stimulated with PMA, or exposed to liquid-grown, exponential-phase FA1090 Gc that were Opa- (MOI 82) or expressed OpaB (OpaB+; MOI 120). ROS production was measured as in Fig. 1A.B. Enhanced ROS production in PMNs infected with agar-grown OpaB+ Gc. ROS production was measured in PMNs that were left uninfected or infected with OpaB+ Gc that were grown in liquid medium to exponential phase (Gcliquid; MOI 160) or on agar-containing medium (Gcplate; MOI 14). C. Enhanced ROS production in PMNs exposed to liquid-grown, heat-killed OpaB+ Gc. ROS production was measured in PMNs that were left uninfected, or infected with MOI 260 of liquid-grown OpaB+ Gc that were live or had been killed by heating (HK).
Nonviable Gc stimulate ROS production in PMNs
The weak ROS response of PMNs to liquid-grown OpaB+ Gc led us to consider whether bacterial growth conditions influenced the ability of PMNs to mount an oxidative burst. Previous work examining Opa+ Gc interactions with PMNs typically used bacteria collected from agar-containing medium or grown in liquid culture for short times (< 3 h) prior to infection (Rest et al., 1982; Virji and Heckels, 1986; Fischer and Rest, 1988; Naids and Rest, 1991; Gray-Owen et al., 1997; Simons et al., 2005). When FA1090 OpaB+ Gc were harvested from agar plates and presented to PMNs, they elicited eight-fold more total LDCL from PMNs than liquid-grown Gc, even though the viable CFU present in the liquid-grown culture outnumbered the plate-grown by a factor of ten (Fig. 3B). ROS production in response to plate-grown bacteria was first detected after ~15 min and reached a maximum after 30–40 min, persisting at that level for > 60 min of total exposure (Fig. 3B). We reasoned that the increased responsiveness of PMNs to plate-grown OpaB+ Gc could be due either to changes in bacterial physiology under different growth conditions or to dead bacteria and bacterial products that are more abundant in plate-grown preparations than in liquid culture. To test the latter possibility, live and dead Opa-expressing Gc were compared in their ability to elicit PMN ROS production. Liquid-grown OpaB+ FA1090 Gc were heated to 56 °C for 30 min, a condition that inhibits autolysis (Hebeler and Young, 1975; Elmros et al., 1976) and results in a complete loss of bacterial viability (data not shown). The heat-killed Gc elicited 12-fold more total ROS production in PMNs than their live counterparts (Fig. 3C), and ROS production in PMNs exposed to heat-killed and plate-grown OpaB+ Gc had similar kinetics (compare Fig. 3B and 3C). These results show that the minimal oxidative burst in PMNs exposed to liquid-grown, exponential-phase OpaB+ Gc can be enhanced by intact, dead bacteria as well as bacterial factors present in agar-grown Gc preparations.
To test whether the stimulation of the PMN oxidative burst by nonviable Gc could also be observed with Opa- bacteria, liquid-grown Opa- Gc were killed by heating. Heat-killed Opa- MS11 Gc stimulated PMN ROS production, while an equal number of live bacteria did not: heat-killed Opa- Gc elicited 39 times greater total ROS produced from PMNs than viable Gc (Fig. 4A). Similar results were obtained for Opa- FA1090 Gc (data not shown). The kinetics of ROS production in PMNs stimulated with Opa- and OpaB+ bacteria were similar (compare Fig. 3B and Fig 4A). Opa- bacteria killed by isopropanol treatment also promoted PMN ROS production, indicating that the stimulatory property of nonviable Gc on PMNs was not specifically released by heating (data not shown). Taken together, these results demonstrate that dead Gc, regardless of their Opa profile, retain the ability to induce the oxidative burst in PMNs, but live bacteria can overcome this effect.
Fig. 4. The absence of substantial ROS production in PMNs exposed to liquid-grown, exponential-phase Gc requires live bacteria capable of de novo protein synthesis.
A. Liquid-grown, heat-killed Opa- Gc stimulate ROS production in PMNs. PMNs were left uninfected or were infected with liquid-grown Opa- MS11 Gc that were live (MOI 120) or killed by heating at 56 °C for 30 min (HK; concentration equivalent to MOI 250). ROS production was measured as in Fig. 1A.B. Viable Opa- Gc treated with antibiotics that inhibit protein synthesis stimulate PMN ROS production. PMNs were infected with liquid-grown Opa- FA1090 Gc at a MOI of 100. Gc were exponentially growing (live), heat-killed (HK), or treated with sublethal concentrations of the protein synthesis inhibitors chloramphenicol (Cm) or tetracycline (Tet). Uninfected PMNs did not generate any detectable ROS (data not shown).
Based upon these observations, we hypothesized that Gc had the ability to stimulate ROS metabolism in PMNs, but liquid-grown, exponential-phase Gc made additional products that prevented PMNs from producing ROS. To test whether bacterial protein synthesis was necessary for viable Gc to prevent the PMN oxidative burst, FA1090 Gc were pretreated with chloramphenicol or tetracycline at concentrations previously determined for this strain to not affect bacterial viability but sufficient to prevent initiation of new rounds of replication (Tobiason and Seifert, 2006). The antibiotic-treated Gc did not exhibit any survival defect (data not shown) but elicited a strong oxidative burst in PMNs compared to untreated bacteria (Fig. 4B). Antibiotic-treated Gc stimulated the release of ROS from PMNs with similar kinetics and potency to heat-killed bacteria, but ROS production did not persist in response to antibiotic-treated Gc as it did for heat-killed bacteria, for reasons that remain obscure (Fig. 4B). This result shows that liquid-grown, exponential-phase Gc prevent PMNs from producing ROS by a process that requires de novo bacterial protein synthesis. These results lead us to conclude that viable Gc undergoing protein synthesis actively prevent PMNs from mounting the oxidative burst.
Live Gc suppress the PMN oxidative burst
If viable Gc actively block PMN ROS production, they should not only fail to induce the oxidative burst on their own but also should inhibit the oxidative burst stimulated by dead bacteria. To directly test this hypothesis, PMNs were exposed to liquid-grown, exponential-phase, Opa- Gc, isogenic heat-killed bacteria, or a combination of the two. Live MS11 Gc failed to elicit ROS production in PMNs at MOI of either 200 or 500 (Fig. 5A; only the results for MOI 500 are presented), while heat-killed bacteria stimulated PMN oxidative metabolism in a MOI-dependent manner. When equivalent numbers of viable and heat-killed Gc were added to PMNs, the PMN oxidative burst was completely abrogated (Fig. 5A). Live Gc retained the ability to inhibit the PMN oxidative burst elicited by heat-killed Gc when outnumbered 2.5-fold by dead bacteria, reducing the burst to 60% of that elicited by heat-killed Gc alone (Fig. 5A). We conclude that live Opa- Gc actively inhibit the PMN respiratory burst elicited by nonviable bacteria.
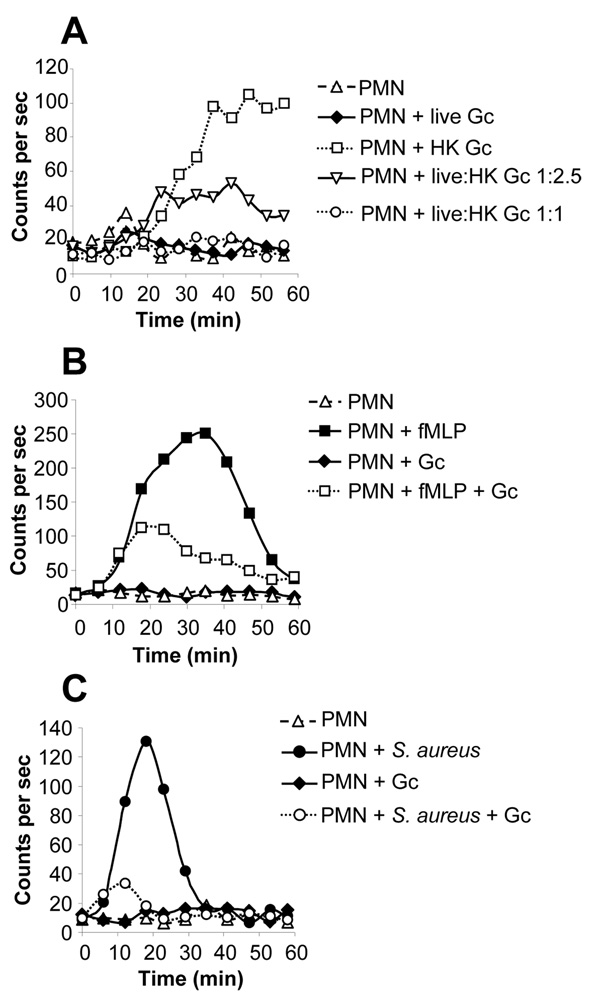
Fig. 5. Live Opa- Gc suppress the oxidative burst of PMNs stimulated with isogenic heat-killed Gc, formylated peptides, and S. aureus.
A. Live Opa- Gc suppress PMN ROS production elicited by heat-killed bacteria. ROS production was measured in PMNs that were left uninfected, exposed to liquid-grown Opa- MS11 Gc that were live (MOI 500) or heat-killed (HK; concentration equivalent to MOI 500), or mixtures of live and heat-killed bacteria at the indicated ratios. ROS production was measured as in Fig. 1A. Viable Gc also failed to elicit ROS production in PMNs at a MOI of 200 (data not shown). B. Gc suppress the fMLP-stimulated PMN oxidative burst. PMNs were left untreated or were exposed to 10 µM fMLP, MS11 Gc at a MOI of 100, or fMLP and Gc simultaneously. C. Gc suppress the PMN oxidative burst elicited by opsonized S. aureus. PMNs were left untreated or were exposed to serum-opsonized S. aureus (MOI 100), FA1090 Gc (MOI 100), or S. aureus and Gc simultaneously (each at MOI 100).
To examine whether the ability of live Gc to inhibit the PMN oxidative burst could be observed for other stimuli, LDCL was monitored in PMNs infected with live Opa- Gc and exposed to factors known to elicit PMN ROS production. PMNs stimulated with 10 µM of formyl-methionylleucylphenylalanine (fMLP) generated ROS that were detectable after 5–10 min, reached a maximum after 30 min, and declined to basal levels after 60 min (Fig. 5B). Infection with Opa- FA1090 or MS11 Gc reduced the fMLP-induced oxidative burst by 60% (Fig. 5B and data not shown). This reduction was not due to bacterial sequestration or degradation of the peptide, since fMLP solution that was preincubated with Gc elicited the same amount of ROS production from PMNs as fresh fMLP (data not shown). Gc also affected the PMN oxidative burst elicited by serum-opsonized S. aureus. At a MOI of 100, S. aureus promoted ROS production in PMNs that peaked after 20 min and declined to basal levels by 35 min (Fig. 5C). Coinfection of PMNs with equivalent numbers of S. aureus and Gc reduced the S. aureus-induced oxidative burst by 84% (Fig. 5C). In experiments where Gc outnumbered S. aureus by 5-fold, the S. aureus-induced burst was almost completely abolished, indicating that the inhibitory effect of Gc was dose-dependent (data not shown). Notably, the suppressive effect of Gc on both the fMLP- and S. aureus-induced PMN oxidative burst required viable, Opa-bacteria, as heat-killed or OpaB+ Gc enhanced the burst elicited by these stimuli (data not shown).
To test whether the suppression of PMN oxidative metabolism extended to all stimulatory factors, the oxidative burst was measured in PMNs infected with Gc and stimulated with PMA, which directly activates protein kinase C isoforms necessary for NADPH oxidase activation (Nauseef et al., 1991). Viable Opa- Gc did not affect the magnitude of the oxidative burst elicited by PMA, even when PMNs were incubated with Gc for 15 min prior to PMA addition, whereas Gc inhibited the S. aureus-induced burst by 65% under these conditions (Fig. 6A). Opa- Gc also had no effect on the small oxidative burst elicited by OpaB+ Gc, even though almost 3-fold more Opa- bacteria were present (Fig. 6B). Therefore, live, Opa- Gc have the ability to suppress the PMN oxidative burst that is elicited by a subset of stimuli, which can be either soluble (fMLP) or particulate (S. aureus).
Fig. 6. Live Opa- Gc do not suppress the oxidative burst of PMNs stimulated with phorbol esters or Opa+ bacteria.
A. Gc do not reduce the PMA-induced PMN oxidative burst. PMNs were left untreated, stimulated with 1 ng ml−1 PMA, or infected with opsonized S. aureus at a MOI of 130. 15 min prior to PMA addition, a subset of PMNs were infected with Opa- MS11 Gc at MOI 100 as indicated. ROS production was measured as in Fig. 1A.B. Opa- Gc do not inhibit the small oxidative burst elicited by OpaB+ Gc. PMNs were left uninfected or were infected with live Opa- FA1090 Gc (MOI 130), live OpaB+ FA1090 Gc (MOI 50), or Opa-and OpaB+ Gc together.
Inhibition of the PMN oxidative burst is contact-dependent
To begin to characterize the mechanism by which viable Opa- Gc suppress the PMN oxidative burst, we examined whether suppression was mediated by secreted Gc products or required direct bacterial contact with PMNs. To test the former possibility, Opa- MS11 Gc were grown to mid-logarithmic phase in a rich defined medium optimized for Gc growth (Morse and Bartenstein, 1980), and the spent supernatant was added to PMNs. The spent supernatant did not itself induce ROS production in PMNs (Fig. 7A), suggesting that any immunomodulatory products of Gc are not present in culture supernatants at sufficient quantities to activate PMNs on their own. Exposure to the spent supernatant had no effect on the PMN oxidative burst induced by S. aureus (Fig. 7A), whereas live, intact Gc grown in the defined medium inhibited the maximal S. aureus-induced burst by nearly 50% (Fig. 7A). Therefore, Gc do not secrete factors that modulate the PMN oxidative burst during growth in rich liquid medium.
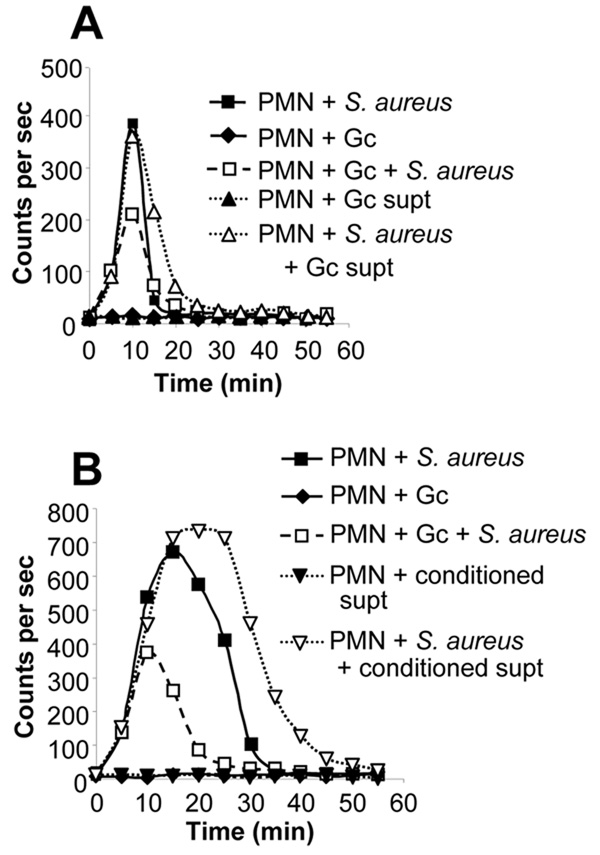
Fig. 7. Gc suppression of the PMN oxidative burst is not mediated by a secreted bacterial factor.
A. The suppressive factor is not directly secreted by live Opa-Gc. PMNs were infected with Opa- MS11 Gc (MOI 35), opsonized S. aureus (MOI 90), or S. aureus and Gc simultaneously. Other PMNs were exposed to the spent supernatant (supt) from liquid-grown, exponential-phase Gc, alone or in the presence of S. aureus.B. The suppressive factor is not released from Gc or PMNs following infection. Conditioned supernatant (supt) was collected from PMNs infected for 1 h with MS11 Gc at a MOI of 100 in the absence of luminol. Naïve PMNs were exposed to the conditioned supernatant in the absence or presence of opsonized S. aureus (MOI 25). Other PMNs were infected with exponentially-growing MS11 Gc (MOI 140), S. aureus, or Gc and S. aureus simultaneously, without the addition of conditioned supernatant. In panels A–B, uninfected PMNs did not generate detectable ROS (data not shown).
In order to examine whether the suppressive factor was secreted specifically in response to PMN infection, PMNs were exposed to Opa- MS11 Gc, and the supernatant from the infection was presented to naïve PMNs. PMN exposure to this conditioned supernatant did not suppress the S. aureus-induced burst and in fact enhanced it, likely due to release of bacterial and/or PMN-derived stimulating factors into the infection medium (Fig. 7B). In the same experiment, intact Gc suppressed the S. aureus-induced burst by 63% (Fig. 7B). These results show that suppression of the PMN oxidative burst is not mediated by a secreted factor derived from either Gc or PMNs.
To test the alternative possibility that Gc-mediated inhibition of the PMN oxidative burst is contact-dependent, ROS production was monitored in PMNs separated from live Opa- Gc by 0.2 µm-pore filter supports. When physically separated from PMNs in this manner, Gc did not inhibit ROS production in response to S. aureus (where S. aureus and PMNs were incubated on the same side of the filter support), and in fact enhanced the S. aureus-induced burst (Fig. 8A). The reason for this potentiation of the S. aureus burst is unclear but may be attributable to factors released by Gc growing in the Transwell system (but not sufficiently present in spent bacterial supernatants), which pass through the filter pores and enhance PMN ROS metabolism. In contrast, the same culture of liquid-grown, exponential-phase Gc inhibited the S. aureus-induced burst by 80% when the filter was absent (Fig. 8A). Therefore, Gc require contact with PMNs to inhibit the phagocyte oxidative burst.
Fig. 8. Gc suppression of the PMN oxidative burst requires bacteria-PMN contact but not phagocytosis.
A. Gc inhibition of PMN ROS production requires bacteria-PMN contact. PMNs were incubated with Opa- MS11 Gc (MOI 200) for 15 min, where Gc and PMNs were allowed to mix in the well (PMN + Gc) or were separated by a 0.2 µm-pore filter support (Gc in Transwell). Opsonized S. aureus (MOI 40) were then added to the same side of the wells as PMNs. ROS production was measured as in Fig. 1A.B. Extracellular Gc suppress the PMN oxidative burst. PMNs were treated with 10 µg ml−1 cytochalasin D (CD) or an equal volume of DMSO carrier. PMNs were then infected with liquid-grown Opa- MS11 Gc (MOI 320), treated with 10 µM fMLP, or exposed to Gc and fMLP simultaneously, in the absence or presence of CD as indicated. In panels A–B, uninfected PMNs did not generate detectable ROS (data not shown).
Since both adherent and phagocytosed Gc are found in association with PMNs during infection, we examined whether Gc needed to be internalized by PMNs to prevent ROS production by treating PMNs with the actin-depolymerizing toxin cytochalasin D (CD). CD shifted the kinetics of ROS production in response to fMLP such that the oxidative burst was initiated slightly earlier and persisted for less time than in untreated PMNs (Fig. 8B). The inhibition of ROS production in Gc-infected, fMLP-stimulated PMNs was similar whether CD was present or not, as infection reduced the fMLP-stimulated LDCL in untreated PMNs by 71% and in CD-treated PMNs by 61%. From this series of experiments, we conclude that live Gc suppress the PMN oxidative burst by production of a factor requiring de novo protein synthesis, whose mechanism of action requires bacterial contact with PMNs but is independent of phagocytosis of Gc.
Discussion
The long-term association of Gc with the human population reflects its successful adaptation to life exclusively in the human urogenital tract. Although the controlled environmental and nutritional conditions of this anatomic site support bacterial growth and provide a direct route for the spread of infection, Gc must also contend with the potent PMN-rich innate immune response initiated in response to bacterial colonization. This work reveals one previously unappreciated means by which Gc subvert PMN antimicrobial activity: the ability of live, exponentially-growing bacteria to actively suppress the PMN oxidative burst. Disarming this critical aspect of PMN defenses may enhance Gc virulence in infectious exudates to facilitate colonization of the current host and transmission to new individuals.
Several lines of evidence indicate that Gc suppression of PMN ROS production is an active process. First, heat-killed or plate-grown bacteria retain the ability to stimulate ROS production in PMNs, even in the absence of serum opsonins. This finding implies that PMNs have the ability to recognize and respond to Gc products; however, live bacteria overcome their stimulatory effects. Stimulatory products of Gc could include lipooligosaccharide, peptidoglycan, porins, and the Lip lipoprotein, which are recognized by Toll-like receptors that are expressed on many cell types including PMNs (Massari et al., 2002; Fisette et al., 2003; Mogensen et al., 2006). Second, bacterial protein synthesis is required for Gc to block the PMN oxidative burst, as demonstrated using sublethal concentrations of antibiotics that impede ribosomal function. Third, PMNs retain viability and the ability to respond to phorbol esters during infection with Gc. The latter observation also implies that live, exponentially-growing Gc did not deplete the assay medium of dissolved oxygen, the substrate for NADPH oxidase activity, even if Gc oxygen consumption is stimulated by the presence of phagocyte-derived lactate (Britigan et al., 1988). Fourth, Gc do not immediately detoxify the ROS produced by PMNs by virtue of their potent catalase activity, since catalase-deficient Gc also fail to elicit the PMN oxidative burst. Taken together, these observations indicate that viable Gc synthesize factors that prevent PMNs from producing ROS in response to pathogen-associated molecular patterns (PAMPs). PAMPs are present not only on Gc but also on the gram-positive S. aureus and are mimicked by the formylated peptide fMLP, two stimuli whose PMN oxidative burst was inhibited by Gc. Given these observations, we hypothesized that S. aureus survival after infection of PMNs would be enhanced if Gc were also present. Using a previously published PMN assay (Stohl et al., 2005), our results suggest that coinfection with Gc does increase S. aureus survival, but the effect is modest (two-fold increase in recovered S. aureus) and variable among experiments, suggesting that only a subset of S. aureus are killed by oxidative means in PMNs (data not shown). It will be intriguing to determine whether Gc modulate the survival of pathogens that commonly co-colonize the urogenital tract when confronted with activated, ROS-producing PMNs.
Gc joins a number of pathogens that are known to modulate PMN oxidative metabolism, but this bacterium appears to utilize a unique mechanism to accomplish this feat. Unlike Helicobacter pylori, Salmonella enterica serovar Typhimurium, Streptococcus pyogenes, and Leishmania donovani, which stimulate the PMN oxidative burst but redirect NADPH oxidase activity away from the phagosome (Vazquez-Torres et al., 2000; Gallois et al., 2001; Allen et al., 2005; Lodge et al., 2006; Staali et al., 2006), viable Opa- Gc prevent PMNs from producing ROS. Anaplasma phagocytophilum and Francisella tularensis also block PMN oxidative metabolism, but these organisms suppress the respiratory burst in response to both fMLP and PMA, while Gc cannot block the PMA-induced burst (Mott and Rikihisa, 2000; Carlyon and Fikrig, 2003; Carlyon et al., 2004; McCaffrey and Allen, 2006). Failure to block the PMA-induced burst implies that Gc suppression of the oxidative burst does not involve proteolysis of NADPH oxidase subunits or transcriptional repression of oxidase genes, as associated with A. phagocytophilum infection (Carlyon et al., 2002; Mott et al., 2002). Since PMA directly activates protein kinase C isoforms, these results also imply that suppression by Gc does not involve direct inhibition of protein kinase C activity, as found for Pseudomonas aeruginosa secreted phospholipase (Terada et al., 1999). Instead, we hypothesize that Opa- Gc infection blocks a signaling pathway that is required for NADPH oxidase activation, which is stimulated by engagement of the formyl peptide and complement receptors but not CEACAM receptors. These pathways could involve mitogen-activated protein kinases or protein kinase A, which are targeted by Bacillus anthracis toxins to suppress the PMN oxidative burst in response to fMLP but not PMA (Crawford et al., 2006), or tyrosine kinases or lipid second messengers that are required for fMLP-mediated NADPH oxidase activation (Le et al., 2002).
Although our results show that exponentially-growing FA1090 OpaB+ Gc elicit a minor respiratory burst in PMNs, others have reported that Opa-expressing Gc induce a potent phagocyte oxidative burst (Rest et al., 1982; Virji and Heckels, 1986; Fischer and Rest, 1988; Naids and Rest, 1991; Gray-Owen et al., 1997; Simons et al., 2005). In this study, Gc were grown to mid-logarithmic phase after successive rounds of subculturing into rich liquid medium, which allows > 95% bacterial viability (data not shown). In previous reports where Gc stimulated the PMN oxidative burst, bacteria were either directly harvested from agar-containing medium or were harvested from agar medium and grown in liquid medium for short times. In both cases, dead bacteria and bacterial products are present along with live Gc. Since we have shown that dead Gc (both Opa− and Opa+) potently stimulate the release of ROS from PMNs, it is likely that previous studies were primarily measuring the effects of nonviable bacteria or shed bacterial products on PMNs. Intriguingly, these results raise the possibility that when PMNs are infected with live Gc in the presence of a stimulus known to induce an oxidative burst (e.g., dead bacteria or PMA), the resulting oxidative damage could cause increased bacterial killing. However, using a previously developed assay for measuring Gc survival after exposure to PMNs (Stohl et al., 2005), we have not found PMA-stimulated PMNs to kill liquid-grown Gc any more effectively than untreated PMNs (data not shown). These results may reflect the extensive and redundant antioxidant defenses possessed by Gc (Seib et al., 2006). Alternatively, they may indicate that PMNs primarily direct non-oxidative antimicrobial mechanisms against Gc. This possibility is supported by reports from the Rest laboratory showing that PMNs maintained under anoxic conditions or lacking NADPH oxidase activity retain some ability to kill Opa+ Gc (Rest et al., 1982; Frangipane and Rest, 1992), as well as the recent observation that Gc mutated in genes encoding various antioxidants are not compromised for survival after PMN challenge (Seib et al., 2005). We are currently reexamining which antimicrobial mechanisms PMNs direct against Gc during infection.
This work provides two key observations about the unknown factor produced by liquid-grown, exponential-phase Gc that inhibits PMN oxidative metabolism. First, since suppression of the PMN oxidative burst requires de novo bacterial protein synthesis, suppression is mediated either by a protein that is rapidly turned over or strongly induced during PMN infection or by a nonprotein factor that requires de novo protein synthesis for its production or release. Second, suppression of the PMN oxidative burst is not mediated by secreted bacterial products, in agreement with the observations that Gc do not produce exotoxins or encode large numbers of functional secreted proteins in their genome (Ulsen and Tommassen, 2006; see http://www.genome.ou.edu/gono.html). Certain strains of Gc possess a gonococcal genetic island (GGI) encoding a type IV secretion apparatus that secretes DNA (Dillard and Seifert, 2001; Hamilton et al., 2005), but the fact that strains MS11 (GGI+) and FA1090 (GGI-) can both suppress the PMN oxidative burst indicates that the type IV secretion system is not involved in modulating PMN activation. Instead, our results strongly suggest that Gc inhibition of the PMN oxidative burst requires bacteria-phagocyte contact. Since the FA1090 genome does not encode secretion systems that deliver virulence-associated proteins into the host cell cytosol, we assume that the Gc suppressive factor is exported to the bacterial surface, where it interacts with the phagocyte plasma membrane. Thus far we have tested and discounted several surface-exposed Gc virulence factors for their potential to suppress ROS production in PMNs, including type IV pili (pilin and PilQ secretin), the MtrCDE efflux pump, IgA protease, and a Gc phospholipase D homolog (Fig. 1B and data not shown). In previous work from our laboratory, mutants in ngo1686 and recN were shown to be more sensitive to PMN killing (Stohl et al., 2005), but these mutants had no effect on PMN ROS production (data not shown). Although we do not currently know the identity of the Gc suppressive factor, one possibility is that ROS production in PMNs is modulated by Gc porin, which has been shown to translocate from the bacterial surface into eukaryotic plasma and internal membranes (Massari et al., 2003). Intriguingly, Lorenzen et al. have shown that purified Gc porin inhibits the oxidative burst of PMNs, but in other studies, porin had no effect on PMN oxidative metabolism (Bjerknes et al., 1995; Bauer et al., 1999; Lorenzen et al., 2000). We are exploring whether porin and other Gc products play a role in suppression of the PMN oxidative burst in this system.
Given that Gc are constantly barraged by the PMN-rich host inflammatory response, it is not surprising that this strict human pathogen has evolved mechanisms to withstand interactions with PMNs, including the ability to suppress the phagocyte oxidative burst. Since the suppressive activity of Gc on PMN ROS metabolism varies depending on the viability of the bacteria and the surface structures present (i.e. Opa proteins), we speculate that Gc suppression of the PMN oxidative burst aids bacterial survival at specific stages of gonorrheal disease. Early in infection, when the majority of Gc may be viable, suppression of the oxidative burst would aid in colonization of the urogenital mucosa during the initial inflammatory response. The ability of Opa- Gc to suppress PMN oxidative metabolism may also allow the bacteria to ascend the urogenital tract and disseminate to secondary sites, since the majority of Gc isolates from the upper urogenital tract and those causing gonococcal arthritis lack Opa expression (O'Brien et al., 1983; Edwards and Apicella, 2004). In contrast, during active lower urogenital tract infection, the predominance of Opa+ Gc (James and Swanson, 1978; Swanson et al., 1988; Jerse et al., 1994), in combination with increasing numbers of dead Gc and proinflammatory bacterial products, could overcome the suppression mediated by live Opa- Gc and stimulate the PMN oxidative burst. However, the intrinsically high resistance of Gc to ROS (Archibald and Duong, 1986; Hassett et al., 1990) as well as the ROS-mediated upregulation of gene products that protect Gc from PMN killing (Stohl et al., 2005) would aid Gc survival when confronted with the resulting PMN oxidative burst. Gc suppression of PMN oxidative metabolism may also help to explain the discrepancies in disease presentation between men and women. Male urethral infection selects for Opa+ Gc that are capable of inducing ROS production in PMNs and may exacerbate the potent inflammatory response that characterizes acute disease in men (James and Swanson, 1978; Zak et al., 1984). In contrast, Opa- Gc predominate in women depending on the stage of the menstrual cycle, and suppression of the PMN oxidative burst under these conditions may contribute to the preponderance of asymptomatic or subclinical disease in the female population (James and Swanson, 1978; Edwards and Apicella, 2004). Given natural variations in Opa expression and the balance between viable and nonviable Gc during the course of human infection, Gc suppression of the PMN oxidative burst can be considered a dynamic process, serving at critical junctures to enhance gonorrheal pathogenesis in the face of the inflammatory host immune response.
Experimental procedures
Bacterial strains and growth conditions
The parent strains of Gc used in this study were piliated, Opa- derivatives of strain FA1090 encoding pilin variant 1-81-S2 (Seifert et al., 1994) and strain MS11 encoding pilin variant VD300 (Koomey et al., 1987). Gc were maintained on gonococcal medium base agar (GCB) plus Kellogg’s supplements (Kellogg et al., 1963) and routinely grown for 20 h at 37 °C, 5% CO2. Constitutively nonpiliated derivatives of FA1090 and MS11 carrying deletions of the pilE locus have been described (Segal et al., 1985; Chen et al., 2004). The deletions were introduced into the FA1090 1-81-S2 or MS11 VD300 chromosome by natural transformation, nonpiliated progeny were identified by colonial morphology, and incorporation of the deletion was confirmed by Southern blotting with a pilE-specific probe. The FA1090 kat::kan mutant was obtained from A. Jerse (Uniformed Services Univ. of the Health Sciences; Soler-Garcia and Jerse, 2004), the strain FA19 mtrC::kan mutant was obtained from W. Shafer (Emory Univ.; Hagman et al., 1995), the strain 1291 pld::kan mutant was obtained from M. Apicella and J. Edwards (Univ. of Iowa and Research Institute at Nationwide Children’s Hosp.; Edwards et al., 2003), and the FA1090 pilQ::cat mutation has been described (Long et al., 2003). The mutations were introduced into FA1090 1-81-S2 Gc and/or MS11 VD300 Gc by natural transformation, and transformants were selected on the appropriate antibiotics at the following concentrations: kanamycin, 40 µg ml−1; chloramphenicol, 0.6 µg ml−1 for FA1090 and 2 µg ml−1 for MS11. Genotypes were confirmed by Southern blot with gene-specific probes. In all strains, pilE genes were sequenced as described (Seifert et al., 1994) to confirm retention of the desired pilin variant.
An OpaB-expressing (OpaB+) derivative of FA1090 1-81-S2 Gc was isolated after in vitro passage. Isolates of FA1090 Gc in which all 11 opa genes were deleted and where opaB was reintroduced at a second site in the chromosome were provided by J. Cannon (Univ. of North Carolina; Fulcher, 2004). Opa expression profiles of FA1090 and MS11 Gc were confirmed by immunoblotting bacterial lysates according to published methods (Black et al., 1984) with a panel of monoclonal and polyclonal antibodies obtained from J. Cannon, M. Blake (FDA), and A. Jerse.
Viable, exponentially-growing Gc were generated in liquid culture using a modification of a previously described technique (Stohl et al., 2005). Gc cultured for 9–10 h on GCB were suspended at OD550 = 0.07 in gonococcal liquid medium containing Kellogg’s supplements and 0.042% Na2HCO3 (GCBL) and grown at 30 °C with rotation for 14–16 h. The cultures were diluted into fresh GCBL and grown at 37 °C to OD550 = 0.6–0.7, then diluted to OD550 = 0.07 and grown at 37 °C to mid-logarithmic phase. The CFU per ml present in each culture was estimated from a previously determined growth curve and validated by serial dilution and plate count. For experiments with plate-grown bacteria, confluent lawns of Gc that had been grown on GCB for 20 h were collected with a Dacron swab and resuspended in liquid medium. To kill liquid-grown Gc, bacteria were heated at 56 °C for 30 min or incubated in 70% isopropanol for 5 min. Gc protein synthesis was inhibited by treating liquid-grown FA1090 bacteria with 2 µg ml−1 of chloramphenicol or 1 µg ml−1 of tetracycline, concentrations that enrich for fully replicated chromosomes in Gc but do not affect bacterial viability (Tobiason and Seifert, 2006, and data not shown).
S. aureus ATCC strain 25923 was obtained from K. Fullner-Satchell (Northwestern Univ.) and grown on Todd-Hewitt agar (Difco). Overnight cultures of S. aureus in Todd-Hewitt broth (Difco) were diluted 1:100 into fresh broth and grown for 4–6 h. S. aureus were opsonized in 10% autologous human serum for 20 min at 37 °C prior to PMN infection.
P. aeruginosa strain PA99 expressing the type III secreted protein ExoU was obtained from A. Hauser (Northwestern Univ.). Overnight cultures of PA99 in Luria-Bertani broth (LB; Difco) were diluted 1:40 into fresh LB and grown for 3 h.
Isolation of human PMNs
Heparinized venous blood was obtained from consented healthy volunteers following a protocol approved by the Northwestern University Institutional Review Board. Dextran-sedimented PMNs were purified on a Ficoll-Hypaque gradient as previously described (Stohl et al., 2005). PMNs were resuspended at 2 × 107 cells per ml in Dulbecco’s PBS (without calcium and magnesium; Mediatech) containing 0.1% dextrose and kept on ice until use. PMN preparations routinely contained > 95% granulocytes, assessed morphologically by phase-contrast microscopy, and were > 99% viable, monitored by trypan blue exclusion.
Luminol-dependent chemiluminescence (LDCL)
106 PMNs were incubated in Morse’s defined medium (MDM; Morse and Bartenstein, 1980) in the presence of 20 µM luminol (Sigma), at a final volume of 1 ml. PMNs were infected with Gc at the indicated MOI; the MOI for heat-killed bacteria was determined by serial dilution and plate count of the culture prior to heat treatment. PMNs were stimulated with opsonized S. aureus at the indicated MOI, 10 µM fMLP (Sigma), and/or 1–10 ng ml−1 PMA (Sigma), in the presence or absence of Gc. LDCL was measured in a LKB-Wallac 1250 luminometer (Perkin Elmer) every 5 min for 1 h at 37 °C, and the counts per sec (CPS) of LDCL were averaged per condition between duplicate wells. Untreated and 10 ng ml−1 PMA-treated PMNs served as negative and positive controls for ROS production, respectively. In some experiments, PMNs were preexposed to 10 µM diphenylidene iodonium hydrochloride (Sigma) or 10 µg ml−1 cytochalasin D (Sigma) for 10 min. Experiments were repeated at least three times, using PMNs from a different donor for each replicate.
For experiments with Transwells, PMNs were separated from Gc using 6.5 mm diameter, 0.2 µm pore Transwell filter supports (Costar). Opsonized S. aureus were added to the same side of the support as the PMNs to stimulate LDCL production. To collect spent Gc supernatants, liquid-grown MS11 VD300 Gc were prepared as described, except Gc were grown in MDM for the final subculture. Bacteria were pelleted from the mid-logarithmic phase culture, and the supernatant was passed through a 0.22 µm pore filter in order to remove residual bacteria. The supernatant was used in place of MDM in the LDCL assay. To collect culture supernatants from previously infected PMNs, 106 PMNs were infected with Gc at a MOI of 100 for 1 h in MDM. The supernatant was collected and passed through a 0.2 µm syringe filter, added to naïve PMNs in the presence of luminol and S. aureus, and LDCL was measured for 1 h as above.
Total LDCL was calculated by approximating the area under the curve by the trapezoidal rule, i.e. the sum of LDCL produced in each interval between the times tn and tn+1, calculated as
where CPStn+1 and CPStn are the background-subtracted CPS of LDCL measured at each time point (background = unstimulated PMNs).
LDH assay
PMNs were resuspended in modified HEPES-buffered saline (15 mM HEPES pH 7.4, 8 mM glucose, 4 mM KCl, 140 mM NaCl, 0.5 mM MgCl2, 0.9 mM CaCl2) and exposed to MS11 Gc at a MOI of 390 for 1 h or left untreated. Release of lactate dehydrogenase from PMNs was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (BioRad) according to the manufacturer’s instructions. PMNs infected for 3 h with ExoU-expressing P. aeruginosa at a MOI of 100 served as a positive control for PMN lysis. Experiments were repeated three times, using PMNs from different donors, with similar results.
Immunofluorescence microscopy
PMNs were infected for 30 min with piliated, Opa- FA1090 Gc at a MOI of 10. PMNs were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) in PBS, and internal and external bacteria were discriminated from one another using a polyclonal anti-N. gonorrhoeae antibody (Biosource) and a differential immunofluorescence procedure as previously described (Criss and Seifert, 2006). Cells were examined using a LSM510 confocal laser scanning microscope (Zeiss). Images were acquired with LSM510 operating software and processed with Adobe Photoshop 7.0.
Acknowledgements
We thank Michael Apicella, Milan Blake, Janne Cannon, Jennifer Edwards, Alan Hauser, Ann Jerse, Karla Satchell, and William Shafer for antibodies and bacterial strains; Alan Hauser, John Carter, Ciara Shaver, and Laurie Tudor (Northwestern University) for assistance with venipuncture; Michael Apicella for advice with PMN experiments; and Elizabeth Stohl and Mark Anderson for reading and editing the manuscript. This work was supported by R01 AI044239, R01 055977, and R37 AI033493 to H.S.S. and F32 AI056681 and K99 TW008042 to A.K.C.
References
- Allen LA, Beecher BR, Lynch JT, Rohner OV, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658–3667. doi: 10.4049/jimmunol.174.6.3658. [DOI] [PubMed] [Google Scholar]
- Archibald FS, Duong MN. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun. 1986;51:631–641. doi: 10.1128/iai.51.2.631-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer FJ, Rudel T, Stein M, Meyer TF. Mutagenesis of the Neisseria gonorrhoeae porin reduces invasion in epithelial cells and enhances phagocyte responsiveness. Mol Microbiol. 1999;31:903–913. doi: 10.1046/j.1365-2958.1999.01230.x. [DOI] [PubMed] [Google Scholar]
- Belland RJ, Chen T, Swanson J, Fischer SH. Human neutrophil response to recombinant neisserial Opa proteins. Mol Microbiol. 1992;6:1729–1737. doi: 10.1111/j.1365-2958.1992.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Bjerknes R, Guttormsen HK, Solberg CO, Wetzler LM. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect Immun. 1995;63:160–167. doi: 10.1128/iai.63.1.160-167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black WJ, Schwalbe RS, Nachamkin I, Cannon JG. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984;45:453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan BE, Klapper D, Svendsen T, Cohen MS. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J Clin Invest. 1988;81:318–324. doi: 10.1172/JCI113323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon JA, Fikrig E. Invasion and survival strategies of Anaplasma phagocytophilum. Cell Microbiol. 2003;5:743–754. doi: 10.1046/j.1462-5822.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Chan WT, Galan J, Roos D, Fikrig E. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J Immunol. 2002;169:7009–7018. doi: 10.4049/jimmunol.169.12.7009. [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Abdel-Latif D, Pypaert M, Lacy P, Fikrig E. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect Immun. 2004;72:4772–4783. doi: 10.1128/IAI.72.8.4772-4783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Tobiason DM, Thomas CE, Shafer WM, Seifert HS, Sparling PF. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J Bacteriol. 2004;186:730–739. doi: 10.1128/JB.186.3.730-739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Gotschlich EC. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci U S A. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MA, Aylott CV, Bourdeau RW, Bokoch GM. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J Immunol. 2006;176:7557–7565. doi: 10.4049/jimmunol.176.12.7557. [DOI] [PubMed] [Google Scholar]
- Criss AK, Seifert HS. Gonococci exit apically and basally from polarized epithelial cells and exhibit dynamic changes in type IV pili. Cell Microbiol. 2006;8:1430–1443. doi: 10.1111/j.1462-5822.2006.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- Dehio C, Gray-Owen SD, Meyer TF. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- Dillard JP, Seifert HS. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol Microbiol. 2001;41:263–277. doi: 10.1046/j.1365-2958.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JL, Entz DD, Apicella MA. Gonococcal phospholipase D modulates the expression and function of complement receptor 3 in primary cervical epithelial cells. Infect Immun. 2003;71:6381–6391. doi: 10.1128/IAI.71.11.6381-6391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins C, Rest RF. Monoclonal antibodies to outer membrane protein PII block interactions of Neisseria gonorrhoeae with human neutrophils. Infect Immun. 1990;58:1078–1084. doi: 10.1128/iai.58.4.1078-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmros T, Burman LG, Bloom GD. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976;126:969–976. doi: 10.1128/jb.126.2.969-976.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Fischer SH, Rest RF. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988;56:1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- Frangipane JV, Rest RF. Anaerobic growth of gonococci does not alter their Opa-mediated interactions with human neutrophils. Infect Immun. 1992;60:1793–1799. doi: 10.1128/iai.60.5.1793-1799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher NB. Thesis. Department of Microbiology and Immunology. Chapel Hill, NC: University of North Carolina; 2004. The role of Neisseria gonorrhoeae opacity proteins in host cell interactions and pathogenesis; pp. 1–202. [Google Scholar]
- Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J Immunol. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. 1997;26:971–980. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- Hamilton HL, Dominguez NM, Schwartz KJ, Hackett KT, Dillard JP. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol. 2005;55:1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Harkness AH. The pathology of gonorrhoea. Br J Vener Dis. 1948;24:137–147. [PMC free article] [PubMed] [Google Scholar]
- Hassett DJ, Charniga L, Cohen MS. recA and catalase in H2O2-mediated toxicity in Neisseria gonorrhoeae. J Bacteriol. 1990;172:7293–7296. doi: 10.1128/jb.172.12.7293-7296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler BH, Young FE. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975;122:385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EW, Holmes KK. Gonococcal infections. Ann Intern Med. 1985;102:229–243. doi: 10.7326/0003-4819-102-2-229. [DOI] [PubMed] [Google Scholar]
- James JF, Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978;19:332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun. 2003;71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, Seifert HS, Cannon JG. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DS, Jr, Peacock WL, Deacon WE, Brown L, Pirkle CI. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey M, Gotschlich EC, Robbins K, Bergstrom S, Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 1987;117:391–398. doi: 10.1093/genetics/117.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- Lodge R, Diallo TO, Descoteaux A. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell Microbiol. 2006;8:1922–1931. doi: 10.1111/j.1462-5822.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- Long CD, Tobiason DM, Lazio MP, Kline KA, Seifert HS. Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect Immun. 2003;71:6279–6291. doi: 10.1128/IAI.71.11.6279-6291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen DR, Gunther D, Pandit J, Rudel T, Brandt E, Meyer TF. Neisseria gonorrhoeae porin modifies the oxidative burst of human professional phagocytes. Infect Immun. 2000;68:6215–6222. doi: 10.1128/iai.68.11.6215-6222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari P, Ram S, Macleod H, Wetzler LM. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 2003;11:87–93. doi: 10.1016/s0966-842x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- McCaffrey RL, Allen LA. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol. 2006;80:1224–1230. doi: 10.1189/jlb.0406287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J Leukoc Biol. 2006;80:267–277. doi: 10.1189/jlb.1105626. [DOI] [PubMed] [Google Scholar]
- Morse SA, Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980;26:13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- Mott J, Rikihisa Y. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect Immun. 2000;68:6697–6703. doi: 10.1128/iai.68.12.6697-6703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J, Rikihisa Y, Tsunawaki S. Effects of Anaplasma phagocytophila on NADPH oxidase components in human neutrophils and HL-60 cells. Infect Immun. 2002;70:1359–1366. doi: 10.1128/IAI.70.3.1359-1366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naids FL, Rest RF. Stimulation of human neutrophil oxidative metabolism by nonopsonized Neisseria gonorrhoeae. Infect Immun. 1991;59:4383–4390. doi: 10.1128/iai.59.12.4383-4390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM, Volpp BD, McCormick S, Leidal KG, Clark RA. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991;266:5911–5917. [PubMed] [Google Scholar]
- O'Brien JP, Goldenberg DL, Rice PA. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine. 1983;62:395–406. [PubMed] [Google Scholar]
- Qu XD, Harwig SS, Oren AM, Shafer WM, Lehrer RI. Susceptibility of Neisseria gonorrhoeae to protegrins. Infect Immun. 1996;64:1240–1245. doi: 10.1128/iai.64.4.1240-1245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest RF, Pretzer E. Degradation of gonococcal outer membrane proteins by human neutrophil lysosomal proteases. Infect Immun. 1981;34:62–68. doi: 10.1128/iai.34.1.62-68.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest RF, Fischer SH, Ingham ZZ, Jones JF. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982;36:737–744. doi: 10.1128/iai.36.2.737-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–1315. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Segal AW. How neutrophils kill microbes. Ann Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E, Billyard E, So M, Storzbach S, Meyer TF. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985;40:293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Seib KL, Wu HJ, Kidd SP, Apicella MA, Jennings MP, McEwan AG. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol Mol Biol Rev. 2006;70:344–361. doi: 10.1128/MMBR.00044-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib KL, Simons MP, Wu HJ, McEwan AG, Nauseef WM, Apicella MA, Jennings MP. Investigation of oxidative stress defenses of Neisseria gonorrhoeae by using a human polymorphonuclear leukocyte survival assay. Infect Immun. 2005;73:5269–5272. doi: 10.1128/IAI.73.8.5269-5272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest. 1994;93:2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer WM, Rest RF. Interactions of gonococci with phagocytic cells. Ann Rev Microbiol. 1989;43:121–145. doi: 10.1146/annurev.mi.43.100189.001005. [DOI] [PubMed] [Google Scholar]
- Shafer WM, Onunka VC, Martin LE. Antigonococcal activity of human neutrophil cathepsin G. Infect Immun. 1986;54:184–188. doi: 10.1128/iai.54.1.184-188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer WM, Qu X, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA. 1998;95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons MP, Nauseef WM, Apicella MA. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect Immun. 2005;73:1971–1977. doi: 10.1128/IAI.73.4.1971-1977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Garcia AA, Jerse AE. A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals. Microb Pathog. 2004;37:55–63. doi: 10.1016/j.micpath.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Soler-Garcia AA, Jerse AE. Neisseria gonorrhoeae catalase is not required for experimental genital tract infection despite the induction of a localized neutrophil response. Infect Immun. 2007;75:2225–2233. doi: 10.1128/IAI.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staali L, Bauer S, Morgelin M, Bjorck L, Tapper H. Streptococcus pyogenes bacteria modulate membrane traffic in human neutrophils and selectively inhibit azurophilic granule fusion with phagosomes. Cell Microbiol. 2006;8:690–703. doi: 10.1111/j.1462-5822.2005.00662.x. [DOI] [PubMed] [Google Scholar]
- Stohl EA, Criss AK, Seifert HS. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol. 2005;58:520–532. doi: 10.1111/j.1365-2958.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Barrera O, Sola J, Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988;168:2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS, Johansen KA, Nowbar S, Vasil AI, Vasil ML. Pseudomonas aeruginosa hemolytic phospholipase C suppresses neutrophil respiratory burst activity. Infect Immun. 1999;67:2371–2376. doi: 10.1128/iai.67.5.2371-2376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiason DM, Seifert HS. The obligate human pathogen, Neisseria gonorrhoeae, is polyploid. PLoS Biol. 2006;4:1069–1078. doi: 10.1371/journal.pbio.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulsen P, Tommassen J. Protein secretion and secreted proteins in pathogenic Neisseriaceae. FEMS Microbiol Rev. 2006;30:292–319. doi: 10.1111/j.1574-6976.2006.00013.x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- Virji M, Heckels JE. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986;132:503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- Warner DM, Folster JP, Shafer WM, Jerse AE. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J Infect Dis. 2007;196:1804–1812. doi: 10.1086/522964. [DOI] [PubMed] [Google Scholar]
- WHO. Geneva: WHO; Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. 2001:1–50. [PubMed]
- Zak K, Diaz J-L, Jackson D, Heckels JE. Antigenic variation during infection with Neisseria gonorrhoeae: Detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984;149:166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]