Abstract
Traumatic brain injury (TBI) results in functional deficits that often are effectively treated clinically with the neurostimulant methylphenidate (MPH). We hypothesized that daily MPH administration would reverse striatal neurotransmission deficits observed in the controlled cortical impact (CCI) model of TBI. CCI or naïve rats received daily injections of MPH (5mg/kg) or saline for 14 days and were assessed on day 15 using fast scan cyclic voltammetry (FSCV). DA transporter (DAT) localization, DA-related proteins, and transcription factor (c-fos) expression were also assessed. CCI resulted in reduced electrically evoked overflow of DA and maximal velocity of DA clearance (Vmax). In contrast, CCI was associated with a decrease in the apparent KM of DAT. Daily MPH after CCI resulted in robust increases in evoked DA overflow and Vmax as well as increased apparent KM. Reductions in total striatal DAT expression occurred after CCI that were not further affected by MPH. In contrast, membrane bound striatal DAT levels were increased in both CCI groups. MPH post-CCI significantly increased striatal c-fos levels compared to saline. These results support the hypothesis that daily MPH improves striatal DA neurotransmission after CCI. DAT expression and transcriptional changes affecting DA protein function may underlie the injury and MPH induced alterations in neurotransmission observed.
Keywords: traumatic brain injury, dopamine, methylphenidate, striatum, voltammetry, neurotransmission, transcription factor
INTRODUCTION
Dopamine (DA) systems have been implicated with brain pathology after traumatic brain injury (TBI) and appear to play a role in the subsequent functional and cognitive deficits typically observed. In fact, clinical studies report improvements with patients in the areas of attention, motor speed, and memory tasks with the administration of the DA reuptake inhibitor methylphenidate (MPH) following injury (Whyte et al. 1997; Whyte et al. 2004). Additionally, clinical treatment guidelines recommend MPH treatment to improve motor processing speed, attention, and general cognition post injury (Warden et al. 2006). In addition to clinical studies, experimental behavioral studies show improvements on specific tasks in those receiving the DAT transporter inhibitor, MPH, after TBI (Kline et al. 2000; Wagner et al. 2007). However, these studies do not investigate how CCI affects striatal DA neurotransmission or how treatment with a DA reuptake inhibitor may affect DA neurotransmission in the injured brain.
Dopaminergic neurotransmission is highly regulated by the Na+/Cl- -dependent DA transporter (DAT), as it terminates the action of vesicular DA release at the synapse via reuptake of extracellular DA (Torres et al., 2003) and through its role in reverse transport of DA under basal conditions (Borland and Michael, 2004). There are many ways in which transporters may be chronically regulated, including gene transcription, post-translational modifications, oligomerization, and trafficking (Zahniser and Doolen 2001). Furthermore, function and expression for many transporters are regulated by transporter substrates (Bernstein 1999; Munir et al. 2000; Quick 2002; Williams and Galli 2006). In addition, DA itself can regulate DAT via its interaction with the transporter or presynaptic autoreceptors (Williams and Galli 2006). Pharmacological treatments may also have long lasting effects on DA protein expression, particularly in juvenile rats (Moll et al. 2001). However, little is known about MPH effects specifically on DA neurotransmission.
Both experimental TBI and MPH administration are associated with multiple striatal protein changes. Our previous studies show decreased total tissue striatal DAT expression 2-4 weeks after experimental TBI using the controlled cortical impact (CCI) injury model (Wagner et al. 2005; Wagner et al. 2005b). Additionally, progressive changes have been noted in tyrosine hydroxylase (TH) expression over time after CCI (Wagner et al. 2005; Yan et al. 2007). By two weeks post-CCI no significant changes have been previously noted with either D2DR or vesicular monoamine transporter (VMAT2) (Wagner et al. 2005). In addition, numerous studies suggest both acute and chronic administration of DAT substrates and inhibitors are correlated with changes in c-Fos expression (Graybiel et al. 1990; Hope et al. 1992; Lin et al. 1996; Canales and Graybiel, 2000; Hawken et al. 2004). These findings suggest the possibility that both CCI and MPH treatment may affect striatal neurotransmission.
Fast scan cyclic voltammetry (FSCV) allows real-time assessment of DA release and clearance (Michael and Wightman 1999), providing a tool to observe the effects of CCI and MPH treatment on DA neurotransmission. Previous work using Western blot and FSCV suggests that striatal DAT levels measured in tissue lysates are decreased after CCI and that decreases in striatal release and clearance, under saturated conditions, occur in this injury model (Wagner et al. 2005). The changes in DAT expression and impaired neurotransmission observed after CCI provide one hypothesis for the clinical effectiveness of DAT inhibitors such as MPH on recovery in patients with TBI. To test this hypothesis, the goal of this study was to use FSCV to examine the effects of chronic daily MPH treatment on striatal DA overflow and clearance using FSCV. In addition, Western blot was performed to identify the effects of injury and daily MPH treatment on c-fos expression, a ubiquitous transcription factor, and other DA proteins.
MATERIALS AND METHODS
Animals
Young adult male, Sprague-Dawley rats (Hilltop Laboratories, Scottsdale, PA, USA) were used in accordance with the regulations of the University of Pittsburgh's Institutional Animal Care and Use Committee. Animals received a CCI injury (n=26) or were naïve controls (n=26). For FSCV studies, CCI animals were given daily injections beginning one day after injury of 5 mg/kg i.p. MPH (Sigma) (n =6) or saline vehicle (n=8) for 14 days. Naïve animals were also given daily injections of 5 mg/kg i.p. MPH (n=7) or saline vehicle (n=7) for 14 days. Dosing was based on previous behavioral studies in CCI demonstrating improved spatial learning with this dosing regime (Kline et al. 2000; Wagner et al. 2007). Western blot was completed using striatal tissue lysates in 24 animals, with group designations (n=6 per group) as described above. A separate biotinylation experiment was carried out with 32 animals, (n=8 per group), with group designations also as previously described. Animals were provided with food and water ad libitum and were housed in a 12 hour light-dark cycle.
Controlled Cortical Impact Injury
The CCI injury device (Pittsburgh Precision Instruments, Inc. Pittsburgh, PA) used for this study has been described previously (Dixon et al., 1991). Under isoflurane anesthesia (4% isoflurane and a 2:1 N2O/O2 initially, followed by 1-1.5% isoflurane), animals were placed in a stereotaxic frame. A midline scalp incision was made and the soft tissues reflected to perform a craniotomy over the left parietal cortex between lambda and bregma and approximately 2 mm lateral to the central suture. The exposed dura was struck to a depth of 2.9 mm with an impact velocity of 4 m/s by the CCI device. Core body temperature was maintained at 37°C during surgery, and post-injury righting reflexes were monitored (Dixon et al. 1991). After CCI, animals were allowed to recover and then were returned to their cages.
Voltammetric Electrodes
Voltammetric electrodes were fabricated using a 7 μm diameter carbon fiber (T-300, Union Carbide, Danbury, CT, USA) threaded through 0.75 mm inner diameter borosilicate glass capillary tubes (Sutton Instruments, Novato, CA, USA) and pulled to a tip using a micropipette puller (Narshige, East Meadow, NY, USA). An epoxy resin (Spurr Polysciences, Warrington, PA, USA) was used to secure the carbon fibers in place. Electrodes were cured for at least 12 h at 80°C. Fiber tips were cut to a length of 400 mm and dip coated with a 2.5% Nafion/isopropanol solution (Aldrich, Milwaukee, WI, USA) as previously described (Wagner et al. 2005) to enhance electrode sensitivity and selectivity. Mercury and a ni-chrome wire were then inserted into the electrodes to establish an electrical connection with the carbon fiber.
Surgical Preparation for Fast Scan Cyclic Voltammetry (FSCV)
FSCV studies were performed one day after the last MPH or saline injection, or, 15 days following injury. At this time, animals were anesthetized with 400 mg/kg chloral hydrate and 0.1 mg/kg atropine (i.p.) and monitored as previously described (Wagner et al. 2005). Once a surgical level of anesthesia was established, the animals were placed in a stereotaxic frame (Kopf, Tujunga, CA, USA). The skull was exposed by making a midline incision on the scalp and reflecting the soft tissues. Portions of skull were removed using a dental drill to expose dura and to allow for placement of the working, reference, and stimulating electrodes.
Carbon fiber microelectrodes were lowered in the striatal hemisphere ipsilateral to the injury site using flat-skull coordinates [1.7 mm anterior/posterior (AP), 2.0 mm medial/lateral (ML), and -4.5 mm dorsal/ventral (DV)] (Paxinos and Watson, 1998). A bipolar stimulating electrode (MS301-1, Plastics One, Roanoke, VA, USA) was lowered into the medial forebrain bundle (MFB) at the stereotaxic coordinates of -4.0 mm AP, 1.7 mm ML, and -7.6 mm DV (Paxinos and Watson, 1998) in the hemisphere ipsilateral to the CCI. A salt bridge was formed by placing an Ag/AgCl reference electrode in direct contact with the dura mater.
Fast Scan Cyclic Voltammetry Protocol
The specific process by which DA was detected using FSCV has been previously described (Yang et al. 1998; Wagner et al. 2005). Briefly, FSCV was performed with a computer-controlled potentiostat (EI-400; Ensman Instruments, Bloomington, IN, U.S.A.). The applied potential was held at 0mV vs. Ag/AgCl between voltammetric scans. Each scan comprised linear potential sweeps to 1,000 mV, then to -500mV, and back to 0 mV vs. Ag/AgCl at a rate of 300 V/s. Scans were repeated at 100-ms intervals during in vivo experiments. The presence of DA was determined by visually inspecting the background-subtracted cyclic voltammograms. Voltammetric currents were converted to DA concentration (μM) through post-calibration of the working electrode following removal from the brain.
Stimulation of the Medial Forebrain Bundle (MFB)
The MFB was stimulated at a frequency of 60 Hz for 10 s every 20 minutes with a 280 μA biphasic constant current pulse and a 2 ms pulse width. The MFB was located using the stereotaxic coordinates described above, with the first stimulation applied at the dorso-ventral position of -7.6 mm. Once a current response of >40 nA was achieved, a series of stimulation responses were obtained by sequentially lowering the stimulating electrode in 100 μm increments and stimulating the MFB at each dorso-ventral coordinate with 20 min between each stimulation response. Stimulations continued until the dorso-ventral coordinate of -9.0 mm was reached or until no DA response was observed in the striatum (Wagner et al. 2005). The maximal stimulation response obtained for each rat was used for kinetic analysis.
Analysis of DA Clearance Kinetics
Methods for extracting DA kinetic parameters from voltammetric stimulus responses have been characterized in several previous studies (Wightman and Zimmerman 1990; Wu et al. 2001; Sabeti et al. 2002; Greco and Garris 2003; Wagner et al. 2005). In this study, the kinetic parameters extracted included the maximum concentration of DA overflow achieved with the stimulation, the maximum rate of DA clearance (Vmax), the release rate of DA, the first order rate constant k, and the [DA] at which clearance kinetics change from zero-order to first-order. By locating the point at which the slope of the first derivative changes, the segments of the concentration vs. time decay curve associated with zero order kinetics and first order kinetics were isolated (Greco and Garris 2003). Vmax was derived by measuring the slope of the zero order portion of the voltammetric signal occurring after the termination of MFB stimulation.
First order decay curves were fit to the equation [DA](t)=A*exp(-kt). Here, k is representative of DAT function in unsaturated conditions and equal to Vmax/KM, where KM is the Michaelis-Menten constant and defined as (k-1+k2)/(k1). k1 and k-1 are rate constants for the binding of DA to DAT when DAT is facing outward, and k2 is the rate constant for the inward dissociation of the DA-DAT complex (Jones et al. 1999). Our approach for measuring k was based on a return of [DA] to zero and not to basal [DA], which has varied reported values (Kulgina et al. 2001; Bungay et al. 2003). Therefore, our first order decay patterns show a slow return of [DA] to baseline and small k values. As such, the first order rate parameter, k, was defined as “apparent k”, and this approach has been used previously (Sabeti et al. 2002).
The Michaelis-Menten constant KM=(k-1+k2)/k1 (assuming k-1 is negligible during reuptake) can be defined as KM=k2/k1. Vmax can also be described as a function of [DAT] and the rate constant k2: Vmax=k2[DAT]. When the first order rate constant k is defined as k=Vmax/KM, it can then be rearranged as k=(Vmax/k2/k1) or k=k2[DAT](k1)/k2 which is then reduced to k=[DAT](k1). The Michaelis-Menten constant KM was calculated using Vmax/k parameters extracted from the maximum stimulus response for each animal. Because extracellular [DA] is influenced by both diffusion and reuptake of DA by DAT (Venton et al. 2003), and because k is an apparent value, we defined KM as “apparent KM” to reflect the ignored effects of diffusion and sensor response time on current decay. The point at which DA clearance behavior changed from zero-order to first-order, the transition concentration, was determined to be the point at which the slope of the first derivative plot of the clearance data changed distinctly.
In addition, traces were made of averaged stimulation responses expressed as percentages such that the amount of time required for the concentration of evoked DA overflow to progress from 100% to 0% of the maximum evoked DA concentration observed after the stimulus was terminated was determined from the decaying portion of the voltammetric signal (normalized decay). DA concentration at the termination of stimulation was set equal to 100% so that the [DA] at all other time points could be expressed as a percentage of this maximum [DA].
Analysis of DA Release Kinetics
Release rate for DA was calculated by subtracting Vmax from the slope of the zero order portion of the rising segment of the voltammetric signal. Traces were also made of averaged stimulation responses expressed as percentages. The amount of time required for the concentration of evoked DA overflow to progress from 0% to 100% of the maximum evoked DA concentration, (normalized release), was plotted over the rising portion of the voltammetric signal. Again, the maximum evoked [DA] was set equal to 100%, and all other [DA] values were expressed as a percentage of the maximum [DA].
Western Blot Analysis
On day 15, rats were anesthetized with pentobarbital and underwent rapid decapitation for Western blot analysis of striatal tissue lysates. Brains were rapidly removed and dissected on a chilled dissection plate to collect the injured striatal hemisphere. In preparation for Western blotting, striatal tissue was placed in 5-10 ml of lysis buffer (0.1M NaCl, 0.01M Tris-Cl, 0.001M EDTA, 1 ug/ml aprotinin, 100ug/ml PMSF). Samples were sonicated for 30 seconds at 30mV, and the lysates were centrifuged at 13,000 rpm for 30 min at 4°C. The supernatant was then divided into aliquots and frozen at -80°C until analysis.
The proteins analyzed were DAT, c-fos, tyrosine hydroxylase (TH), vesicular monoamine transporter (VMAT), D1 receptor (D1DR) and D2 receptor (D2DR). The protein concentration was measured using a bicinchoninic acid (BCA) assay kit (Pierce, Rockfold, IL, USA). Aliquots of 50 μg (for TH, D1DR, D2DR, and c-Fos) or 100 μg (for DAT) of protein from each sample were mixed with 2× sample buffer and boiled for 5 min. The proteins were resolved on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to Hybond-polyvinylidene difluoride (PVDF) membranes (Amersham, Arlington Heights, IL, USA). Membranes were blocked, using Tris-buffered saline with Tween-20 (TBST-20) (150 mm NaCl, 10 mm Tris-HCl, pH 8.0, and 0.05% Tween-20) containing 5% non-fat milk for 1 hour, then probed with either anti-DAT (1:300 Chemicon International, Temecula, CA, USA) overnight, or anti-TH (1:20 000 Chemicon International), anti-VMAT (1:1000 Santa Cruz Biotechnology, Inc.), anti-D2DR/D1DR (1:2000 Santa Cruz Biotechnology, Inc.), or anti-c-fos (1:1000 Santa Cruz Biotechnology, Inc.) antibody for 1 hour. After washing three times in Tris-buffered saline with Tween 20, the membranes were probed with anti-rabbit horseradish peroxidase-conjugated antibody (1:5000) or anti-mouse horseradish peroxidase-conjugated antibody (1:20,000) to allow detection of the appropriate bands using enhanced chemiluminescence (Amersham). The band densities were semi-quantitated using NIH Scion image (Scion, Frederick, MD, USA).
To ensure equal loading of samples, membranes used to measure DA protein expression were stripped in stripping buffer (100mM glycine, pH 2.3) at 55°C for 30 minutes. Membranes were blocked using TBST containing 5% nonfat milk for 1hr and probed with anti-actin antibody (1:20,000 Sigma) for one hour. After washing 4 times with TBST, the membranes were probed with anti-mouse horseradish peroxidase-conjugated antibody (1:20,000) to allow detection of the appropriate bands using enhanced chemiluminescence (Perkin Elmer Life Science, Boston, MA)
Biotinylation
Synaptosomal preparations of entire striatal hemisphere ipsilateral to the CCI were obtained to determine cell surface DAT expression. The use of the impermeant biotinylation reagent sulfo-NHS-biotin for isolation of plasma membrane-associated proteins in brain synaptosomes is characterized by Zhu et al. 2005. Synaptosomes were resuspended in ice-cold phosphate-buffered saline supplemented with 0.1 mM CaCl2 and 1 mM MgCl2 (PBS/Ca/Mg) and treated with sulfosuccinimidobiotin (sulfo-NHS-biotin) diluted with ice-cold PBS/Ca/Mg at a final concentration of 1 mg of protein/ml. Samples were shaken for 1 h at 4°C. The free sulfo-NHS-biotin was then removed by rapidly washing 2 times with ice-cold 100-mM glycine containing PBS/Ca/Mg followed by 2 washes with PBS/Ca/Mg. Biotinylated samples were solubilized while shaking for 30 min at 4°C in 1 ml of RIPA buffer containing protease inhibitors. Samples were centrifuged at 20,000g to pellet nonsolubilized material, and protein determinations were performed on the supernatant with the DC protein assay (Bio-Rad) and with bovine serum albumin as a standard. Biotinylated proteins in supernatant were separated from nonbiotinylated proteins by batch affinity chromatography with monomeric avidin beads. The beads were then incubated with supernatant overnight at room temperature with consistent rotation and then centrifuged. The beads were then washed 3 times in 1 ml of RIPA buffer. The biotinylated proteins were eluted from the beads by incubation for 30 min at room temperature with an equal volume of SDS-PAGE sample buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 0.05% mercaptoethanol, and 0.05% bromphenol blue). The biotinylated proteins were stored at -20°C for later assessment of biotinylated DAT. Samples were subjected to gel electrophoresis, Western blotting, and band quantification as described above. Each blot was stripped using Tris buffer and reprobed for detection of tubulin (1:10,000 Sigma). Amount of tubulin, a plasma membrane resident protein, was used to normalize for protein loading between treatment groups.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows 14.0. Means and standard error of the mean (SEM) are reported. Multi-factor analysis of variance (MANOVA) was used to assess the main effects of injury and MPH treatment, as well as the interaction of MPH treatment in the context of injury on kinetic parameters, DA protein expression and cell surface DAT expression. Descriptive pair-wise comparisons between injury and treatment groups were performed using Fisher's LSD. A p-value <0.05 was considered significant for all analyses.
RESULTS
Striatal Evoked Overflow with Fast Scan Cyclic Voltammetry
Figure 1 main panel displays experimental data from a naïve animal showing the DA concentration vs. time plot obtained with a single MFB stimulation data collection, and the Figure 1 inset shows the cyclic voltammogram (CV) acquired. The post-calibration (CV) for DA overlaid on the experimental data and the position of the oxidation and reduction peaks in relation to the applied potential confirm that the substance detected is DA.
Figure 1.
Sample plot of experimental data of naïve animal. Current vs. time plot was transformed into DA concentration vs. time via flow cell post-calibration. 60Hz stimulation was initiated 10 s into data collection and lasted for a period of 10 s. Inset: Sample cyclic voltammogram corresponding to the data in main panel. The positions of the oxidation and reduction peaks verify the identity of the species as DA.
Striatal DA clearance is affected by MPH treatment and CCI
There was a significant MPH treatment effect (F=6.04; p=0.022) and an interaction effect between injury and MPH treatment on Vmax (F=8.78; p=0.007). Pair-wise comparisons show that saline treated CCI rats had a reduced Vmax compared to naïve controls (p=0.009) and MPH treated naïve rats (p=0.020). In contrast, MPH treated CCI rats showed an increase in Vmax compared to saline treated CCI rats (p=0.001). There was no significant difference in Vmax between saline treated and MPH treated naïve groups, and the MPH treated TBI group had a Vmax value similar to both naïve groups (Figure 2a). These trends are illustrated graphically by examining the average normalized decay curve for each group (Figure 3). The curves represent group averaged [DA] expressed as a percentage of the maximum [DA] as it returns to baseline concentration over time. The decreased Vmax in CCI saline rats is evident when evaluating the curves in that it takes this group about one second longer than the other groups, all having similar decay curves, to reach 80% of maximum [DA] following termination of the MFB stimulation.
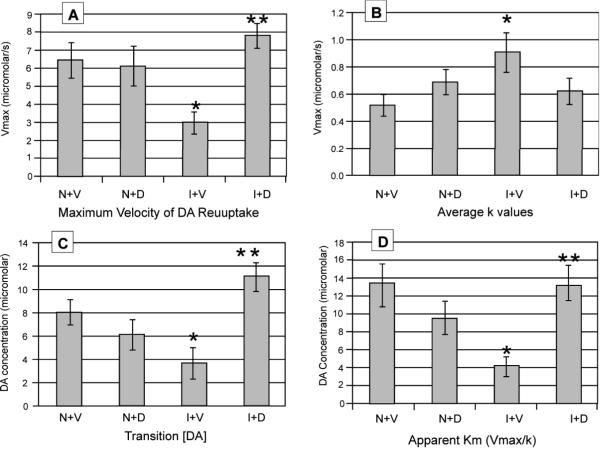
Figure 2.
Kinetic parameters of reuptake for evoked DA overflow data calculated from maximum stimulation response current versus time plots for naïve saline (N+V), naïve MPH (N+D), CCI saline (I+V), and CCI MPH (I+D) rats. a) maximum velocity of DA reuptake [n=7 (N+V), n=8 (I+V), n=7 (N+D), n=6 (I+D)] b) average k values [n=7 (N+V), n=8 (I+V), n=7 (N+D), n=6 (I+D)] c) [DA] where clearance transitions from zero order to first order decay. [n=5 (N+V), n=6 (I+V), n=6 (N+D), n=5 (I+D)] d) Apparent Km calculated from Vmax/k. [n=7 (N+V), n=8 (I+V), n=7 (N+D), n=6 (I+D)]; *p<0.05 N+V vs I+V. **p<0.05 I+V vs. I+D.
Figure 3.
Decay curves based upon the average percentage of the maximum concentration of DA overflow after discontinuation of the stimulus versus time since maximum [DA].
There was a trend for the combination of injury and MPH to effect the apparent k value (F=3.93; p=0.059). Naïve controls have the smallest k value on average, with both MPH treated groups having intermediate k values. Pair-wise analyses show an increase in apparent k for CCI rats treated with saline compared to the naïve saline group (p=0.021). Also, there was a trend for CCI MPH rats to have a decreased k compared with CCI saline rats (p=0.092) (Figure 2b).
Results show that treatment alone (F=4.50; p=0.048) and the combination of injury and MPH (F=13.01; p=0.002) had a significant effect on transition [DA]. Notably, the approximated transition [DA] concentrations are roughly similar to the calculated apparent KM values (Figure 2c). Pair-wise analyses show that CCI saline rats had a decreased transition [DA] compared to naïve controls (p=0.028) and MPH treated CCI rats (p=0.001). Further, MPH treated CCI rats had an increased transition [DA] compared with MPH treated naïve rats (p=0.015).
The combination of injury and MPH treatment significantly affected apparent KM (F=12.69; p=0.002). Similar to other kinetic analyses, pair-wise comparisons show that CCI saline rats have a decreased apparent KM value compared with naïve controls (p=0.001), and there is a trend for CCI saline treated rats to have a decreased apparent KM value compared with naïve MPH rats (p=0.061). In contrast, there was a robust increase in the apparent KM for CCI MPH rats compared to CCI controls (p=0.002) (Figure 2d).
DA evoked overflow and release rate of DA are affected by MPH treatment after CCI
Similar to that noted with DA clearance parameters, there was a significant interaction between CCI and MPH treatment with regard to maximum evoked overflow of DA (F=7.48; p=0.012). Pair-wise analysis shows that saline treated CCI rats had lower evoked DA compared to saline treated naïve animals (p=0.052). There was no significant difference in evoked DA overflow between naive saline and naive MPH rats. However, compared to saline treated CCI rats, there was an increase in evoked DA overflow for CCI rats receiving daily MPH treatment (p=0.012). Additionally, MPH treated CCI rats showed a trend for increased maximum evoked overflow of DA compared with their naïve counterparts (p=0.079) (Figure 4a). There was also a trend (F=3.77; p=0.068) for the combination of injury and MPH treatment to confer a significant effect upon release rate. Pair-wise comparisons show DA release rates for MPH treated CCI rats increased compared to CCI controls (p=0.024) (Figure 4b).
Figure 4.
Kinetic parameters of release for evoked DA overflow data calculated from maximum stimulation response current versus time plots for naïve saline (N+V), naïve MPH (N+D), CCI saline (I+V), and CCI MPH (I+D) rats. a) maximum [DA} [n=7 (N+V), n=8 (I+V), n=7 (N+D), n=6 (I+D)] b) release rate of DA [n=5 (N+V), n=6 (I+V), n=6 (N+D), n=5 (I+D)]. *p<0.05 I+V vs. I+D. **p<0.05 I+V vs. I+D.
Figure 5 displays normalized, group averaged [DA] expressed as a percentage of the maximum [DA] as it rises to peak concentration over time. On average, MPH treated naïve and saline treated CCI rats had a faster relative rise with the stimulus response when compared with naïve saline rats and CCI MPH rats. When comparing these groups, it is evident that there is about a 0.5 s difference in time to reach both 20% and 80% of maximum [DA], with saline treated naives and MPH treated CCI rats taking longer to reach each point. Additionally, peak concentrations are reached much earlier for MPH naïve and saline CCI rats.
Figure 5.
Average normalized release curves versus time since stimulation began. The stimulation lasts for 10 seconds. Normalized release curves represent the effects of both release and reuptake. MPH=methylphenidate; CCI=control cortical impact
Western blot analysis: TH, VMAT, DRD2, DAT, and c-Fos expression
Western blot analysis of striatal TH, D1DR, and D2DR expression (Figure 6a-c) showed no significant effects of injury, MPH treatment, or injury and MPH in combination. However, there was a trend for treatment alone (F=3.58; p=0.073) as well as combined injury and MPH treatment (F=3.49; p=0.077) to effect TH expression. For VMAT (figure 6d), there was an injury effect (F=5.36; p=0.031) with injured groups having a small, but significant increase in VMAT compared to naïve groups. Pair-wise comparisons suggest trends for small increases in CCI MPH rats compared to naïve rats treated with saline (p=0.092) and MPH (p=0.076).
Figure 6.
Striatal Western blot analysis for naïve saline (N+V), naïve MPH (N+D), CCI saline (I+V), and CCI MPH (I+D) rats (n=6 per group). a) Tyrosine hydroxylase (TH) b) D1 dopamine receptor (D1DR) c) D2 dopamine receptor (D2DR) d) Vesicular monamine transporter (VMAT). Percentage change in optical density is referenced against the N+V condition.
There was a significant effect of injury on DAT protein expression striatum lysates (F = 36.62; p<0.0001). DAT expression in saline treated CCI rats was 64% of that in saline treated naïve rats (p=0.001) and 69% of that in MPH treated naïve rats (p=0.005). Additionally, MPH treated CCI rats showed a decrease in DAT expression compared to both naïve groups (p<0.0001 all comparisons). Interestingly, DAT expression was not further decreased significantly by MPH treatment for either naïve or CCI rats, and there was no interaction between injury-and MPH treatment on DAT expression (Figure 7a). In contrast, there was a significant injury effect on the proportion of synaptosomal DAT that is membrane bound (F=11.02; p=0.003). In this case, membrane bound [DAT] was increased in CCI saline rats compared with naïve saline (p=0.017) and naïve MPH (p=0.037) rats. Additionally, MPH treated CCI rats showed an increase in [DAT] membrane bound compared to both naïve groups (p<0.05 all comparisons) (Figure 7b).
Figure 7.
Striatal Western blot analysis for naïve saline (N+V), naïve MPH (N+D), CCI saline (I+V), and CCI MPH (I+D) rats a) Dopamine transporter (DAT) concentration from tissue lysates (n=6 per group). b) membrane-bound DAT from synaptosome preparations (n=8 per group) c) c-fos concentration (n=6 per group). Percentage change in optical density is referenced against the N+V condition. *p<0.05 N+V vs. I+V; **p<0.05 N+V vs. I+D, ***p<0.05 I+V vs. I+D.
Results show a significant interaction with the combination of CCI and MPH treatment on c-fos expression in the striatum (F=6.36; p= 0.020). The significant c-fos relationships mirror the significant relationships identified for kinetic parameters. In this case, there was a trend for decreased c-Fos expression in CCI rats treated with saline (p=0.076). However, c-Fos expression in the MPH treated CCI rats was 147% of that in saline treated CCI rats (p=0.007) (Figure 7c).
DISCUSSION
Although less well characterized compared to other structures, CCI causes damage to the striatum (Dunn-Meynell and Levin 1997; Tong et al. 2002), a subcortical structure enriched with dopaminergic terminals. The striatum is associated with cognition, attention, and motor processing speed, and TBI patients often show deficits in these areas. While MPH has shown some therapeutic potential in treating these symptoms in the population with TBI (Whyte et al. 1997; Whyte et al. 2004) and in experimental models (Kline et al. 2000; Wagner et al. 2007; Kline et al. 2002), a mechanistic relationship between dopaminergic disruption, TBI related impairments, and DA agonists in alleviating these impairments has not been well characterized. However, our work shows that CCI results in deficits in evoked striatal DA neurotransmission. Further, this is the first report to show that daily MPH after CCI restores striatal DA neurotransmission and is associated with MPH induced changes in the transcription factor c-fos. Given the role of the striatum in cognition, these findings provide support for how MPH might mediate improvements in learning after CCI and provides insight regarding how MPH works clinically after TBI.
Our study is consistent with previous work characterizing deficits in striatal DA neurotransmission after CCI (Wagner et al. 2005). We found that release as well as clearance parameters associated with DA neurotransmission were altered after CCI. However, daily administration of the DA reuptake inhibitor MPH for two weeks after CCI resulted in significant improvements in kinetic parameters associated with striatal DA neurotransmission such that there were no statistically significant differences noted between this group and naïve controls. Injury and MPH treatment appeared to primarily affect DAT and c-fos protein expression, findings which may underlie the functional changes observed with FSCV. In contrast, daily MPH treatment had minimal effects on DA neurotransmission for naïve rats.
Our results show that in CCI vehicle treated rats, Vmax was significantly decreased compared to naïves treated with vehicle, and decrements in Vmax were roughly proportional to injury related decreases noted with total tissue DAT expression. One interpretation of this finding is that if Vmax is proportional to total [DAT], then decreases in Vmax may be attributable to the decrease in [DAT]. One working hypothesis for this interpretation is that decreases in DAT (and Vmax) after CCI are compensatory in nature and result in a relative prolongation of extracellular DA in the synaptic cleft (Wagner et al. 2005). Indeed, DAT knockdown models have demonstrated decreased DAT expression in conjunction with smaller Vmax values (Zhuang et al. 2001), and changes in the cell surface [DAT] have been associated with changes in Vmax (Pristupa et al. 1998). However, Western blots of biotinylated DAT taken from synaptosomes ex vivo show that membrane bound DAT expression are higher after CCI despite lower levels of total tissue DAT. Prior work suggests that DAT is located both on axons and at synaptic terminals (Hercsh SM 1997), but that DAT is not typically found in synaptic active zones (Hercsh SM 1997; Hoffman BJ 1998). The extrasynaptic localization of DAT may allow for diffusion to influence the extracellular half-life of DA and contribute to cell signaling. However, the specific contribution of injury induced increases in synaptosomal membrane bound [DAT] vs. decreases in total tissue [DAT] to Vmax are not known. Since Michaelis-Menten kinetics describes Vmax as k2[DAT], one explanation for our findings is that decreases in extrasynaptic [DAT] are primarily responsible for injury induced decreases observed Vmax. Alternatively, decreases in the k2 rate constant for the dissociation of the DA-DAT complex inside the terminal may decrease Vmax after CCI. However, we observed a significant increase in Vmax for CCI MPH rats, without a change in total tissue or membrane bound DAT expression, when compared to CCI saline rats. These findings imply that a change in DAT function (k2) could be central to the changes in Vmax observed with CCI and MPH.
To further examine the effects of CCI and MPH treatment on DA clearance, we evaluated signal decay behavior during unsaturated conditions (k) and also apparent KM. Interestingly, CCI rats treated with saline had a higher apparent k value compared to the other treatment groups, suggesting that first order striatal DA clearance operates more effectively in CCI rats compared to naives. Since Michaelis-Menten kinetics describes k as a function of [DAT]k1, then the observed increase in k in CCI saline rats, compared to saline treated naives, could theoretically be due to the increase in membrane bound synaptosomal [DAT], an increase in k1 (the association constant for DA and outwardly facing DAT), or both. However, in CCI MPH rats, the apparent k is reduced while minimal changes in [DAT] are noted in CCI saline rats. Therefore it is possible that k1 decreases contribute significantly to an overall decrease in DAT efficiency during unsaturated conditions in CCI-MPH rats. When calculating the apparent KM for CCI-saline rats, the decrease in Vmax, in conjunction with an increase in apparent k, resulted in a reduced apparent KM value (Vmax/k) compared with CCI MPH rats or with naïve saline rats, suggesting that overall transporter efficiency is greater in CCI-saline rats. The transition [DA] for each group shows similar relationship as that observed with the calculated apparent KM.
Although ex vivo [DAT] may be helpful with interpreting voltammetry findings, the surface expression of DAT during stimulation should be also considered when interpreting CCI induced decreases in Vmax and increases in clearance during unsaturated conditions (k). Previous work suggests that a SNARE-mediated mechanism of glycine transporter trafficking to the plasma membrane exists via an interaction with syntaxin 1. Under conditions where there is stimulated vesicular glycine release, the glycine transporter is trafficked to the plasma membrane and then internalized (Geerlings et al., 2001). Interestingly the SNARE protein, syntaxin 1, also interacts with DAT (Lee K et al., 2004). Michaelis Menten kinetics assumes a fixed [DAT] during and after MFB stimulation. However, we speculate that plasma membrane [DAT] during and after vesicular DA release could be dynamic. If true, a delay or lag in DAT trafficking to and from the membrane may contribute to decreases in Vmax, measured shortly after termination of the stimulus, as well as increases in k in the CCI condition. Similarly, MPH may affect DA neurotransmission by influencing DAT trafficking associated with MFB stimulation
Total [DAT] was decreased in both injured groups compared to naïve groups. This decrease in striatal [DAT] is consistent with other studies, both in rats (Wagner et al. 2005; Wilson et al. 2005) and humans (Donnemiller et al. 2000). This study and previous work (Wagner et al. 2005) also suggest that VMAT levels, an indicator of DAergic terminal density (Kilbourn et al., 1996; Vander Borght et al., 1995), remained largely constant. Therefore, CCI related changes in [DAT] are likely due to altered DAT regulation and not terminal loss. However, electron microscopy may be helpful in definitively assessing striatal DA terminal loss after TBI. The fact that our data shows membrane bound synaptosomal [DAT] is increased after CCI, in the setting of decreased total [DAT] expression, further supports the hypothesis of regulatory influences on DAT after CCI. DAT is subject to post translational modifications (Foster et al. 2002; Miranda et al. 2007; Yi et al. 2005; Mortensen and Amara 2003; Li et al. 2004), that may affect DAT function, expression, and localization with CCI and MPH. Changes in cell signaling (Pristupa et al., 1998; Crawford et al., 1998; Batchelor and Schenk, 1998) and protein-protein interactions (Torres et al. 2003) may influence DAT function either in basal or stimulated release conditions. However, ex vivo DAT localization and expression were not substantially influenced by MPH. As such, post-translational modifications with MPH in the CCI condition may affect DAT function or trafficking during stimulation given the robust changes in neurotransmission noted with MPH in CCI rats.
Our kinetic modeling approach to assess first order decay (k) and transporter efficiency (KM) is a single curve analysis previously discussed in the literature (Greco et al., 2003; Sabeti et al., 2004) that assumes a return to zero instead of a return to basal dopamine levels and results in relatively small k values and relatively large, non-physiological KM values. Despite these limitations, our approach allowed for between group assessments of changes in DA kinetics with MPH and CCI. Further work using quantitative methods that explicitly estimate these parameters and changes with CCI and MPH is warranted. Additionally, our voltammetry methods only measured DA overflow and clearance within a small region of the striatum. The striatum is heterogeneous with varied profiles of DA kinetics reported regionally (May and Wightman 1989; Wightman et al., 2007). Thus, other striatal regions may be affected by CCI and MPH treatment.
DAT localization and expression, as well as other DA proteins, were not affected by MPH treatment. However, there was a significant effect of MPH after CCI for c-Fos expression, a finding which implies a role for c-Fos in restoring DA neurotransmission. Studies suggest both acute and chronic administration of DAT substrates and inhibitors are correlated with changes in c-Fos expression (Graybiel et al. 1990; Hope et al. 1992; Lin et al. 1996; Canales and Graybiel, 2000; Yatin et al. 2002; Yatin et al. 2005). However, since c-fos was measured in tissue harvested approximately 24 hours after the MPH last dose, the acute affects of the last MPH dose are minimal. While regional increases in c-fos and other immediate early gene expression have been reported very early after CCI (Dash et al., 1995; Yang et al., 1994), chronic reductions in striatal c-fos expression have not been previously reported. Reductions in c-fos and other transcription factors after CCI may contribute to hypo-functioning of neurotransmitter systems chronically and warrant further examination. Since DA protein expression was not changed with MPH treatment, MPH induced changes in c-fos after CCI may be more likely to influence gene transcription for other cell signaling and regulatory proteins affecting DAT and other striatal protein functions.
One next step is to identify how, independent of expression, DAT function is regulated. Because this study indicates that c-fos expression is decreased after injury and restored to near normal levels with MPH, future work will focus on identifying cell types within the striatum in which these changes occur. Correlating MPH mediated improvements with behavioral performance to DAT expression and MPH induced changes in striatal neurotransmission will be important to better understand how MPH impacts recovery after TBI. Additionally, characterizing physiological changes in DA transmission like that observed with spontaneous DA transients (Robinson and Wightman, 2004) or decay patterns associated with lower stimulation frequencies (Garris and Wightman, 1994; Garris et al, 1997) may also inform how CCI and MPH influence striatal DA transmission.
Despite modest changes in TH, VMAT2, or D2DR protein levels, evoked DA overflow and release rates after CCI were impaired. These findings corroborate previous reports on DA protein expression and striatal DA neurotransmission (Wagner et al. 2005). Further, MPH therapy after CCI did not affect protein expression for these DAergic markers despite robust changes in neurotransmission. However, functional changes in DA release may be the result of changes in vesicular trafficking, the amount of DA per vesicle, and/or altered D2DR mediated presynaptic autofeedback, each of which can be influenced by DAT inhibitors (Fleckenstein et al., 2008; Volz et al., 2008; Volz et al., 2007). Indeed smaller release rates in CCI rats could be due to less DA per vesicle. The faster time to peak [DA] noted in naives treated with MPH and in CCI-saline rats displayed in Figure 3 could be due to tighter D2 autoregulation. Other changes in DA protein function may influence striatal DA neurotransmission with CCI and MPH, despite no changes in protein levels being noted. For example, phosphorylation of TH, a critical enzyme in DA synthesis (Kumer and Vrana, 1996), can be manipulated by D2DR autoreceptors (Lindgren et al., 2001) as well as striatal NMDA receptors (Lindgren et al., 2000).
While significant changes occurred in a number of kinetic parameters for neurotransmission as well as in c-fos expression in CCI rats treated with MPH, the effects of MPH treatment on naïve rats is minimal. MPH effects on DAT density in naïve rats this study are consistent with other studies assessing post-pubertal rats (Moll et al., 2001). Our results suggest that in naïve rats, there may be changes in cell signaling, post translational modification of DA proteins, and gene expression occurring in an effort to maintain DA homeostasis. These results are, in part, supported by studies in which naïve rats chronically treated with MPH have attenuated c-fos expression after a cocaine challenge, suggesting that naïve rats are able to maintain homeostasis in the context of repeated MPH treatment (Brandon and Steiner., 2003; Chase et al., 2003). A loss of homeostatic potential after TBI may make the striatum amenable to MPH effects on DA neurotransmission.
In conclusion, our results suggest that there is dopaminergic disruption following CCI, and that the clinical efficacy of DAT inhibitors in patients with TBI may be explained, in part, by the positive association between daily MPH treatment and restored striatal DA neurotransmission after CCI. Additionally, the interaction of CCI and MPH treatment on DA transmission and c-fos expression suggest that MPH therapy is acting beyond a DA replacement therapy to affect plastic changes in striatal neurotransmission. The data provide further insight into how DAT inhibitors restore DA neurotransmission and alleviate injury related cognitive and motor deficits.
Acknowledgements
This work was supported by NIH K08HD40833 (AKW) and R01NS40125 (CED). Special thanks to Xiecheng Ma and Scott Kunkel for their technical support on this project
Abbreviations
- (TBI)
Traumatic brain injury
- (DA)
Dopamine
- (MPH)
Methylphenidate
- (CCI)
controlled cortical impact
- (MFB)
Medial forebrain bundle
- (FSCV)
Fast scan cyclic voltammetry
- (DAT)
DA transporter
- (TH)
Tyrosine hydroxylase
- (VMAT2)
Vesicular monoamine transporter
- (D1DR)
D1 receptor
- (D2DR)
D2 receptor
- (BCA)
Bicinchoninic acid
- (SDS)
Sodium dodecyl sulfate
- (PVDF)
Hybond-polyvinylidene difluoride
- (TBST-20)
Tris-buffered saline with Tween-20
- (PBS)
phosphate buffered solution
References
- Batchelor M, Schenk JO. Protein kinase A activity may kinetically upregulate the striatal transporter for dopamine. J. Neuroscience. 1998;18:10304–10309. doi: 10.1523/JNEUROSCI.18-24-10304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein EM, Quick MW. Regulation of gamma-aminobutyric acid (GABA) transporters by extracellular GABA. J. Biol. Chem. 1999;274:889–895. doi: 10.1074/jbc.274.2.889. [DOI] [PubMed] [Google Scholar]
- Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J. Neurochem. 2004;91(1):220–9. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Steiner H. Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Euro. J. of Neurosci. 2003;18:1584–1592. doi: 10.1046/j.1460-9568.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J. Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. Patterns of gene expression and behavior induced by chronic dopamine treatments. Ann. Neurol. 2000;47:S53–S59. [PubMed] [Google Scholar]
- Chang M, Lee S, Kim J, Lee K, Kim Y, Son H, Lee Y. Protein kinase C-mediated functional regulation of dopamine transporter is not achieved by direct phosphorylation of the dopamine transporter protein. J. Neurochem. 2001;77:754–761. doi: 10.1046/j.1471-4159.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- Chase TD, Brown RE, Carrey N, Wilkinson M. Daily methylphenidate administration attenuates c-fos expression in the striatum of prepubertal rats. Neuroreport. 2003;14(5):769–72. doi: 10.1097/00001756-200304150-00022. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Meier TL, Collins RL, Watson JB. Repeated methylphenidate treatment induces behavioral sensitization and decreases protein kinase A and dopamine-stimulated adenylyl cyclase activity in the dorsal striatum. Psychopharmacology. 1998;136:34–43. doi: 10.1007/s002130050536. [DOI] [PubMed] [Google Scholar]
- Dash PK, Moore AN, Dixon CE. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J. Neurosci. 1995;15:2030–9. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaqhmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Donnemiller E, Brenneis C, Wissel J, Scherfler C, Poewe W, Riccabona G, Wenning GK. Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur. J. Nucl. Med. 2001;27:1410–1414. doi: 10.1007/s002590000308. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Levin BE. Histological markers of neuronal, axonal and astrocytic changes after lateral rigid impact traumatic brain injury. Brain Res. 1997;761:25–41. doi: 10.1016/s0006-8993(97)00210-2. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: Neurotoxic and therapeutic implications. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.07.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J. Biol. Chem. 2002;277(28):25178–86. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Garris PA, Walker QD, Wightman RM. Dopamine release and uptake rates both decrease in the partially denervated striatum in proportion to the loss of dopamine terminals. Brain Res. 1997;753:225–234. doi: 10.1016/s0006-8993(97)00003-6. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J. Neurosci. 1994;14(1):442–50. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings A, Núñez E, López-Corcuera B, Aragón C. Calcium- and syntaxin 1-mediated trafficking of the neuronal glycine transporter GLYT2. J. Biol. Chem. 2001;276(20):17584–90. doi: 10.1074/jbc.M010602200. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc. Natl. Acad. Sci. USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco GG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur. J. Pharmacol. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Hawken CM, Brown RE, Carrey N, Wilkinson M. Long-term methylphenidate treatment down-regulates c-fos in the striatum of male CD-1 mice. Neuroreport. 2004;15:1045–8. doi: 10.1097/00001756-200404290-00022. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J. Comp. Neurol. 1997;388(2):211–27. [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front. Neuroendocrinol. 1998;19(3):187–231. doi: 10.1006/frne.1998.0168. Review. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc. Natl. Acad. Sci. USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomphe C, Lemelin PL, Okano H, Kobayashi K, Trudeau LE. Bidirectional regulation of dopamine D2 and neurotensin NTS1 receptors in dopamine neurons. Eur. J, Neurosci. 2006;24:2789–2800. doi: 10.1111/j.1460-9568.2006.05151.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Joseph JD, Barak LS, Caron MG, Wightman RM. Dopamine neuronal transport kinetics and effects of amphentamine. J. Neurochem. 1999;73:2406–2414. doi: 10.1046/j.1471-4159.1999.0732406.x. [DOI] [PubMed] [Google Scholar]
- Kilbourn MR, Frey KA, VanderBorght R, Sherman PS. Effects of dopaminergic treatment on in vivo radioligand binding to brain vesicular monoamine transporters. Nuclear Med. Biol. 1996;23:467–471. doi: 10.1016/0969-8051(96)00023-6. [DOI] [PubMed] [Google Scholar]
- Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci. Lett. 2000;280:163–166. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- Kulgina NV, Zigmond MJ, Michael AC. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 2001;19:121–128. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem. Res. 2004;29(7):1405–9. doi: 10.1023/b:nere.0000026404.08779.43. [DOI] [PubMed] [Google Scholar]
- Li LB, Chen N, Ramamoorthy S, et al. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 2004;279(20):21012–20. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc. Natl. Acad. Sci. USA. 1996;93:14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Herrera-Marschitz M, Haycock J, Hökfelt T, Fisone G. Dopamine D(2) receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur. J. Neurosci. 2001;13(4):773–80. doi: 10.1046/j.0953-816x.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Lindskog M, Herrera-Marschitz M, Goiny M, Haycock J, Goldstein M, Hökfelt T, Fisone G. Regulation of tyrosine hydroxylase activity and phosphorylation at Ser(19) and Ser(40) via activation of glutamate NMDA receptors in rat striatum. J. Neurochem. 2000;74(6):2470–7. doi: 10.1046/j.1471-4159.2000.0742470.x. [DOI] [PubMed] [Google Scholar]
- May LJ, Wightman RM. Heterogeneity of stimulated dopamine overflow within rat striatum as observed with in vivo voltammetry. Brain Res. 1989;487(2):311–20. doi: 10.1016/0006-8993(89)90835-4. [DOI] [PubMed] [Google Scholar]
- Michael DJ, Wightman RM. Electrochemical monitoring of biogenic amine neurotransmission in real time. J. Pharm. Biomed. Anal. 1999;19:33–46. doi: 10.1016/s0731-7085(98)00145-9. [DOI] [PubMed] [Google Scholar]
- Miranda M, Dionne KR, Sorkina T, Sorkin A. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Molecular Biology of the Cell. 2007;18(1):313–23. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Hause S, Ruther E, Rothenberger A, Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J. Child Adolesc. Psychopharmacol. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- Munir M, Correale DM, Robinson MB. Substrate-induced up-regulation of Na(+)-dependent glutamate transport activity. Neurochem. Int. 2000;37:147–162. doi: 10.1016/s0197-0186(00)00018-8. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur. J. Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Page G, Barc-Pain S, Pontcharraud R, Cante A, Piriou A, Barrier L. The up-regulation of the striatal dopamine transporter's activity by cAMP is PKA-, CaMK II-and phosphatase-dependent. Neurochem. Int. 2004;45:627–632. doi: 10.1016/j.neuint.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. Academic Press; San Diego: 1998. [Google Scholar]
- Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJS, Wang YT, Niznik HB. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Quick MW. Substrates regulate gamma-aminobutyric acid transporters in a syntaxin 1A-dependent manner. Proc. Natl. Acad. Sci. USA. 2002;99:5686–5691. doi: 10.1073/pnas.082712899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. J. Neurochem. 2004;90(4):894–903. doi: 10.1111/j.1471-4159.2004.02559.x. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Adams CE, Burmeister J, Gerhardt GA, Zahniser NR. Kinetic analysis of striatal clearance and exogenous dopamine recorded by chronoamperometry in freely-moving rats. J. Neurosci. Methods. 2002;121:41–52. doi: 10.1016/s0165-0270(02)00229-7. [DOI] [PubMed] [Google Scholar]
- Tong W, Igarashi T, Ferriero DM, Noble LJ. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp. Neurol. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- Torres GE, Carneiro A, Seamans K, et al. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J. Biol. Chem. 2003;278(4):2731–9. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- Vander Borght T, Kilbourn M, Desmond T, Kuhl D, Frey K. The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. J. Eur. Pharmacol. 1995;294:577–583. doi: 10.1016/0014-2999(95)00594-3. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Philips PE, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J. Neurochem. 2003;87:1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, King JL, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate administration alters vesicular monoamine transporter-2 function in cytoplasmic and membrane-associated vesicles. J. Pharmacol. Exp. Ther. 2007;323(2):738–745. doi: 10.1124/jpet.107.126888. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR, Fleckenstein AE. Methylphenidate induced increases in vesicular dopamine sequestration and dopamine release in the striatum: the role of muscarinic and D2 receptors. J. Pharmacol. Exp. Ther. 2008 doi: 10.1124/jpet.108.139386. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem. 2005;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Chen X, Kline AE, Li Y, Zafonte RD, Dixon CE. Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exp. Neurol. 2005;195:475–83. doi: 10.1016/j.expneurol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Ren D, Willard LA, Wenger MK, Zafonte RD, Dixon CE. Gender associations with chronic methylphenidate treatment and behavioral performance following experimental TBI. Behav. Brain Res. 2007;181(2):200–9. doi: 10.1016/j.bbr.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J. Neurotrauma. 2006;23:1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- Whyte J, Hart T, Schuster K, Fleming M, Polansky M, Coslett HB. Effects of methylphenidate on attentional function after traumatic brain injury. A randomized, placebo-controlled trial. Am. J. Phys. Med. Rehab. 1997;76:440–450. doi: 10.1097/00002060-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Whyte J, Hart T, Vaccaro M, Grieb-Neff P, Risser A, Polansky M, Coslett HB. Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am. J. Phys. Med. Rehabil. 2004;83:401–420. doi: 10.1097/01.phm.0000128789.75375.d3. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res. Brain Res. Rev. 1990;15(2):135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur. J. Neurosci. 2007;26(7):2046–54. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Williams JM, Galli A. The dopamine transporter: a vigilant border control for psychostimulant action. Handb. Exp. Pharmacol. 2006;175:215–232. doi: 10.1007/3-540-29784-7_11. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Chen X, Ma X, Ren D, Wagner AK, Reynolds IJ, Dixon CE. Synaptosomal dopamine uptake in rat striatum following controlled cortical impact. J. Neurosci. Res. 2005;80:85–91. doi: 10.1002/jnr.20419. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Meth. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Ma X, Chen X, Li Y, Shao L, Dixon CE. Delayed increase of tyrosine hydroxylase expression in rat nigrostriatal system after traumatic brain injury. Brain Res. 2007;1134:171–179. doi: 10.1016/j.brainres.2006.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Peters JL, Michael AC. Coupled effects of mass transfer and uptake kinetics on in vivo microdialysis of dopamine. J. Neurochem. 1998;71:684–692. doi: 10.1046/j.1471-4159.1998.71020684.x. [DOI] [PubMed] [Google Scholar]
- Yang K, Mu XS, Xue JJ, Whitson J, Salminen A, Dixon CE, Liu PK, Hayes RL. Increased expression of c-fos mRNA and AP-1 transcription factors after cortical impact injury in rats. Brain Res. 1994;664(1-2):141–7. doi: 10.1016/0006-8993(94)91964-x. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Miller GM, Norton C, Madras B. Dopamine Transporter-dependent induction of c-Fos in HEK cells. Synapse. 2002;45:52–65. doi: 10.1002/syn.10084. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Miller GM, Madras BK. Dopamine and norepinephrine transporter-dependent c-Fos production in vitro: relevance to neuroadaptation. J. Neurosci. Methods. 2005;143:69–78. doi: 10.1016/j.jneumeth.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Ehlers MD. Ubiquitin and protein turnover in synapse function. Neuron. 2005;47(5):629–32. doi: 10.1016/j.neuron.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Doolen S. Chronic and acute regulation of Na+/Cl- dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol. Ther. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J. Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc. Natl. Acad. Sci. USA. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]