Abstract
We developed Therapeutic Interactive Voice Response (TIVR) as an automated, telephone-based tool for maintenance enhancement following group cognitive-behavioral therapy (CBT) for chronic pain. TIVR has four components: a daily self-monitoring questionnaire, a didactic review of coping skills, prerecorded behavioral rehearsals of coping skills, and monthly personalized feedback messages from the CBT therapist based on a review of the patient’s daily reports. The first three components are pre-recorded and all four can be accessed remotely by patients via touch-tone telephone on demand.
Following 11-weeks of group CBT, fifty-one subjects with chronic musculoskeletal pain were randomized to one of two study groups. Twenty-six subjects participated in 4 months of TIVR, while a control group of twenty-five subjects received standard care only. The TIVR group showed maximum improvement over baseline at the 8-month follow-up for 7 of the 8 outcome measures; improvement was found to be significant for all outcomes (p ≤ .001). Between-group analysis of covariance (ANCOVA) revealed significantly greater improvement for the experimental group at both 4 and 8 month follow-ups for most of the outcomes. Results demonstrate that TIVR can be used to decrease pain, improve coping and decrease likelihood of relapse into pain behavior. Preliminary analysis of medication usage suggests that the superior outcome of the TIVR group was unlikely to be a consequence of differential medication use.
Keywords: Chronic Pain, Coping, IVR, Automated Telephone, Cognitive Behavioral Therapy
INTRODUCTION
When cure is not possible and pain becomes chronic, self-management of pain is a valuable treatment option. There is considerable evidence that a course of 8–12 weekly group cognitive-behavioral therapy (CBT) offers significant therapeutic benefit to patients with persistent pain (Basler 1993; Basler et al 1997; Connally & Sanders 1991; Vlaeyen et al 1995). In their review of the behavioral and cognitive behavioral therapy (BT-CBT) literature, McCracken and Turk (2002) conclude that “BT-CBT for chronic pain reduces patients’ pain, distress, and pain behavior, and improves their daily functioning”.. In their meta-analysis, Morley et al (1999) more specifically concluded that patients in BT-CBT treatments demonstrated greater improvement in pain experience, positive coping strategy use, and pain behavior. Recently converging lines of evidence suggest that systematic training in pain coping skills training (CST) may represent a particularly valuable addition to chronic pain management (Keefe & Van Horn 1993; Compas et al 1998).
While these findings are encouraging, maintenance of coping skills after completing behavioral training appears to be variable and many patients experience decline in therapeutic benefit within several weeks (Turk & Rudy 1991; Lanes et al 1995; Basler 1993). Initial gains in work performance and job retention also decline over time (Davis et al 1991). CBT protocols emphasize the importance of regular coping skills practice in the development and maintenance of effective pain control. But the transition from initially learning skills, while working with a therapist and with considerable group support, to mastering and maintaining those skills on one’s own can be difficult.
The development of strategies for enhancing maintenance and extending the initial treatment gains patients obtain during CBT is one of the most neglected topics in the pain management literature (Turk & Rudy 1991; Keefe & Van Horn 1993). Our telephone-based intervention tool, Therapeutic Interactive Voice Response (TIVR) was designed to meet this goal (Naylor et al 2002). We initially tested the feasibility of the TIVR following 11 weeks of group CBT in a convenience pilot sample of ten subjects consecutively assigned to four months of TIVR following CBT. We also recruited eight subjects for a comparison sample who received CBT with no subsequent TIVR. Both samples improved after CBT. However, between subjects analysis showed that relevant outcome scores in the comparison sample worsened in the four months after CBT whereas the same outcomes in the TIVR group improved still further during this period (Naylor et al 2002).
In the study reported herein, we extend that work by testing in a larger randomized controlled trial (RCT) whether a TIVR-based intervention could increase treatment compliance and adherence in chronic pain patients and improve outcome at follow-up. The TIVR was designed to enhance group CBT by providing automated access to self-monitoring, didactic review of coping skills, guided behavioral rehearsals of skills including prompts for regular practice, and personalized encouragement and reinforcement. To our knowledge, this is the first (RCT) using automated telephonic technology as an intervention for chronic pain.
1. METHODS
1. 1. Overview of the design
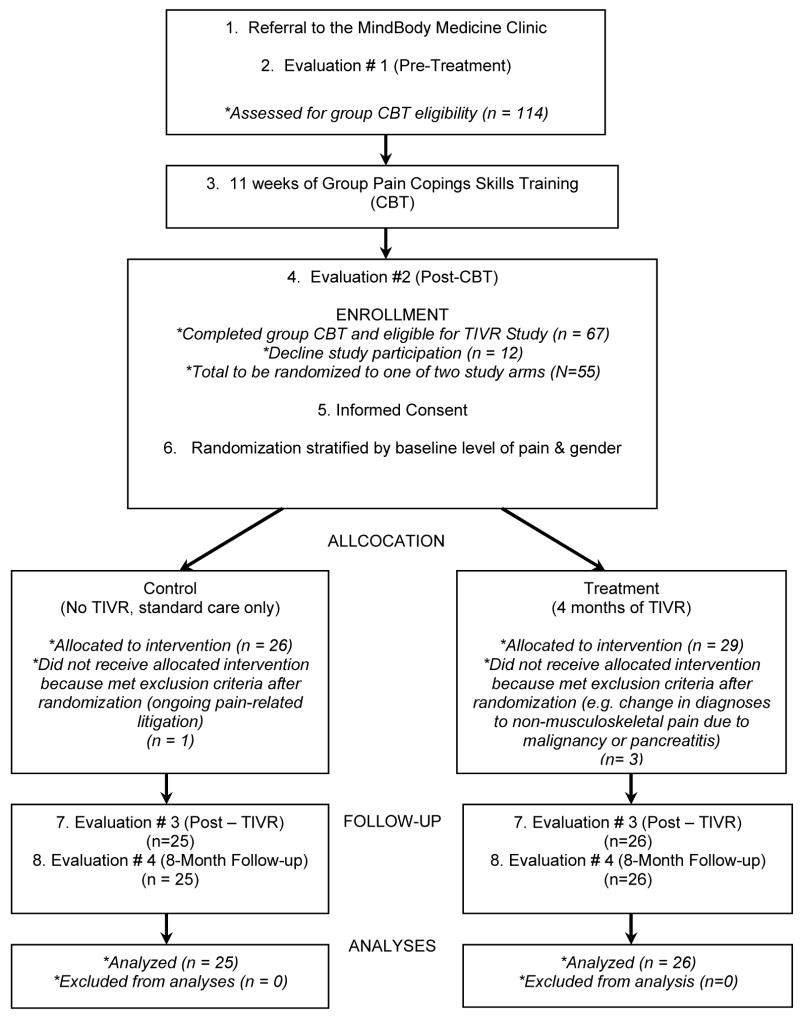
The overall study design is illustrated in Figure 1. The design is a two-group, prospective (RCT) trial to examine whether Therapeutic Interactive Voice Response (TIVR) is an effective relapse prevention intervention for patients with chronic musculoskeletal pain. All study subjects completed group pain coping skills training (CBT) (Keefe et al 1990a; Keefe et al 1990b) which consisted of weekly, 90-minute sessions over 11 weeks. Therapy groups consisted of 7–9 patients. Each CBT group was based on a protocol and was led by the author of this study (MRN), who has extensive training in administering CBT (see group CBT description below).
Figure 1.
STUDY DESIGN
During the study, subjects in both study conditions received “treatment as usual”, for example medications, massage therapy or steroid injections, managed by their primary care physicians. After completing the 11 weeks of CBT, participants were randomly assigned to one of two study conditions: control or experimental. The experimental group received four months of maintenance enhancement via the TIVR (see Treatment Procedures below). The control group received “treatment as usual” only. Subjects in both study conditions were assessed at four time-points: prior to starting CBT, the conclusion of CBT, four months following CBT (corresponding to completion of the TIVR calls in the experimental group), and eight months following CBT.
1. 2. Participants
Subjects for this study were a consecutive sample of patients with chronic musculoskeletal pain referred to the MindBody Medicine Clinic (MBMC) at the university medical center during the period of study.
The MBMC group therapy is ongoing as a service to the community. Therefore referrals were not considered as potential candidates for this investigation unless they had successfully completed the standard 11 weeks of group CBT. Successful completion of group CBT was defined as attending at least 3 of the first 4 group meetings and at least 8 of the 11 total sessions. Those who met the inclusion/exclusion criteria detailed below were offered the opportunity to participate in the research project if they signed formal consent after being fully informed of the study details. There was no difference between patients in TIVR and Control groups in session attendance during the 11-weeks CBT.
Inclusion Criteria were as follows: at least 6 months of musculoskeletal pain (such as back pain, osteoarthritis, or fibromyalgia); met study threshold for severity of pain “over the past four weeks” of 4 or more on a 10-point scale measured at baseline on the McGill Pain Questionnaire short form (Melzack 1975); able to perform usual self-care; had ongoing health care from a physician; age 18 or older, had a touch-tone phone in the home. Exclusion Criteria were: patients with malignancy, radiation, or chemotherapy causing or influencing chronic pain; awaiting a pain-related surgical procedure; involved in pain-related litigation; psychosis, an uncontrolled Axis I disorder, or a severe personality disorder that would interfere with participation in group therapy; inability to use the telephone-based TIVR due to cognitive or hearing impairment.
1. 3. Procedure
The University of Vermont Institutional Review Board approved the research protocol and informed consent was obtained from each subject.
Patients with chronic musculoskeletal pain who successfully completed 11 weeks of group CBT were offered the opportunity to participate in this research project. After the last group meeting, the study was described and informed consent obtained for those who were interested in participating. Consenting subjects were stratified by level of pain and by gender, and then randomized to one of the two study groups. In the consent form we ask for permission to use baseline and post-CBT data for research. Randomization was done after group therapy was completed in order to avoid the risk of differential CBT exposure based on group assignment.
1. 4. Assessment Measures
The following measures of pain, function/disability, and coping were used. All of the formal questionnaires measures are self-administered.
Measures of Pain
Two measures were used to assess pain: 1) the short form of the McGill Pain Questionnaire (MPQ) (Melzack 1975), and 2) the physical experience of pain dimension (Pain Symptoms) from the Enhanced SF-36 TOPS (Total Pain Symptoms - see below for TOPS description) (Rogers et al 2000a). The short form of the McGill Pain Questionnaire (MPQ) includes two Likert-type subscales: Pain Now and Pain Typical with the range from zero (no pain) to ten (worst pain). MPQ reliability and validity has been established previously (Melzack 1975).
Measures of Function/Disability
Three instruments were used to assess patients’ functioning and disability: 1) the SF-36 Mental Function scale, 2) the SF-36 Physical Function Scale, 3) The TOPS Total Pain Experience scale.
Scores on the Mental Function and Physical Function Scales of the SF-36 were computed using TOPS data. The Short Form-36 questionnaire was originally developed for the Medical Outcomes Study as a general instrument to evaluate health status in a broad range of medical populations (Ware & Sherbourne 1992). The SF-36 is brief, has considerable normative data, and has been validated worldwide (Gandek & Ware 1998).
The TOPS is a 120-item inventory that includes the original SF-36 items along with other items designed for use in pain treatment outcome studies. The TOPS is intended for use with patients having persistent pain. Recent research has provided strong support for the reliability and validity of this pain measure. (Rogers et al 2000a; Rogers et al 2000b). We report the Total Pain Experience score which represents a summary of five dimensions: (1) the physical experience of pain (Pain Symptoms), (2) lower body functional limitations (Lower Body Limitations), (3) patient-perceived limitations on the ability to perform family and social roles (Perceived Social Disability), (4) limitations in engaging in specific social activities (Objective Social Disability), and (5) the degree of work disability (Objective Work Disability). The scores for these five dimensions are reported in Tables because of their clinical relevance but they are not considered as main outcomes.
Measure of Pain Coping Strategies
The Coping Strategies Questionnaire (CSQ) (Lawson et al 1990; Keefe et al 1991) measures: 1) the extent to which subjects report using each of six different cognitive coping strategies and one behavioral strategy when they feel pain, and 2) the degree to which subjects perceive themselves as able to use these strategies to control and decrease pain. The decrease and control pain scores are each a single rating while the catastrophizing score is the mean of six ratings.
Medication Intake
In addition to the above primary outcome measures, we also assessed medication use including opioid analgesics, benzodiazepines, NSAIDs and antidepressants intake. A structured interview was carried out by a study psychiatrist at each of the four evaluations to assess patients’ intake of medication. Subjects were requested to report their medications, the dosage and average frequency of use in the two weeks preceding the evaluation. These medications were classified into four categories and standardized doses were calculated within each category: 1) opioid analgesic doses were translated into an equivalent morphine dose, 2) benzodiazepines into an equivalent diazepam dose, and 3) NSIADs into an equivalent aspirin dose.
1. 4.1 Treatment Procedures
Group Cognitive Behavioral Therapy (CBT)
Cognitive behavioral therapy was delivered in eleven, 90-minute weekly group sessions. Our CBT intervention for pain management was designed to: 1) change cognition and decrease maladaptive catastrophizing, 2) enhance patients’ ability to use attention diversion, and 3) change activity patterns to better control pain.
Below are the five treatment components emphasized in our program, and the associated cognitive-behavioral interventions to achieve the goals described above. During the first group session, a simplified version of Melzack and Wall’s gate control model of pain is presented to show that pain is a complex experience affected by thoughts, feelings and behaviors. Throughout the program, therapists demonstrate examples of each coping skills and patients are encouraged to practice s kills as homework assignments. Feedback and reminders of effective coping strategies are provided by therapists on a weekly basis.
Cognitive Coping Strategies
In order to help reconceptualize chronic pain, patients are taught how to recognize problematic thoughts and how to reframe their thinking in a process termed cognitive restructuring. Cognitive restructuring helps patients recognize the relationships between thoughts, feelings and behavior. With the help of the therapist, patients challenge their maladaptive assumptions in order to develop more objective, adaptive thoughts. Catastrophizing is one such maladaptive strategy used by chronic pain patients which should be challenged in order to achieve improvement (Keefe et al 1989; Keefe et al 1991, Sullivan et al 1995; Sullivan et al 1997). One therapeutic goal emphasized in our program is helping patients recognize catastrophizing, challenge negative thoughts, and replace them with positive self-statements (Jensen & Karolny 1991; Jensen et al 1991, Haythornthwaite et al 1998; Haythornthwaite et al 1999; Thorn et al 1999; Stroud et al 2000). In addition to cognitive restructuring we teach other adaptive coping skills like problem solving techniques and communication skills. Pain diaries are used as a weekly self-education tool. Patients are encouraged to keep a paper and pencil daily pain diary which enables therapists to monitor treatment progress and provide a tool for patient self-education. Patients are taught to recognize connections between their life events and the daily fluctuations in their pain. Use of the patient’s own self-reported information helps to emphasize the importance of cognitive appraisal and emotion. Pain diaries also reveal the relationship of over- or under-exertion and exacerbation of pain.
Self-regulatory Skills (Relaxation Techniques)
Self-regulatory techniques allow patients to decrease muscle tension, reduce anxiety, and in general diminish autonomic arousal. Patients are trained to use progressive muscle relaxation, imagery, and autogenic training. Brief relaxation methods are taught so that patients can utilize them during routine daily activities. In addition, patients are provided with audio CDs of recorded relaxation techniques to be practiced daily at home (Davis et al 2006).
Attention Diversion Methods
Attention diversion (distraction) techniques (Gatchel & Robinson, 2003) are taught during our group program in order for patients to focus on physical or auditory stimuli or other thoughts and feelings unrelated to their pain. Patients learn how to use daily activities such as watching a movie, having a telephone conversation, listening to music or reading a book as distraction techniques.
Changing Activity Patterns (Activity Pacing)
The goal of pacing is to learn how to be physically active in order to meet previously set goals, rather than being engaged in physical activity to the point of exhaustion. Activity-rest cycling and pleasant activity scheduling is also used to increase patient activity levels and therefore reduce pain.
Enhancing Social Support
At week eight, spouses or significant others are invited to attend the group session. In this study 80% of partners attended with no difference between control and TIVR groups. The purpose is to educate partners about the program, to provide an opportunity for them to ask questions, to obtain collateral feedback regarding patient’s progress, and to share their experiences with the other patients and partners.
Treatment-as-usual
The control group was offered treatment as usual only after the completion of group CBT. This did not include any TIVR but further treatment was available from their usual care sources if sought. The TIVR group also had free access to treatment-as-usual. We did not monitor the frequency of doctor visits.
Description of Therapeutic Interactive Voice Response (IVR)
The technology underlying the TIVR is Interactive Voice Response (IVR). IVR is a method for interaction between an individual and a computer through the medium of a telephone using the touch-tone keypad. Typically an automated script poses questions following a branching logic format and the caller keys in responses using the telephone keypad. IVR offers potential benefits as a self-monitoring and/or intervention method. These include convenience, simplicity of use, and a high level of patient comfort in reporting even highly sensitive material (Kobak et al 1997; Turner et al 1998). IVR systems have been successfully used to supplement behavioral interventions in obsessive-compulsive disorder (Bear & Greist 1997; Marks et al 1998) and major depression (Osgood-Hynes et al 1998) and alcohol abuse (Helzer et al, in review). We developed the Therapeutic IVR system (TIVR) to enhance the maintenance of treatment gains following pain coping skills training. We pilot tested the TIVR in a pilot study of patients with chronic musculoskeletal pain and found evidence that the tool enhanced and prolonged the therapeutic benefit of CBT. (Naylor et al 2002)
The TIVR as four components:
1. Daily Self Monitoring Questionnaire
This is a 21-item questionnaire the patient is asked to complete each day by calling our toll-free number. A recorded voice asks a series of questions to assess daily coping, daily perceived pain control, and daily mood used in our prior research. It also includes items asking about medication use and stress. With a few practice sessions, this part of the call takes approximately two to three minutes to complete. The remaining TIVR components are optional and patients use them at will, as frequently or infrequently as they like. Component 1 is designed to improve self-monitoring of pain behavior. Since the questionnaire includes items about coping skills used that day, it also serves as a regular skills reminder that may improve adherence.
2. Didactic Review of Skills
Participants are able to access a verbal review of eight different pain management skills they learned during the 11 weeks of CBT (relaxation response, diaphragmatic breathing, positive self-talk, cognitive restructuring, activity-rest pacing, distraction techniques, reappraisal of pain, and defusing catastrophizing). Each review is approximately 3 minutes in length. The didactic review messages are recorded in the voice of an experienced therapist with a soothing telephone voice.
3. Guided Behavioral Rehearsal of Pain Coping Skills (Practice Sessions)
Patients can access guided behavioral rehearsals of eight of the coping skills taught during CBT (body scan relaxation, diaphragmatic breathing, visualization, autogenic training, brief relaxation techniques [“minis”], cognitive restructuring, and sleep induction). For example, a patient who is feeling very tense or cannot fall asleep can call the TIVR to access a 10-minute relaxation message. The guided behavioral rehearsal messages are recorded in the same voice as the skills reviews. Components 2 and 3 are designed to help patients master coping skills and to make adherence to the use of skills a part of their daily routine.
4. Monthly Therapist Feedback Message
Once a month the group therapist analyzes computer-collated patient-specific data and calls the TIVR to record a personalized message for each participant. These messages contain a summary of that participant’s daily reports to the TIVR for the past month; insight into possible relationships between use of coping skills, mood, stress and pain levels based on these daily data; suggestions for other pain management tactics; and verbal encouragement. Patients often report these personalized monthly messages to be both valuable feedback and a continuing positive connection with the therapist. They also claim that the value of the messages increases with the frequency of their own use of the TIVR, especially the Daily Questionnaire. Therefore, we speculate that an important effect of the Monthly Message is to increase self-monitoring and adherence to pain management skills and to improve overall motivation to remain engaged in the TIVR.
1.4.2 Statistical Procedures
A power analysis for this study was based on data from a pilot study (Naylor et al, 2002). Effect sizes greater than 0.5 standard deviation had been found for 4 outcomes. The current study was powered to detect an effect size of 0.5 using ANCOVA for the endpoint comparisons between the two groups.
The effect of the 11-week CBT program was evaluated by comparing the first follow-up scores of all subjects combined to the baseline scores using the paired t test. The effect sizes for these analyses were calculated using the mean difference scores (post-CBT scores subtracted from the baseline scores) divided by the standard deviation of the difference scores. A repeated measures analysis of variance (ANOVA) model was used to evaluate the treatment effect for each group separately (within-group comparisons to baseline): This enabled us see at which point of the study the largest treatment effect was observed. Group comparisons were made for the 4- and 8-month follow-ups using ANCOVA with the post-CBT score entered as the covariate. Because the TIVR group was found to be significantly more responsive to the CBT treatment for two measures before the start of the TIVR interventions, an additional covariate was added to the group comparison models for the last two follow-ups, specifically the difference score measuring the improvement during CBT (post-CBT score subtracted from the baseline). The effect sizes reported in table 3 were calculated using the residuals from regressions of the outcomes adjusted by the covariates included in the ANCOVAS (Cohen, 1988). Confirmatory analyses were run with changes in benzodiazepines, opioid, antidepressant and NSAIDs medication use as additional time-varying covariates. Analyses were done using SAS version 9 (SAS Institute Inc., Cary, NC). All tests were two-tailed with alpha set at 0.05.
Table 3.
Effect Sizes of Group Differences in Improvement
| Test | Post CBT (1) | Four month follow-up (2) | Eight month follow-up (2) |
|---|---|---|---|
| SF-36 Mental Composite | .64* | .70* | .80* |
| SF-36 Physical Composite | .08 | .71* | 1.0** |
| MPQ Pain Now | .57* | .72* | 1.2*** |
| MPQ Pain Typical | .11 | .90* | .92* |
| CSQ Ability to Control Pain | .05 | 1.1** | 1.2*** |
| CSQ Ability to Decrease Pain | .31 | 1.1** | .94* |
| CSQ Catastrophizing | .44 | .72* | .60* |
| CSQ Divert Attention | .23 | .23 | .64* |
| CSQ Reinterpret Pain | .19 | .15 | .03 |
| CSQ Self Talk | .21 | .37 | .37 |
| CSQ Ignore Sensations | .06 | .12 | .13 |
| CSQ Pray/Hope | .19 | .52 | .37 |
| CSQ More Active | .07 | .03 | .37 |
| TOPS Total Pain Experience | .26 | 1.3*** | 1.3*** |
| • Pain Symptoms | .33 | 1.1** | 1.3*** |
| • Lower Body Limitations | .31 | .74* | .65* |
| • Perceived Social Disability | 0.7 | 1.0** | .98* |
| • Objective Social Disability | .44 | .76* | .70* |
| • Objective Work Disability† | .53 | .51 | .36 |
Post CBT group means were compared after adjusting for baseline scores. Group differences were not expected to be different.
Group means were compared after adjusting for post-CBT scores and responsiveness to CBT treatment (difference score: Post-CBT score – baseline).
Dichotomous outcome. Fisher’s exact test used for group comparison of actual counts (no adjustment for prior scores). Cohen’s effect size index h is reported.
Significance
p<.05,
p<.001,
p<.0001 Where significant, TIVR group showed more improvement
An intent-to-treat approach was used. All subjects who successfully completed CBT and who agreed to be randomized were retained for the primary analyses. For three cases with missing data at the second or third follow-ups the average of the scores from the prior and following time points were used. Two subjects from the TIVR group who were missing the final set of questionnaires were assumed to have regressed to the baseline.
2. Results
Subjects
Fifty-five subjects met criteria, agreed to participate in the study, and were randomly assigned to one of the two study groups (Figure 1). Four subjects met exclusion criteria soon after the randomization (e.g. diagnosed with cancer or admitted to be involved in pain related litigation). Most of the enrollees were Caucasian (96%), were women (84%), and the mean age of the sample was 46. For further details of the demographic characteristics, see Table 1.
Table 1.
Demographics for each group and total sample
| TIVR Group N=26 |
Control Group N=25 |
Total Sample N= 51 |
|
|---|---|---|---|
| Age | x̄ = 47 ± 10.42 | x̄ =46 ± 12.42 | x̄ = 46 ± 11.47 |
| Gender | |||
| • Females | 23 (88%) | 21 (84%) | 44 (86%) |
| Race | |||
| • White/Caucasian | 25 (96%) | 24 (96%) | 48 (96%) |
| Duration of Pain in Years | x̄ =13.60 ± 9.53 | x̄ =8.60 ± 8.45 | x̄ =11.15 ± 9.27 |
| Martial Status | |||
| • Never Married | 6 (24%) | 0 | 6 (12%) |
| • Married/Living Together | 17 (64%) | 20 (80%) | 37 (72%) |
| • Divorced/Separated | 3 (12%) | 5 (20%) | 8 (16%) |
| Living Situation | |||
| • 3+ person household | 5 (16%) | 12 (48%) | 17 (32%) |
| • 2 person household | 13 (52%) | 11 (44%) | 24 (48%) |
| • Living alone | 8 (32%) | 2 (8%) | 10 (20%) |
| Education in Years | x̄ =14.12 ± 1.83 | x̄ =14.29 ± 1.76 | x̄ =14 ± 1.80 |
| • Did not report education | 0 | 1 (4%) | 1 (2%) |
| • 9–12 years | 9 (32%) | 6 (24%) | 15 (28%) |
| • 13–16 years | 14 (56%) | 16 (64%) | 30 (60%) |
| • 17+ years | 3 (12%) | 2 (8%) | 5 (10%) |
The Efficacy of the CBT Group Therapy
The effectiveness of the CBT program was evaluated by a paired t-test with all subjects’ scores. There was a statistically significant improvement over baseline for all outcomes Effect sizes ranged from 0.29 of a standard deviation (SF-36 Physical Composite) to 0.94 (CSQ Catastrophizing). Within-group comparisons to baseline are listed in Table 2. There are some group differences at the end of CBT. The control group did not show significant improvement in three outcomes (SF-36 Mental Health Composite, MPQ Pain Now, and CSQ Ability to Decrease Pain) while the TIVR group did not show significant improvement in two outcomes (SF-36 Physical Composite and MPQ Pain Typical). The groups differed significantly in two of the outcomes, namely SF-36 Mental Health Composite (p=.03) and MPQ Pain Now (p=.03; Table 3). Group comparisons at later follow-ups included adjustments for the differences existing at the end of CBT.
Table 2.
Comparisons of Baseline Scores to Follow-up Scores
| CONTROL GROUP Sample size = 25 |
Change from baseline Mean difference (S.D) |
||||
|---|---|---|---|---|---|
| Test | Range of possible Scores (Norms) | Baseline Mean | Post CBT | Post TIVR | 8th month Follow-up |
| SF-36 Mental Health Composite [+]† | 0–100 (74.9) | 38.7 (11.6) | 2.0 (9.5) | 0.2 (12.2) | 1.1 (12.0) |
| SF-36 Physical Composite score [+] | 0–100 (66.3) | 28.6 (7.9) | 2.3 (4.4)* | 2.0 (7.7) | 2.6 (7.3) |
| MPQ Pain Now [−] | 0–10 (N/A) | 5.6 (2.0) | −0.2 (2.7) | −0.4 (2.4) | −0.2 (2.5) |
| MPQ Pain Typical [−] | 0–10 (N/A) | 6.8 (1.5) | −1.2 (2.1)* | −1.1 (1.9)* | −1.0 (1.8) |
| CSQ Ability to Control Pain [+] | 0–6 (1.2) | 2.3 (1.2) | 1.1 (1.2)*** | 0.8 (1.4)* | 1.0 (1.2)** |
| CSQ Ability to Decrease Pain [+] | 0–6 (2.8) | 2.5 (1.2) | 0.5 (1.2) | 0.2 (1.3) | 0.4 (1.0)* |
| CSQ Catastrophize [−] | 0–36 (17.8) | 16.4 (7.2) | −5.9 (6.5)** | −5.2 (7.6)* | −6.8 (7.5)** |
| CSQ Divert Attention [+] | 0–36 (13.6) | 11.0 (8.3) | 4.3 (6.6)* | 1.9 (6.5) | 0.6 (6.2) |
| CSQ Reinterpret Pain [+] | 0–36 (10.6) | 4.3 (5.3) | 2.7 (5.5)* | 1.8 (5.8) | 1.8 (4.6) |
| CSQ Self-Talk [+] | 0–36 (22.4) | 19.2 (5.7) | 1.6 (4.9) | 2.3 (5.8) | 2.2 (5.5) |
| CSQ Ignore Sensations [+] | 0–36 (15.5) | 10.8 (7.2) | 2.0 (7.8) | 0.8 (7.2) | 1.5 (6.3) |
| CSQ Pray/Hope [−] | 0–36 (11.8) | 15.4 (6.3) | −1.2 (5.7) | −1.8 (6.6) | −1.8 (7.9) |
| CSQ More Active [+] | 0–36 (16.7) | 13.6 (7.0) | 3.5 (7.0)* | 1.8 (6.6) | 0.8 (5.6) |
| TOPS Total Pain Experience [−] | 0–100 (63.4) | 60.2 (12.2) | −5.3 (7.0)** | −4.1 (10.3) | −3.5 (11.4) |
| • Pain Symptoms (−) | 0–100 (72.0) | 68.7 (12.3) | −4.0 (9.2)* | −3.7 (13.1) | −5.3 (15.7) |
| • Lower Body Limitations (−) | 0–100 (58.8) | 59.4 (33.0) | −1.9 (19.4) | −1.1 (26.0) | −5.2 (17.0) |
| • Perceived Social Disability (−) | 0–100 (62.1) | 56.5 (13.5) | −7.6 (12.4)* | −6.9 (12.7)* | −5.2 (15.2) |
| • Objective Social Disability (−) | 0–100 (24.2) | 68.5 (21.3) | −4.8 (19.3) | 0.7 (19.0) | −0.3 (17.4) |
| • Objective Work Disability (−)‡ | yes/no | 36% | −8 | 4 | 0 |
|
| |||||
|
TIVR GROUP Sample size = 26 |
Change from baseline Mean difference (S.D) |
||||
|
| |||||
| Test | Range of possible Scores (Norms) | Baseline Mean | Post CBT | Post TIVR | 8th month Follow-up |
|
| |||||
| SF-36 Mental Health Composite [+]† | 0–100 (74.9) | 38.7 (13.4) | 7.3 (10.6)* | 10.1 (14.9)* | 10.4 (14.2)* |
| SF-36 Physical Composite score [+] | 0–100 (66.3) | 31.4 (9.0) | 1.3 (7.6) | 6.0 (6.7)** | 8.9 (10.1)** |
| MPQ Pain Now [−] | 0–10 (N/A) | 6.1 (1.7) | −1.8 (2.3)** | −2.8 (2.3)*** | −3.5 (2.2)*** |
| MPQ Pain Typical [−] | 0–10 (N/A) | 5.7 (1.9) | −0.2 (2.1) | −1.7 (2.3)* | −2.3 (2.3)*** |
| CSQ Ability to Control Pain [+] | 0–6 (1.2) | 2.5 (1.4) | 0.9 (1.5)* | 1.7 (1.3)*** | 1.8 (1.3)*** |
| CSQ Ability to Decrease Pain [+] | 0–6 (2.8) | 2.5 (1.2) | 0.7 (1.0)* | 1.4 (1.3)*** | 1.3 (1.3)*** |
| CSQ Catastrophize [−] | 0–36 (17.8) | 16.5 (9.4) | −8.7 (8.7)*** | −10.5 (6.9)*** | −11.5 (8.1)*** |
| CSQ Divert Attention [+] | 0–36 (13.6) | 10.4 (7.0) | 5.8 (6.7)* | 3.9 (6.4)* | 5.0 (7.0)* |
| CSQ Reinterpret Pain [+] | 0–36 (10.6) | 2.5 (3.5) | 4.5 (7.3)* | 4.2 (7.5)* | 3.5 (7.4)* |
| CSQ Self-Talk [+] | 0–36 (22.4) | 19.7 (8.3) | 0.3 (6.6) | −0.8 (9.8) | −0.4 (8.1) |
| CSQ Ignore Sensations [+] | 0–36 (15.5) | 11.1 (7.2) | 1.4 (7.7) | −0.2 (8.5) | 0.5 (7.1) |
| CSQ Pray/Hope [−] | 0–36 (11.8) | 14.6 (8.0) | −1.9 (7.9) | −4.6 (7.1)* | −4.3 (7.1)* |
| CSQ More Active [+] | 0–36 (16.7) | 12.5 (5.6) | 4.4 (5.0)** | 2.5 (5.2)* | 3.0 (5.4)* |
| TOPS Total Pain Experience [−] | 0–100 (63.4) | 56.8 (12.1) | −6.7 (11.3)* | −16.0 (12.8)*** | −18.1 (13.5)*** |
| • Pain Symptoms (−) | 0–100 (72.0) | 70.6 (16.0) | −8.8 (16.7)* | −21.0 (19.6)*** | −26.8 (19.2)*** |
| • Lower Body Limitations (−) | 0–100 (58.8) | 52.1 (31.3) | −6.1 (22.9) | −16.0 (20.1)** | −18.8 (29.1)* |
| • Perceived Social Disability (−) | 0–100 (62.1) | 52.7 (12.4) | −6.9 (14.9)* | −17.1 (17.6)*** | −17.3 (15.1)*** |
| • Objective Social Disability (−) | 0–100 (24.2) | 64.2 (21.8) | −9.5 (15.4)* | −11.3 (17.1)* | −10.7 (17.7)* |
| • Objective Work Disability (−)‡ | yes/no | 62% | −8 | 4 | −8 |
SF-36 Normative Values are for the general population (Ware and Sherbourne, 1992); TOPS and CSQ Normative Values are for chronic pain patients (Rogers et all, 2000) (Stewart et all, 2001)
Sign in parentheses indicates the direction test results should go in order to satisfy expected outcome
Percentage of those not claiming work disability. Exact test for dependent proportions was used to assess the significance of change from baseline
Bolded results indicate the follow-up with the maximum improvement from baseline
Significance
p<.05,
p<.001,
p<.0001
Of the 8 measures, the control group showed greater improvement in 3 by the end of CBT. However, the TIVR group did show the greater improvement on the two measures where the difference was significant (SF36 MCS and MPQ Now). We reran the group comparisons at the last two follow-ups including a covariate for responsiveness to CBT (difference between post-CBT and baseline). There was some decrease in the p-values, but the TIVR effect did remain significant.
Within Group Analysis
Within-group changes for all three follow-ups are summarized in Table 2. For the control group the largest treatment effect was seen at post-CBT evaluation, where five of the eight main outcomes had p-values less than 0.05. At the eight-month follow-up, four of the eight outcomes were significantly better than baseline. The subjects in control group appear to have maintained some of the CBT gains at four and eight-month follow-up, although there was a decrease in the TOPS scores.
In contrast, the TIVR group had significant improvements over baseline at both 4 and 8 months in all of the outcomes. What is important to emphasize is that the TIVR group showed maximum improvement over baseline at the last follow-up for seven of the eight outcome measures, indicating continued improvement after of the TIVR intervention already ended.
Between-Group Analysis
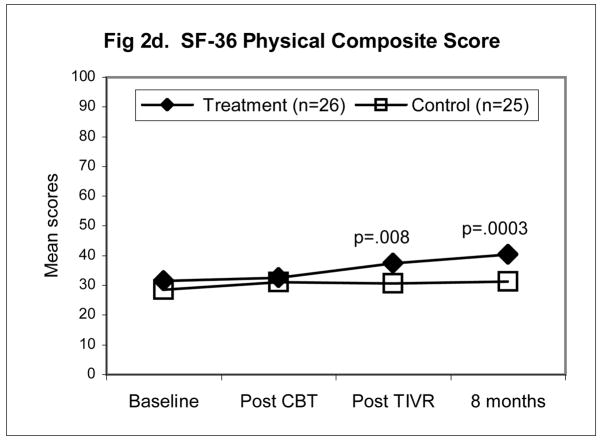
Between-group analysis (ANCOVA) (Table 3) revealed significant differences at both 4 and 8 month follow-ups for all of the outcomes, notably MPQ Pain Typical (p<.0001), CSQ Ability to control Pain (p<.0001), TOPS Total Pain Experience (p<.0001), and SF-36 Physical Composite (p=.0003). At the last follow-up, the TIVR group scores differed by as much as one standard deviation from the scores of the control group, after adjusting for post-CBT differences.
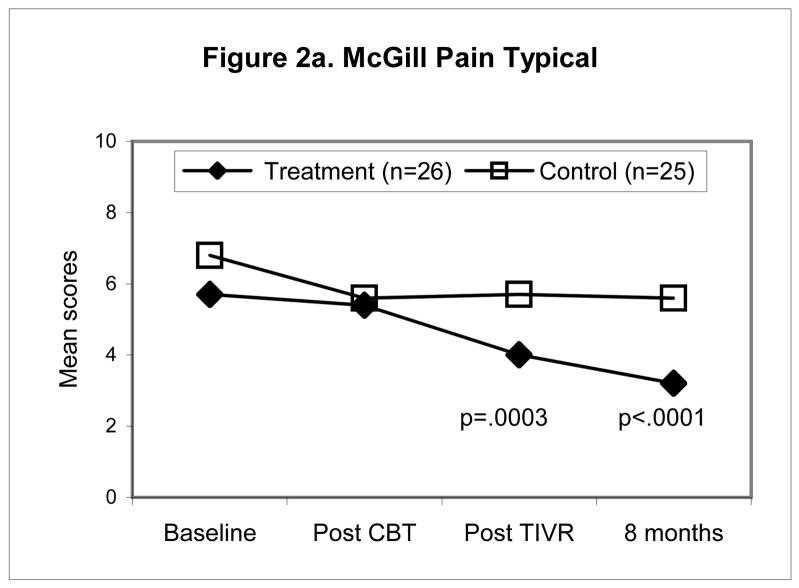
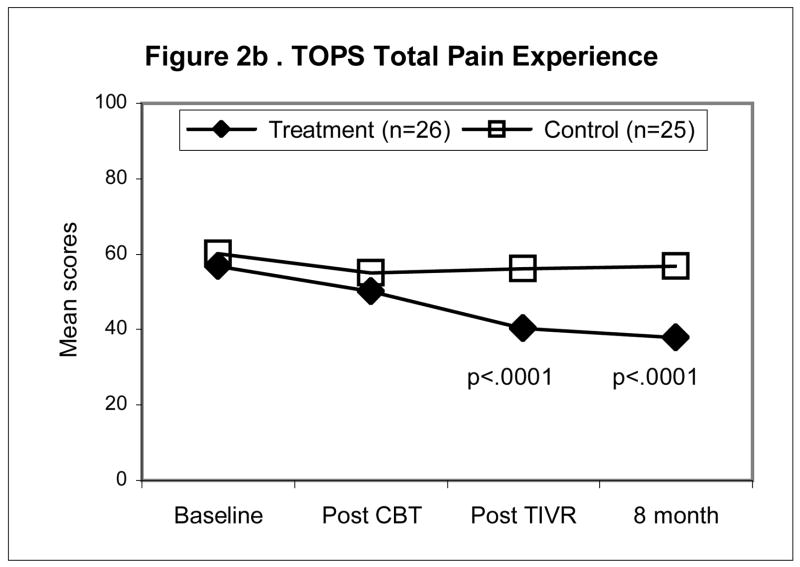
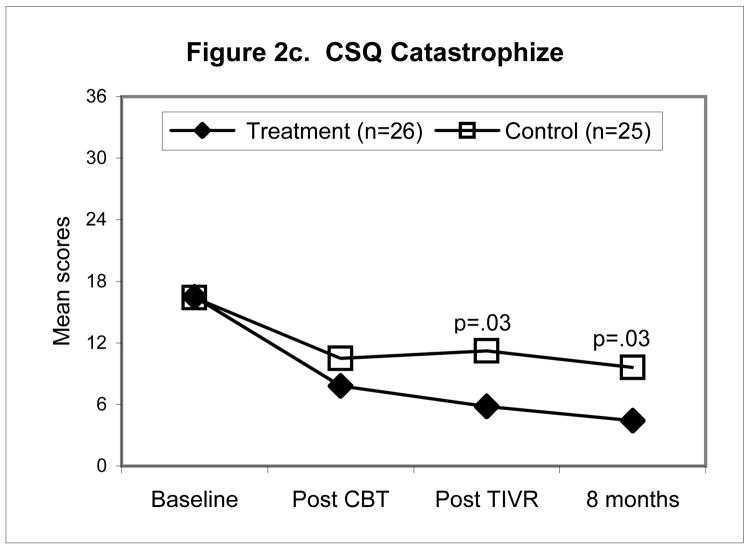
Figures 2a–d illustrate the pattern of change for four of the outcomes namely, McGill Pain Typical (Fig. 2a), TOPS Total Pain Experience (Fig. 2b), CSQ Catastrophizing (Fig. 2c), the SF-36 Physical Composite Score (Fig. 2d). All four outcomes scores were significantly improved at the end of the four-month maintenance program in the TIVR group while scores in the non-TIVR control group stayed the same as post CBT. The final evaluation (eight months post CBT) shows that scores in the TIVR group continued to improve. In contrast, scores in the control sample remained unchanged as compared to post CBT.
Figure 2.
Mean scores for the control and TIVR groups at four time points. The two groups’ scores were similar at post-CBT but became significantly different by the end of the TIVR intervention and more so by the last follow-up since the TIVR group continued to improve. The p-values are reported for the between-group comparisons, adjusted for post-CBT differences (ANCOVA), at the last two assessments.
Medication Use
We considered the possibility that changes in medication use may have contributed to the observed improvements in pain and function. Some of the statistical analyses summarized in Table 3 were rerun with the addition of four covariates reflecting the changes in medication use in each of the four categories. At the 8th month follow-up, the TIVR group still had significantly better outcomes for the SF-36 and McGill subscales, CSQ ability to decrease and control pain, as well as the TOPS Total Pain Experience after adjusting for changes in medication use.
DISCUSSION
This randomized, controlled study demonstrates that use of the TIVR is associated with decreases in ratings of pain, improved coping and decreased likelihood of relapse into pain behavior. Our findings support the use of TIVR as an option for self-directed treatment as an adjunct to behavioral group therapy in order to sustain patients with chronic musculoskeletal pain.
Using the TIVR for four months post-group CBT resulted in improvements not only in measures of pain, mental health and coping but also in physical activity and performance as measured by the SF36 Physical Composite Score. The TIVR prevented relapse not only while patients used the tool but treatment gains were maintained or improved further four months after access to the TIVR was terminated. This suggests two things. First, patients were able to use the TIVR to incorporate the skills they learned in group CBT into their personal lives. Second, four months of TIVR is a sufficient duration for this adaptation process to occur.
As demonstrated in previous studies short term (9–16 weeks) group CBT for chronic pain is clearly beneficial (Basler 1993; Basler et al 1997; Vlaeyen et al 1995). Patients learn new coping skills, learn from the process under the guidance of a trained therapist, and with the support of other group members. However, in many cases, beneficial effects gradually decline after the groups have finished. In our experience patients appear to recognize this vulnerability and often request to continue groups or repeat the program, and at times try to arrange follow-up support groups on their own. Continuing a group program with a skilled CBT leader as a long term process is not feasible due to cost and not necessarily desirable for adaptive functioning, as patients may benefit more from becoming independent and integrating the newly acquired skills into their daily routines.
We began this research with the hope of testing a tool to prevent relapse into pain behavior by bolstering the gains made in group CBT. Results of our pilot study suggested that the TIVR might actually enable patients to continue improving with minimal professional time investment (Naylor et al 2002). However, that study reported results only from the TIVR intervention. The current study examines TIVR effectiveness in a randomized controlled trial utilizing a larger sample. Results are similarly positive and thus replicate the earlier pilot study.
In the present study the TIVR and Control showed similar therapeutic gains from CBT. However, outcome scores were significantly improved at the end of the four-month maintenance program in the TIVR group while scores in the non-TIVR control group remained static. Even more remarkable is that the scores in the TIVR group continue to improve further four months after TIVR program was over while scores in the control sample remain unchanged, declined, or in some instances returned to baseline (Total Pain Experience and SF 36 Physical Composite Score). Since all but one of the group comparisons were significant at the final follow-up even after taking into account changes in medication dosages, we concluded that the superior outcome of the TIVR group was unlikely to be a consequence of differential use of narcotic analgesics or benzodiazepine medication. We plan to look into the medication effect in more detail in subsequent analysis.
There are several possible mechanisms of efficacy related to positive outcome among those patients using the TIVR. First, the TIVR may serve to encourage consistent use of skills learned in CBT. Included in the TIVR Daily Questionnaire (Component 1) is a listing of skills learned in CBT with a query about whether each was used that day. This serves both as a daily reminder of the skills repertoire and an implicit reminder to review or rehearse skills as necessary (Components 2 and 3, respectively). The daily questionnaire also enables the therapist to monitor skills use and remind patients about skills that are poorly utilized. During the follow-up interviews patients in the TIVR condition often reported that the monthly therapist feedback improved their motivation to practice skills daily. We speculate that more consistent skills use in vivo likely helps patients incorporate in to their own personal lives skills first presented in the more artificial context of CBT group therapy. Such incorporation along with consistent therapist encouragement could also lead to skill mastery. This could help explain the continued improvement in clinical status even after the four months of TIVR was terminated. While we do not have a systematic measure of skills use in the no-TIVR condition, we suspect that skills most important use declines over time. We are testing for a possible differential in skills use in a new, ongoing study. We believe that the reason for the positive outcome of TIVR is patients’ adaptation of skills to personal lives.
A second potential mechanism of TIVR efficacy is improved self-monitoring skills. Self-monitoring is believed to be one of the most important components of maintenance enhancement (Marlatt & Gordon 1985). Relapse prevention models emphasize the utility of self-monitoring in helping patients evaluate the effects of specific coping skills, identify early warning signs of setbacks in coping efforts, and become aware of both problems and successes in dealing with setbacks (Marlatt & Gordon 1985; Keefe & Van Horn 1993). Consistent with this literature, we believe it likely that the TIVR daily self-monitoring was one of the contributing factors in improvement of symptoms.
A third element of efficacy is the monthly therapist feedback. Component 1 of the TIVR (pain diaries) enabled the therapist to monitor daily skills use and treatment progress. The therapist could then use this information to help patients recognize, in vivo, interactions between life events, and daily fluctuations in pain and emotions in relationship to the frequency of skills practice. We found that the use of the patient’s own self-reported information also provided opportunities for the therapist to emphasize the importance of cognitive appraisal and emotion as well as the relationship of physical activity and exacerbation of pain. During the follow-up interviews patients consistently reported that the monthly therapist feedback improved their self-awareness.
Study Limitations
There are a few limitations that must be considered in interpreting our results. First is the relatively small sample size of only 25 subjects in each group. The demographic composition is also skewed since the sample is predominantly female and there are only two minority group members. The latter is reflective of the demographic composition of the state of Vermont.
Second, we are unable to disaggregate continued therapist support from either the informational feedback or from simple attention control. We do not know what aspects of the monthly feedback are necessary for a therapeutic effect. Could simple motivational messages unrelated to the subjects’ data be as effective?
Third, there was only 4 month follow-up post TIVR so we are not able to see if the TIVR group continued to show a superior therapeutic effect or if their clinical status began to decline over time.
A current ongoing RCT addresses many of these limitations. It includes a larger study population, an attention control condition, and a 12 month post-CBT follow-up. Future research plans include an assessment of whether the monthly message can be automated and whether the TIVR could be used as a substitute for group therapy in patients without access to CBT.
Conclusion
In summary, to our knowledge, there is no other self-directed treatment program that has demonstrated efficacy as a tool for pain coping skills maintenance enhancement. We created the TIVR program with the hope to prevent relapse into pain behavior after the successful completion of group CBT. We were surprised to see that patients using this tool not only did not relapse but continued to improve for four months after the TIVR was completed. We believe that if our findings are replicable then using the TIVR as a coping skill consolidation and relapse prevention program could be efficacious and cost-effective addition to any health care providers.
Acknowledgments
Acknowledgement of Support: This research was supported by grants from the National Institute of Drug Addiction (NIDA) R21 DA016115, National Institute of Arthritis, Musculoskeletal Diseases (NIAMS) R01 AR052131, and National Institute on Alcohol Abuse and Alcoholism (NIAAA) R01 AA014270. The Authors thank Michele Comette, B.A., for study coordination and support in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baer L, Greist JH. An interactive computer-administered self-assessment and self-help program for behavior therapy. J Clin Psychiatry. 1997;58:23–28. [PubMed] [Google Scholar]
- Basler HD. Group treatment for pain and discomfort. Patient Educ Couns. 1993;20:167–175. doi: 10.1016/0738-3991(93)90130-o. [DOI] [PubMed] [Google Scholar]
- Basler HD, Jakle C, Kroner-Herwig B. Incorporation of cognitive-behavioral treatment into the medical care of chronic low back patients: A controlled randomized study in German pain treatment. Patient Educ Couns. 1997;31:113–124. doi: 10.1016/s0738-3991(97)00996-8. [DOI] [PubMed] [Google Scholar]
- Compas BE, Haaga DAF, Keefe FJ, Leitenberg H, Williams DA. A sampling of empirically supported psychological treatments from health psychology: Smoking, chronic pain, cancer, & bulimia nervosa. J Consult Clin Psychol. 1998;66:89–112. doi: 10.1037//0022-006x.66.1.89. [DOI] [PubMed] [Google Scholar]
- Connally GH, Sanders SH. Predicting low back pain patients’ response to lumbar sympathetic nerve blocks and interdisciplinary rehabilitation: The role of pretreatment overt pain behavior and cognitive coping strategies. Pain. 1991;44:139–146. doi: 10.1016/0304-3959(91)90127-J. [DOI] [PubMed] [Google Scholar]
- Davis MA, Ettinger WH, Neuhaus JM, Mallon KP. Knee osteoarthritis and physical functioning: evidence from the NHANES I epidemiologic follow-up study. J Rheumatol. 1991;18:591–598. [PubMed] [Google Scholar]
- Gandek B, Ware J., Jr Translating functional health and well-being: IQOLA Project studies of the SF-36 health survey. J Clin Epidemiol. 1998;51:953–959. [Google Scholar]
- Haythornthwaite JA, Menefee LA, Heinberg LJ, Clark MR. Pain coping strategies predict perceived control over pain. Pain. 1998;77:33–39. doi: 10.1016/S0304-3959(98)00078-5. [DOI] [PubMed] [Google Scholar]
- Haythornthwaite JA, Heinberg LJ. Coping with pain: what works, under what circumstances, and in what ways? Pain Forum. 1999;8:172–175. [Google Scholar]
- Helzer JE, Rose GL, Badger GJ, Searles JS, Thomas C, Lindberg S, Guth S. Utilizing Interactive Voice Response to Enhance Brief Alcohol Intervention in Primary Care Settings. J Stud Alcohol. doi: 10.15288/jsad.2008.69.251. In review. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karolny P. Control beliefs, coping efforts, and adjustment to chronic pain. J Consult Clin Psychol. 1991;59:431–438. doi: 10.1037//0022-006x.59.3.431. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM. Self-efficacy and outcome expectancies: Relationship to chronic pain coping strategies and adjustment. Pain. 1991;44:263–269. doi: 10.1016/0304-3959(91)90095-F. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8:163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R, Sellers W. Four year follow-up of a meditation-based program for the self-regulation of chronic pain: Treatment outcomes and compliance. Clinical J Pain. 1986;2:159–173. [Google Scholar]
- Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: Catastrophizing as a maladaptive strategy. Pain. 1989;37:51–56. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Caldwell DS, Williams DA, Gil KM, Mitchell D, Robertson C, Martinez S, Nunley J, Beckham JC, Crisson JE, Helms M. Pain coping skills training in the management of osteoarthritic knee pain: A comparative study. Behav Ther. 1990a;21:49–62. [Google Scholar]
- Keefe FJ, Caldwell DS, Williams DA, Gil KM, Mitchell D, Robertson C, Martinez S, Nunley J, Beckham JC, Crisson JE, Helms M. Pain coping skills training in the management of osteoarthritic knee pain: Follow-up results. Behav Ther. 1990b;21:435–448. [Google Scholar]
- Keefe FJ, Caldwell DS, Martinez S, Nunley J, Beckham J, Williams DA. Analyzing pain in rheumatoid arthitis patients: Pain coping strategies in patients who have had knee replacement surgery. Pain. 1991;46:153–160. doi: 10.1016/0304-3959(91)90070-E. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Van Horn Y. Cognitive-behavioral treatment of rheumatoid arthritis pain: Understanding and enhancing maintenance of treatment gains. Arthritis Care Res. 1993;6:213–222. doi: 10.1002/art.1790060408. [DOI] [PubMed] [Google Scholar]
- Kobak KA, Taylor LH, Dottl SL, Greist JH, Jefferson JW, Burroughs D, Mantle JM, Katzelnick DJ, Norton R, Henk HJ, Serlin RC. A computer-administered telephone interview to identify mental disorders. J Am Med Assoc. 1997;278:905–946. [PubMed] [Google Scholar]
- Lanes TC, Gauron E, Spratt KF, Wernimont TJ, Found EM, Weinstein JN. Long-term follow-up of patients with chronic back pain treated in a multidisciplinary rehabilitation program. Spine. 1995;20:801–806. doi: 10.1097/00007632-199504000-00012. [DOI] [PubMed] [Google Scholar]
- Lawson K, Reesor K, Keefe FJ, Turner J. Dimensions of pain-related coping: Cross validation of the factor structure of the Coping Strategy Questionnaire. Pain. 1990;43:195–204. doi: 10.1016/0304-3959(90)91073-R. [DOI] [PubMed] [Google Scholar]
- Marks IM, Baer L, Greist JH, Park JM, Bachofen M, Nakagawa A, Wenzel KW, Parkin JR, Manzo PA, Dottl SL, Mantle JM. Home Self-Assessment of Obsessive-Compulsive Disorder. Br J Psychiatry. 1998;172:406–412. doi: 10.1192/bjp.172.5.406. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse Prevention. New York, NY: Guilford Press; 1985. [Google Scholar]
- McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain. Spine. 2002;27:2564–2573. doi: 10.1097/00007632-200211150-00033. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: Major Properties and Scoring Methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- Naylor MR, Helzer JE, Naud S, Keefe FJ. Automated Telephone as an Adjunct for the Treatment of Chronic Pain: A Pilot Study. J Pain. 2002;3:429–438. doi: 10.1054/jpai.2002.129563. [DOI] [PubMed] [Google Scholar]
- Osgood-Hynes DJ, Gresit JH, Marks IM, Baer L, Heneman SW, Wenzel KW, Manzo PA, Parkin JR, Spierings CJ, Dottl SL, Vitse HM. Self-Administered Psychotherapy for Depression Using a Telephone-Accessed Computer System Plus Booklets: An Open U.S.-U.K. Study. J Clin Psychiatry. 1998;59:358–365. doi: 10.4088/jcp.v59n0704. [DOI] [PubMed] [Google Scholar]
- Rogers WH, Wittink HM, Ashburn MA, Cynn D, Carr DB. Using the TOPS an Outcomes Instrument for Multidisciplinary Outpatient Pain Treatment. Pain Med. 2000a;1:55–67. doi: 10.1046/j.1526-4637.2000.99101.x. [DOI] [PubMed] [Google Scholar]
- Rogers WH, Wittink HM, Wagner A, Cynn D, Carr DB. Assessing Individual Outcomes during Outpatient Multidisciplinary Chronic Pain Treatment by Means of an Augmented SF-36. Pain Med. 2000b;1:44–54. doi: 10.1046/j.1526-4637.2000.99102.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc., Cary, NC; http://www.sas.com/)
- Stroud MW, Thorn BE, Jensen MP, Boothby JL. The relation between pain beliefs, negative thoughts, and psychosocial functioning in chronic pain patients. Pain. 2000;84:347–352. doi: 10.1016/s0304-3959(99)00226-2. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- Sullivan MJL, Rouse D, Bishop S, Johnston S. Thought suppression, catastrophizing, and pain. Cog Ther Res. 1997;21:555–568. [Google Scholar]
- Thorn BE, Rich MA, Boothby JL. Pain beliefs and coping attempts: conceptual model building. Pain Forum. 1999;8:169–171. [Google Scholar]
- Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science. 1998;280:867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- Turk DC, Rudy TE. Neglected topics in the treatment of chronic pain patients – Relapse, noncompliance, and adherence enhancement. Pain. 1991;44:5–28. doi: 10.1016/0304-3959(91)90142-K. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JWS, Haazen IWC, Schuerman JA, Kole-Snijders AMJ, Eek H. Behavioral rehabilitation of chronic low back pain: Comparison of an operant treatment, an operant-cognitive treatment and an operant-respondent treatment. Br J Clin Psychol. 1995;34:95–118. doi: 10.1111/j.2044-8260.1995.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]