Abstract
The one-bead one-compound (OBOC) combinatorial library method enables the rapid generation and screening of millions of discrete chemical compounds on beads. Most of the OBOC screening methods require the library compounds to remain tethered to the bead during screening process. Methods have also been developed to release library compounds from immobilized beads for in situ solution phase or “lawn” assays. However, this latter approach, while extremely powerful, is severely limited by the lack of suitable solid supports for such assays. Here we report on the development of a novel hydrogel TentaGel shell-core (HTSC) bead in which hydrogel is grafted onto the polystyrene-based TentaGel® (TG) bead as an outer shell (5–80 µm thick), via free radical surface-initiated polymerization. This novel shell-core bilayer resin enables the preparation of encoded OBOC combinatorial small molecule libraries such that the library compounds reside on the highly hydrophilic outer layer and the coding tags reside in the polystyrene-based TG core. Using fluorescein as a model small molecule compound, we have demonstrated that fluorescein molecules that have been linked covalently to the hydrogel shell via a disulfide bond, could readily diffuse out of the hydrogel layer into the bead surrounding after reduction with dithiothreitol. In contrast, under identical condition, the released fluorescein molecules remained bound to unmodified TG bead. We have prepared an encoded OBOC small molecule library on the novel shell-core beads and demonstrated that the beads can be readily decoded.
Keywords: One-bead one-compound (OBOC) combinatorial library, high-throughput screening, in situ solution phase releasable assay, Hydrogel-TentaGel shell-core bead, TentaGel bead, free radical surface-initiated polymerization (SIP)
Introduction
The "one-bead one-compound" (OBOC) combinatorial library approach,1 and the “split-mix synthesis” method1–4 allow the efficient synthesis of thousands to millions of chemical compounds, such that each bead displays only one compound entity, with 1013 copies of the same compound on one single bead. The OBOC concept first recognized by Lam et al.1 made possible the screening of peptide and small molecule chemical libraries in an ultra-high throughput fashion using an on-bead screening assay. Literally millions of compounds can be screened in a matter of a few days.1 Many ligands or substrates for a number of biological targets have been discovered with this approach.5 Most of the published work in OBOC combinatorial libraries involves on-bead screening, in which the library compound remains tethered to an individual bead during screening. In the last few years, we have developed simple method to prepare topologically segregated bilayer beads,6–8 as well as several encoding methods to encode “non-sequenceable” peptide, peptidomimetic and even small molecule libraries. In our encoding systems, the library compounds reside on the outer layer of the bead and the coding tags remain in the bead interior. Such a configuration facilitates not only the encoding process but also minimizes interference by the coding tags during screening. Furthermore, the bilayer configuration enables us to easily increase the screening stringency to identify high-affinity ligands, by down-substituting the chemical loading on the outer layer but retaining full substitution in the bead interior for decoding. Very recently, we reported the discovery of a high affinity high specificity in vivo imaging ligand (IC50 ~ 2 picomolar) for lymphoma xenograft in nude mice, by screening peptide-encoded OBOC peptidomimetic combinatorial libraries with a on-bead cell binding assay.9 Application of the OBOC approach to solution phase assay, however, is problematic. We10, 11 and others12–15 have described the use of an in situ release solution phase assay to screen OBOC combinatorial libraries, with limited success. In these methods, beads were immobilized in soft agar and the compounds on each bead were cleaved and released to the bead surrounding, where biological activity occurred. The main problem of this approach is that many hydrophobic or heterocyclic compounds remain inside the bead matrix and therefore are not released to the bead surroundings. TG bead, a polystyrene-based bead grafted with polyethylene glycol (PEG) linkers developed by Rapp Polymer Inc (Tübingen, Germany), has been used in this work. This bead is compatible with aqueous and organic conditions. While its properties are ideal for on-bead screening assays, the porosity of the bead is too small, and the hydrophobic or heterocyclic library compounds tend to bind to the aromatic groups of the polystyrene inside the bead. To fully exploit this potentially very powerful in situ release approach, we wanted to develop a novel solid support that can overcome these limitations. An ideal solid support for such application should have the following properties (i) uniform in size, shape, and substitution (ii) physically robust so that fragmentation during the synthesis steps does not occur, (iii) compatible with both organic and aqueous condition and be able to withstand harsh acidic condition such as 100% TFA, (iv) compatible with our chemical encoding system, and (v) the library compounds (both hydrophilic and hydrophobic) to be released should be able to diffuse out of the bead readily under an aqueous environment.
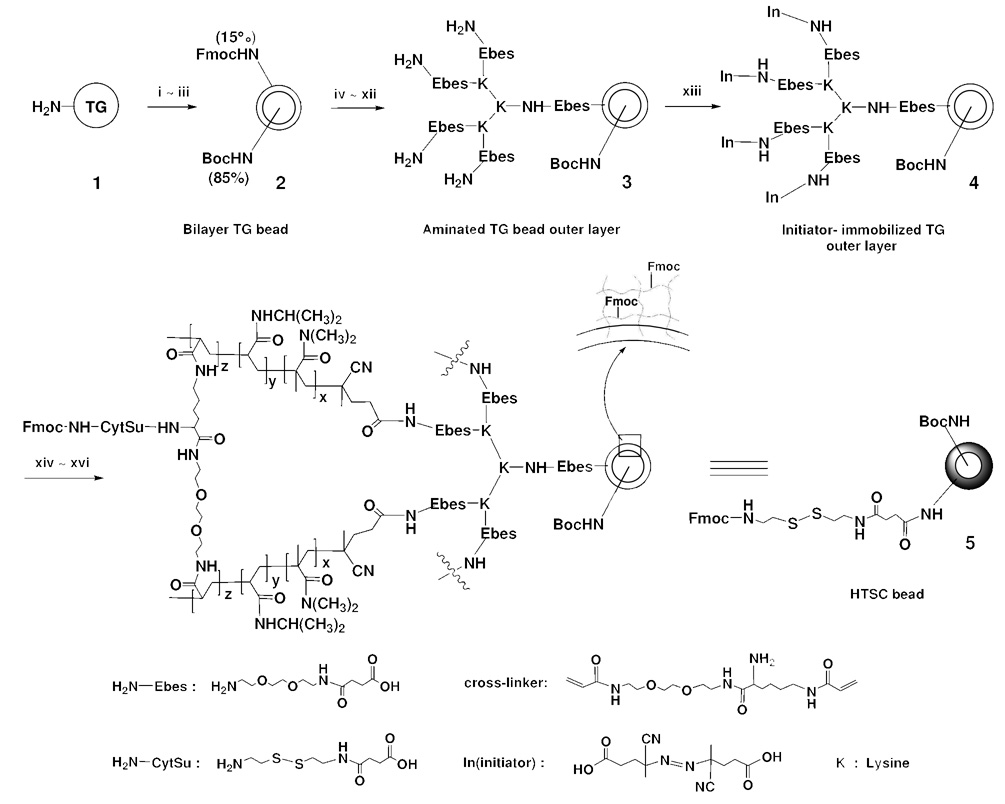
Lee et al reported the preparation of core-shell-type resin for solid-phase peptide synthesis.16–18 They first treated the aminomethyl polystyrene resin (1% cross-linked) with 2,4,6-trichloro-1,3,5-triazine, followed by reaction with excess O,O’-bis(2-aminopropryl) poly(ethylene glycol) 500, and finally the remaining chloride on the resins was capped with dimethylamine. In this paper, we detail the synthesis and characterization of a different type of core-shell beads (HTSC beads), which comprise of TG bead as the core covered by a shell of hydrogel. The hydrogel layer, prepared by polymerization of NIPAAm, NDMAAm, and a cross-linker ((5-acryloylamino-1-{2-[2-(2-acryloylamino-ethoxy)-ethoxy]-ethylcarbamoyl}-pentyl)-carbamic acid) that contains an Fmoc-amino group, was grafted to the surface of topologically segregated TG beads (Scheme 1). The azo initiator, first linked covalently to the outer layer of TG bilayer beads, was thermally activated, and a hydrogel layer was then formed in situ by free radical surface-initiated polymerization (SIP).19–22 The loading of the resulting HTSC beads was determined by quantifying the amount of Fmoc released from a known weight of resin. To study the compound releasing properties of the resin, six hydrophobic compounds including two fluorescent dyes, FITC or Abz, were covalently linked to the hydrogel shell of the shell-core beads via a cleavable disulfide linker, while the interior core of the beads was capped by Boc. For comparison, the same compounds were also coupled to the outer layer via a disulfide linker of TG bilayer beads (80% Boc-NH-inside / 20% NH2-outside). The beads were first immobilized in agarose. Then DTT was added and the release of compounds was quantified using HPLC. The release of fluorescent compounds (FITC and Abz) was also monitored under a fluorescent microscope. In addition, we have also prepared OBOC combinatorial peptidomimetic library on the above-mentioned novel shell-core beads with releasable library compound on the outer hydrogel shell and coding tags in the TG core.
Scheme 1.
Synthetic scheme of the preparation for HTSC bead. Reagents and conditions : (i) H2O, 24h; (ii) Fmoc-OSu, DIEA, Et2O/DCM(45:55, v/v), 40min; (iii) (Boc)2O, DIEA, Et2O/DCM(45:55, v/v); (iv) 20% piperidine/DMF; (v). Fmoc-Ebes-OH, HOBt, DIC, DMF; (vi) 20% piperidine/DMF; (vii) Fmoc-Lys(Fmoc)-OH, HOBt, DIC, DMF; (viii) 20% piperidine/DMF; (ix) Fmoc-Lys(Fmoc)-OH, HOBt, DIC, DMF; (x) 20% piperidine/DMF; (xi) Fmoc-linker-OH, HOBt, DIC, DMF; (xii) 20% piperidine/DMF; (xiii) 4,4'-azobis(4-cyanovaleric acid), EEDQ, DMF, 25°C, 5h; (xiv) NIPAAm, NDMAAm, Fmoc-cross-linker, MeOH, 80°C; (xv) 20% piperidine/DMF; (xvi) Fmoc-CytSu-OH, HOBt, DIC, DMF.
Results and Discussion
Design and synthesis of HTSC bead
Typically, TG bead is produced by a process in which PEG units of approximately 3kDa are grafted onto low-cross-linked polystyrene bead. This results in a relatively hydrophilic support making the bead swells both in water and in most polar organic solvents. These properties are suitable for (i) combinatorial libraries synthesis in which organic solvents are used, and (ii) bioassays under aqueous conditions. For on-bead binding assay, the screening stringency can be tuned by controlling the number or concentration of library compounds accessible to the interacting molecular probes at the bead surface.23 For in situ solution phase releasable assays, not only is the loading of the library compounds inside each bead is important, but the ability of library compounds to diffuse out of the bead into the surrounding is even more important. This latter property depends greatly on the nature of the library compounds and the physicochemical properties, swelling properties, and porosity of the bead matrix under aqueous conditions. TG resin is not suitable for OBOC solution phase releasable assays because it is polystyrene-based and not very porous, and heteocyclic and hydrophobic compounds tend to be trapped inside the polystyrene matrix.
We have recently developed a simple, inexpensive, and highly reproducible bi-phasic solvent approach to topologically segregate TG beads.6–8 TG resin beads with free amino group were first swollen in water and the outer layer of the beads was exposed to a mixture of DCM and Et2O (55:45), as well as an amine-derivatizing reagent (e.g. Fmoc-OSu). Meanwhile, the bead interior remains in water without any derivatizing reagent. By adjusting the amount of derivatizing reagents used, the thickness of the outer layer can be readily controlled. Subsequently, the interior of beads can be protected with a desirable orthogonal protecting group such as Boc.
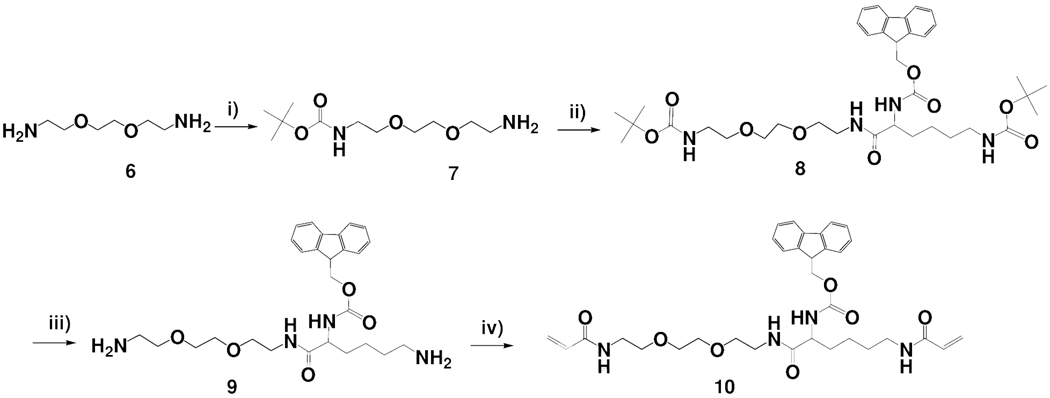
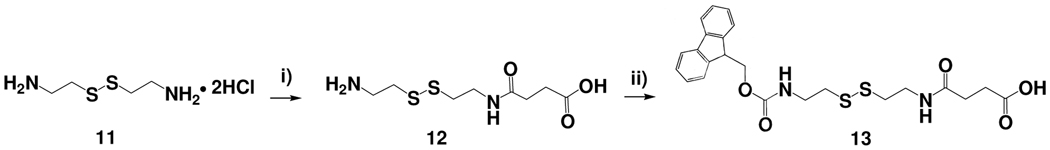
Library beads for in situ solution phase releasable screening must include a hydrophilic and porous outer layer that allows easy release of the library compounds from the beads. As shown in Scheme 1, the conversion of the bilayer TG beads (2) to HTSC beads (5) was achieved by grafting a hydrogel layer onto the surface of the TG beads. Combinatorial peptide, peptidomimetic or small molecule libraries together with the encoding tags can be readily built on such solid-support. The coding tag will reside in the TentaGel core and the library compounds which will be later released for solution phase assays, will be tethered to the hydrogel shell. The three main considerations for the preparation of HTSC bead include: (i) synthesis of the hydrophilic cross-linker containing functional group for library compound synthesis, (ii) synthesis of cleavable linker, for the library compounds to release from hydrogel layer under an aqueous environment, and (iii) choice of suitable polymer chemistry so that the resulting hydrogel layer is physically robust but porous, and does not fracture during synthesis steps. We first designed a new hydrophilic Fmoc-cross-linker containing a Fmoc-protected amino group (site for library compound synthesis) and two acryloy groups for crosslinking [(10) in Scheme 2]. The synthetic route is shown in Scheme 2. Briefly, 2,2'-(ethylenedioxy)-bis(ethylamine) (Ebe) (6) was allowed to react with 1 equivalent (Boc)2O in dioxane to form mono Boc-protected diamine (7). Fmoc-lysine(Boc)-OH was coupled to (7) to give bis Boc-protected diamine (8). The tert-Boc-protecting group was removed by TFA in DCM to recreate free diamine (9). The acryloyl chloride was coupled to the diamine to give the hydrophilic cross-linker (10) with an Fmoc-protected amino group. In addition, we also synthesized a new cleavable linker (13) containing a disulfide bond. The synthetic route is illustrated in Scheme 3. Cystamine dihydrochloride (11) was allowed to react with the succinic anhydride in a mixture of CHCl3 / MeOH in the presence of DIEA to form the mono-acylated intermediate (12), which was subsequently protected with Fmoc-OSu in situ. The disulfide bonds can be cleaved by a well-known thiol-disulfide exchange reaction using reducing agents such as DTT.24–26 Accordingly, the peptide or small molecule connected with disulfide linker could readily diffuse out from the hydrogel layer into the bead surrounding after reduction with DTT.
Scheme 2.
Synthetic scheme of the new Fmoc-cross-linker. Reagents and reaction conditions: (i) (Boc)2O, dioxane; (ii) Fmoc-Lys (Boc)-OH, DCC, HOBt, THF; (iii) TFA / DCM; (iv) acryloxy chloride, DCM, TEA, 0°C.
Scheme 3.
Synthetic scheme of a disulfide cleavable linker. Reagents and reaction conditions: (i) succinic anhydride, DIEA, CHCl3/MeOH, (ii) Fmoc-OSu, DIEA, DCM.
To generate HTSC beads, we first prepared bilayer TG bead with a thin outer layer (15% segregation) protected by Fmoc and the inner core (85% segregation) protected by Boc (Scheme 1). Using the standard HOBt/DIC coupling method, we then introduced a Fmoc-hydrophilic linker,27 Fmoc-Ebes (structure is shown in Scheme 1) on the outer layer of the bead increases hydrophilicity and acts as a spacer. Sequentially, after Fmoc-deprotection, each N-terminus of the outer layer was amplified four times with three lysines to generate four branches to which four hydrophilic linkers were added serially, followed by an azo initiator (4,4’-Azobis(4-cyanovaleric acid)). To initiate radical polymerization at the surface of TG at the initiator site, the modified resin was mixed with a solution of 1:1 (v/w) NIPAAm and NDMAAm plus a varying amount of Fmoc-cross-linker (compound 10 in Scheme 2) in MeOH at 80°C. Azo initiator immobilized on TG outer layer was easily decomposed to create a radical in MeOH solution.
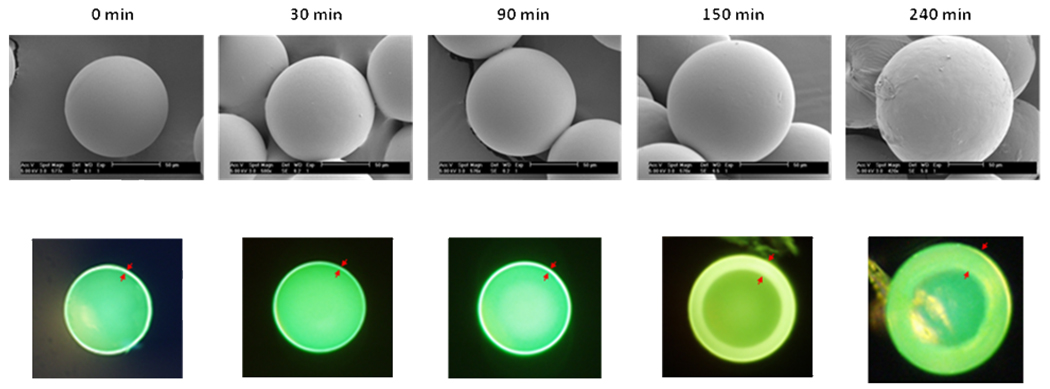
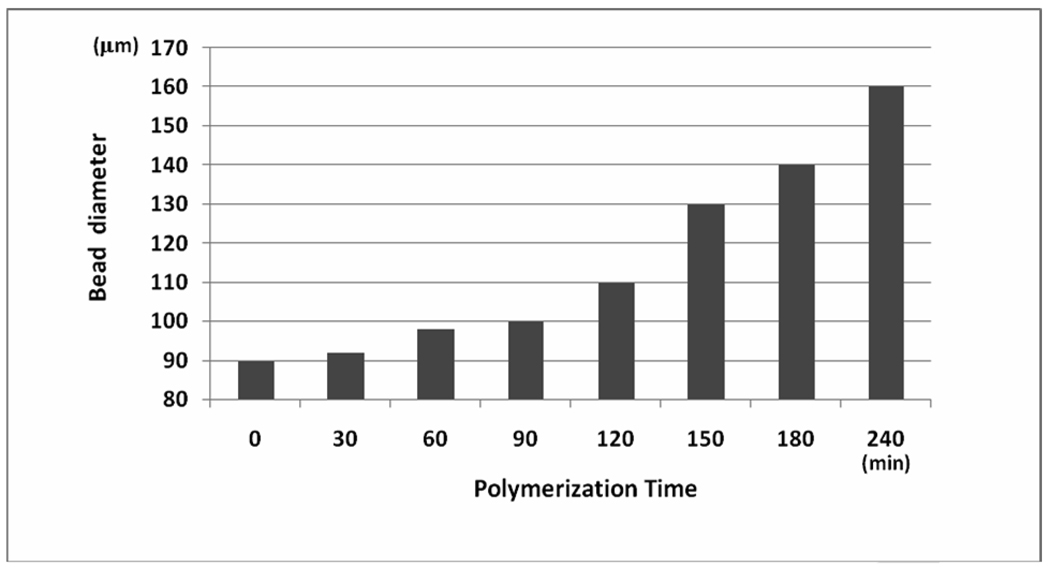
Both fluorescent microscopy and scanning electron microscopy (SEM) were used to characterize the HTSC beads. Figure 1 (upper panel) shows the SEM micrographs of unmodified TG bead (~80µm) and the HTSC beads (100 –160µm). To facilitate the characterization of the hydrogel shell, we labeled this layer with FITC after Fmoc deprotection. The beads were then sliced into two-halves under a dissecting microscope with a sharp scalpel, and visualized under a fluorescent microscope (Figure 1, lower panel). It is clear that the thickness of the hydrogel layer increases as a function of the polymerization time (Figure 2).
Figure 1.
Scanning electron microscopy (SEM) (upper panels) and fluorescent microscopy (lower panels) of sliced HTSC beads prepared at 80°C in MeOH (5mL) under various polymerization time. The red arrows marked the thickness of the hydrogel layer of the shell-core bead. The bead interior was capped with Boc. The outer layer was labeled with FITC.
Figure 2.
Effect of polymerization time on final diameter of HTSC bead.
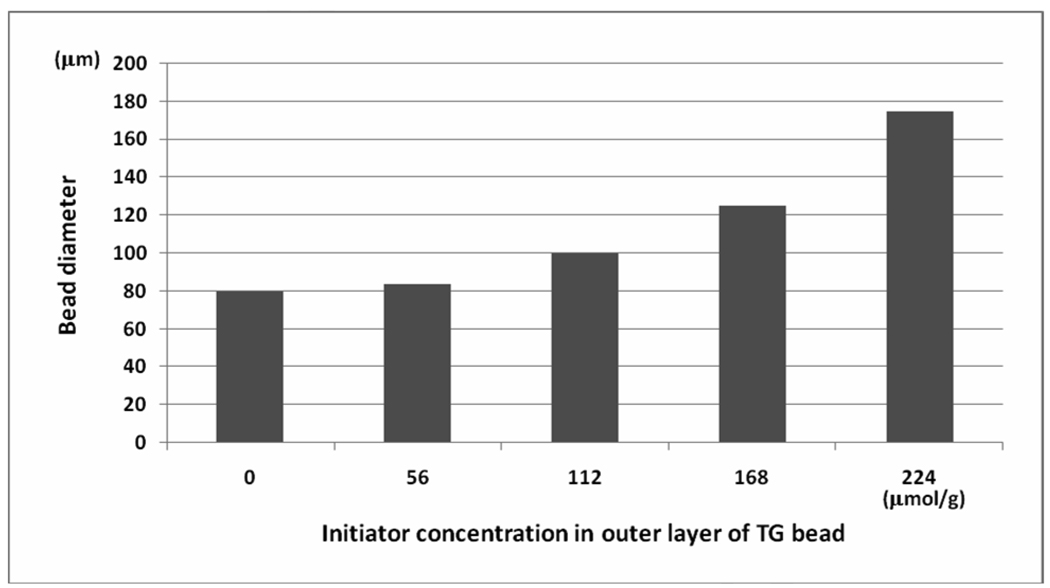
We prepared several types of topologically segregated bi-functional TG bead with varying degree of segregation (with 5, 10, 15 and 20% outer layer and corresponding 95, 90, 85 and 80% inner core) according to our published procedure.6,7 By adjusting the amount of Fmoc-OSu used, the thickness, or % segregation, of the outer layer of the TG bead can be controlled. Polymerization of the hydrogel layer on the surface of the TG bead was performed according to the method shown in Scheme 1. Depending on the concentration of initiator grafted to the bead outer layer and the polymerization time (30–240 minutes), the diameter of the resulting HTSC beads was found to vary between 85–175µm (Figure 2 and Figure 3). Fmoc loading of the hydrogel shell was controlled by the concentration of Fmoc-cross-linker used.
Figure 3.
Effect of concentration of initiator on the diameter of HTSC bead. Polymerization condition: initiator-grafted TG bead (300mg), Fmoc-cross-linker (300mg), NDMAAm (300µL), NIPAAm (300mg); polymerization continued for 120min at 80°C in MeOH (5mL); inside core of the beads was block by Boc.
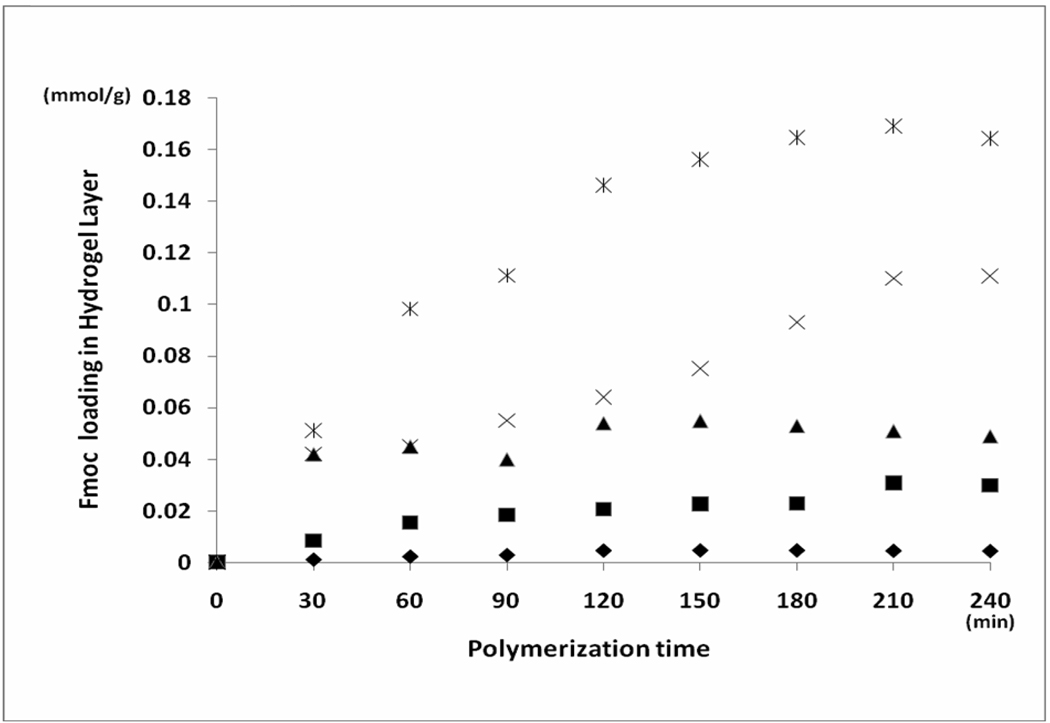
Loading capacities of the hydrogel shell prepared under different conditions were determined by Fmoc-derivatization followed by quantification of the released piperidine-dibenzylfulvene adduct.28 The result is shown in Figure 4. The Loading capacity was found to increase as a function of polymerization time and the concentration of cross-linker used.
Figure 4.
Fmoc group loading capacities in hydrogel layer according to different amount of Fmoc-cross-linker used [10 0(♦), 50 (∎), 100 (▲), 300 (×) and 500 (ж) mg]. In addition, to the cross-linker, the polymerization reaction mixture contained NDMAAm (300µL), NIPAAm (30mg), and initiator-immobilized TG beads [300mg, (85%, Boc-protected) / outer-layer (15%, initiator conc.)] at 80°C in MeOH (5mL).
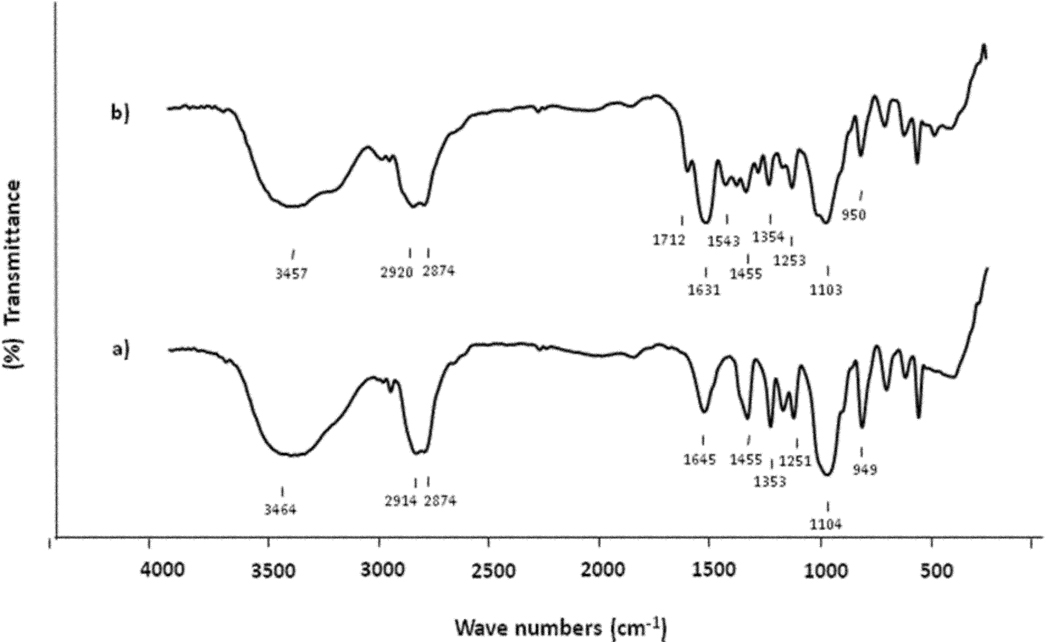
Progress of hydrogel grafting can be monitored by FT-IR. As shown in Figure 5, a broad and strong absorption peak for the ether linkage (1104 cm−1, due to the PEG chain) and an absorption peak for N-H bend (1645 cm−1, due to the primary amine in the TG bead) were found in FT-IR spectra of both TG and HTSC beads. The characteristic strong absorption bands of hydrogel layer (poly (NIPAAm-co-NDMAA)) with an amide carbonyl band (1631 cm−1) and strong absorption peak for the ether linkage (around 1103 cm−1, due to the PEG chain and Fmoc-cross-linker) in the spectra of HTSC beads confirmed the successful grafting of a hydrogel layer.
Figure 5.
FT-IR spectra of TG beads a), and HTSC beads b) in KBr.
To compare the compound release property of the novel HTSC bead with the standard TG (80 % Boc-inner core / 20 % NH2-outer layer) beads, FITC and Abz were first each conjugated onto each of these beads via a disulfide bond containing linker. The beads were then immobilized in soft agar containing PBS, and the kinetics of the release of fluorescein (hydrophobic) and Abz (hydrophilic) from the beads, after addition of 0.05% DTT, were followed under an inverted fluorescent microscope. Figure 6 (top rows of both panels) clearly shows that fluorescein, which is larger, hydrophobic and heterocyclic, remained inside the TG bead over 2 hours without any sign of diffusing out into the bead exterior. The smaller hydrophilic Abz, however, did diffuse out of the TG bead over the two hour period (top row of lower panel). This observation can be explained by the fact that TG bead is not very porous and polystyrene based; and released hydrophobic and heterocyclic compounds tend to be trapped in the polystyrene matrix. The HTSC bead was designed to overcome this problem. The hydrogel shell of the HTSC bead is highly hydrophilic and porous. We expect the released compounds to be able to diffuse out of the bead rapidly. This is indeed the case. As demonstrated in Figure 6 (bottom row of both upper and lower panels), both FITC and Abz rapidly diffused out of the HTSC to the bead exterior within 25 minutes.
Figure 6.
Fluorescent micrograph of release of (I) Fluorescein and (II) Abz from TG bead (upper panels of I and II, 80% Boc-inside / 20% FITC-outside) and HTSC beads (low Panels of I and II) as a function of time after the addition of 0.05% DTT. The core of the shell-core beads was capped by Boc.
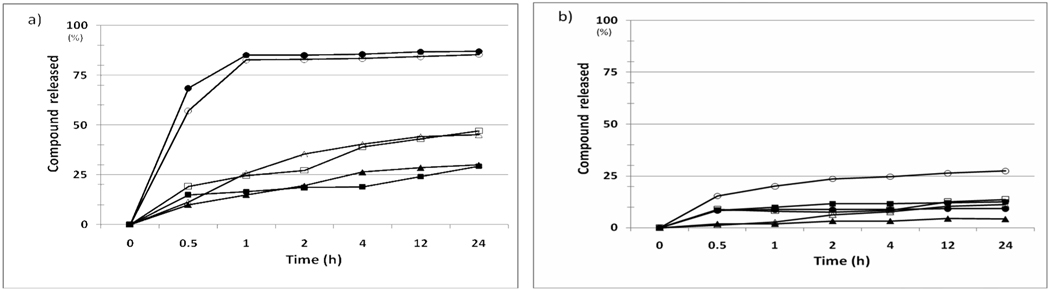
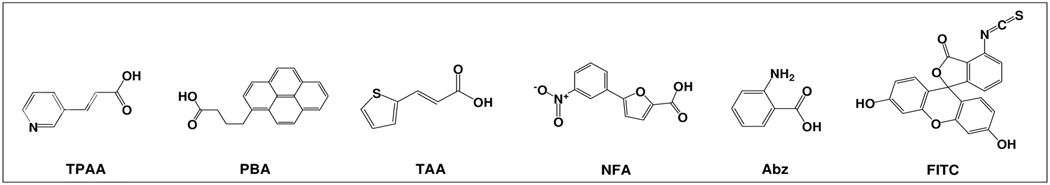
In addition to Fluorescein and Abz, we have also used four additional organic molecules as model compounds. These include trans-3-(3-pyridyl) acrylic acid (TPAA), 1-pyrenebutyric acid (PBA), 5-(3-nitrophenyl)-2-furoic acid (NFA), 3-(2-thienyl) acrylic acid (TAA). These four compounds together with FITC and Abz were each chemically coupled to the outer layer of the HTSC beads or TG beads via a disulfide linker. These compound beads were then added to PBS solution containing 0.2% DMSO and 0.05% DTT at 25°C. The supernatants were then sampled as a function of time (0–24 hours) and quantified with HPLC. This compound release buffer was chosen because it is compatible with cell-based assay. As shown in Figure 7, although the release profile of each compound was found to be quite different, the amount of compounds released from the HTSC bead (Figure 7a) was consistently much higher than those released from the TG beads (Figure 7b).
Figure 7.
Release of model compounds from (a) HTSC beads, and (b) TG bead (50% Boc-inside / 50% compounds-outside) as a function of time in 0.2% DMSO/PBS solution after the addition of 0.05% DTT at 25°C : (□) TPAA, (■) PBA, (∆) TAA, (▲) NFA, (○) Abz, (●) FITC. The core of the shell-core beads was capped by Boc.
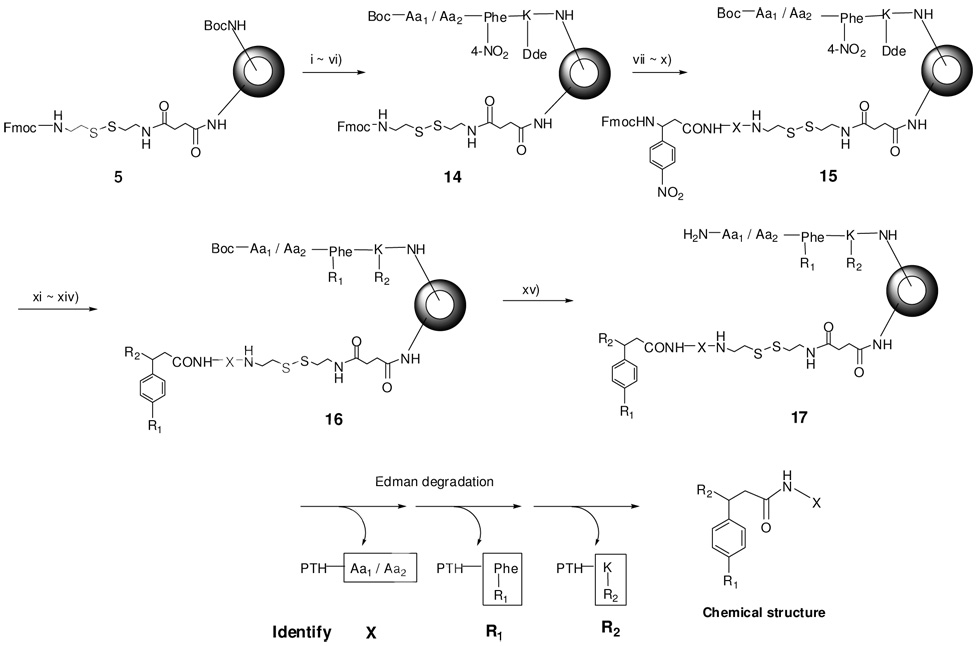
After the HTSC beads were characterized, we prepared a peptide-encoded OBOC small molecule library according to Scheme 4, using our previously reported encoding/decoding approach.7 First, a coding arm precursor, Boc-Aa1/Aa2-(4-NO2)phenylalnine-lysine(Dde) (15) was built in the interior of the bead (wherein Aa1 and Aa2 represent sequenceable amino acids). Then, a testing arm precursor, Fmoc-β-(4-NO2)phenylalanine-X (16), was built in the hydrogel layer of bead. X includes 96 amino acids which are encoded by 96 combinations of Aa1 and Aa2, respectively. β-(4-NO2)phenylalanine is a scaffold for library synthesis with three points of diversities. Aryl nitro groups at both coding and testing arms were easily converted to amino groups by reduction with SnCl2, followed by acylation at R1 position with 30 carboxylic acids, acyl chlorides or sulfonyl chlorides. Then, Fmoc at the testing arm and Dde at the coding arm were removed concurrently with 2% hydrazine in DMF, followed by acylation with 45 carboxylic acids or acids anhydrides at R2 position. For decoding, positive beads from screening will be isolated and subjected to automatic microsequencing using our modified sequencing method.29 The signals of Aa1 and Aa2 (residing in inner core of the bead) in the first cycle correspond to X of the testing compound. In the second cycle, the signal of Phe(4-NHCOR1) corresponds to the acylated aniline derivative (R1). The signal of the lysine derivative in the third cycle corresponds to the last coupling moiety (R2). By combing all the decoded structural information, the complete chemical structure of the testing molecule can be easily determined.7
Scheme 4.
Synthetic scheme of the encoded model library using hydrogel TentaGel shell-core bead. Reagents and conditions : (i) 50% TFA/DCM, 30min; (ii) Boc-Lys(Dde)-OH, HOBt, DIC, DMF; (iii) 50% TFA/DCM, 30min; (iv) Boc-Phe(4-NO2)-OH, HOBt, DIC, DMF; (v) 50% TFA/DCM, 30min ; (vi) 96 Boc-Aa1 / Boc-Aa2 HOBt, DIC, DMF; (vii) 20% piperidine / DMF ; (viii) 96 Fmoc-x-OH, HOBt, DIC, DMF; (ix) 20% piperidine / DMF; (x) Fmoc-Scaffold(NO2)-OH {Fmoc-β-D,L-Phe(NO2)-OH}, HOBt, DIC, DMF; (xi) 2M SnCl2 • 2H2O; (xii) R1COOH or R1COCl or R1SO2Cl; (xiii) 2 % hydrazine / DMF; (xiv) R2COOH or R2COCl or R2SO2Cl; (xv) TFA: phenol: water: TIS (88:5:5:2, v/v %), 30min.
The decoding process can be straightforward since the testing molecule is stable to the Edman condition, and the coding molecule consists of peptides with α-amino acids that can be readily sequenced by an automatic protein micro-sequencer. To verify the success of our encoding scheme for this library, twelve beads were randomly picked from the library and all were decoded with microsequencing without any ambiguity. Alternatively, the coding tag can be released (if a cleavable linker is used) and decoded unambiguously by mass spectrometry, if each of the coding subunit(s) has a unique molecular mass. This work has also demonstrated that the HTSC beads are stable chemically and physically, and suitable for synthesis of OBOC combinatorial library with our encoding system. Work is currently under way in our laboratory to evaluate the library of first generation hydrogel coated solid support for in situ release solution phase assay.
Conclusions
We have successfully developed a novel method to construct shell-core hydrogel TentaGel beads for in situ release solution phase assay. A poly (NDMAAm-co-NIPPAm) hydrogel layer with a thickness of about 5 to 80 µm was prepared through free radical surface-initiated polymerization on the outer layer of topologically segregated bilayer TentaGel bead. Such hydrogel-coated TentaGel beads allow efficient release of both hydrophilic and hydrophobic compounds into the surroundings of each bead under aqueous environment, after the linker between the compounds and solid support is cleaved. In addition, the hydrogel-coated beads are chemically and physically stable. All these properties render these new beads excellent solid supports for synthesis of OBOC libraries for in situ releasable screening which can be adapted to many existing biological assays currently being used for drug screen, including the multi-parametric cell-based assays.
Experimental Section
Material and General Methods
TentaGel S NH2 resin ~ 80 µm, 0.28 mmol/g) was purchased from Rapp Polymere GmbH (Tübingen, Germany). HOBt (1-hydroxybenzotriazole) and Fmoc-OSu were purchased from GL Biochem (Shanghai, China). Fmoc-OSu, (Boc)2O, HOBt, Fmoc-2-Abz and Fmoc-protected L-amino acids were purchased from Chem-Impex international, Inc. (Wood Dale, IL) and Advanced ChemTech (Louisville, KY). Boc-protected amino acids were obtained from SynPep Corporation (Dublin, CA). N-Fmoc-Lys(Fmoc)-OH, and N-Boc-Lys (Fmoc)-OH were purchased from Advanced ChemTech (Louisville, KY). DIC, DIEA, NIPAAm, NDMAAm, 4,4’-Azovis(4-cyanovaleric acid), EEDQ, Cystamine • 2HCl, FITC, and Ebe were purchased from Aldrich (Milwaukee, WI) and used after drying at 25°C in vacuum. All organic solvent, and other chemical reagents were purchased from Fisher Scientific (Huston, TX), and Aldrich. All the experiments were carried out at room temperature unless otherwise noted. For Fmoc deprotection, the resin was treated with 20% piperidine/DMF for 15 min and then washed thoroughly with DMF, MeOH, and DCM 3 time each. All infrared spectra were determined on a Genesis II Mattson FT-IR. NMR was recorded on a Bruker DRX spectrometer (Billerica, MA) in DMSO-d6 at 25°C (500 MHz for 1H NMR and 125 MHz for 13C NMR spectra). HRMS was performed with Finnigan LTQ FT. MS was performed with Finnigan LCQ. UV-Visible absorbances were obtained using Spectra Max M2 spectrometer of Molecular Devices (Sunnyvale, CA). Column chromatography was performed on silica gel using a mixture of MeOH and DCM as the eluent. Analytical HPLC analyses (Vydac column; 4.6 mm × 250 mm; 5 µm; 300 Å; C18; 1.0 mL/min; 25 min gradient from 100% aqueous H2O (0.1% TFA) to 100% CH3CN(0.1% TFA); 214, 220, 254, and 280 nm) were performed on a Beckman System Gold HPLC system (Fullerton, CA) or on Waters 2996 photodiode array detector, a Waters 2525 binary gradient module, and a Waters 2767 sample manager equipped with a 4.6 × 150 mm Waters Xterra MS C18 5.0 µm column employing a 20 min gradient from 100% aqueous H2O (0.1% TFA) to 100% CH3CN (0.1% TFA) at a flow rate of 1.0 mL/min. Scanning Electron Microscopy (SEM) studies were performed on a JEOL JSM 6400 scanning electron microscope. High-resolution fluorescence data and images were obtained using an Olympus fluorescence microscope (IX70). Fluorescence images were opened into Adobe Photoshop CS2 for processing, and reproduced as laser prints. Samples were dried on adhesive carbon disks and sputter-coated with a thin layer of gold prior to examination.
Preparation of Fmoc-cross-linker (10)
To a solution of Ebe (6) (5.0g, 33.74 mmol) in 1,4-dioxane (100 mL) cooled to 5 °C in an ice bath was added a solution of (Boc)2O (7.0 g, 32.1 mmol) in 1,4- dioxane (100 mL) dropwise over a period of 1 hr. The reaction mixture was allowed to warm to room temperature and stirred for two more hours. The solvent was removed under reduced pressure and the residue dissolved in DCM (100 mL), washed with water (100 mL × 3), dried over magnesium sulfate (MgSO4), filtered, and the solvent evaporated under reduced pressure to give Boc-Ebe (7) (5.90g, yield 70%). Fmoc-Lys (Boc)-OH (11.09g, 23.67mmol) was added with DCC (4.88g, 23.67mmol), HOBt (3.20g, 23.67mmol) and THF (100mL), to a dried 250mL one-necked round-bottomed flask immersed in a ice bath. This solution was stirred for 30min, and the Boc-Ebe (5.90g, 23.67mmol) in 50mL of THF was then added dropwise to the flask over 1h. The reaction mixture was stirred at 20°C for 5h and the solvent evaporated under reduced pressure to yield crude solid product. Purification by flash chromatography on silica gel (elution with EtOAc) gave 13.4g (85% yield) of Fmoc-Lys(Boc)-Ebe-Boc (8) as a white powder.
1H NMR (DMSO-d6, 500MHz) δ: 7.93(t, J = 5 Hz, 1H,), 7.88(m, J = 7.5 Hz, 3H,), 7.72(q, J = 3.5, 3H,), 7.43(m, 3H), 7.32(m, J = 7.5, 3H), 6.77(t, 1H), 4.27(m, J = 5 Hz, 2H), 4.22(t, J = 7.5 Hz, 1H), 3.92(q, J = 5 Hz, 1H), 3.42~3.30(m, 4H), 3.24~3.15(m, 2H), 3.05~3.04(m, 2H), 2.88~2.86(m, 2H), 1.56~1.43(m, 4H), 1.35~1.33(m, 18H), 1.23~1.10(m, 4H). 13C NMR (DMSO-d6, 75.45MHz) δ: 172.7, 157.5, 156.6, 156.2, 144.6, 144.5, 141.4, 128.3, 127.7, 126.9, 126.0, 121.7, 120.8, 78.2, 78.0, 70.2, 70.1, 69.8, 69.7, 66.3, 55.3, 47.3, 41.3, 39.3, 32.4, 29.9, 28.98, 28.92, 24.0, 23.5. ; HR ESI-FTMS (M+1) m/z Calcd for C37H54N4O9: 698.3891. Found: 699.4.
Compound 8 (13.4g, 20.04mmol) was stirred with 50% TFA / DCM for 30min in the ice bath and subsequently the temperature was allowed to rise to room temperature. The reaction was continued under stirring for 1h and the solvent was evaporated thoroughly. The resulting yellow oil (compound 9) was dissolved with TEA (13.15mL, 94.5mmol) in 150mL of CHCl3. After cooling to −10°C, a solution of acryloyl chloride (3.84mL, 47.23mmol) in 50mL of CHCl3 was then added dropwise over 1h. The reaction mixture was warmed at room temperature and stirred for 5hr. The solvent was evaporated under reduced pressure to give solid. The resulting solid was re-dissolved by the addition of CHCl3 (150mL) and added water (100mL). The organic layer was washed a second time with 60mL of deionized water and dried over MgSO4. The solution was concentrated to a small volume and diluted with Et2O to give white solid compound. The crystallized product was collected, washed with Et2O and dried in vacuum. A white solid (compound 10) was obtained with a yield of 70%.
1H NMR (DMSO-d6, 500MHz) δ: 8.19(s, 1H, NH), 8.09(s, 1H, NH), 7.95(s, 1H, NH), 7.90(d, 2H), 7.73(d, 2H), 7.47(d, 1H, NH), 7.43(d, 2H), 6.23~6.17(m, 2H), 6.09(m, 2H), 5.57(m, 2H), 4.27~4.21(b, 3H), 3.92(m, 1H), 3.49(m, 4H), 3.34(m, 4H), 3.23(m, 4H), 3.26(m, 2H), 3.18(m, 2H), 1.59~1.54(b, 2H), 1.41(m, 2H), 1.18~1.10(b, 2H). 13C NMR (DMSO-d6, 75.45MHz) δ: 172.2, 164.8, 164.6, 156.0, 143.9, 143.8, 140.8, 131.9, 131.7, 127.7, 127.1, 125.4, 125.2, 124.9, 120.1, 69.6, 69.1, 67.9, 65.7, 56.7, 54.7, 46.7, 38.7, 38.6, 31.8, 28.8, 23.1, 18.6. ; HR ESI-FTMS (M+1) m/z Calcd for C33H42N4O7: 606.3054. Found: 607.4.
Synthesis of disulfide cleavable linker, Fmoc-CytSu-OH (13)
A suspension of cystamine dihydrochloride 11 (5.0 g, 22.202 mmol, 1equiv.) in 40mL of MeOH / CHCl3 (50:50, v/v) was treated with DIEA (15.31 mL, 88.81 mmol, 4equiv.) and stirred for 30min at room temperature. A solution of succinic anhydride (2.22 g, 22.202 mmol, 1equiv.) in 10mL of CHCl3 was then added dropwise over 1h at 0°C; and the temperature was allowed to rise to room temperature. The reaction was continued under stirring for 5h. After cooling to 0°C again, a solution of Fmoc-OSu (7.43g, 22.20 mmol, 1equiv.) with DIEA (7.76mL, 44.40 mmol, 2equiv.) in 40mL of DCM was then added dropwise over 1h. The reaction mixture was warmed to room temperature and stirred for 12h. The solution was filtered and removed in vacuum to give solid product. A residue was re-dissolved by the addition of DCM (100mL) and added water (100mL). The solution mixture was then acidified with 1M HCl to pH 2 and extracted with DCM (60mL × 2). The organic layer was washed a second time with 60mL of deionized water and dried over MgSO4. The solution was concentrated to a small volume and diluted with Et2O to give white solid compound. The crystallized product was collected, washed with Et2O and dried in vacuum. A white solid (compound 13) was obtained with a yield of 47.6%.
1H NMR (DMSO-d6, 500MHz) δ: 12.08(s, 1H, OH), 8.05(d, 1H, NH), 7.88(d, 2H), 7.69(d, 2H), 7.49(d, 1H, NH), 7.42(d, 2H), 7.34(d, 2H), 4.32(d, 2H), 4.21(t, 1H), 3.29(m, 2H), 3.27(m, 2H), 2.77(m, 2H), 2.59(t, 2H), 2.50(t, 2H), 2.43(t, 2H). ). 13C NMR (DMSO-d6, 75.45MHz) δ: 173.9, 171.2, 156.1, 143.9, 140.8, 127.6, 127.1, 125.2, 120.1, 65.4, 46.7, 40.3, 38.0, 37.4, 37.2, 30.0, 29.1. HR ESI-FTMS (M +1) m/z Calcd for C23H26N2O5S2: 474.1283. Found: 475.1.
Synthesis of hydrophilic linker, Fmoc-Ebes-OH
This compound prepared from Ebe (5.86 mL, 40 mmol), succinic anhydride (4.0 g, 40 mmol) and Fmoc-OSu (13.83 g, 41 mmol) according to literature.30, 31
1H NMR (500 MHz, DMSO-d6) δ/ppm 7.91 (s, 1H), 7.89 (d, J = 7.5 Hz, 2H), 7.69 (d, J = 7.5 Hz, 2H), 7.41 (t, J = 7.5 Hz, 2H), 7.33 (m, 2H), 6.28 (s, 1H), 4.29 (d, J = 6.8 Hz, 2H), 4.21 (t, J = 6.8 Hz, 1H), 3.5–3.6 (m, 4H), 3.3–3.4 (m, 4H), 3.1–3.2 (m, 4H), 2.40 (t, J = 7.0 Hz, 2H), 2.30 (t, J = 7.0 Hz, 2H); 13C NMR (125 MHz, DMSO-d6) δ/ppm 174.6, 171.9, 156.9, 144.6, 141.5, 128.3, 128.0, 125.9, 120.8, 70.2, 69.8, 66.0, 47.4, 40.8, 39.3, 30.8, 30.1; HR ESI-FTMS (M + 1) m/z Calcd for C25H30N2O7: 470.2053. Found: 471.3.
General procedure for preparation of two layer TG beads [inner (85% Boc-protected), outer-layer (15%, Fmoc-protected)]
For preparation of topologically segregated bifunctional TG beads (2) with 15% Fmoc outside and 85% Boc inside, TG beads (1.0 g, 0.28 mmol) were swollen in water for 48 h. The water was drained, and a solution of Fmoc-OSu (14.12 mg, 42.0 µmol) in 50mL of DCM / Et2O mixture (55:45, v/v) was added to the resin, followed by addition of DIEA (14.68 µL, 84.0 µmol). The resulting mixture was shaken vigorously for 1 h. The resin was washed with DCM (5 mL × 3) and DMF (5 mL × 6). A solution of (Boc)2O (207.82 mg, 0.95 mmol) in 10 mL of DCM was then added to the beads, followed by the addition of DIEA (332.12 µL, 1.9 mmol). The mixture was shaken until the ninhydrin test was negative. The obtained outside-Fmoc-inside-Boc-bifunctional beads (2) were washed with DCM (5 mL × 3), DMF (5 mL × 3), DCM (5 mL × 3) and MeOH (5 mL × 3) each and dried in vacuum. The percentage of outer region was determined to be 15% using quantitative UV absorption analysis of the dibenzofulvene-piperidine adduct released by treatment with piperidine.
Bifunctional beads (2) (1g, 15% Fmoc-outside: ~ 42.0µmol, 85% Boc-inside: ~ 0.238 mmol) were swollen in DMF overnight, followed by Fmoc deprotection. A mixture of Fmoc-Ebes-OH (59.27mg, 126 µmol), HOBt (17.02 mg, 126 µmol), DIC (19.73 µL, 126 µmol), and 5 mL DMF was added to the resin beads. The reaction mixture was agitated until the ninhydrin test was negative. The resin was washed with DMF (5 mL × 3), MeOH (5 mL × 3), and DCM (5 mL × 3) and followed by Fmoc deprotection. A mixture of Fmoc-Lys (Fmoc)-OH (74.43 mg, 126 µmol), HOBt (17.02 mg, 126 µmol), DIC (19.73 µL, 126 µmol), and 5 mL DMF was added to the resin beads. The reaction mixture was agitated until the ninhydrin test was negative. The resin was washed with DMF (5 mL × 3), MeOH (5 mL × 3), and DCM (5 mL × 3) and followed by Fmoc deprotection. A mixture of Fmoc-Lys (Fmoc)-OH (148.86 mg, 252.0 mmol), HOBt (34.0 mg, 252.0 mmol), DIC (39.49 µL, 252.0 mmol), and 5 mL DMF was added to the resin beads. The reaction mixture was agitated until the ninhydrin test was negative. The resin was washed with DMF (5 mL × 3), MeOH (5 mL × 3), and DCM (5 mL × 3) and then followed by Fmoc deprotection. A mixture of Fmoc-Ebes-OH (237.13 mg, 504.0 mmol), HOBt (68.09 mg, 504.0 mmol), DIC (78.91 µL, 504.0 mmol), and 5 mL DMF was added to the resin beads. The reaction mixture was agitated until the ninhydrin test was negative. The resin was washed with DMF (5 mL × 3), MeOH (5 mL × 3), and DCM (5 mL × 3) and then followed by Fmoc deprotection. After the ninhydrin test was positive, the beads (3) were washed thoroughly with DMF, MeOH, and DCM three times each and then dried in vacuum.
Procedure for preparation of HTSC beads (5)
To introduce the initiator on TG bead (3), 4,4’-Azobis(4-cyanovaleric acid) (87.4 mg, 0.312 mmol) and EEDQ (154.3 mg, 0.62 mmol), an initiator and a condensing agent, respectively, were dissolved in 5ml of DMF in each vial. The solution was bubbled with N2 gas for 10min. TG beads (3) were then immersed in the solution, and the mixture was degassed again for 10min before the reaction was started. The reaction was carried out at room temperature for 6hr under an N2 gas atmosphere. Initiator-immobilized TG beads (4) were washed with DMF and EtOH, consecutively, and dried in vacuum overnight.
Hydrogelation of the beads 4 was carried out by the following method to avoid incomplete deoxygenation of beads: NIPAAm (300mg 2.65mmol) and NDMAAm (300µL, 2.47mmol), and Fmoc-cross-linker (300mg) including Fmoc-group were dissolved in 2ml methanol in a glass ampule. Beads 4 (300 mg) were added to the monomer solution. The reaction mixture was then bubbled with N2 gas for 1hr, and polymerization was carried out at 80°C for 30 to 240 min under an N2 gas atmosphere. Hydrogel-coated TG beads were filtered and washed three times with methanol and DMF respectively and then followed by Fmoc deprotection. Finally to introduce the linker containing disulfide bond, A mixture of Fmoc-CytSu-OH (142.2mg, 0.3 mmol), HOBt (40.5 mg, 0.3mmol), DIC (47.0µL, 0.3mmol), and 5 mL DMF was added to the resin beads. The reaction mixture was agitated until the ninhydrin test was negative. The beads (5) were washed with DMF (5 mL × 3), MeOH (5 mL × 3), and DCM (5 mL × 3) and dried in vacuum overnight.
Synthesis of FITC or Abz-labeled HTSC beads and release test
HTSC Beads (5) (1g, Fmoc capped hydrogel layer-outer layer, ~ 0.1mmol/g; Boc capped core, 0.238mmol/g, ~130µm) were swollen in DMF for 5h, followed by Fmoc deprotection. Half of the beads were treated with fluorescein-5-isothiocyanate (3 equiv) in the presence of DIEA (6 equiv) and agitated for 5 h. The other half of the beads was coupled with Fmoc-Abz (3equiv), using HOBt (3equiv), and DIC (3equiv). The reaction mixture was shaken until the ninhydrin test was negative. The resin was washed with DMF (5 mL × 3), MeOH (5 mL × 3), and DCM (5 mL × 3) and followed by Fmoc deprotection. After washing with DMF and MeOH 5 times each, the beads were dried in vacuum overnight.
Also to make two layer TG bead (20 % FITC or Abz-outside, 80 % Boc-inside), TG beads (1.0 g, 0.28 mmol) were swollen in water for 48 h. The water was drained, and a solution of Fmoc-OSu (9.44 mg, 28µmol) in a DCM / Et2O mixture (50 mL, v/v = 55:45) was added to the resin, followed by addition of DIEA (9.79µL, 56µmol l). The resulting mixture was shaken vigorously for 1 h. The resin was washed 6 times with DMF and 3 times with DCM. A solution of (Boc)2O (165.1mg, 0.76 mmol) in 10 mL of DCM was added to the bead, followed by the addition of DIEA (264.3µL, 1.51 mmol). The mixture was shaken until the ninhydrin test was negative. The obtained outside-Fmoc-inside-Boc-bifunctional resin was washed with DCM, DMF, DCM and MeOH 3 times each, followed by Fmoc deprotection. Fmoc-CytSu-OH, FITC or Abz were coupled in the manner described above.
The each batch of beads were mixed and immobilized by preparing a solution consisting of 1% low EEO agarose in 1mL of PBS buffer, separately. The bead-agarose solutions were cooled to room temperature in the Petri dish. After the bead agarose solution had hardened, 0.05% DTT was added to cleave the disulfide bond. The beads were visualized under a fluorescent microscope according to time dependence.
Synthesis of releasing compound-labeled HTSC and TG beads and release studies
HTSC Beads (5) (1.2g, Fmoc capped hydrogel layer-outer layer, ~ 0.1mmol/g; Boc capped core, 0.238mmol/g, ~130µm) were swollen in DMF for 5h, followed by Fmoc deprotection. The beads were divided into 6 portions, and 5 portion of beads were coupled with 5different carboxylic acids (TPAA, PBA, NFA, TAA, and Abz, 3eqs. each) using HOBt (3equiv), and DIC (3equiv). One portion beads were treated with FITC (3 equiv) in the presence of DIEA (6 equiv). The reaction mixture were shaken until the ninhydrin test was negative. The resin was washed with DMF (5 mL × 3), MeOH (5 mL × 3), and DCM (5 mL × 3) and followed by Fmoc deprotection. After washing with DMF and MeOH 5 times each, the beads were dried in vacuum overnight.
Also to make two layer TG bead (50 % Fmoc-outside, 50 % Boc-inside), TG beads (1.2 g, 0.336 mmol) were performed in general procedure described above. The obtained outside-Fmoc-inside-Boc-bifunctional resin was washed with DCM, DMF, DCM and MeOH 3 times each, followed by Fmoc deprotection. 5Carboxylic acids, and FITC were coupled in the manner described above.
Quantification of model compounds released from beads was conducted in PBS containing 0.2% DMSO at room temperature as follows: Beads (10 mg, each) were separately placed into the eppendorf tube. To start the release experiment, DTT was added to a final concentration of 0.05%. The tubes were mechanically shaken at a room temperature. At 0.5, 1, 2, 4, 12 and 24 hr, 100µL solution were taken from the each release medium for measurements of compounds released by HPLC using calibration curves for different concentration.
Slicing of FITC-labeled HTSC beads
The HTSC bead taken from polymerization reaction step according to time were followed by Fmoc deprotection and treated with FITC (3 equiv) in the presence of DIEA (6 equiv) for 5 h. After washing with DMF and MeOH 5 times each, one bead was retrieved and placed in a Petri dish. The bead was sliced with a scalpel under a dissecting microscope and then visualized under a fluorescent microscope.
Synthesis of a peptide-encoded small molecule library using HTSC bead
The library synthesis was carried out according to literature procedures.7
Fmoc/ Boc orthogonal protecting strategy was employed in the synthesis of the coding and testing arms on the HTSC beads 5 (3.0g, Fmoc capped hydrogel layer-outer layer, ~ 0.1mmol/g; Boc capped core, 0.25 mmol/g, ~130µm). The coding-precursor strand in the interior of the bead was first constructed: A solution of Boc-Lys (Dde) (930mg, 2.27 mmol) and HOBt (306mg, 2.27 mmol) in DMF (8mL) was added to the beads followed by addition of DIC (355.2µL, 2.27 mmol). The coupling reaction was carried out at room temperature for 2h. The beads were then washed with DMF (10mL × 3), MeOH (10mL × 3) and DCM (10mL × 3), respectively. The Boc group was removed with 50% TFA in DCM for 30 min, followed by neutralization with 2% DIEA / DMF twice. A mixture of Boc-Phe (4-NO2) (705mg, 2.27 mmol) and HOBt (306mg, 2.27 mmol) in DMF (8mL) was added to the beads followed by addition of DIC (355.2µL, 2.27 mmol). The coupling reaction was conducted at room temperature for 2h. The Boc group was deprotected in the same manner. The beads were split into 96 equal portions in a 96-well plate, and 96 different coding molecules (single Boc-amino acids or combination of two Boc-amino acids, one in each well, 3eqs.) together with DIC/HOBt were added. The Boc-amino acids condensation proceeded at room temperature for 2h.
The Fmoc group was removed in 96-well plate with 20 % piperidine in DMF. 96 Fmoc-amino acids (9.3µmol, each) were dissolved in equal volumes of HOBt solution (total 121mg HOBt, 0.89 mmol) in DMF (28.8 mL) and was added to the corresponding well (i.e. one amino acid in each well), followed by the addition of DIC (1.46µL, 9.3µmol). The coupling reaction was carried out at room temperature for 2h. After the coupling was completed, all beads in 96 wells were combined, mixed and the Fmoc deprotected. A solution of Fmoc-β-D, L-Phe (3-NO2) (130mg, 0.3mmol) and HOBt (41mg, 0.3mmol) in DMF (8mL) were added to the bead, followed by addition of DIC (47µL, 0.3mmol). The coupling was performed at room temperature for 3h. The beads were washed thoroughly with DMF, MeOH, and DMF. Aryl nitro groups in both the coding and testing arms were reduced by 2M SnCl2 • 2H2O in DMF at room temperature for 2h, twice. The bead was split into 30 equal portions. Acylation of amino group in aniline was achieved by using 25 carboxylic acids, 2 acyl chlorides, 3 sulfonyl chlorides. After randomization of all 30 portions of beads, Fmoc in the testing arm and Dde in the coding arm were removed in a single step by 2% hydrazine/DMF. The beads were divided into 45 portions, and 45 different carboxylic acids (20eqs. each) were pre-activated by BOP in DMF/DIEA and added to the corresponding wells. This final acylation was carried out at room temperature for 2h and repeated once. After combination of all 45 portions of bead, the beads were washed with DMF (10mL × 3), methanol (10mL × 3) and diethyl ether (10mL × 3). The beads were then dried under vacuum for 1h. Side chain deprotection was achieved with a solution of TFA: phenol: water: Tis (88:5:5:2, v/w/v/v) for 30min. After neutralization with 10% DIEA/DMF twice, the resin was washed sequentially with DMF (10mL × 3), methanol (10mL × 3), DCM (10mL × 3), DMF (10mL × 3), DMF/water (10mL × 3), water (10mL × 3), and finally PBS (10 mL × 3).
Ninety-six Fmoc amino acids used as the first building blocks for the testing molecules are shown in Table 1. Thirty carboxylic acids, acyl chlorides, and sulfonyl chlorides for acylation or sulfonylation of aniline are shown in Table 2. The structure of forty-five carboxylic acids or anhydrides for acylation of ε-NH2 of lysine are shown in Table 3. Table 1, 2, and 3 are shown in supporting material section.
Supplementary Material
1H NMR, 13C NMR and MS spectra for compounds 8, 10, 13, and Fmoc-Ebes-OH. Building blocks for synthesis of a model OBOC library on HTSC beads. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
The authors wish to thank Dr. Jong Soo Lee and Bei Xang for assistance with the scanning electron microscopy. This work was supported by the National Institutes of Health (R33CA-86364, R33CA-99136, R01CA098116, and U19CA113298) and the National Science Foundation (CHE-0302122). The 500 MHz NMR spectrometer was purchased in part with the grant NSF 9724412.
Abbreviations
- EtOAc
ethyl acetate
- MeOH
methanol
- EtOH
ethanol
- Et2O
diethyl ether
- DCM
dichloromethane
- CHCl3
chloroform
- THF
tetrahydrofuran
- NaOAc
sodium acetate
- Fmoc-OSu
N-(9-fluorenylmethoxycarbonyloxy) succinimide
- Boc
tert-butyloxycarbonyl
- (Boc)2O
Boc anhydride or di-tert-butyl-dicarbonate
- Fmoc-Abz
N-(9-fluorenylmethoxycarbonyloxy)-2-aminobenzoic acid
- FITC
fluorescein-5-isothiocyanate
- TPAA
trans-3-(3-pyridyl)acrylic acid
- PBA
1-pyrenebutyric acid
- NFA
5-(3-nitrophenyl)-2-furoic acid
- TAA
3-(2-thienyl)acrylic acid
- HPLC
high performance liquid chromatography
- DMF
N,N-dimethylformamide
- HOBt
N-hydroxybenzotriazole
- DIC
diisopropylcarbodiimide
- DCC
dicyclohexylcarbodiimide
- NIPAAm
N-isopropylacryl-amide
- NDMAAm
N,N-dimethylacrylamide
- EEDQ
1-ethoxycarbonyl-2-ethoxy-1,2-dihydro-quinoline
- Ebe
2,2'-(ethylenedioxy)-bis(ethylamine)
- BOP
(benzotriazol-1-yl-oxy) tris-(dimethylamino)phos-phonium hexafluorophosphate
- TIS
triisopropylsilane
- TFA
trifluoro-acetic acid
- TEA
triethylamine
- DIEA
diisopropylethylamine
- PTH
phenylthiohydantoin
- PBS
phosphate-buffered saline
- Dde
1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl
- Fmoc-Lys(Boc)-Ebe-Boc
(5-tert-Butoxy-carbonylamino-1-{2-[2-(2-tert-butoxycarbonylaminoethoxy)-eth-oxy]-ethylcarbamoyl}-pentyl)-carbamic acid 9H-fluor-en-9-ylmethyl ester
- Fmoc-CytSu-OH
(N-Fmoc-2,2’2,2’-dithio-bisethanamine)-mono-succinamide
- Fmoc-Ebes-OH
N-Fmoc-2,2’-(ethylenedioxy)bis(ethyl-amine) mono-succinamide
References
- 1.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 2.Houghten RA, Pinilla C, Blondelle SE, Appel JR, Dooley CT, Cuervo JH. Nature. 1991;354:84–86. doi: 10.1038/354084a0. [DOI] [PubMed] [Google Scholar]
- 3.Furka A, Sebestyén F, Asgedom M, Dibó G. Int. J. Pept. Protein Res. 1991;37:487–493. doi: 10.1111/j.1399-3011.1991.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 4.Lam KS, Krchnak V, Lebl M. Chem. Rev. 1997;97:411–448. doi: 10.1021/cr9600114. [DOI] [PubMed] [Google Scholar]
- 5.Lam KS. Anticancer Drug Des. 1997;12:145–167. [PubMed] [Google Scholar]
- 6.Lebl M, Lam KS, Salmon SE, Krchnak V, Sepetov P, Kocis K. U.S. Patent. 1998:5840485. [Google Scholar]
- 7.Liu R, Marik J, Lam KS. J. Am. Chem. Soc. 2002;124:7678–7680. doi: 10.1021/ja026421t. [DOI] [PubMed] [Google Scholar]
- 8.Liu R, Wang X, Song A, Bao T, Lam KS. QSAR Comb. Sci. 2005;24(No10):1127–1140. [Google Scholar]
- 9.Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Nat. Chem. Biol. 2006;2(7):381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- 10.Lebl M, Patek M, Kocis P, Krchnak V, Hruby VJ, Salmon SE, Lam KS. Int. J. Pept. Protein Res. 1993;41:201–203. doi: 10.1111/j.1399-3011.1993.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 11.Salmon SE, Liu-Stevens RH, Zhao Y, Lebl M, Krchnak V, Wertman K, Sepetov N, Lam KS. Mol. Diversity. 1996;2:57–63. doi: 10.1007/BF01718701. [DOI] [PubMed] [Google Scholar]
- 12.Evans B, Pipe A, Clark L, Banks M. Bioorg. Med. Chem. Lett. 2001;11:1297–1300. doi: 10.1016/s0960-894x(01)00201-3. [DOI] [PubMed] [Google Scholar]
- 13.Hiemstra HS, Benckhuijsen WE, Amons R, Rapp W, Drijfhout JW. J. Pept. Sci. 1998;4:282–288. doi: 10.1002/(sici)1099-1387(199806)4:4<282::aid-psc145>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Jayawickreme CK, Sauls H, Bolio N, Ruan J, Moyer M, Burkhart W, Marron B, Rimele T, Shaffer JJ. Pharmacol. Toxicol. Methods. 1999;42:189–197. doi: 10.1016/s1056-8719(00)00083-6. [DOI] [PubMed] [Google Scholar]
- 15.Silen JL, Lu AT, Solas DW, Gore MA, MacLean D, Shah NH, Coffin JM, Bhinderwala NS, Wang Y, Tsutsui KT, Look GC, Campbell DA, Hale RL, Navre M, DeLuca-Flaherty CR. Antimicrob. Agents Chemother. 1998;42:1447–1453. doi: 10.1128/aac.42.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Cho JK, Chung W, Lee Y. Org. Lett. 2004;6:3273–3276. doi: 10.1021/ol048815q. [DOI] [PubMed] [Google Scholar]
- 17.Lee T, Ryoo S, Byun J, Lee S, Lee Y. J. Comb. Chem. 2005;7:170–173. doi: 10.1021/cc0498635. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Cho JK, Aimoto S, Lee Y. Org. Lett. 2006;8:1149–1151. doi: 10.1021/ol0530629. [DOI] [PubMed] [Google Scholar]
- 19.Advincula R. J. Dispersion. Sci. Technol. 2003;24:343–361. [Google Scholar]
- 20.Fan X, Xia C, Advincula R. In: Dekker Encyclopedia of Nanoscience and Nanotechnology. Schwarz J, Contescu C, Putyera K, editors. New York: Marcel Dekker; 2004. p. 2959. [Google Scholar]
- 21.Fan X, Xia C, Advincula RC. Langmuir. 2005;21:2537–2544. doi: 10.1021/la048126n. [DOI] [PubMed] [Google Scholar]
- 22.Kanazawa H, Sunamoto T, Ayano E, Matsushima Y, Kikuchi A, Okano T. Anal. Sci. 2002;18:45–48. doi: 10.2116/analsci.18.45. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Peng L, Liu R, Lam KS. J. Peptide Res. 2005;65:130–138. doi: 10.1111/j.1399-3011.2005.00192.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsarevsky NV, Matyjaszewski K. Macromolecules. 2002;35:9009–9014. [Google Scholar]
- 25.Aliyar HA, Hamilton PD, Ravi N. Biomacromolecules. 2005;6:204–211. doi: 10.1021/bm049574c. [DOI] [PubMed] [Google Scholar]
- 26.Plunkett KN, Berkowski KL, Moore JS. Biomacromolecules. 2005;6:632–637. doi: 10.1021/bm049349v. [DOI] [PubMed] [Google Scholar]
- 27.Song A, Wang X, Zhang J, Marik J, Carlito B, Lebrilla, Lam KS. Bioorg. Med Chem. Lett. 2004;14:161–165. doi: 10.1016/j.bmcl.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 28.Atherton E, Sheppard RC. Solid Phase peptide Synthesis A practical Approach. Oxford: IRL press; 1989. [Google Scholar]
- 29.Liu R, Lam KS. Anal. Biochem. 2001;295:9–16. doi: 10.1006/abio.2001.5172. [DOI] [PubMed] [Google Scholar]
- 30.Zhao ZG, Im JS, Lam KS, Lake DF. Bioconjugate Chem. 1999;10:424–430. doi: 10.1021/bc980120k. [DOI] [PubMed] [Google Scholar]
- 31.Song A, Jhang J, Lebrilla CB, Lam KS. J. Am. Chem. Soc. 2003;125:6180–6188. doi: 10.1021/ja034539j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1H NMR, 13C NMR and MS spectra for compounds 8, 10, 13, and Fmoc-Ebes-OH. Building blocks for synthesis of a model OBOC library on HTSC beads. This material is available free of charge via the Internet at http://pubs.acs.org.