Abstract
Objectives/Hypothesis
The majority of congenital airway anomalies arise from deficits in the respiratory tract cartilage, emphasizing the importance of this cartilage to the form and function of the upper airway. The primary objective of this study was to characterize molecular mechanisms that regulate rate and direction of chondrocyte growth in the larynx and trachea. Our specific hypothesis for this study was that fibroblast growth factor 18 (FGF18) provides proliferative and directional cues to the developing laryngeal and tracheal cartilage in the mouse by up-regulating the cartilage specifying gene, Sox9.
Study Design
Molecular genetic and histological analyses of gene expression and cartilage growth in a mouse model.
Methods
Controlled mating of wild-type FVB/N (Friend Virus B-type/NIH mouse) mice and fibroblast growth factor 18 (FGF18) over-expressing mice were carried out, and embryos ranging from embryonic (E) day 10.5 to E18.5 were obtained. The respiratory tract, including the larynx, trachea, and lung, was removed through meticulous dissection, and subjected to whole-mount in situ hybridization with RNA probes, or was sectioned and subjected to immunohistochemistry. Respiratory tracts from FVB/N mice were grown in culture in the presence of exogenous FGF18 or known inhibitors of the FGF pathway, and then subjected to quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) to measure the expression of cartilage-specific genes.
Results
The upper respiratory tract begins as a simple out-pouching from the ventral foregut endoderm at E10.5. The chondrocytes that form the cartilaginous structures of the upper respiratory tract are located at the junction of the respiratory tract out-pouching and the ventral foregut endoderm. This population of chondrocytes then undergoes directional proliferation to eventually assume the mature 3-dimensional configuration of the upper respiratory tract cartilaginous framework. Immunohistochemical localization of extracellular signal-regulated kinases (ERKs), a known modulator of FGF signaling, demonstrated the presence of this enzyme at the periphery of growing cartilage. Explants of larynx-trachea-lung grown in culture with exogenous FGF18, demonstrated hyperplastic growth and directed growth towards the FGF18 source. Finally, both FGF18 over-expressing tracheas and tracheas cultured with exogenous FGF18 demonstrated increased expression of the cartilage- specifying gene, Sox9.
Conclusions
FGF18 provided both directional and proliferative cues to chondrocytes in the developing upper respiratory tract. FGF18 exerted this effect on developing chondrocytes by up-regulating Sox9 expression.
Keywords: Larynx, trachea, cartilage, development, respiratory tract, airway, fibroblast growth factor
II. INTRODUCTION
The cartilaginous support structure represents a key sub-element of the larynx and trachea, providing the integrity to keep the laryngeal tracheal lumen patent and provide attachments for associated muscles. The physiological importance of the cartilaginous support structure is emphasized by the fact that most clinically relevant congenital anomalies of the upper airway involve defects in the cartilaginous subcomponent1. Congenital malformations of the thyroid, cricoid and tracheal cartilage can lead to profound respiratory distress, dysphonia, dysphagia, and even death in the newborn period 1,2 .
The current understanding of congenital laryngeal and tracheal malformations is fairly rudimentary. The anatomy and embryology of the normal upper respiratory tract, including cartilaginous support structures, has been described in detail3. The gross anatomical configuration of various congenital malformations of the upper respiratory tract has also been described1. Unknown, however, are the molecular mechanisms that give rise to abnormal development of the upper respiratory tract. This lack of knowledge has hindered the ability to develop more efficacious diagnostic and treatment modalities for congenital airway anomalies. To understand the molecular mechanisms of congenital airway anomalies, it is reasonable to start by characterizing the genetic pathways of normal upper respiratory tract development. Subsequently hypotheses can be developed regarding the mechanisms that give rise to congenital malformations. Given that cartilage anomalies are commonly the cause of congenital airway anomalies, it seems appropriate to first understand the genes that pattern the normal development of upper respiratory tract cartilage.
Recent studies have identified a gene, Sox9, essential for the commitment of cells to the chondrocyte lineage expressed during development of the upper respiratory tract cartilage4. Sox9 is a member of the Sox (Sry-type HMG box) subfamily of proteins containing a DNA-binding high-mobility-group (HMG) domain. The Sox9 gene, located on human chromosome 17 and mouse chromosome 11, is expressed prominently in chondrogenic precursor cells 5,6. Sox9 binds and strongly activates the 48-bp chondrocyte-specific collagen II (Col2A1) enhancer element 7. The identification of a heterozygous mutation in the Sox9 gene in a rare human genetic disease called campomelic syndrome (or campomelic dysostosis; MIM *114290) first suggested a role for this gene in chondrocyte differentiation 8-11. To test this hypothesis, Bi et al constructed a heterozygous (+/-) Sox9 mutant mouse 12. The Sox9+/- mice died perinatally, showing evidence of hypoplasia and bending of many skeletal and cartilaginous structures. Sox9 expression in the upper respiratory tract is thought to recruit cells to the chondrocyte lineage, and initiate the expression of cartilage specific genes such as Col2A14. Mechanisms that regulate Sox9 expression are therefore essential to the normal development of upper respiratory tract cartilage.
In addition to Sox9, fibroblast growth factors (FGFs) have been shown to play a role in cartilage development 13-17. The FGF family consists of 22 members that can be grouped into subfamilies based on greater sequence similarity and ability to bind and activate 1 of 4 high-affinity FGF receptor (FGFR) tyrosine kinases18-20. FGFR1, FGFR2 and FGFR4, are expressed in chondrocyte primordia, and FGFR3 is expressed in the resting and proliferating zones of the growth plate in long bones 21,22. Binding of FGF to a FGFR activates mitogen activated protein kinase (MAP kinase), the classical form of which is extracellular signal related kinase (ERK)23. Genetic linkage analysis has implicated three members of the FGFR family (FGFR1 to 3) as the underlying cause of several skeletal dysplasias and autosomal dominant craniosynostosis syndromes 24.
Recently, FGF18 has gained attention as potentially playing a key role in upper airway chondrogenesis25. Mice homozygous for a targeted disruption of FGF18 26 or that conditionally over-express FGF1825 had an abnormal cartilage phenotype. In fact mice that over-expressed FGF18 displayed abnormally shaped, hypertrophic tracheal cartilage rings25. To further validate FGF’s action on chondrocytes, Murakami et al recently demonstrated that FGF could up-regulate Sox9 expression via a mitogen-activated protein (MAP) kinase pathway27. These studies suggest that FGF18, like Sox9, plays a role in tracheal cartilage development. Furthermore, these data suggest that FGF18 works upstream of Sox9, and directly regulates the expression of Sox9.
The objective of this study is to characterize molecular mechanisms that regulate rate and direction of chondrocyte growth in the larynx and trachea. Based on the above previously published data, we hypothesize that FGF18 regulates the expression of Sox9 in the developing upper respiratory tract, therefore regulating the rate and direction of cartilage growth. Cells expressing Sox9 are recruited to the chondrocyte lineage, forming the cartilaginous structures of the upper respiratory tract. The rationale for the proposed research was that, once the sequence of molecular events involved in cartilage development are characterized, then testable biological hypotheses and animal models can be developed to examine the pathophysiology of congenital upper respiratory tract anomalies. Such studies will in turn lead to the development of more efficacious diagnostic and treatment modalities.
To test our hypothesis, the following specific aims and sub-hypothesis will be tested: 1. Define the temporal and spatial development of upper respiratory tract cartilage. We will test the hypothesis that the initial chondrocytes in the developing tracheal cartilage must undergo directional proliferation to form the intricate cartilaginous framework of the upper respiratory tract. 2. Characterize the effect of FGF18 on cartilage growth. We will test the hypothesis that FGF18 provides directional and proliferative cues to developing chondrocytes. 3. Define the effect of FGF18 on the expression of the cartilage specifying gene, Sox9. We will test the hypothesis that FGF18 provides directional and proliferative cues to developing chondrocytes by up-regulating Sox9 expression.
III. METHODS
Please note that the animal protocol used in this study was approved by the Animal Care Committee at our institution (protocol 6C10076)
A. Whole-mount in situ hybridization of embryonic larynx-trachea-lung with probes to collagen 2A1 (Col2A1) demonstrates directional proliferation of chondrocytes in the larynx and trachea to form the mature 3-dimensional cartilaginous framework of the upper respiratory tract
Controlled mating of wild-type FVB/N (Friend Virus B-type/NIH mouse)28 mice was carried out. Timing of conception was determined by daily vaginal plug checks. Mouse gestation is 19 days in duration, with days 0 to 16 designated as embryonic (E) and days 17 to 19 designated as fetal (E). Identification of a vaginal plug was designated as E0.5 day of gestation. Pregnant dams were sacrificed at gestational ages between E10.5 to E13.5, and embryos were harvested from the pregnant dam through a hysterotomy. Whole-mount tissue was prepared by dissecting the upper airway (larynx and trachea) and lung from the embryo. The efficacy of dissecting the embryonic larynx, trachea, and lung has been previously demonstrated in our laboratory 4 and by others25,29,30. In short, the dissection is performed by placing the embryo in a watch glass (Apple Scientific Inc, Chesterland, OH) filled with normal saline. Dissection is performed under a dissecting microscope (Leica Microsystems, Bannockburn, Il) using a magnification of 2.5x or 5x and a pair of dissecting knifes (Fine Scientific Tools, Foster City, CA). The thorax is entered via a vertical incision through the mandible, chest and abdomen. In E10.5 embryos, the ventral foregut endoderm and respiratory tract out-pouching is identified. The upper respiratory tract (larynx and trachea), heart, and lungs are then removed en bloc, by making a transverse cut between the ventral foregut endoderm and the developing respiratory tract. The heart is then teased away from the ventral surface of the trachea and lung, and discarded. In E11.5 to F18.5 embryos, the anatomy of the upper aerodigestive tract is more similar to that of the post-natal animal. At this stage in development, the esophagus and trachea are almost completely separate structures and are separated with a longitudinal cut between the posterior aspect of the larynx and proximal esophagus. The heart is easily teased away from the ventral surface of the trachea and tongue. The tongue which is removed along with the respiratory tract, as it helps define the superior aspect of the larynx, is finally removed with a transverse cut between the base of tongue and the superior aspect of the larynx.
Harvested larynx-trachea-lung tissue is then fixed in 4% paraformaldehyde, followed by dehydrating the tissue in methanol. Whole-mount tissue was subjected to in situ hybridization with the digoxegenin labeled RNA probes as described previously 4,31. Digoxegenin labeled RNA probes were generated using cDNA prepared from E11.5 mouse embryos. Polymerase chain reaction (PCR) primers to mouse Col 2A1 were designed using MacVector 7.0 software for MacIntosh, and nucleotide sequence data downloaded from the National Institutes of Health genetic sequence database (GenBank). PCR primers were chosen to amplify a unique fragment of Col2A1 about 884 base pairs (bp) in length4. The PCR-generated Col2A1 fragment was cloned into PCR II vector (Invitrogen, Carlsbad, CA) using the manufacturer’s protocols. Both strands of the cloned insert were sequenced to confirm the identity and fidelity of the cloned insert. After cloning Col2A1, RNA probes were generated by in vitro transcription (Promega Riboprobe system, Madison, WI) using a nucleotide mix containing a set ratio of digoxegenin-11-UTP (Roche Molecular Biochemicals, Indianapolis, IN). Both strands of the cloned insert were transcribed separately and quantitated using spectrophotometry. Antisense RNA probes were used to detect expression of Col2A1, whereas sense probes were used as a negative control. Alkaline phosphatase tagged anti-digoxegenin Fab (fragment, antigen binding; Roche Diagnostics, Germany) was used to detect the hybridized digoxegenin labeled RNA probe. Experiments were performed in triplicate to insure reproducibility. Results were photo-documented using a dissecting microscope equipped with a digital camera (Leica Microsystems, Bannockburn, Illinois).
B. Extracellular signal-regulated kinase (ERK), a mediator of the FGF pathway, is present in cells surrounding the trachea, allowing a mechanism for the action of FGF in tracheal cartilage development
Controlled mating of wild-type FVB/N mice was carried out. E12.5 embryos were collected, fixed in 4% paraformaldehyde, and imbedded in Tissue-Tek OCT (Optimal Cutting Temperature Compound; Ted Pella, CA). Twelve micron frozen sections through the embryo at the level of the trachea were cut using a cryostat and subjected to hematoxylin-eosin (H&E) staining and immunohistochemistry using antibodies to ERK (1:250 rabbit polyclonal antibody; Cell Signaling Technology, Danvers, MA) using the manufacturer’s protocol. Biotinylated goat anti-rabbit antibody (1:200) was used as the secondary antibody, and bound antibody-antigen complexes were detected using the Vectastain ABC kit (Vector laboratories, Burlingame, CA). Results were photo-documented using a compound microscope equipped with a digital camera (Leica Microsystems, Bannockburn, Il).
C. Blocking MAP kinase activity in larynx-trachea explant cultures inhibits the expression of the cartilage- specifying gene, Sox9
Controlled mating of wild-type FVB/N mice was carried out as previously described. Ell.5 embryos were collected and the developing larynx-trachea was removed by meticulous dissection. The trachea was separated from the lung at the level of the carina. Larynx-trachea explant cultures were established by placing the dissected tissue on 8.0 uM Whatman Nucleopore membrane (Whatman International, Clifton, NJ) floating on DMEM/F12 media (Invitrogen, Carlsbad, CA) with 5% Fetal Bovine Serum (Sigma, St. Louis, MO) in a 3 well spot dish. One of the following two inhibitors of FGF pathway was added at the initiation of each culture: SU5402 (10 uM), or U0126 (20 uM). All inhibitors (EMD Biosciences, La Jolla, CA) were dissolved in dimethylsulfoxide (DMSO, Sigma Chemicals, St. Louis, MO) resulting in a final concentration of 0.1% DMSO in the final culture medium. As a control larynx-trachea explants were cultured in media containing 0.1% DMSO only. Explants were cultured at 37°C for 48 hours in a humidified 5% C02-95% air incubator. The explant tissue was then removed from culture, and used to isolate RNA (Qiagen-RNeasy Micro Kit, Quiagen Inc, Valencia, CA). RNA isolated from the cultured larynx-trachea was reverse-transcribed (Invitrogen Superscript III First Strand cDNA Synthesis for RT-PCR; Invitrogen Corp, Carlsbad, CA), and Sox9 expression levels were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) using Taqman gene expression assay and the 7300 Realtime PCR System (Applied Biosystems, Foster City, CA) 32. Data from each experiment were normalized to the house keeping gene, ribosomal 18s. All above experiments and controls were performed in triplicate.
To determine if the effect of the FGF pathway inhibitors was secondary to an indirect toxic effect, larynx-trachea explants were cultured with SU5402 for 24 hours, the medium was exchanged for fresh media without SU5402 and cultured for another 24 hours. As controls, larynx-trachea explants were cultured in 0.1% DMSO or SU5402 for 48 hours. Sox9 expression was measured as above using qRT-PCR. All above experiments and controls were performed in triplicate. Quantitative real-time PCR p values from experimental and control tissue were analyzed by using the Student t-test assuming unequal variance to determine the statistical significance.
Larynx-tracheal-lung explants could also be grown in the same culture medium and under the same conditions enrobed in growth-factor reduced Matrigel (BD Biochemical, Franklin Lakes, NJ) as opposed to filter paper, allowing a more 3-dimensional growth pattern. In addition, larynx-trachea-lung explant cultures were performed with tissue harvested from Ell.5 transgenic mice expressing glial fibrillary protein under the control of the Col2A1 promoter 33 allowing cartilage development to be monitored in real-time.
D. Whole-mount in situ hybridization of larynx-trachea-lung from transgenic mice over-expressing FGF18 demonstrates increased expression of the cartilage-specifying gene, Sox9
As previously described 25, a double transgenic mouse containing the (teto) 7 CMV-FGF18 and SP-C-rtTA transgenes were generated. The reverse tetracycline transactivator (rtTA) developed by Gossen and Bujard34 was used to specifically activate and over-express (teto)7CMV-FGF18 transgene. The rtTA is a fusion protein containing a DNA binding element from Escherichia coli that binds doxycycline, and a protein from herpesvirus (VP16). The rtTA activates a minimal promoter from the cytomegalovirus (CMV) when bound to a defined upstream enhancer element consisting of a concatmer of seven (teto) binding sites34. Binding of doxycycline activates the rtTA, leading to binding of the rtTA to the target (teto)7 CMV sequence. Binding of rtTA to the (teto)7 element recruits VP16, activating the CMV promoter and downstream gene transcription. The surfactant protein-C reverse tetracycline transactivator (Sp-C-rtTA) transgenic mouse line has been extensively characterized25,35,36. The Sp-C-rtTA construct is highly and specifically expressed in the endodermal cells of the developing upper respiratory tract as early as day E937,25,37.
A double transgenic mouse containing the (teto)7 CMV-FGF18 and SP-C-rtTA transgenes were generated using a mating scheme previously described25. Transmission of both transgenes follows typical Mendelian inheritance patterns 25. Pregnant dams were administered doxycycline in food pellets (25 mg/g; Harlen Teklar, Madison, Wisconsin) at the time of conception. Pregnant Dams were sacrificed on day F18.5 of gestation, and the collected embryos were genotyped by PCR analysis of tail clippings. Embryos homozygous or heterozygous at the (teto)7CMV-FGF18 transgene locus, and heterozygous at the SP-C-rtTA transgene locus were used in this experiment. The larynx-trachea-lung of these animals was removed by meticulous dissection and subjected to whole-mount in situ hybridization using a 745 bp digoxegenin labeled RNA probe to Sox9 according to the methodology described in Section IIIA4. Alcian blue staining on larynx-trachea-lung tissue was performed following the manufacturer’s protocols (Poly Scientific R&D, Bay Shore, NY).
E. Quantitative RT-PCR demonstrates increased expression of Sox9 in the larynx and trachea of FGF18 over-expressing mice
As described in subsection IIID, a double transgenic mouse containing the (teto)7 CMVFGF18 and SP-C-rtTA transgenes were generated. Pregnant dams were sacrificed on day E11.5 through E13.5 of gestation, and the collected embryos were genotyped by PCR analysis of tail clippings. Only embryos homozygous or heterozygous at the (teto)7 CMV-FGF18 transgene locus and heterozygous at the SP-C-rtTA transgene locus were used in this experiment. The larynx and trachea from these embryos were collected by meticulous dissection and S0x9 expression was analyzed by qRT-PCR (See section IIIC). The larynx and trachea were separated from the lung at the level of the carina. As a control, Sox9 expression in E11.5 through E13.5 larynx-trachea from wild-type FVB/N mice was measured. All experiments and controls were performed in triplicate.
F. Exogenous FGF18 provides proliferative and directional cues to chondrocytes in the developing larynx and trachea
Controlled mating of wild-type FVB/N mice was carried out as previously described. E11.5 embryos were collected and the developing larynx-trachea-lung was removed by meticulous dissection. Larynx-trachea-lung explant tissue was enrobed in zgrowth-factor reduced Matrigel (BD Biochemical, Franklin Lakes, NJ). Heparin-acrylic beads with a diameter of 200 um (Sigma, St. Louis, Missouri) were soaked for 4 hours in 50 ul of 20 ng/ul recombinant human FGF18 (Pepro Tech Inc, Rocky Hills, NJ) 38. The FGF18-coated beads were then placed directly next to the trachea of the matrigel enrobed larynx-trachea-lung explant tissue. Larynx-trachea-lung explants with FGF18 beads were cultured at 37°C for 4 days in a humidified 5% C02-95% air incubator. The larynx-trachea-lung explant tissue was then removed from the matrigel, fixed in 4% paraformaldehyde, dehydrated in ethanol and embedded in paraffin blocks. Six micron sections through the trachea were cut using a microtome and subjected to H&E staining and immunohistochemistry using antibodies to Sox9 (1:300, rabbit polyclonal antibody; Santa Cruz Biotechnology, Inc, Santa Cruz, CA) and Col2A1 (undiluted, mouse monoclonal antibody; Lab Vision Products, Fremont, CA). Biotinylated goat anti-rabbit IgG antibody (1:200) was used as the secondary antibody to detect the Sox9 antibody, and bound antibody-antigen complexes were detected using the Vectastain ABC kit (Vector laboratories, Burlingame, CA). A mouse on mouse kit (Vector laboratories, Burlingame, CA) was used to detect the Col2A1 antibody. Antigen detection for both antibodies was enhanced with nickel-diaminobenzidine and tris-cobalt, followed by counter staining with nuclear fast red. Results were photo-documented using a compound microscope equipped with a digital camera. As a control, larynx-trachea-lung explants were cultured next to beads soaked in normal saline solution. All experiments and controls were performed in triplicate.
G. Quantitative RT-PCR demonstrates increased expression of Sox9 in the larynx, trachea and lung of explants cultured in the presence of exogenous FGF18
Controlled mating of wild-type FVB/N mice was carried out as previously described. E11.5 and E12.5 embryos were collected and the developing upper respiratory tract and lung was removed by meticulous dissection. Larynx-trachea-lung explant cultures were established by placing the dissected tissue on 8.0 uM Whatman Nucleopore membrane (Whatman International, Clifton, NJ) floating on DMEM/F12 media (Invitrogen) with 5% Fetal Bovine Serum (Sigma) in a 3 well spot dish. Heparin-acrylic beads coated with FGF18 or normal saline solution as previously described (subsection IIIF) were placed directly next to the developing trachea. Explants were cultured at 37°C for either 48, 72, or 96 hours in a humidified 5% C02-95% air incubator. The explant tissue was then removed from culture, and RNA was isolated. Levels of Sox9 expression in the isolated RNA were then analyzed by qRT-PCR (see section IIIC). All experiments and controls were performed in triplicate.
IV. Results
A. Whole-mount in situ hybridization of embryonic larynx-trachea-lung with probes to collagen 2A1 (Col2A1) demonstrates directional proliferation of chondrocytes in the larynx and trachea to form the mature 3-dimensional cartilaginous framework of the upper respiratory tract
At E10.5, the respiratory tract is simple in overall anatomical configuration (Figure 1). The lumen of the ventral foregut endoderm and the lumen of the respiratory tract out-pouching meet at a wide interface, creating a cleft along the dorsal aspect of the upper airway from the level of the larynx to the carina. At the distal end of the out-pouching are the lung buds, which will form the multi-lobed right and left lungs. At E10.5, chondrocytes, identified by Col2A1 staining, were present exclusively at the interface of the ventral foregut endoderm and the upper respiratory tract out-pouching.
Figure 1.
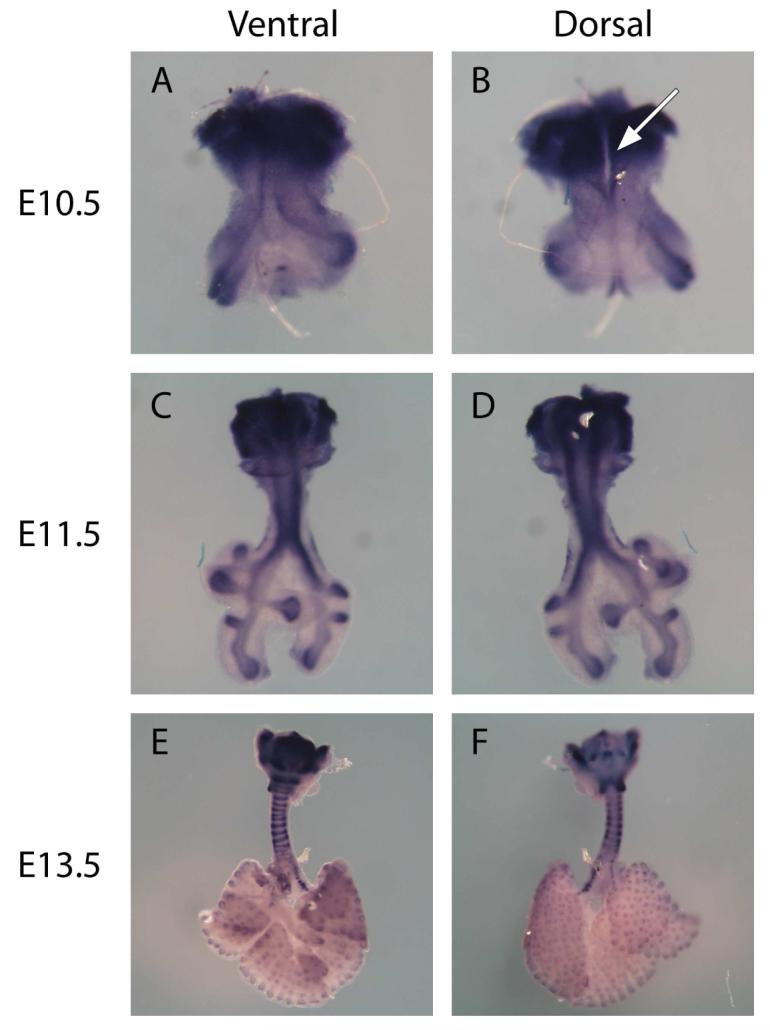
Whole mount in situ hybridization of Larynx-trachea-lung explants from E10.5 (A & B), E11.5 (C & D), and E13.5 (E & F) using RNA probes to Col2A1. Both ventral (A, C, E) and dorsal (B, D, F) views are presented. Col2A1 is expressed specifically by chondrocytes, and therefore binding of the Col2A1 anti-sense RNA probe denoted by blue staining identifies chondrocytes in the developing upper respiratory tract. At E10.5, the upper respiratory tract begins as a simple out-pouching of the ventral foregut endoderm. At this stage of development there is a cleft along the posterior aspect of the trachea from the level of the larynx to the carina (see arrow, figure 1B). To form the mature configuration of the thyroid, arytenoid, cricoid, and tracheal cartilage, the chondrocytes present at E10.5 must expand directionally. Col2A1 expression is also present in the distal aspects of the lung where it is known to be expressed in the endodermal compartment. Sense probes used to verify non-specific binding, showed no stain (data not shown). (A & B were taken at 10X, C & D at 5X, and E & F at 2.5X)
At E11.5, the larynx and trachea had a near mature configuration and the cleft along the dorsal aspect of the trachea has completely closed. Chondrocytes were present at the circumference of what will eventually be the thyroid cartilage. Additionally, chondrocytes were found in the pattern of two longitudinal stripes along the posterior-lateral aspect of the trachea and main stem bronchi.
At E13.5, chondrocytes were present throughout the cartilage of the upper respiratory tract. At this stage of development, the chondrocytes had assumed the mature 3-dimensional configuration of the thyroid, arytenoid, cricoid, and tracheal cartilage. Chondrocytes along the periphery of the thyroid cartilage had now more diffusely covered the developing thyroid cartilage. Chondrocytes that were present in a longitudinal stripe-like pattern along the posterior-lateral aspect of the trachea had grown from its posterior-lateral position anteriorly to form the C-shaped tracheal cartilage rings. In addition, alternating groups of chondrocytes along the posterior-lateral longitudinal stripe-like pattern of the trachea had terminated their chondrocyte programming, and had gone on to form the membranous areas between the tracheal cartilage rings. In summary, chondrocytes present at E10.5 had expanded directionally to achieve the final mature configuration of the laryngeal and tracheal the simple configuration of cartilaginous framework at E13.5.
As evident in figure 1, Col2A1 was also expressed in the lung in the very distal aspects of the airway. Previous studies have demonstrated that Col2A1 expression occurs in the endodermal compartment of the lung, 4 where its role is unknown 39. This is in contrast to Col2A1 expression in the larynx and trachea, which occurs in the mesenchymal compartment, consistent with its role in cartilage development4.
B. Extracellular signal-regulated kinase (ERK), a mediator of the FGF pathway, is present in cells surrounding the trachea, allowing a mechanism for the action of FGF in tracheal cartilage development
Hematoxylin-eosin staining of an axial section through the E12.5 midtrachea demonstrates mesenchymal cells surrounding the trachea (Figure 2). Likely, some of these mesenchymal cells have already differentiated into chondrocytes, while others are in the process of differentiating into chondrocytes. Directly posterior to the trachea, the lumen of the esophagus is apparent. On each of the lateral sides of the trachea are vascular structures. Immunohistochemistry using antibodies to ERK, demonstrated expression of this known FGF mediator in sporadic mesenchymal cells around the entire periphery of the trachea. ERK-positive cells also appeared in the mesenchymal cells surrounding the esophagus and other vascular structures. These results suggest that ERK is expressed in the mesenchyme of the upper aerodigestive tract both in areas of cartilage development and in areas where no cartilage is expected to develop.
Figure 2.
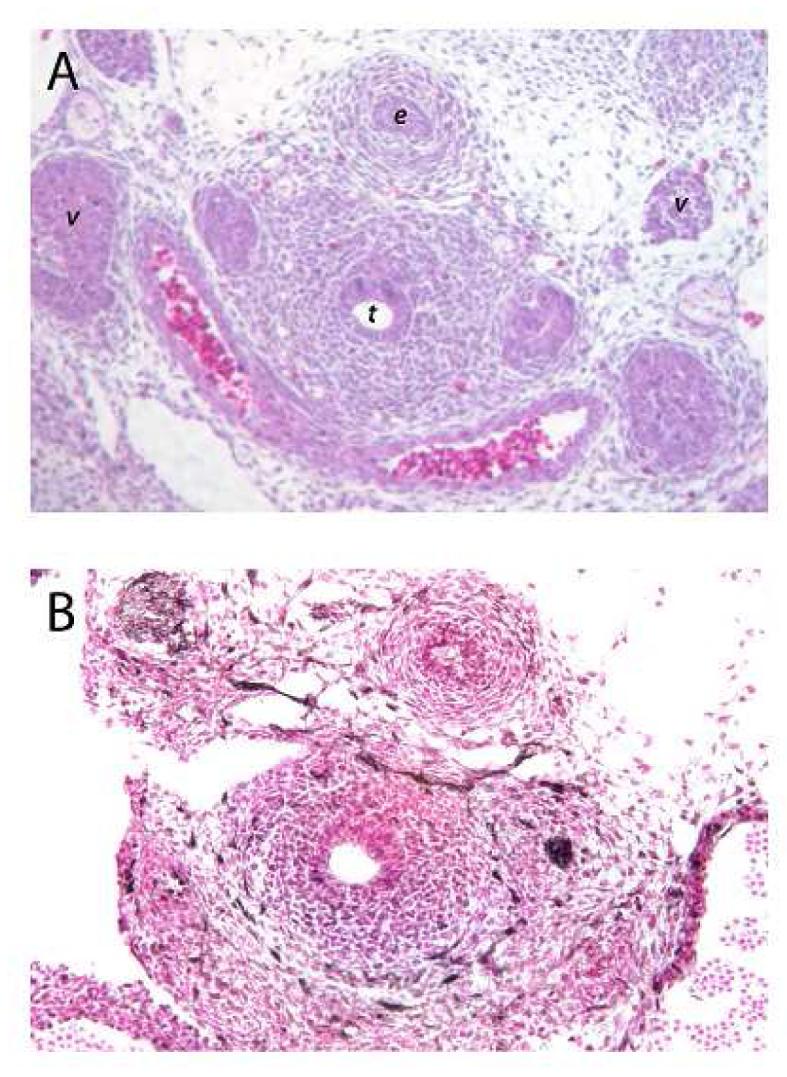
(A) H&E stained axial section through the mid trachea of an E12.5 mouse embryo. The tracheal lumen (t) and surrounding mesenchymal cells are readily apparent. Posterior to the trachea is the esophageal lumen (e). Lateral to the trachea are the lumen of vascular structures (v). (B) Antibodies to ERK highlight (dark brown stain) cells at the periphery of the mesenchymal cells surrounding the trachea. ERK positive cells are also seen in the mesenchymal cells surrounding the esophagus, and adjacent vascular structures. (This section of tissue does have a tear artifact on the left side between the trachea and adjacent vascular structure. Controls with secondary antibody alone showed not staining (data not shown). (Figures are taken at 20X)
C. Blocking MAP kinase activity in larynx-trachea-lung explant cultures inhibits the expression of the cartilage specifying trachea-lung explants could be maintained in culture for up to 4 days enrobed gene, Sox9
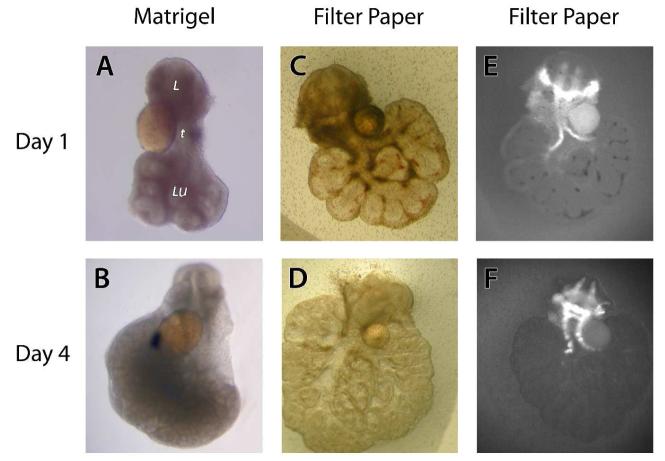
Ell.5 larynx-in matrigel or on filter paper (Figure 3). Explants grown in matrigel were able to grow in three-dimensions, whereas explants grown on filter paper assumed a two-dimensional growth pattern. The larynx-trachea-lung explants grew avidly in culture and the sequential branching of the distal airways could be readily appreciated with increasing time in culture. Larynx-trachea-lung explants from transgenic animals expressing glial fibrillary protein under the Col2A1 promoter demonstrated fluorescent green cartilage. The fluorescent nature of the cartilage in these transgenic animals allowed visualization of cartilage development in real-time.
Figure 3.
Appearance of larynx-trachea-lung explants after 24 (A, C, E) and 96 hours (B, D, F) in culture. Explants were grown enrobed in matrigel (A & B) or on filter paper (C, D, E, F). The larynx (l), trachea (t), and lung (lu) are denoted. Glass beads are noted in direct contact with the trachea. Explants grown in matrigel assume more of a 3-dimensional configuration compared to explants grown on filter paper. Chondrocytes in larynx-trachea-lung explants from transgenic mice expressing GFP under the control of the Col2A1 promoter glow when stimulated with a fluorescent light source (E & F). Please note the avid growth of the larynx, trachea and lung in these explant cultures. (All pictures taken at 5X)
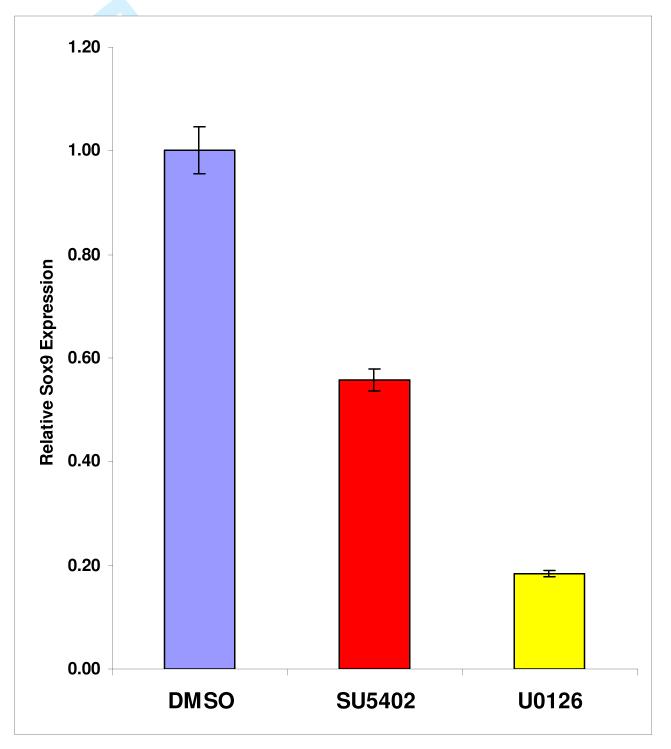
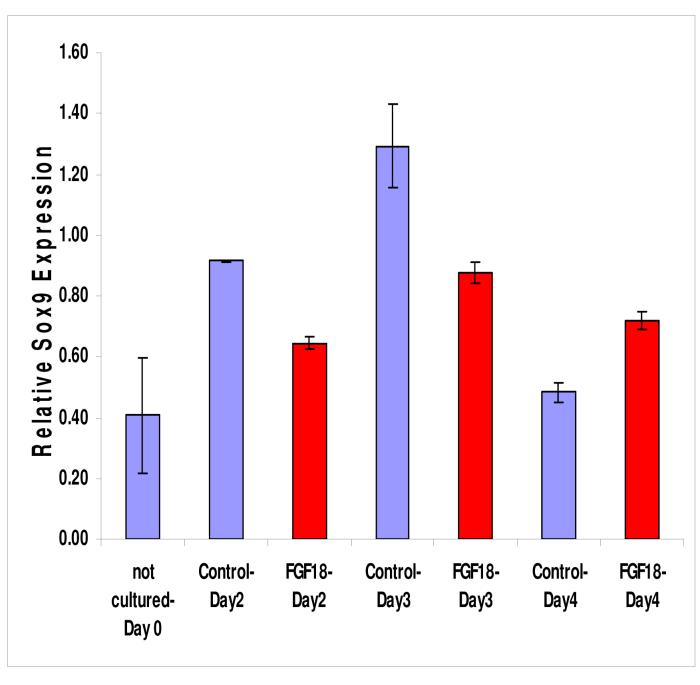
To determine if FGF pathways were important in tracheal cartilage development, inhibitors of FGF signaling were added to the culture medium of larynx-trachea explants grown on filter paper. Both the FGF receptor antagonist (SU5402) and the MAP Kinase/ERK inhibitor (U0126) caused a statistically significant (p<0.05) reduction in the expression of the cartilage-specifying gene Sox9 compared to DMSO control (Figure 4). Of the two inhibitors, U0126 was the most potent inhibitor, causing a five-fold decrease in Sox9 expression.
Figure 4.
Larynx-trachea explants grown in culture in the presence of inhibitors of the FGF pathway, SU5402 (red) and U0126 (yellow), demonstrated a significant decrease (p<0.05) in Sox9 expression compared to DMSO control (blue).
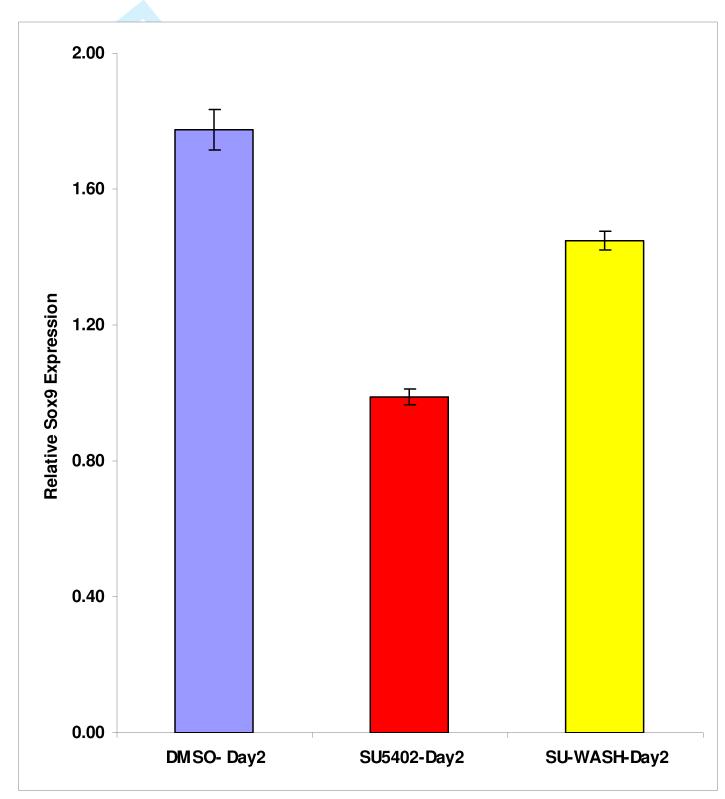
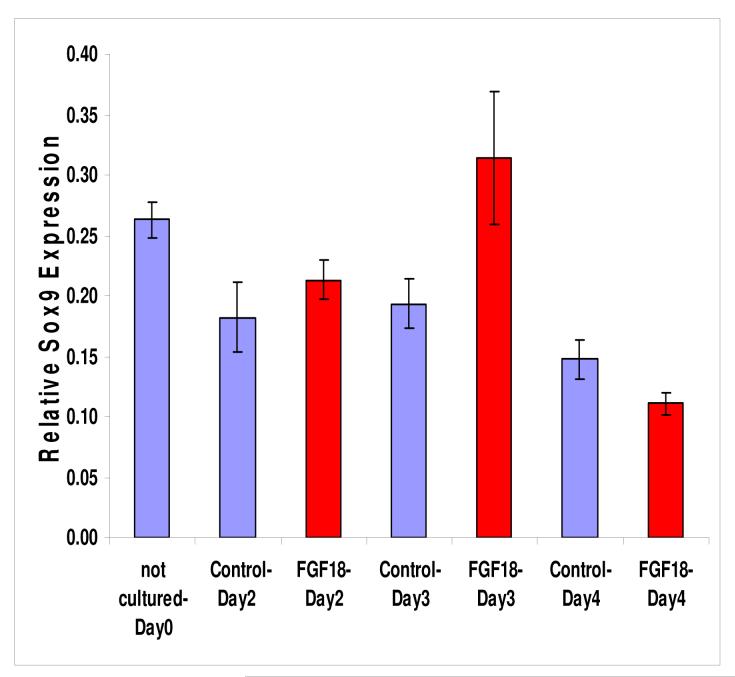
To determine if the FGF pathway inhibitors were indirectly toxic to the explant cultures, Ell.5 larynx-trachea explants were grown in culture with SU5402 for 24 hours. The explants were then repeatedly washed with fresh culture medium and cultured in medium not containing SU5402 for another 24 hours. After removing SU5402 from the culture medium, there was a significant recovery of Sox9 expression when compared to the control (Figure 5). Therefore, the effect of SU5402 on Sox9 expression appeared to be reversible and largely independent of cell toxicity.
Figure 5.
To determine if SU5402 was toxic to explant cultures, larynx-trachea explants were cultured in this FGF pathway inhibitor for 24 hours. The media was changed and the SU5402 removed, and the tissue cultured for another 24 hours. Removal of the SU5402 demonstrated a significant (p<0.05) increase in Sox9 expression (yellow) compared to control cultures in which the SU5402 was not removed (red). Sox9 expression in both SU5402 treated explant cultures was significantly decreased (p<0.05) compared to the DMSO control (blue).
D. Whole-mount in situ hybridization of larynx-trachea from transgenic mice over-expressing FGF18 demonstrates increased expression of the cartilage specifying gene, Sox9
Previous work by Whitsett et al 25 demonstrated that transgenic mice over-expressing FGF18 in the epithelial compartment of the airway formed abnormal tracheal cartilage rings. Indeed, alcian blue staining of the larynx and trachea from mice over-expressing FGF18 showed hyperplastic and malformed tracheal cartilage rings (Figure 6). When larynx-trachea-lung explant tissue from these transgenic animals was subjected to whole-mount in situ hybridization with RNA probes to Sox9, the pattern of Sox9 expression was also disorganized compared to that in wild-type controls. Although, it appeared subjectively that Sox9 expression was increased in the FGF18 over-expressing larynx and trachea, a definitive statement as to differences could not be made.
Figure 6.
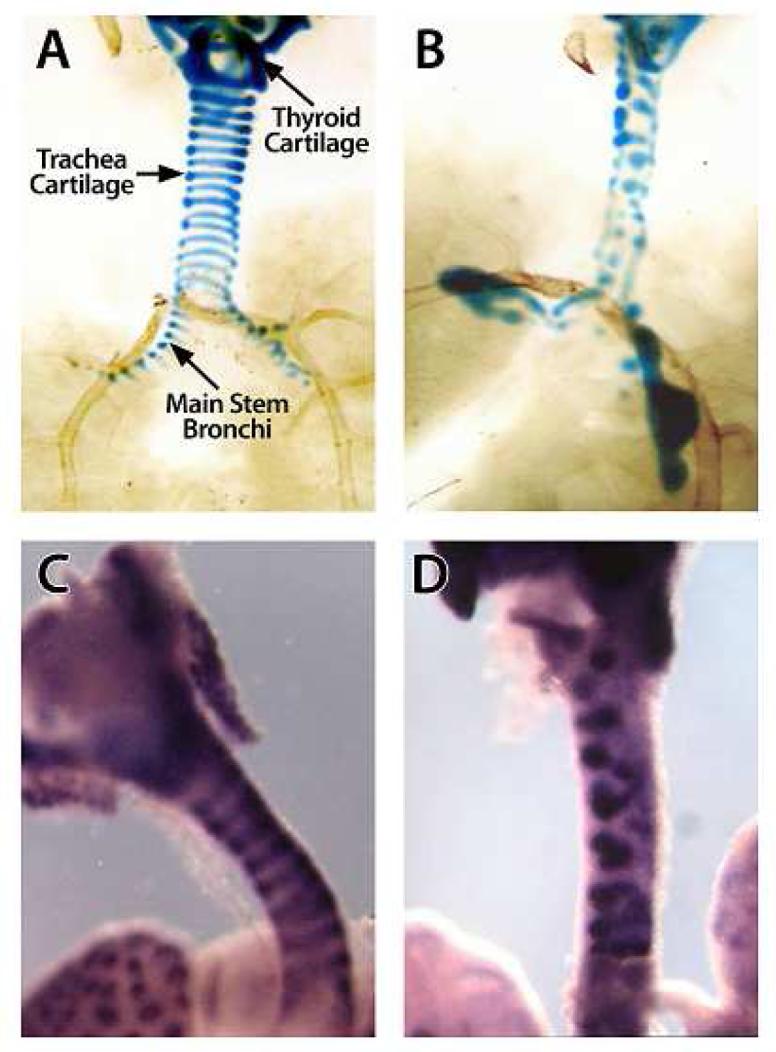
Alcian blue staining highlights the cartilaginous structures of the larynx and trachea from E18 wild-type FVB/N (A) and FGF18 over-expressing (B) embryos. Apparent in the FGF18 over-expressing larynx and trachea are hypertrophic and malformed cartilaginous structures compared to the wild-type controls. Whole-mount in situ hybridization using anti-sense probes to Sox9 confirms the disorganization of chondrocytes in the FGF18 over-expressing larynx-trachea (D) compared to a wild-type control (C). Finally Sox9 expression seems subjectively more intense in the FGF18 over-expressing larynx-trachea. (Pictures were taken at 10X).
E. Quantitative RT-PCR demonstrates increased expression of Sox9 in the larynx and trachea of FGF18 over-expressing mice
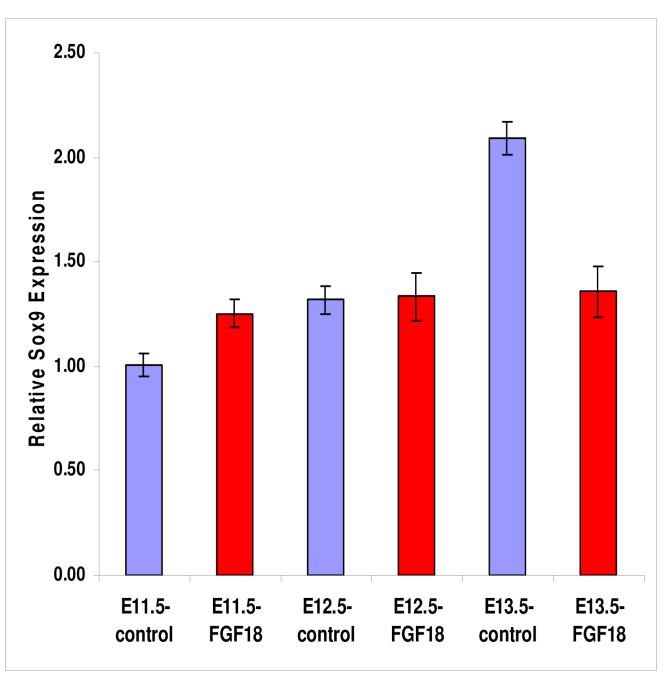
E11.5, E12.5, and E13.5 embryos over-expressing FGF18 in the epithelium of the upper respiratory tract were used to quantify levels of expression of Sox9 in the developing upper respiratory tracts. The larynx-trachea from E11.5, E12.5 and E13.5 FGF18 over-expressing mouse embryos were removed and subjected to qRT-PCR for Sox9 expression. Only in E11.5 was there a statistically significant increase in Sox9 expression in the FGF18 over-expressing mice compared to wild-type controls (Figure 7). Interestingly, in E13.5 there was a statistically significant decrease in Sox9 expression in the FGF18 over-expressing mice compared to controls.
Figure 7.
Sox9 expression in Ell.5, E12.5, and E13.5 larynx-trachea from FGF18 over-expressing transgenic mice was analyzed by qRT-PCR. Only at E11.5 did there appear to be a statistically significant (p<0.05) increase in Sox9 expression in FGF18 over-expressing larynx-trachea. Interestingly, at E13.5 there appears to be a statistically significant (p<0.05) decrease in Sox9 expression in the FGF18 over-expressing larynx-trachea.
F. Exogenous FGF18 provides proliferative and directional cues to cartilage growth in larynx-trachea-lung grown in culture
As previously demonstrated (Section IVC), larynx-trachea-lung explants grow avidly in culture for up to 4 days. In this experiment, heparin-coated glass beads soaked in recombinant FGF18 or normal saline were placed directly next to the trachea of cultured larynx-trachea-lung tissue at the initiation of the culture. After 4 days in culture, the tissue was harvested, sectioned, and subjected to H&E staining as well as immunohistochemistry using antibodies to Col2A1 and Sox9. Larynx-trachea-lung explants treated with normal saline soaked glass beads demonstrated the development of the classical C-shaped tracheal cartilage ring (Figure 8). Sox9 was expressed in a patchy manner through the tracheal cartilage ring consistent with its intracellular localization. Col2A1 on the other hand appeared diffusely expressed through the tracheal cartilage ring, consistent with its expression in the extracellular matrix. Larynx-tracheal-lung explants exposed to FGF18- soaked beads demonstrated the development of a malformed and hypertrophic tracheal cartilage ring. Furthermore, there appeared to be preferential growth of the developing tracheal cartilage towards the FGF18- soaked bead. Col2A1 and Sox9 are expressed throughout the malformed cartilage ring. Interestingly, Sox9 expression appeared the highest along the periphery of the malformed cartilage ring closest to the FGF18- soaked bead. Sox9 was also expressed in the epithelium of adjacent lung tissue, where as previously mentioned, its role in is unknown4,39.
Figure 8.
Larynx-trachea-lung explants grown in culture for 96 hours along with beads soaked in either normal saline or FGF18 were fixed, sectioned, and analyzed by H&E stain and immunohistochemistry. Larynx-trachea-lung explant grown with normal saline soaked beads ( *) developed a normal C-shaped tracheal cartilage ring (A, B, C), whereas a similar explant grown next to a FGF18 soaked bead (*) formed a malformed and hypertrophic cartilage ring (D, E, F). Figures A and D are H& E stains, B and E demonstrates Col2A1 expression, and C and F demonstrate Sox9 expression. Tracheal cartilage grown next to a normal saline soaked bead demonstrates a punctuate pattern of expression of Sox9 and a diffuse extracellular pattern expression of Col2A1. Tracheal cartilage grown next to a FGF18 soaked bead demonstrates the similar punctuate expression of Sox9 and diffuse extracellular staining with Col2A1. The one significant difference is that Sox9 expression in the FGF18 exposed tracheal cartilage seems to be higher at the edge of the cartilage closest to the FGF18 soaked bead. Sox9 expression can also be noted in the endoderm of adjacent lung tissue where its role is unknown. In this example the normal saline soaked bead was lost during processing of tissue. The (*) denotes the position of the bead relative to the trachea during culture. (t -trachea, e -esophagus, lu -lung)(All pictures are taken at 20X)
G. Quantitative RT-PCR demonstrates increased expression of Sox9 in the larynx, trachea and lung of explants cultured in the presence of exogenous FGF18
E11.5 and E12.5 larynx-trachea-lung from wild-type FVB/N were cultured in the presence of normal saline or FGF18- soaked beads, juxtaposed to the trachea, for 48, 72 or 96 hours. RNA was harvested from the explant tissue and analyzed by qRT-PCR for Sox9 expression. Ell.5 explant tissue exposed to exogenous FGF18, demonstrated a significant (p<0.05) increase in Sox9 expression compared to controls after 96 hours in culture (Figure 9). At 48 and 72 hours in culture, there was actually a decrease in Sox9 expression in the explant tissue exposed to exogenous FGF18. In E12.5 explant tissue exposed to exogenous FGF18, there was a significant (p<0.05) increase in Sox9 expression after 72 hours in culture (Figure 10). At 48 and 96 hours there was not a significant difference in Sox9 expression in explant tissue exposed to exogenous FGF18 versus controls.
Figure 9.
Histogram demonstrating expression levels of Sox9 in E11.5 larynx-trachea-lung tissue grown in culture for 48, 72 and 96 hours in juxtaposition to heparin coated glass beads soaked in normal saline or FGF18. Sox9 expression is significantly increased (p<0.05) in the trachea of explants grown in culture with FGF18 only after 96 hours in culture compared to controls. At 48 and 72 hours in culture there is actually a decrease in Sox9 expression in the FGF18 exposed trachea compared to controls. Sox9 expression in E11.5 trachea that is not culture is also included as a control.
Figure 10.
Histogram demonstrating expression levels of Sox9 in E12.5 larynx-trachea-lung tissue grown in culture for 48, 72 and 96 hours in juxtaposition to heparin coated glass beads soaked in normal saline or FGF18. . Sox9 expression is significantly increased (p<0.05) in the trachea of explants grown in culture with FGF18 after 72 hours in culture compared to controls. At 48 and 96 hours in culture there is no significant difference in Sox9 expression in the FGF18 exposed trachea compared to controls. Sox9 expression in E12.5 larynx-trachea that is not culture is also included as a control.
IV. Discussion
The cartilaginous support structure of the human upper respiratory tract has a very precise configuration, and is composed of multiple subunits strung together in series and suspended from the skull base and mandible (Figure 11A). The thyroid cartilage is composed of two halves fused anteriorly at a sharp angle (90 degrees in males, and 120 degrees in females). The superior cornu attaches to the thyrohyoid ligament, while the inferior cornu articulates with the cricoid cartilage. The cricoid cartilage is the skeletal support of the subglottis, which is the portion of the larynx below the vocal folds. The subglottis is the only point in the airway with a completely rigid diameter. Anteriorly, the cricoid is about 1 cm high, with a smooth, curved surface. Posteriorly, it is 2 to 3 cm high, and the superior surface is flattened centrally to provide an area of articulation for the arytenoid cartilages. Two other small sesamoid cartilages, the corniculate and the cuneiform, are located superior to the arytenoid and support the aryepiglottic fold. Attached to the inferior aspect of the cricoid are the tracheal cartilage rings. Tracheal cartilage is a C-shaped structure, with the incomplete part of the ring facing posteriorly. The two free edges of the tracheal cartilage ring are bridged by the muscular trachealis. The trachealis is one of several components that compose the tracheal-esophageal septum, the soft tissue separating the respiratory and digestive tracts. There are on average 17 tracheal cartilage rings in the human trachea interconnected by intervening fibroelastic tissue. C-shaped cartilaginous support structures, similar to that found in the trachea, continue to the level of the main stem bronchi. 40
Figure 11.
(A) Schematic of the cartilaginous framework of the upper respiratory tract. Note the intricate and precise 3-dimensional configuration of the cartilaginous structures. (B) Schematic representation of the embryonic stages of development of the upper respiratory tract.
The current understanding of the embryology of the larynx and trachea is limited to descriptive anatomic studies in the mouse and post-mortem studies in human embryos 3,41-45. In the mouse, development is divided into the embryonic (E) period (first 16 days of gestation) and the subsequent fetal (F) period (last 3 days of gestation). According to the Carnegie staging system (CS), the embryonic and fetal periods have 23 stages. Laryngeal and tracheal development begins at stage 11 and can be divided into eight phases (Figure 11B). During phase I (CS 11, E9.5), the first sign of the respiratory system is seen as an epithelial thickening along the ventral aspect of the foregut, known as the respiratory primordium. The respiratory primordium is a rest of mesenchymal cells, which will give rise to the muscle and cartilaginous components of the upper airway and lung. During phase II (CS 12, E10) the respiratory diverticulum forms as an out-pouching of the foregut endoderm. The respiratory diverticulum expands into the respiratory primordium and is enveloped in mesenchymal cells. During the later aspect of this phase, bronchopulmonary buds appear from the lateral aspect of the respiratory diverticulum, and eventually form the lower respiratory tract. The site of origin of the respiratory diverticulum is called the primitive pharyngeal floor; this eventually develops into the glottic region of the adult larynx. In phase III (CS 13-14, E10.5-11), there is caudocranial elongation of the foregut and a concomitant dorsocaudal elongation of both the respiratory diverticulum and bronchopulmonary buds. The carina, which represents the location of the bifurcation of the main stem bronchi, originates from the caudal aspect of the respiratory diverticulum. With the continued caudal descent of the bronchopulmonary buds, the main stem bronchi and distal airways are formed and the carina appears as a separate and distinct region from the respiratory diverticulum. The trachea is formed from the increasing area between the respiratory diverticulum and the carina. Towards the end of this phase, the primitive laryngo-pharynx becomes compressed bilaterally by enlarging laryngeal mesodermal anlagen. The mesodermal anlagen, derived from the second, third, fourth and fifth branchial arches, gives rise to laryngeal cartilage and musculature. During phase IV (CS 15, E11.5-12), the bilateral compression of the laryngopharynx results in obliteration of the ventral aspect of the primitive laryngopharynx and the creation of the epithelial lamina. In phase V (CS 16, E12-12.5), the laryngeal mesodermal anlagen consolidates into two distinct masses, the hyoid anlage and the cricothyroid anlage complex. The cricothyroid anlage is composed of a U-shaped thyroid anlage superiorly and the cricoid anlage inferiorly. The cricoid anlage is composed of three parts, one ventromedian component and two dorsolateral components. Growth and fusion of these three components during the next phase is required to form the complete ring of the adult cricoid. During phases VI (CS 17-18, E13-14) and VII (CS 19-23, E15-16), the epithelial lamina begins to recanalize. The last portion of the primitive laryngopharynx to recanalize is at the glottic level. The final step, phase VIII (fetal day17-19), entails completion of the recanalization, and establishment of a complete connection between the supraglottis and infraglottis.
Knowledge of the molecular mechanisms that orchestrate this complex algorithm of cellular differentiation and spatial organization, and give rise to the upper laryngeal and tracheal cartilage is essentially non-existent. FGFs are known to play a significant role in skeletal development as described previously. A recent study has demonstrated that over-expression of FGF18 in the epithelium of the airway perturbs tracheal cartilage development 25. Therefore, the purpose of this study was to understand the role of FGF18 in the development of upper respiratory tract cartilage.
The hypothesis of this study was that FGF18 regulates the expression of Sox9 in the developing upper respiratory tract therefore regulating the rate and direction of cartilage growth. Our specific aims were to: 1) Characterize the growth pattern of chondrocytes in the developing larynx and trachea. The hypothesis tested was that chondrocytes have to proliferate and grow directionally to form the 3-dimensional configuration of the cartilaginous airway. 2) Determine the effect of FGF18 on the growth of developing chondrocytes. The hypothesis tested was that FGF18 provides proliferative and directional cues to developing chondrocytes. 3) Determine the effect of FGF18 on the expression of Sox9. The hypothesis tested was that FGF18 promotes cartilage growth by increasing the expression of the transcription factor Sox9, which is known to recruit cells to the chondrocyte lineage.
The current study demonstrates that the organization of chondrocytes at the inception of the upper respiratory tract is simple, and that this pool of chondrocytes undergoes directional proliferation to form the final configuration of the laryngeal and tracheal cartilage. At E10.5, the initiation of the upper respiratory tract development, chondrocytes were found only at the junction of the ventral foregut endoderm and the respiratory tract out-pouching. At E11.5, chondrocytes surround the developing thyroid cartilage and formed two longitudinal stripe-like patterns along the posterior-lateral aspect of the trachea. By E13.5 chondrocytes had completely filled the thyroid cartilage. Furthermore, chondrocytes along the posterior-lateral aspect of the trachea have had migrated anteriorly meeting at the anterior midline of the trachea to form the C-shaped tracheal cartilage rings. It was also evident that alternating levels of chondrocytes along the posterior-lateral aspect of the trachea terminated their chondrocyte programming, possibly by apoptosis, creating membranous spaces between the cartilage rings. Col2A1 expression was also expressed in the distal lung, where it plays a yet unknown role in the epithelium4,39. Additional studies are needed to determine the mechanism(s) by which intermittent levels of chondrocytes terminate their chondrocyte differentiation program to form the fibro-elastic tissue between tracheal cartilage rings.
An important finding is that at E10.5 there was a cleft along the entire posterior aspect of the larynx and trachea, similar to that of a Type IV laryngeal-tracheal-esophageal cleft (LTEC) observed in human to infants. This is the first description of such a cleft during upper aerodigestive tract development. By E11.5, this cleft completely filled in, separating the respiratory tract from the esophagus. It is tempting to hypothesize that failure of the genetic signals to completely fill in this developmental cleft and separate the trachea from the esophagus during development give rise LTECs. Equally important to note is that the posterior-lateral positioning of the stripe-like patterns of chondrocytes along the trachea is essential for the development of a normal C-shaped tracheal cartilage ring. For example, if the longitudinal stripe-like pattern of chondrocytes was moved more anteriorly, the tracheal cartilage rings that would develop would be smaller and have a wide U-shaped configuration. The wide U-shaped tracheal cartilage rings and the widened trachealis would have an anatomy predisposed to tracheomalacia. Therefore, genetic signals that direct the organization and growth of chondrocytes are critical to the formation of normal upper respiratory tract cartilage.
Data from the current study suggest that FGF18 can provide proliferative and directional signals to developing chondrocytes, suggesting that this cytokine has a key role in the normal development of upper respiratoy tract cartilage. The first set of studies showed that the classical MAP kinase and known mediator of FGF signaling, ERK, was expressed along the periphery of developing trachea cartilage. The presence of ERK in developing cartilage establishes a mechanism by which FGF18 could effect cartilage growth. However, ERK-positive cells were also found in areas where there is no known cartilage development, such as the posterior aspect of the trachea, and surrounding the esophagus and adjacent vascular structures. A plausible explanation for these results is that the expression of ERK alone is insufficient for cartilage development. Therefore, cartilage does not develop adjacent to the esophagus or adjacent vascular structures because other cartilage specifying factors such as Sox9 and/or FGF18 are not present in these areas. In these non-cartilaginous areas of ERK expression, this MAP kinase may be mediating signals from other growth factors involved in development of other mesenchymal structures.
In order to further scrutinize the role of FGF18 in upper respiratory tract cartilage development, we developed an in vitro culture system for mouse larynx, trachea and lung. When inhibitors of FGF binding, SU5402, and inhibitors of MAP kinase/ERK, U0126, were added to these cultures there was a significant reduction in the expression of the cartilage specifying gene, Sox9, in the larynx and trachea. As Sox9 is essential for recruiting cells to the chondrocyte lineage, these results suggest that inhibitors of the FGF pathway inhibit the differentiation of chondrocytes. Conversely, FGFs appear to increase the expression of Sox9, and therefore differentiation of mesenchymal cells to chondrocytes.
Indeed, when larynx-trachea-lung explants were grown in culture with exogenous FGF18 there appeared to be a more robust proliferation of chondrocytes, with directional growth towards the FGF18 source. Furthermore, Sox9 expression appeared to be higher at the periphery of the trachea cartilage ring, closest to the FGF18 source. The observation that FGF18 can up-regulate expression of Sox9 was verified using both transgenic mice that over-express FGF18 in the epithelium of the larynx and trachea, and larynx-trachea-lung explants exposed to exogenous FGF18. Quantitative RT-PCR analysis demonstrated a small though significant increase in Sox9 expression in the larynx and trachea from Ell.5 FGF18 over-expressing transgenic mice compared to that in E11.5 wild-type larynx-trachea. Similarly, in cultured E11.5 and E12.5 larynx-trachea-lung explant tissue, exogenous FGF18 up-regulated Sox9 expression at 96 and 72 hours in culture, respectively.
There was no change in Sox9 expression at E12.5 and a reduction in Sox9 expression at E13.5 in the larynx-trachea from FGF18 over-expressing transgenic mice. FGF18 appeared to have no effect or even an inhibitory effect on cartilage development at later time-points in development. In E11.5 larynx-trachea-lung cultured in the presence of exogenous FGF18, Sox9 expression was unchanged or down-regulated after 96 hours in culture. Similarly, in E12.5 larynx-trachea-lung cultured with exogenous FGF18, Sox9 expression was unchanged or down regulated after 72 hours in culture. Therefore, FGF18 appears to up-regulate Sox9 expression and promote cartilage development, though only during a finite temporal window during development. This temporal “lock” limiting the effect of FGF18 on Sox9 expression and cartilage development may serve to block aberrant FGF18 signals which could disrupt normal upper respiratory tract cartilage formation. Further experimentation is needed to determine the mechanism by which the actions FGF18 are temporally confined.
VII. Conclusions
Congenital anomalies of laryngeal and tracheal cartilage cause considerable morbidity and mortality in the pediatric population. An understanding of the pathophysiology of congenital anomalies of the larynx and trachea is limited by the sparse knowledge available regarding normal upper respiratory tract cartilage development. The present study describes for the first time the histological development of the cartilaginous support structures of the upper respiratory tract in vertebrate mammals. The model of development of respiratory tract cartilage that we have presented has implications regarding the pathophysiology of congenital airway anomalies seen in humans. We also have described a novel methodology for growing the larynx and trachea in culture. This culture system allows the study of specific developmental pathways in larynx and tracheal development using pharmacologic agents. Finally, this study shows that upper respiratory tract cartilage is formed through an orderly and directional growth of chondrocytes mediated by FGF18 and Sox9.
Acknowledgments
The authors of the paper would like to thank Dr. Jeffrey Whitsett at Cincinnati Children’s Hospital Medical Center for giving us the (teto)7CMV-FGF18 and SP-C-rtTA mice transgenic mice, and for his invaluable advice during this study. We would also like to thank Aliza Cohen for help in editing the manuscript.
Funding: National Institute of Child Health and Human Development (RGE) K08 HD045703
VIII. References
- 1.Hartnick CJ, Cotton RT. Congenital laryngeal anomalies. Laryngeal atresia, stenosis, webs, and clefts. Otolaryngol Clin North Am. 2000;33:1293–1308. doi: 10.1016/s0030-6665(05)70282-6. [DOI] [PubMed] [Google Scholar]
- 2.Hartnick CJ, Rutter M, Lang F, Willging JP, Cotton RT. Congenital high airway obstruction syndrome and airway reconstruction: an evolving paradigm. Arch Otolaryngol Head Neck Surg. 2002;128:567–570. doi: 10.1001/archotol.128.5.567. [DOI] [PubMed] [Google Scholar]
- 3.Henick DH. Three-dimensional analysis of murine laryngeal development. Ann Otol Rhinol Laryngol Suppl. 1993;159:3–24. doi: 10.1177/00034894931020s301. [DOI] [PubMed] [Google Scholar]
- 4.Elluru RG, Whitsett JA. Potential role of Sox9 in patterning tracheal cartilage ring formation in an embryonic mouse model. Arch Otolaryngol Head Neck Surg. 2004;130:732–736. doi: 10.1001/archotol.130.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright E, Hargrave MR, Christiansen J, et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster JW, Dominguez-Steglich MA, Guioli S, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 9.Kwok C, Weller PA, Guioli S, et al. Mutations in SOX9, the gene responsible for Campomelic dysplasia and autosomal sex reversal. Am J Hum Genet. 1995;57:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner T, Wirth J, Meyer J, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 11.Meyer J, Sudbeck P, Held M, et al. Mutational analysis of the SOX9 gene in campomelic dysplasia and autosomal sex reversal: lack of genotype/phenotype correlations. Hum Mol Genet. 1997;6:91–98. doi: 10.1093/hmg/6.1.91. [DOI] [PubMed] [Google Scholar]
- 12.Bi W, Huang W, Whitworth DJ, et al. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki F. Effects of various growth factors on a chondrocyte differentiation model. Adv Exp Med Biol. 1992;324:101–106. doi: 10.1007/978-1-4615-3398-6_10. [DOI] [PubMed] [Google Scholar]
- 15.Savage MP, Fallon JF. FGF-2 mRNA and its antisense message are expressed in a developmentally specific manner in the chick limb bud and mesonephros. Dev Dyn. 1995;202:343–353. doi: 10.1002/aja.1002020404. [DOI] [PubMed] [Google Scholar]
- 16.Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- 17.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 18.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- 20.Ornitz DM, Herr AB, Nilsson M, Westman J, Svahn CM, Waksman G. FGF binding and FGF receptor activation by synthetic heparan-derived di- and trisaccharides. Science. 1995;268:432–436. doi: 10.1126/science.7536345. [DOI] [PubMed] [Google Scholar]
- 21.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 22.Naski MC, Ornitz DM. FGF signaling in skeletal development. Front Biosci. 1998;3:D781–794. doi: 10.2741/a321. [DOI] [PubMed] [Google Scholar]
- 23.L’Hote CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Experimental cell research. 2005;304:417–431. doi: 10.1016/j.yexcr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 24.De Moerlooze L, Dickson C. Skeletal disorders associated with fibroblast growth factor receptor mutations. Curr Opin Genet Dev. 1997;7:378–385. doi: 10.1016/s0959-437x(97)80152-9. [DOI] [PubMed] [Google Scholar]
- 25.Whitsett JA, Clark JC, Picard L, et al. Fibroblast growth factor 18 influences proximal programming during lung morphogenesis. J Biol Chem. 2002;277:22743–22749. doi: 10.1074/jbc.M202253200. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami S, Kan M, McKeehan WL, de Crombrugghe B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2000;97:1113–1118. doi: 10.1073/pnas.97.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taketo M, Schroeder AC, Mobraaten LE, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development. 2008;135:1049–1058. doi: 10.1242/dev.013359. [DOI] [PubMed] [Google Scholar]
- 30.Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In situ Hybridization. Oxford University Press; Oxford: 1992. pp. 75–83. [Google Scholar]
- 32.Lutfalla G, Uze G. Performing quantitative reverse-transcribed polymerase chain reaction experiments. Methods in enzymology. 2006;410:386–400. doi: 10.1016/S0076-6879(06)10019-1. [DOI] [PubMed] [Google Scholar]
- 33.Cho JY, Grant TD, Lunstrum GP, Horton WA. Col2-GFP reporter mouse--a new tool to study skeletal development. Am J Med Genet. 2001;106:251–253. [PubMed] [Google Scholar]
- 34.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glasser SW, Korfhagen TR, Wert SE, et al. Genetic element from human surfactant protein SP-C gene confers bronchiolar-alveolar cell specificity in transgenic mice. Am J Physiol. 1991;261:L349–356. doi: 10.1152/ajplung.1991.261.4.L349. [DOI] [PubMed] [Google Scholar]
- 36.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 37.Clark JC, Tichelaar JW, Wert SE, et al. FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am J Physiol Lung Cell Mol Physiol. 2001;280:L705–715. doi: 10.1152/ajplung.2001.280.4.L705. [DOI] [PubMed] [Google Scholar]
- 38.Mandler M, Neubuser A. FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev Biol. 2001;240:548–559. doi: 10.1006/dbio.2001.0490. [DOI] [PubMed] [Google Scholar]
- 39.Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. 2005;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- 40.Hollinshead WH. The pharynx and larynx Anatomy for surgeons: The head and neck. J. B. Lpincott; Philadelphia: 1982. pp. 389–442. [Google Scholar]
- 41.Tucker JA, O’Rahilly R. Observations on the embryology of the human larynx. Annals of Otology, Rhinology & Laryngology. 1972;81:520–523. doi: 10.1177/000348947208100410. [DOI] [PubMed] [Google Scholar]
- 42.Tucker GF, Tucker JA, Vidic B. Anatomy and development of the cricoid: serial-section whole organ study of perinatal larynges. Ann Otol Rhinol Laryngol. 1977;86:766–769. doi: 10.1177/000348947708600609. [DOI] [PubMed] [Google Scholar]
- 43.Zaw-Tun HA. The tracheo-esophageal septum--fact or fantasy? Origin and development of the respiratory primordium and esophagus. Acta Anat (Basel) 1982;114:1–21. [PubMed] [Google Scholar]
- 44.Zaw-Tun HA, Burdi AR. Reexamination of the origin and early development of the human larynx. Acta Anat (Basel) 1985;122:163–184. doi: 10.1159/000145998. [DOI] [PubMed] [Google Scholar]
- 45.Zaw-Tun HI. Development of congenital laryngeal atresias and clefts. Ann Otol Rhinol Laryngol. 1988;97:353–358. doi: 10.1177/000348948809700405. [DOI] [PubMed] [Google Scholar]