Abstract
Background and Purpose
Our previous studies found that deferoxamine reduces intracerebral hemorrhage (ICH)-induced brain injury in rats. The current study examined whether deferoxamine reduces brain injury in a piglet ICH model.
Methods
Pigs received an injection of autologous blood into the right frontal lobe. Deferoxamine (50 mg/kg, IM) or vehicle was administered 2 hours after ICH and then every 12 hours up to 7 days. Animals were killed 3 or 7 days later to examine iron accumulation, white matter injury and neuronal death.
Results
ICH resulted in development of a reddish perihematomal zone, and iron accumulation, ferritin upregulation and neuronal death within that zone. Deferoxamine reduced the perihematomal reddish zone, white matter injury and the number of Perls’, ferritin and Fluoro-Jade C positive cells.
Conclusions
Iron accumulation occurs in the piglet brain after ICH. Deferoxamine reduces ICH-induced iron buildup and brain injury in piglets.
Keywords: deferoxamine, intracerebral hematoma, iron, neuronal death
Introduction
Iron overload occurs in the brain after intracerebral hemorrhage (ICH) and causes brain damage 1, 2. Deferoxamine (DFX), an iron chelator, reduces brain edema, neuronal death and neurological deficits following ICH in rats 3–5.
Experimental ICH has been studied in many species including mouse, rat, cat, rabbit, dog, pig and monkey2. Rats are the most frequently used species for ICH studies, and the pig ICH model is a very useful large animal model with more white matter 6–8.
We hypothesized that systemic DFX treatment reduces brain iron accumulation, neuronal death and white matter injury in the piglet model of ICH.
Materials and Methods
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of 16 male piglets (8–10 kg, Michigan State University) were used in this study. Piglets were sedated with ketamine (25 mg/kg, IM) and anesthetized with isoflurane. After a surgical plane of anesthesia was reached, animals were orotracheally intubated. The right femoral artery was catheterized for monitoring of blood pressure, blood gases and glucose concentrations. Body temperature was maintained at 37.5 ± 0.5 °C.
A cranial burr hole (1.5−mm) was then drilled 11-mm to the right of the sagittal and 11−mm anterior to the coronal suture. An 18 mm long 20-gauge sterile plastic catheter was placed stereotaxically into the center of the right frontal cerebral white matter at the level of the caudate nucleus and cemented in place. One ml of autologous arterial blood was infused over 15 minutes with an infusion pump. After a five-minute break, another 1.5-ml of blood was injected over 15 minutes 7.
Piglets were treated with DFX (50 mg/kg; administered intramuscularly every 12 hours for 3 or 7 days, starting 2 hours post-ICH) or vehicle by a blinded investigator. Piglets were reanesthetized on day 3 or day 7 and the brains perfused in situ with 10% formalin. Brains were sectioned coronally. Paraffin embedded brain was cut into 10 µm thick sections. Enhanced Perls’ staining was performed to detect iron accumulation4. Ferritin was examined by immunohistochemistry (primary antibody: polyclonal rabbit anti-human ferritin IgG; DACO; 1:400 dilution), neuronal death was measured by Fluoro-Jade C staining and white matter injury was determined by Luxol fast blue staining. For ferritin and Fluoro-Jade cell counting, four adjacent hematoma fields (0.14 mm2 each) were taken to count positive cells. Luxol fast blue staining was assessed as the ipsilateral stained area/contralateral area (%)
Data from different animal groups and brain sites were expressed as mean±SD and analyzed by unpaired Student t-test. Differences were considered significant at p<0.05.
Results
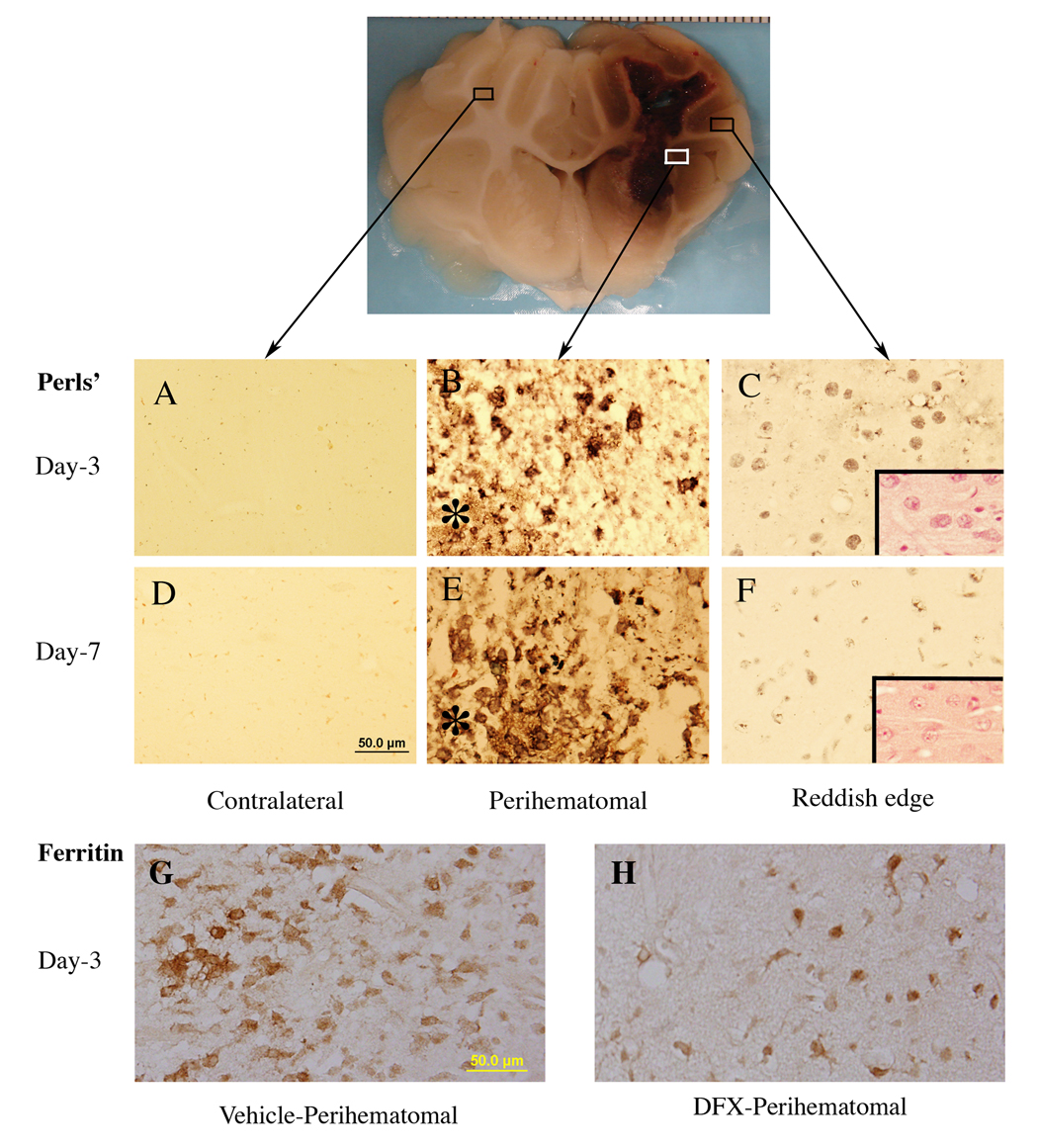
A reddish zone developed around the hematoma in all 16 ICH pigs. DFX treatment significantly reduced this zone at both three days or seven days after ICH (Figure 1). Using enhanced Perls’ reaction to examine iron accumulation, we observed a good spatial correlation between the Perls’ positive zone and the reddish zone (Figure 2). DFX also reduced the number of perihematomal Perls’ positive cells (e.g., day 7: 64±38 vs. 213±35 cells/mm2 in the vehicle, p<0.01).
Figure 1.
Deferoxamine reduces reddish zone around hematoma at day 3 and day 7 in a pig ICH model. Values are means±SD, n=4, # p<0.01 vs. vehicle.
Figure 2.
Iron histochemistry (Perls’ staining) and ferritin immunoreactivity in the brain after ICH. Asterisk indicates the hematoma. Insets in C and F: hematoxylin and eosin staining. Scale bar(A–H)=50 µm.
Ferritin, an iron storage protein, was upregulated in the perihematomal zone. Many glialike ferritin positive cells were detected around the hematoma(Figure 2). Ferritin positive cells were markedly less in DFX-treated animals at day 3 (92±43 vs. 155±23 cells/mm2 in the vehicle, p<0.05) and day 7 (101±46 vs. 232±42 cells/mm2 in the vehicle, p<0.01).
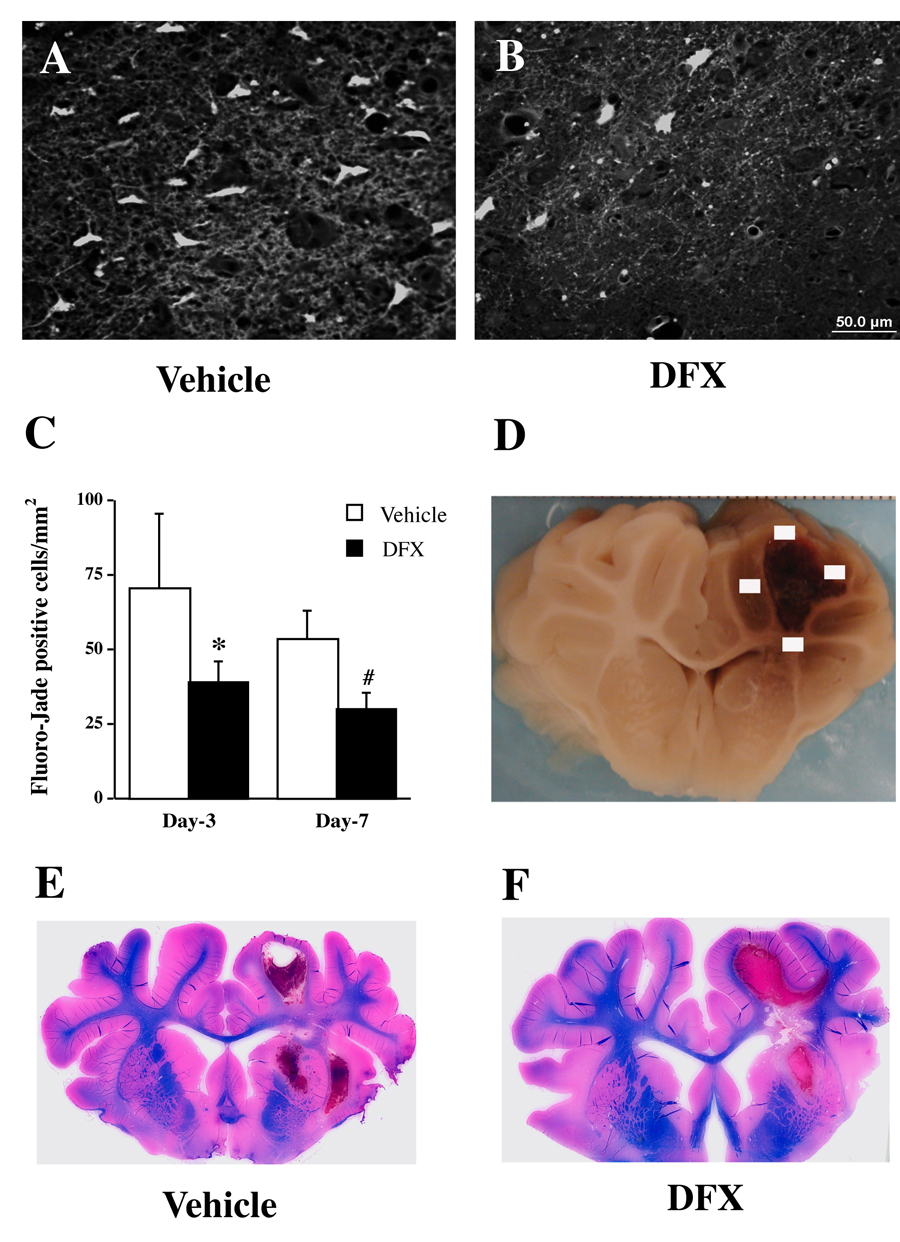
Fluoro-Jade C staining was used to detect neuronal degeneration. DFX reduced Fluoro-Jade C positive cells in the perihematomal area (Figure 3). There was a reduction in Luxol fast blue-stained white matter in the ipsilateral hemisphere at day 7 after ICH. DFX reduced that loss (79±10 and 62±5% of contralateral area in DFX- and vehicle-treated pigs, respectively; p<0.05, Figure 3).
Figure 3.
Fluoro-Jade C positive cells in the perihematomal area (A–C) and Luxol fast blue staining (E & F) after ICH. Part D shows four sampled fields for Fluoro-Jade C cell counting. Pigs had ICH and were treated with either vehicle or deferoxamine. Values are means±SD, n=4, *p<0.05, #p<0.01 vs. vehicle. Scale bar (A & B)=50 µm.
Discussion
The current study demonstrated that DFX reduces perihematomal iron accumulation, neuronal death and white matter injury in a pig ICH model. Our previous studies have found DFX is neuroprotective in rat ICH models 3, 4. The fact that DFX has protective effects in ICH models in two species increases the speculation that the drug may work in humans.
An interesting finding in this study is the development of a reddish zone around the hematoma at three and seven days after ICH (Figure 1) and that this reddish zone matched the Perls’ positive area. Although the precise mechanism for the reddish zone development is unclear, we believe that erythrocyte lysis with hemoglobin/heme release may contribute to the development. Heme from hemoglobin then can be degraded by heme oxygenases in the brain into iron, carbon monoxide and biliverdin. Many Perls’ positive cells were detected in the reddish zone (Figure 2). Future studies should investigate how DFX reduces the perihematomal reddish zone. There is evidence that DFX can inhibit hemin-induced erythrocyte lysis 9.
Iron overload is toxic to the brain. The duration over which clot lysis and iron release occur are likely to be dependent on clot size (i.e. 1–3 days for an 100 µl clot in rats and 3–7 days for a 2.5 ml clot in pigs). Our previous results in rats 3, 4 and current data in pigs showed that DFX is effective in reducing injury in models with different sized clots. While DFX may work directly by chelating iron, it can also inhibit prolyl 4-hydroxylase activity which may lead to protection from oxidative-stress induced cell death 10.
There are some limitations to this study. Because of size constraints, we used piglets (~5 weeks), where brains may not be fully mature, rather than aged pigs. Second, a method of assessing ICH-induced neurological deficits in the pig has yet to be devised.
In conclusion, intracerebral hematoma causes elevated iron in the piglet brain and DFX reduces the resulting neuronal death suggesting iron chelation may have potential as a treatment for ICH patients.
Acknowledgement
This study was supported by grants NS-017760, NS-039866, NS-047245, and NS-052510 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: Role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 4.Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: The role of iron. J Neurosurg. 2006;104:305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- 5.Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: Effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. 2007;38:2861–2863. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- 6.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE, Broderick JP, Brott TG. Lobar intracerebral hemorrhage model in pigs: Rapid edema development in perihematomal white matter. Stroke. 1996;27:490–497. doi: 10.1161/01.str.27.3.490. [DOI] [PubMed] [Google Scholar]
- 7.Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, Brott TG, Hoff JT. The role of blood clot formation on early edema development following experimental intracerebral hemorrhage. Stroke. 1998;29:2580–2586. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 8.Wagner KR. Modeling intracerebral hemorrhage: Glutamate, neclear factor-kappa b signaling and cytokines. Stroke. 2007;38:753–758. doi: 10.1161/01.STR.0000255033.02904.db. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan SG, Baysal E, Stern A. Inhibition of hemin-induced hemolysis by desferrioxamine: Binding of hemin to red cell membranes and the effects of alteration of membrane sulfhydryl groups. Biochim Biophy Acta. 1992;1104:38–44. doi: 10.1016/0005-2736(92)90129-a. [DOI] [PubMed] [Google Scholar]
- 10.Siddiq A, Aminova LR, Ratan RR. Prolyl 4-hydroxylase activity-responsive transcription factors: From hydroxylation to gene expression and neuroprotection. Front Biosci. 2008;13:2875–2887. doi: 10.2741/2892. [DOI] [PMC free article] [PubMed] [Google Scholar]