Abstract
Background & Aims
Recommendations for patients with Barrett’s esophagus (BE) include endoscopic surveillance with esophagectomy for early-stage cancer, although new technologies to ablate dysplasia and metaplasia are available. This study compares the cost-utility of ablation with that of endoscopic surveillance strategies.
Methods
A decision analysis model was created to examine a population of patients with BE (mean age 50), with separate analyses for patients with no dysplasia, low-grade dysplasia (LGD), or high-grade dysplasia (HGD). Strategies compared were: no endoscopic surveillance; endoscopic surveillance with ablation for incident dysplasia; immediate ablation followed by endoscopic surveillance in all patients or limited to patients in whom metaplasia persisted, and esophagectomy. Ablation modalities modeled included radiofrequency, argon plasma coagulation, multipolar electrocoagulation and photodynamic therapy.
Results
Endoscopic ablation for patients with HGD could increase life expectancy by 3 quality-adjusted years at an incremental cost of < $6,000, compared with no intervention. Patients with LGD or no dysplasia can also be optimally managed with ablation, but continued surveillance after eradication of metaplasia is expensive. If ablation permanently eradicates at least 28% of LGD or 40% of non-dysplastic metaplasias, ablation would be preferred to surveillance.
Conclusions
Endoscopic ablation could be the preferred strategy for managing patients with BE with HGD. Ablation might also be preferred in subjects with LGD or no dysplasia, but the cost-effectiveness depends on the long-term effectiveness of ablation and whether surveillance endoscopy can be discontinued following successful ablation. As further post-ablation data become available, the optimal management strategy will be clarified.
Background
Barrett’s esophagus (BE), the premalignant condition associated with esophageal adenocarcinoma, is a highly prevalent condition, affecting up to 10% of those with chronic reflux symptoms.1 While the standard management approach to decrease cancer mortality involves endoscopic surveillance with random biopsy of metaplastic tissue to detect cancer at a curable stage,2–4 endoscopic mucosal resection, photodynamic therapy (PDT), radiofrequency ablation (RFA), multipolar electrocoagulation (MPEC) and argon plasma coagulation (APC) are emerging endoscopic interventions that are efficacious in eliminating dysplasia and metaplasia.5–14
Under conditions of uncertainty, decision analysis may be helpful to compare competing strategies of management.15 We conducted a study to determine whether endoscopic ablation of BE is a cost-effective option to reduce mortality from esophageal adenocarcinoma. In particular we were interested in identifying the minimal efficacy required for endoscopic ablation to surpass endoscopic surveillance as the preferred method of management. We chose to examine ablation in different populations of patients with BE, including patients with high-grade dysplasia (HGD), low-grade dysplasia (LGD), and no dysplasia in order that our findings could be applied to these specific patient groups.
Methods
Patients
The hypothetical cohort consisted of 50 year old patients with a diagnosis of BE, defined as endoscopically identifiable abnormal mucosa with histologically confirmed intestinal metaplasia. In separate analyses, the cohort was modeled to consist of patients with BE with HGD, LGD or no dysplasia. It was assumed that all patients would be candidates for diagnostic and therapeutic procedures, including endoscopy, PDT, RFA, MPEC, APC and esophagectomy, and would consent to the assigned interventions. The analysis followed the cohort until age 80 years or death.
Model
A decision analytic model was constructed in TreeAge Pro (TreeAge, Williamstown, Massachusetts) in the form of a hybrid decision tree (Figure 1A) and Markov process (Figure 1B).15,16 The natural history of the cohort was modeled to examine the costs and outcomes related to the management of esophageal adenocarcinoma in the absence of surveillance or other interventions, and compared to various strategies of management proposed for patients with BE with HGD, LGD or no dysplasia in separate analyses.17–20
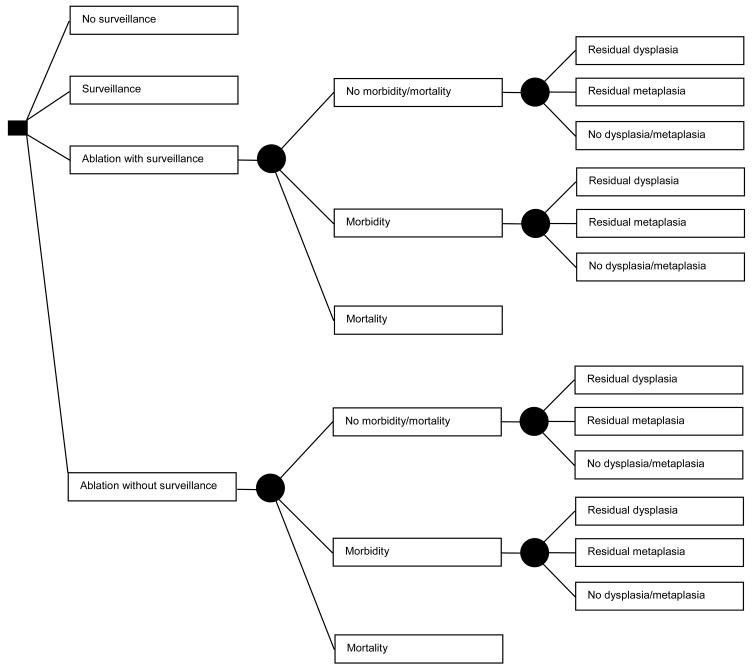
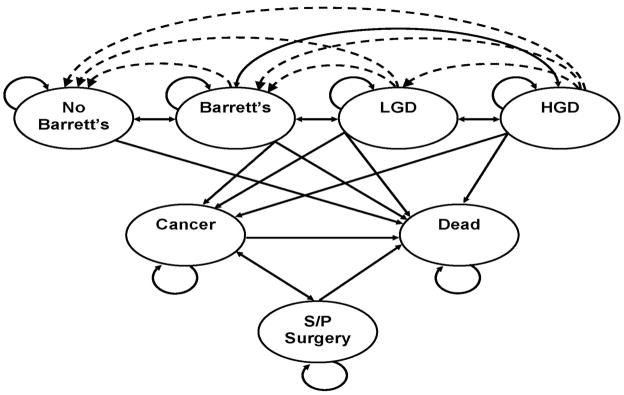
Figure 1.
Figure 1A. Decision Tree: Patient options at the decision node (square) include: no surveillance, surveillance, ablation with surveillance for all patients, or ablation without surveillance for those in whom complete ablation of metaplasia is achieved. Among patients treated with ablation, complications leading to either morbidity or mortality may occur, dictated by the probabilities at the chance nodes (circles). Mortality results in termination of the simulation. All other patients may have persistent dysplasia, no dysplasia but persistent metaplasia, or complete ablation of dysplasia and metaplasia, dictated by the probabilities at the chance nodes, which differ according to patient category (high-grade, low-grade or no dysplasia) and ablation type. Patients not experiencing mortality from ablation enter the Markov Model (Figure 1B), as do patients who are managed by the surveillance or no surveillance strategies.
Figure 1B. Markov Model: Patients can reside in one of seven major states: Patients without Barrett’s esophagus (BE); patients with BE, without dysplasia or cancer; patients with BE and low-grade dysplasia (LGD); patients with BE and high-grade dysplasia (HGD); patients with adenocarcinoma of the esophagus; patients who have had esophagectomy; and death from adenocarcinoma of the esophagus, complications of surgery, endoscopy, or other causes. The arrows indicate the possible transitions from one state to another in each cycle. Dashed lines indicate transition allowed only with ablative therapies.
Natural History
Costs and discounted quality-adjusted life-years (dQALYs) without surveillance or other interventions for BE were determined. Cancer would be diagnosed if symptoms prompted endoscopic evaluation. Depending on the stage of cancer at the time of diagnosis, esophagectomy or palliation would be performed. Patients with unresectable cancer would be treated through hospice or palliative care. The outcome of surgery included peri-operative mortality or morbidity, and survivors of surgery could be cured of cancer or experience persistent or recurrent disease, rates of which depended upon the stage of cancer at the time of diagnosis. Death from causes other than esophageal adenocarcinoma was incorporated in an age-dependent fashion, based on life-tables of U.S. residents for the year 2007.
Transitions
Transitions for the model were derived from published literature. To assess key variables a systematic review of the literature was conducted in the MEDLINE and EMBASE databases from 1970 to 2008 and abstracts from major gastroenterology scientific meetings from 1999 to 2008 were searched using the terms Barrett’s esophagus, esophageal neoplasm, esophageal adenocarcinoma, precancerous conditions, and gastroesophageal reflux disease. If no data existed for a specified transition the authors reached consensus for the best estimate of the parameter. The details regarding the references from which data were abstracted can be found in the supplemental information for this article on the journal website.
Ablation Efficacy and Complications
The efficacy and complications associated with ablative therapy for BE were based on published data of PDT, RFA, MPEC and APC. Details of the systematic reviews of the literature performed to obtain these data are available in the supplemental information. Complete remission (CR) was defined as the absence of dysplasia (CR-D) or intestinal metaplasia (CR-IM) at the specified follow-up point based on histopathological interpretation of biopsy specimens obtained during specialized surveillance endoscopies. The definition of CR-IM excluded primary failure of ablation to eliminate all metaplasia as well as recurrence of metaplasia after ablation, either superficial or subsquamous to account for “buried glands.” All results were reported on an intention to treat analysis.
Natural History of Barrett’s Esophagus
The NIH sponsored health outcomes research workshop “Advancing an Evidence-Based Approach to Barrett’s Esophagus” conducted April 9–10, 2007 in Boston, MA, identified discrepancies between published decision models of BE management. A major cause for these differences was that published rates of progression and regression between various states of dysplasia do not produce the cancer incidence reported in national health statistics when used as inputs in mathematical models. This is likely due to multiple factors including referral bias, publication bias, stochastic variation in small populations, confounding by indication and the poor discriminatory value of dysplasia. The current study utilizes methods to ensure that the model accurately reflects current knowledge of the natural history of BE with respect to development of esophageal adenocarcinoma. We chose to accomplish this by retaining the proportional relationship between transitions while altering the absolute values to fit the overall cancer incidence to the national health statistics. Thus, individual transition rates between health states were abstracted from published literature and subjected to a conversion factor that enabled the overall cancer incidence to reflect best estimates derived from published meta-analyses and the United States National Health Statistics.
In addition to the base-case scenario, extensive sensitivity analyses were conducted using variations around each of the model transitions supported by published literature (Table 1). The simulation was carried out until age 80 years or death, and transitions between various states of metaplasia, dysplasia and cancer were assumed to require at least one year.
Table 1.
Variables
| Description | Base | Low | High | |
|---|---|---|---|---|
| Costs ($U.S. 2008) | ||||
| cost of cancer (annual) | $47,064 | $10,000 | $50,000 | |
| cost of surveillance EGD (with biopsies) | $886 | $350 | $1,000 | |
| cost of surgical morbidity | $35,398 | $17,000 | $70,000 | |
| cost of cancer palliation | $1,652 | $1,000 | $5,000 | |
| cost of perforation during ablation | $54,220 | $10,000 | $100,000 | |
| cost of post surgery state (annual) | $1,426 | $500 | $2,000 | |
| cost of RFA | $7,506 | $3,500 | $15,000 | |
| cost of APC | $5,629 | $2,500 | $12,000 | |
| cost of PDT | $20,000 | $10,000 | $40,000 | |
| cost of MPEC | $6,568 | $3,500 | $14,000 | |
| cost of ablation-related stricture | $2,112 | $900 | $3,000 | |
| cost of esophagectomy | $24,665 | $10,000 | $40,000 | |
| cost of clinic visit | $60 | $25 | $100 | |
| cost of generic proton pump inhibitor (annual) | $91 | $76 | $157 | |
| cost of high-dose proton pump inhibitor (annual) | $3,652 | $599 | $4,529 | |
| discount rate | 0.03 | 0.03 | 0.05 | |
| Transition Rates | ||||
| HGD to cancer | 0.0303 | 0.5 | 0.9 | |
| HGD to LGD | 0.0385 | 0.05 | 0.1 | |
| HGD to ND BE | 0.0055 | 0.01 | 0.15 | |
| LGD to cancer | 0.0138 | 0.005 | 0.05 | |
| LGD to HGD | 0.0275 | 0.01 | 0.078 | |
| LGD to ND BE | 0.3465 | 0.5 | 0.8 | |
| ND BE to cancer | 0.0028 | 0.001 | 0.1 | |
| ND BE to HGD | 0.0055 | 0.0028 | 0.083 | |
| BE to LGD | 0.0275 | 0.01 | 0.078 | |
| mortality in unresectable cancer (annual) | 0.9 | 0.8 | 1 | |
| mortality from non-cancer causes | Varies with age | |||
| Efficacy of Therapy | ||||
| % complete ablation of metaplasia with PDT | 0.60 | 0.00 | 0.80 | |
| In dysplasia* | 0.58 | 0.00 | 0.71 | |
| % complete ablation of metaplasia with RFA | 0.82 | 0.10 | 0.90 | |
| In dysplasia* | 0.64 | 0.10 | 0.90 | |
| % complete ablation of metaplasia with APC | 0.65 | 0.32 | 1.00 | |
| In dysplasia* | 0.62 | 0.28 | 0.97 | |
| % complete ablation of metaplasia with MPEC | 0.75 | 0.62 | 0.81 | |
| % cancer patients who undergo esophagectomy | 0.80 | 0.50 | 1.00 | |
| % cancer cure with esophagectomy | 0.80 | 0.60 | 0.90 | |
| % residual metaplasia with esophagectomy | 0.04 | 0.03 | 0.10 | |
| % residual HGD with esophagectomy | 0.01 | 0.00 | 0.03 | |
| % residual HGD with PDT | 0.12 | 0.00 | 0.50 | |
| % residual HGD with RFA | 0.15 | 0.00 | 0.33 | |
| % residual HGD with APC | 0.19 | 0.00 | 0.56 | |
| % residual LGD with PDT | 0.08 | 0.00 | 0.43 | |
| % residual LGD with RFA | 0.03 | 0.00 | 0.05 | |
| % residual LGD with APC | 0.19 | 0.00 | 0.28 | |
| Complications of Therapy | ||||
| mortality with EGD | 0.000021 | 0 | 0.00005 | |
| mortality from esophagectomy | 0.05 | 0.025 | 0.1 | |
| mortality from esophagectomy with perforation | 0.08 | 0.05 | 0.15 | |
| morbidity from esophagectomy | 0.15 | 0.05 | 0.4 | |
| morbidity from esophagectomy with perforation | 0.2 | 0.1 | 0.5 | |
| perforation with PDT | 0.003 | 0.001 | 0.01 | |
| perforation with APC | 0.0045 | 0.001 | 0.01 | |
| perforation with RFA | 0.0005 | 0.0001 | 0.001 | |
| perforation with MPEC | 0.0005 | 0.0001 | 0.001 | |
| stricture with PDT | 0.185 | 0.1 | 0.3 | |
| stricture with RFA | 0.025 | 0.01 | 0.05 | |
| stricture with APC | 0.026 | 0.01 | 0.05 | |
| stricture with MPEC | 0.025 | 0.01 | 0.05 | |
| Misdiagnosis | ||||
| Actual State | Diagnosed State | |||
| No Dysplasia | LGD | 0.145 | 0.01 | 0.2 |
| No Dysplasia | HGD | 0.01 | 0 | 0.02 |
| No Dysplasia | Cancer | 0.01 | 0 | 0.02 |
| LGD | No Dysplasia | 0.175 | 0.01 | 0.2 |
| LGD | HGD | 0.083 | 0.01 | 0.1 |
| LGD | Cancer | 0.05 | 0.01 | 0.1 |
| HGD | No Dysplasia | 0 | 0 | 0.01 |
| HGD | LGD | 0.115 | 0.01 | 0.2 |
| HGD | Cancer | 0.11 | 0.01 | 0.2 |
| Cancer | LGD | 0.05 | 0.01 | 0.1 |
| Cancer | HGD | 0.175 | 0.01 | 0.2 |
| Utilities | ||||
| utility of Barrett’s without dysplasia | 1 | 0.79 | 1 | |
| utility of LGD | 1 | 0.8 | 1 | |
| utility of HGD | 1 | 0.8 | 1 | |
| utility of cancer | 0.5 | 0 | 1 | |
| utility of post-esophagectomy state | 0.97 | 0.46 | 1 | |
One-way sensitivity analyses used 0% complete ablation with endoscopic ablation EGD: esophagogastroduodenoscopy; RFA: radiofrequency ablation; APC: argon plasma coagulation; MPEC: multipolar electrocoagulation; PDT: photodynamic therapy; HGD: high-grade dysplasia; LGD: low-grade dysplasia; ND: no dysplasia; BE: Barrett’s esophagus
Costs and Utilities
The perspective of the analysis was that of a third-party payer, namely the Center for Medicare and Medicaid Services (CMS). Although CMS generally administers healthcare reimbursement for enrollees 65 years of age and older, the costs incurred by CMS can also be used to benchmark reimbursements for other populations since they represent a national standard that other healthcare insurers follow. Costs, not charges, were the basis for resource inputs in the analysis.15 Costs were limited to the direct health care costs for delivery of services related to BE and esophageal adenocarcinoma and excluded direct non-health care costs (borne by the patient) and productivity or indirect costs (borne by society). Costs were derived from 2007 CMS data using diagnosis-related groups and Current Procedural Terminology codes for inpatient resource expenditures and ambulatory payment classification and Current Procedural Terminology codes for outpatient services rendered. The source for these cost estimates can be found on the CMS website: http://www.cms.hhs.gov. Medication costs were derived from Pharmacy Redbook (base case) and varied in the sensitivity analysis by Internet retail sources. All costs were updated to 2008 estimates using the consumer price index for medical care. Table 1 presents the cost estimates used in the model.
Utilities, or patient preferences for different health states, were derived from published literature and used to convert absolute life-years experienced by cohort members to quality-adjusted life-years (QALYs).21–24 Costs and utilities were discounted at the standard annual rate of 3% (0–5% in sensitivity analysis).
Outcomes
The primary outcome of the study was the incremental cost per discounted and quality-adjusted life year (dQALY) between competing strategies of management, also known as the incremental cost-effectiveness ratio (ICER). The ICER is defined as the difference in cost when moving from one less expensive but less effective strategy to a more expensive but more effective strategy, divided by the change in dQALYs between the same two strategies. Additional outcomes included the mean cost per patient, mean dQALYs per patient and number of cases of esophageal adenocarcinoma estimated to occur with each modeled strategy.
Sensitivity analyses
All assumptions for the model were varied over a wide range of values in a series of one-way sensitivity analyses. Ranges were based on published data: in the absence of published data baseline rates were halved and doubled with adjustment by consensus from the authors. Parameters associated with greater than 25% variation in outcome and clinically important parameters were presented in tornado diagrams. Multivariable sensitivity analysis was performed through Monte-Carlo simulation in which all variables were simultaneously varied over 1,000 trials (second-order simulations). Results of sensitivity analyses were stratified by thresholds for dominance (more effective yet less expensive than comparator strategies) and cost-effectiveness at two levels of willingness to pay (WTP): $50,000 per dQALY gained or $100,000 per dQALY gained, which allowed identification of strategies yielding the greatest benefit without exceeding these cost thresholds. WTP indicates the amount that society is willing to pay in order to achieve a desired health outcome, in this case quality adjusted life-years.
Patient populations
Current management of patients with BE is stratified by the presence and severity of dysplasia diagnosed through histopathological examination of esophageal tissue.2–4 All subjects were assumed to have a correct diagnosis of BE (a change in the esophageal epithelium of any length that can be recognized at endoscopy and is confirmed to have intestinal metaplasia by biopsy of the tubular esophagus and excludes intestinal metaplasia of the cardia) on entry to the study.2 Dysplasia was assumed to consist of cytological and architectural changes in the epithelium of the esophagus, and was divided into low-grade and high-grade dysplasia based on guideline recommendations that stratify clinical management based on these features.2–4 Diagnostic error is problematic in the classification of dysplasia associated with BE and was extensively modeled to reflect the inter- and intra-observer variation in diagnosis.25–30
High-grade dysplasia
The management options for patients with BE and HGD were modeled to consist of no intervention, endoscopic surveillance, PDT, RFA, APC or immediate esophagectomy. No reliable estimates were identified in the literature in which MPEC was used to treat HGD; therefore this modality was not modeled for this patient group. In the absence of endoscopic surveillance, cancer would be detected only through development of symptoms at which time the management of cancer (esophagectomy or palliation) depended on the stage of cancer at the time of diagnosis. Surveillance utilized endoscopy with 4-quadrant biopsies every 1–2 cm along the Barrett’s segment every 3 months. After 12 months surveillance intervals were reassessed based on the highest grade of dysplasia diagnosed during that time period. If HGD was diagnosed at any time surveillance continued at 3 month intervals, whereas LGD or no dysplasia extended surveillance to a 12 month interval. Esophagectomy or endoscopic palliation (stent placement) was utilized for a diagnosis of cancer, depending on the stage of disease at the time of diagnosis. In the base-case scenario, ablative techniques could be either ineffective, ablate dysplasia but have persistent intestinal metaplasia (including subsquamous metaplasia or “buried glands”), or ablate dysplasia and metaplasia. In order to bias the model against interventions, in sensitivity analyses (including Monte Carlo simulations) we assumed complete ablation of metaplasia was never achieved and therefore patients were at continued risk of progression to cancer. Complications of ablation were modeled and included perforation and stricture formation, in addition to mortality. Endoscopic surveillance was assumed to be necessary after ablation for HGD and was conducted based on the diagnosed grade of dysplasia. Esophagectomy was modeled to completely or incompletely remove the Barrett’s segment, and carried risks of mortality or morbidity at published rates.
Low-grade dysplasia
LGD could be managed by no intervention, endoscopic surveillance, PDT, RFA or APC ablation with continued surveillance after complete ablation of intestinal metaplasia, or ablation with surveillance reserved for patients with residual intestinal metaplasia. The base-case transition inputs for the latter strategy were based on RFA data. No reliable estimates were identified in which MPEC was used to treat LGD; therefore this modality was not modeled for this patient group. In the absence of endoscopic surveillance, cancer would be detected only through development of symptoms at which time the management of cancer (esophagectomy or palliation) depended on the stage of cancer at the time of diagnosis. Surveillance utilized endoscopy with 4-quadrant biopsies every 1–2 cm along the Barrett’s segment every 12 months, with esophagectomy reserved for diagnosis of cancer. In the base-case scenario, ablation could be either ineffective, ablate dysplasia but have persistent intestinal metaplasia (including subsquamous metaplasia or “buried glands”), or ablate dysplasia and metaplasia. Again, in order to bias the model against ablation the sensitivity analyses, including Monte Carlo simulations, assumed complete ablation of metaplasia was never achieved.
No dysplasia
Patients with BE without dysplasia could be managed by no intervention, endoscopic surveillance using ablation to treat incident dysplasia, immediate endoscopic ablation with RFA, APC or MPEC with continued endoscopic surveillance after complete ablation of intestinal metaplasia, or immediate ablation with surveillance reserved for patients with residual intestinal metaplasia. The base-case transition inputs for the strategy of surveillance with ablation for incident dysplasia, in addition to the strategy in which ablation was followed by surveillance limited to those with residual metaplasia were based on RFA data. PDT was not modeled due to the lack of suitable published data. The modeling of cancer detection, ablation complications and endoscopic surveillance was identical to the LGD scenario, except that endoscopic surveillance was modeled on current recommendations published by the American Gastroenterological Association that propose a 5-year interval after two endoscopies confirmed the absence of dysplasia.3,4 Ablation could either be ineffective or ablate intestinal metaplasia. The presence of subsquamous metaplasia or “buried glands” was incorporated as a subset of ineffective ablation, which included persistent or recurrent metaplasia after ablation.
Role of the funding source
Support for this study was provided by the National Cancer Institute (R01 CA106773) and National Institute of Diabetes and Digestive and Kidney Diseases (K24 DK080941), National Institutes of Health and an unrestricted research grant from BARRX, the company that developed, manufactures and distributes the radiofrequency ablation device modeled in one arm of this study. The study was designed, conducted, analyzed and reported without influence from BARRX. All sensitivity analyses were performed using a deliberately biased model, in which the least favorable conditions for ablation were used as inputs for the model. Articles in press were provided by the authors or the company.
Results
Natural History
To calibrate the model and compare it with cohorts reporting natural history data in BE, separate runs were conducted simulating populations of patients with BE with HGD, with LGD, and without dysplasia in the absence of interventions to decrease cancer incidence. The model calculated an annual incidence of esophageal adenocarcinoma among patients with BE and HGD equal to 2%, among BE and LGD equal to 0.7% and among BE without dysplasia equal to 0.5%. These results correlated well with recent published estimates of cancer incidence in these cohorts.17,19,29,31–33
Base-Case Results
High-grade dysplasia
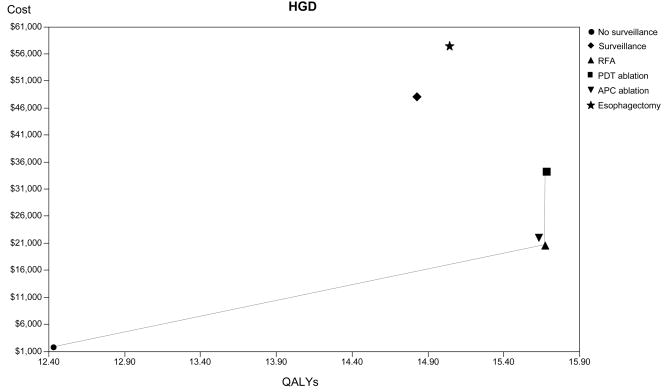
In the absence of interventions to prevent or detect cancer, 50 year old patients with BE and HGD are predicted to live an additional 12.4 dQALYs and consume on average $1,859 in discounted health care resources (Table 2). Compared to no surveillance, strategies employing ablation with PDT, RFA or APC with post-ablation endoscopic surveillance are calculated to extend life by 3.2 dQALYs. APC and RFA are associated with cost increases of $19,000–$20,000 per person, while PDT costs are more than $30,000 greater than no surveillance. Comparison of RFA to no surveillance yields an acceptable ICER of $5,839 per dQALY gained, while the increase in benefit calculated with PDT requires more than $32 million per dQALY gained. The strategy of APC is numerically dominated by RFA; however, the costs and benefits between these ablation techniques are comparable (Figure 2A). Per 100 patients, 33 fewer cancers are predicted to develop with any of the ablation strategies compared to no surveillance. While surveillance or immediate esophagectomy increases life over a strategy of no surveillance, they are dominated by the ablative techniques, since they yield fewer dQALYs at higher cost.
Table 2.
Base-Case Analysis
| Strategy | Cost | dQALYs | Cost-Effectiveness* | ICER | Cancers per 100 population | |
|---|---|---|---|---|---|---|
| HGD | No surveillance | $1,859 | 12.43 | $150 | 37.3 | |
| RFA with surveillance | $20,776 | 15.67 | $1,326 | $5,839 | 4.0 | |
| APC ablation with surveillance | $22,117 | 15.62 | $1,416 | (Dominated) | 4.1 | |
| PDT ablation with surveillance | $34,580 | 15.67 | $2,207 | $32,588,150 | 4.3 | |
| Surveillance | $48,354 | 14.82 | $3,263 | (Dominated) | 7.9 | |
| Esophagectomy | $58,973 | 15.02 | $3,926 | (Dominated) | 1.8 | |
| LGD | No surveillance | $687 | 14.71 | $47 | 14.7 | |
| Ablation without surveillance+ | $12,540 | 15.78 | $795 | $11,147 | 4.5 | |
| APC ablation with surveillance | $13,881 | 15.73 | $882 | (Dominated) | 4.8 | |
| RFA with surveillance | $14,409 | 15.78 | $913 | (Dominated) | 4.4 | |
| Surveillance | $16,210 | 15.38 | $1,054 | (Dominated) | 10.4 | |
| PDT ablation with surveillance | $28,017 | 15.75 | $1,779 | (Dominated) | 4.9 | |
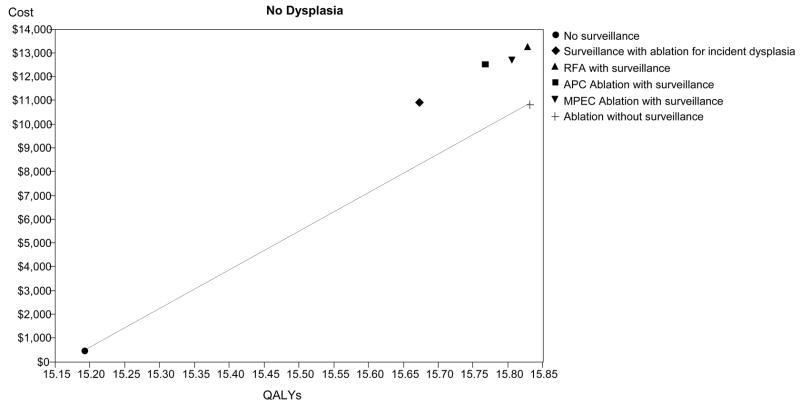
| No Dysplasia | No surveillance | $471 | 15.19 | $31 | 11.0 | |
| Ablation without surveillance+ | $10,876 | 15.83 | $687 | $16,286 | 3.2 | |
| Surveillance with RFA for incident dysplasia | $10,933 | 15.67 | $698 | (Dominated) | 5.8 | |
| APC ablation with surveillance | $12,512 | 15.77 | $794 | (Dominated) | 4.3 | |
| MPEC ablation with surveillance | $12,691 | 15.81 | $803 | (Dominated) | 3.6 | |
| RFA with surveillance | $13,268 | 15.83 | $838 | (Dominated) | 3.1 |
dQALYs: discounted quality-adjusted life-years; ICER: incremental cost-effectiveness ratio; HGD: high-grade dysplasia; LGD: low-grade dysplasia; APC: argon plasma coagulation; RFA: radiofrequency ablation; PDT: photodynamic therapy; MPEC: multipolar electrocoagulation
Ablation without surveillance refers to a strategy in which surveillance is not performed for patients in whom complete ablation of metaplasia has been achieved.
Compared to a common baseline
Ablation modeled using RFA parameter estimates
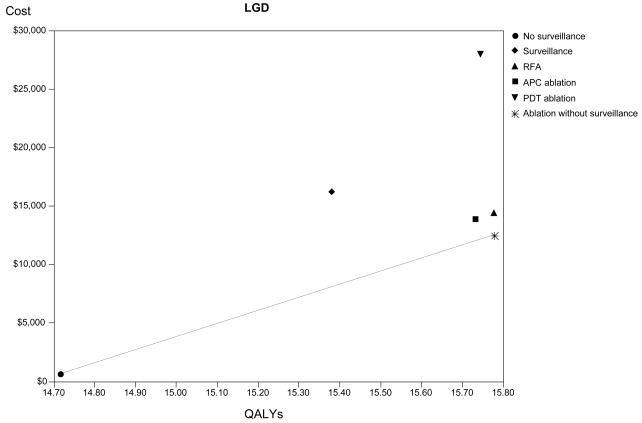
Figure 2.
Cost-effectiveness analysis: The base-case results are depicted for high-grade dysplasia (HGD - Panel A), low-grade dysplasia (LGD - Panel B) and no dysplasia (Panel C). The abscissa depicts discounted quality-adjusted life-years (dQALYs) and the ordinate depicts the costs per patient. The lines connect the non-dominated strategies, and the slope of each line represents the incremental cost-effectiveness ratio (ICER) between strategies.
Low-grade dysplasia
Without intervention, a cohort of patients with BE and LGD is calculated to accrue 14.7 dQALYs and cost $687 per person in healthcare resources (Table 2). Ablation with PDT, RFA or APC followed by surveillance in all patients is predicted to increase life by 1 dQALY compared to no surveillance, with concomitant increases in resource utilization of approximately $13,000 using APC or RFA, or $27,000 with PDT. Numerically, RFA followed by surveillance limited to patients in whom metaplasia persists after ablation dominates all other interventions. However, similar costs and benefits are achieved with this strategy and APC ablation or RFA with surveillance in all patients (Figure 2B). Per 100 patients, 10 fewer cancers are predicted to develop after ablation of any type compared to no surveillance. Compared to ablation, a strategy of surveillance alone is dominated, being both more expensive and less effective than RFA or APC ablation.
No dysplasia
Following the natural history of patients with BE without dysplasia, 15.2 dQALYs are expected with costs of managing incident cancers averaging $471 per person (Table 2). The most cost-effective strategy consists of endoscopic ablation (using RFA parameters) with surveillance limited to patients for whom ablation fails to eradicate metaplasia, yielding an ICER of $16,286 per dQALY gained over no surveillance. Endoscopic surveillance using RFA to treat incident dysplasia is dominated by this strategy, as are strategies employing APC, MPEC or RFA that continue surveillance in patients for whom ablation successfully eradicates metaplasia. However, if discontinuation of surveillance after successful ablation is not a clinically viable option, a strategy of surveillance using ablation for incident dysplasia is equally cost-effective compared to strategies of ablation with APC, MPEC or RFA with surveillance continued in all patients (Figure 2C).
Sensitivity analysis
High grade dysplasia
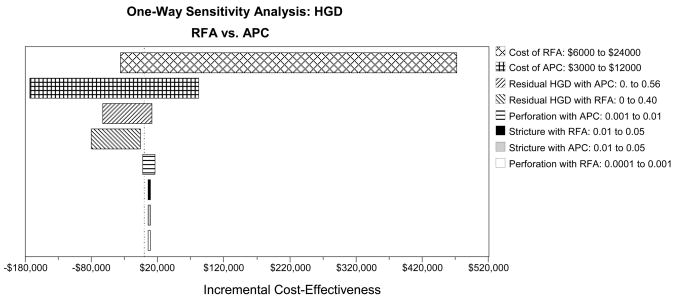
All sensitivity analyses conducted in the population of patients with BE and HGD were biased against ablation by assuming that complete elimination of metaplasia was never achieved. The remaining variables in the model were subject to one-way sensitivity analysis using the range of values indicated in Table 1. The results of these analyses (Table 3) illustrate that ablation is cost-effective as long as at least 25% of patients achieve complete remission from dysplasia. RFA is the preferred ablation strategy as long as the proportion of patients with residual HGD after RFA is less than 17%–18%, otherwise APC is preferred. If the residual HGD after RFA or APC is greater than 23% (WTP $100,000) or 30% (WTP $50,000), PDT is a cost-effective alternative. The costs of competing ablation strategies impact the preferred technique, and the range of variation is well within the plausible boundaries of clinical practice. Figure 3A illustrates the change in the ICER comparing strategies of RFA versus APC among patients with BE and HGD. The greatest variation in ICER results from changes in the cost of ablation therapy and the proportion of patients in whom HGD persists after ablation therapy. Minor changes occur with variation in the complications of ablation therapy.
Table 3.
Threshold Analyses
| HGD | Strategy Change |
|---|---|
| Residual HGD after Ablation | Assume No Complete Ablation of Metaplasia |
| RFA: ≤ 0.17 | RFA Cost-Effective |
| RFA: > 0.17 | APC Cost-Effective (WTP $100,000) |
| RFA: > 0.18 | APC Cost-Effective (WTP $50,000) |
| RFA/APC: > 0.23 | PDT Cost-Effective (WTP $100,000) |
| RFA/APC: > 0.30 | PDT Cost-Effective (WTP $50,000) |
| RFA/APC/PDT: > 0.75 | Surveillance Cost-effective (WTP $100,000) |
| Cost of Ablation | |
| RFA: ≤ $8,500 | RFA Cost-Effective |
| RFA: > $9,000 | APC Cost-Effective (WTP $100,000) |
| RFA: > $11,000 | APC Cost-Effective (WTP $50,000) |
| RFA/APC: > $17,500 | PDT Cost-Effective (WTP $100,000) |
| RFA/APC: > $18,500 | PDT Cost-Effective (WTP $50,000) |
| LGD | |
| Residual LGD after Ablation+ | Assume No Complete Ablation of Metaplasia |
| < 54% | Ablation Cost-Effective (WTP $50,000) |
| < 72% | Ablation Cost-Effective (WTP $100,000) |
| ≥72% | Surveillance Cost-Effective |
| Cost of Ablation+ | |
| ≤$15,000 | Ablation Cost-Effective (WTP $50,000) |
| < $26,000 | Ablation Cost-Effective (WTP $100,000) |
| ≥$26,000 | Surveillance Cost-Effective |
| Cost of EGD+ | |
| No threshold identified | Ablation Cost-Effective or Dominant |
| Perforation or Stricture with Ablation+ | |
| No threshold identified | Ablation Cost-Effective or Dominant |
| No Dysplasia | |
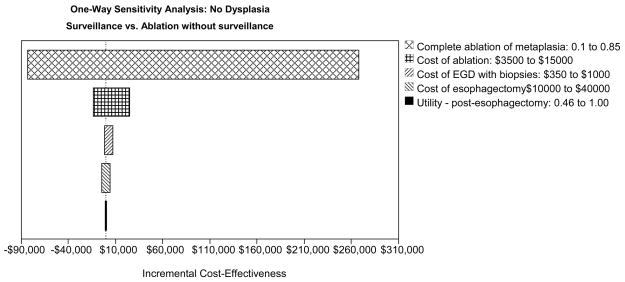
| Complete Ablation of Metaplasia+ | |
| ≤40% | Surveillance Cost-Effective |
| > 40% | Ablation Without Surveillance Cost-Effective (WTP $100,000) |
| > 47% | Ablation Without Surveillance Cost-Effective (WTP $50,000) |
| Ablation without surveillance always preferred over ablation with surveillance | |
| Cost of Ablation+ | |
| No threshold identified | Ablation without surveillance cost-effective |
HGD: high-grade dysplasia; LGD: low-grade dysplasia; RFA: radiofrequency ablation; APC: argon plasma coagulation; PDT: photodynamic therapy; EGD: esophagogastroduodenoscopy; WTP: willingness to pay
Ablation based on RFA parameter estimates
Figure 3.
One-way sensitivity analyses: Tornado diagrams for high-grade dysplasia (HGD - Panel A), low-grade dysplasia (LGD - Panel B) and no dysplasia (Panel C) depict the range of incremental cost-effectiveness ratios by varying each of the examined variables. APC: argon plasma coagulation; PDT: photodynamic therapy; RFA: radiofrequency ablation; MPEC: multipolar electrocoagulation; EGD: esophagogastroduodenoscopy.
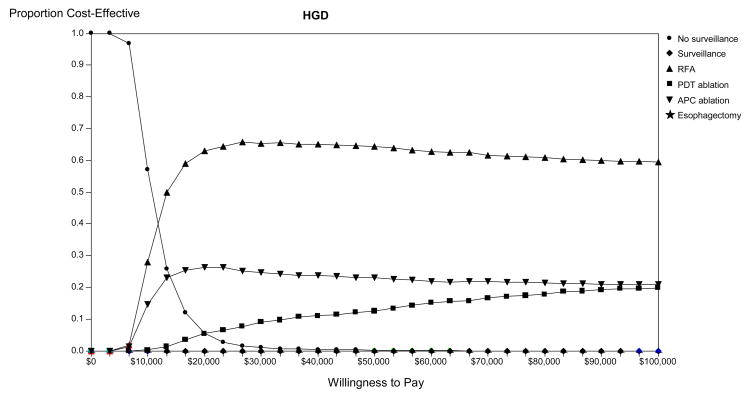
Figure 4A presents the results of the Monte Carlo simulation illustrating the proportion of trials in which each strategy is preferred for management of patients with BE and HGD. Using a threshold WTP between $0 and $100,000 per dQALY gained, ablation is the preferred strategy for the majority of trials. RFA, APC and PDT are preferred to surveillance or esophagectomy in the majority of simulations at even low thresholds, and are preferred in all trials above a WTP threshold of $50,000 per QALY. No surveillance is viable when the WTP less than $30,000, while surveillance alone or esophagectomy are preferred in less than 1% of trials overall.
Figure 4.
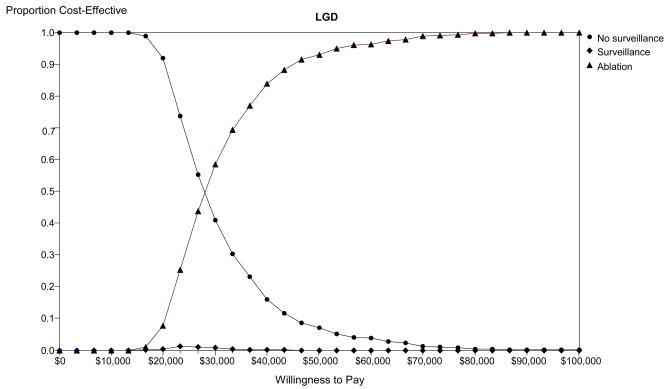
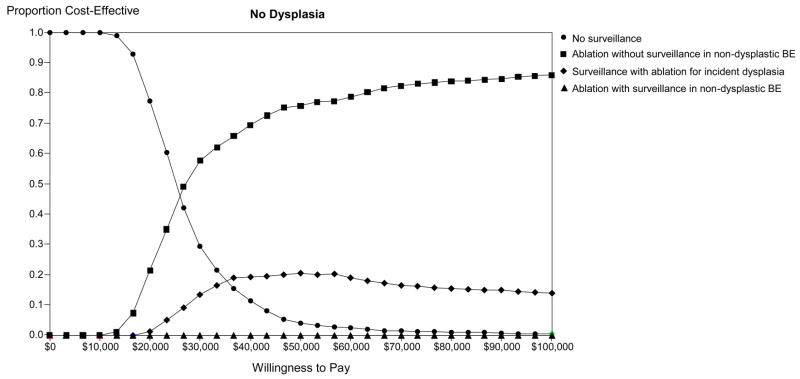
Monte-Carlo simulation: Optimal strategy stratified by willingness to pay in populations comprised of high-grade dysplasia (HGD - Panel A), low-grade dysplasia (LGD - Panel B) and no dysplasia (Panel C). The lines illustrate the proportion of trials in which each strategy was calculated to comprise the optimal strategy, defined as the strategy associated with the greatest dQALYs obtainable within a willingness to pay of $0 to $100,000 per discounted quality-adjusted life-year gained. BE: Barrett’s esophagus; APC: argon plasma coagulation; PDT: photodynamic therapy; RFA: radiofrequency ablation.
Low grade dysplasia
As with HGD, all sensitivity analyses conducted in the population of patients with BE and LGD were biased against ablation by assuming complete elimination of metaplasia is never achieved. Note that with this assumption, the strategy in which surveillance is halted after complete ablation of metaplasia becomes identical to the strategy in which surveillance continues despite absence of metaplasia. Since little difference in outcomes exists between different ablation techniques these are collectively modeled and compared to surveillance or no surveillance strategies. The model is sensitive to the proportion of patients in whom residual LGD persists after ablation. The preferred strategy changes from ablation to surveillance if the proportion is greater than 54% (WTP $50,000) or greater than 72% (WTP $100,000) (Table 3). When the cost of ablation exceeds $15,000 (WTP $50,000) or $26,000 (WTP $100,000), surveillance also becomes cost-effective. No variation in the cost of surveillance endoscopy or the rate of complications associated with ablation within the ranges identified in Table 1 change the preferred strategy (Figure 3B).
Figure 4B presents the results of the Monte Carlo simulation illustrating the proportion of trials in which each strategy was preferred for management of patients with BE and LGD. Using a threshold WTP between $0 and $100,000 per dQALY gained, ablation is the preferred strategy in the majority of trials. No surveillance is preferred in the majority of trials when the WTP drops below $30,000 per QALY. Surveillance is preferred in less than 1% of trials.
No dysplasia
In the base case scenario the optimal strategy requires discontinuation of surveillance after successful ablation of metaplasia. Sensitivity analysis reveals this to be a robust finding as long as complete ablation of metaplasia is achieved in at least 40% (WTP $100,000 per dQALY gained) to 47% (WTP $50,000 per dQALY gained) of cases (Table 3). If ablation is completely successful in less than 40% of cases then surveillance is optimal. Comparing endoscopic surveillance to ablation with no surveillance after complete ablation, the influential variables include the proportion of patients in whom complete ablation of metaplasia is achieved and the cost of ablation. The cost of surgery or endoscopy influences the cost-effectiveness to a smaller degree, while patient preference (utility) of the post-esophagectomy state has little impact on the ICER (Figure 3C). The effectiveness of surgery or complications of surgery and ablation also do not impact the preferred strategy (data not shown).
Monte Carlo simulation reveals that ablation followed by surveillance limited to those with residual metaplasia is optimal in up to 85% of trials, increasing with higher levels of willingness to pay (Figure 4C). Surveillance using ablation for incident dysplasia is preferred in 20% or fewer trials, depending on the WTP. More importantly, ablation with continued surveillance in all patients is optimal in 1% or less of trials. No surveillance is preferred in this population more often than either of the two latter strategies, and is optimal among all strategies if the WTP is less than $25,000.
Discussion
Our analysis of the cost-effectiveness of endoscopic ablation of BE demonstrates that ablation of HGD in subjects with BE is probably cost-effective, capable of providing over three years of additional quality-adjusted life expectancy at a cost of less than $6,000 per dQALY gained. Despite biasing the model against ablation, this strategy is estimated to be more effective and less costly than surveillance or esophagectomy for patients with BE and HGD. The effectiveness of the different ablation techniques appears similar, with variation only in costs. In LGD, ablative therapy may be cost-effective compared to surveillance; however, continued endoscopic surveillance of patients after successful reversion to normal squamous epithelium yields little benefit. In the setting of non-dysplastic BE, ablation may be the preferred strategy if the proportion of patients in whom complete and permanent ablation is achieved is greater than 40% (WTP $100,000 per dQALY gained), and endoscopic surveillance can be discontinued in patients in whom ablation is successful.
A goal of decision analysis is to illuminate important gaps in our knowledge and to highlight key questions that must be answered in order to determine whether a proposed intervention should be implemented. In the present analysis, perhaps the single most important “unknown” is the prognosis of subjects who have undergone ablation, and are now endoscopically and histologically normal. Are these patients no longer at risk for developing cancer, and can they be released from endoscopic surveillance? Currently insufficient data exist to answer this question and presently, we and others are retaining these subjects in endoscopic surveillance programs. Because of uncertainty regarding the prognosis of these patients, we modeled both the currently practiced strategy of maintaining surveillance in these subjects, as well as the strategy of releasing successfully ablated patients from surveillance. Recent studies have questioned the benefit of endoscopic surveillance and our analyses illustrate only a nominal benefit of endoscopic surveillance.34 In all scenarios continuation of surveillance after successful ablation is not a preferred strategy. Whether a strategy of selective follow-up of patients based on ablation results can be justified awaits further outcomes data from cohorts having undergone ablative therapy.
Ablative strategies may appear cost-effective either by being superior modes of therapy, or because the comparator strategies are poor interventions. This analysis suggests that endoscopic surveillance may be an especially inefficient and cost-ineffective strategy. Previous studies have demonstrated the poor cost-effectiveness of surveillance to decrease mortality from esophageal adenocarcinoma.35 From a broad perspective, it is more effective (and cost-effective) to prevent cancer than to treat cancer. It is the intent of this analysis not to promote one specific preventive or ablative technique, but rather to highlight the fundamental advantages of interventions that reduce cancer incidence, especially if the complications associated with the intervention are uncommon. This concept is exemplified in the model of no dysplasia. Ablation is preferred in this group, but only if we are willing to forego continued surveillance for patients in whom metaplasia has been eliminated, even if there remains a small risk of progression to malignancy.
The sensitivity analyses illustrate the key factors influential in the decisions regarding management of patients with BE. Common to all scenarios is the dependence of the results on the efficacy of ablation, the durability of these effects and ultimately whether cancer incidence is reduced after ablation is achieved. Thus, the effectiveness and cost-effectiveness of ablative methods will not be fully understood until such data can be accumulated from patients enrolled in research protocols. The costs of various interventions are also influential but rarely change the order of preferred strategies. Other variables, including the risk or cost of complications, the effectiveness of surgery and the preference of patients for various health states (utilities) do not appear to influence the outcomes.
Our analysis has some important limitations. The values used to populate the model were derived from selected groups of patients in most cases referred to specialized centers and prone to selection and publication bias. Because we felt it important to have our model conform to national cancer statistics we calibrated the natural history of the model to the lowest rates of cancer incidence reported in published literature, thereby biasing our results against the use of any intervention. Also, we have assumed that subjects in whom ablation was unsuccessful or in whom metaplasia recurred after ablation retained the cancer risk of their initial level of dysplasia. Some data suggest that a failed attempt at ablation may actually result in the expression of precancerous markers that could potentially put these patients at a higher risk for neoplasia.36
We chose to examine the management of patients with established BE in this study while excluding the question of whether screening to identify this group is cost-effective. Our earlier work compared the relative impact of screening vs. surveillance and found that while screening 50-year old white men with heartburn to detect cancer and dysplasia would likely be cost-effective, continued surveillance of patients without dysplasia was an expensive strategy.35 The current study expands upon this theme in which surveillance is illustrated to be both ineffective and costly, due to the relatively infrequent development of dysplasia and cancer among patients not diagnosed with dysplasia during the initial screening and the inaccuracy of dysplasia as a marker of progression to malignancy. Development and validation of biomarkers that better predict the development of cancer among people with BE would improve the effectiveness of surveillance, as well as enhance selection of patients most appropriate for ablation.37 A greater question raised through these studies is whether any intervention for patients diagnosed with BE without dysplasia is warranted. Based on the marginal differences in cost-effectiveness between ablation and surveillance, perhaps even ablation in non-dysplastic BE requires resources that are beyond the threshold society is willing to expend; however, this study focuses on optimizing management of patients with established BE while our previous work focused comparing the benefit of the initial screen vs. subsequent surveillance to detect early cancer. More broadly, the results of this study are not limited to valuation of endoscopic ablation but rather provide a set of general principles upon which interventions may be compared to current standards of care. The threshold analyses provided in this paper may be used as a benchmark for any future intervention developed to reduce mortality from esophageal adenocarcinoma. The main concept we wish to convey is that strategies that reduce cancer development should be preferred over strategies that rely on early diagnosis of cancer.
Our data are in line with previous studies of cost-effectiveness of ablation for BE compared to either surveillance or esophagectomy.38–41 Although absolute results have varied across studies, these have shown ablation to be superior to comparator arms in the treatment of HGD. Even when ablation is used to treat incident dysplasia identified through surveillance, the recurrent costs of endoscopy, poor reproducibility of histological interpretation and low incidence of cancer in subjects under surveillance combine to make endoscopic surveillance strategies unfavorable, especially in those with non-dysplastic disease.
In conclusion, our data suggest that endoscopic ablation may be the preferred strategy for the management of BE with HGD. Ablation may also be preferred in subjects with LGD and non-dysplastic disease, but the cost-effectiveness of this strategy is highly dependent on the long-term effectiveness of ablation therapy and whether surveillance endoscopy can be discontinued following a successful ablative procedure. As further post-ablation data become available, the optimal strategy to be implemented will be clarified.
Acknowledgments
Grant Support: National Cancer Institute (R01 CA106773 - JMI) and National Institute of Diabetes and Digestive and Kidney Diseases (K24 DK080941 - JMI), National Institutes of Health Unrestricted research grant from BARRX
Abbreviations
- AE
adverse event
- BE
Barrett’s esophagus
- CA
cancer
- CR
complete remission
- CR-D
complete remission of dysplasia
- CR-IM
complete remission of intestinal metaplasia
- dQALY
discounted quality adjusted life years
- EGD
esophagogastroduodenoscopy
- EMR
endoscopic mucosal resection
- HGD
high-grade dysplasia
- ICER
incremental cost-effectiveness ratio
- IM
intestinal metaplasia
- IMC
intramucosal cancer
- LGD
low-grade dysplasia
- NA
not applicable
- ND BE
non-dysplastic Barrett’s esophagus
- PDT
photodynamic therapy
- QALYs
quality adjusted life years
- Quad
quadrant
- RFA
radiofrequency ablation
- WTP
willingness to pay
- 4 quad q1(−2)cm
4 quadrant biopsies every 1 (to 2) cm
Footnotes
Financial Disclosures: R. Madanick has received an honorarium and N. Shaheen and J. Inadomi have received research support from Barrx Medical, maker of the radiofrequency ablation device; the other authors do not have conflicts to disclose.
Writing Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Lieberman DA, Oehlke M, Helfand M. Risk factors for Barrett’s esophagus in community-based practice. GORGE consortium. Gastroenterology Outcomes Research Group in Endoscopy. Am J Gastroenterol. 1997;92:1293–1297. [PubMed] [Google Scholar]
- 2.Wang KK, Sampliner RE. Updated Guidelines 2008 for the Diagnosis, Surveillance and Therapy of Barrett’s Esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang KK, Wongkeesong M, Buttar NS. American Gastroenterological Association technical review on the role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology. 2005;128:1471–1505. doi: 10.1053/j.gastro.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 4.Wang KK, Wongkeesong M, Buttar NS. American Gastroenterological Association medical position statement: Role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology. 2005;128:1468–1470. doi: 10.1053/j.gastro.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ. Advances in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology. 2005;128:1554–1566. doi: 10.1053/j.gastro.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Reed MF, Tolis G, Jr, Edil BH, et al. Surgical treatment of esophageal high-grade dysplasia. Ann Thorac Surg. 2005;79:1110–1115. doi: 10.1016/j.athoracsur.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Spechler SJ. Dysplasia in Barrett’s esophagus: limitations of current management strategies. Am J Gastroenterol. 2005;100:927–935. doi: 10.1111/j.1572-0241.2005.41201.x. [DOI] [PubMed] [Google Scholar]
- 8.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc. 2007;66:460–468. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Pech O, Gossner L, May A, et al. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett’s cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc. 2005;62:24–30. doi: 10.1016/s0016-5107(05)00333-0. [DOI] [PubMed] [Google Scholar]
- 10.Hage M, Siersema PD, van Dekken H, et al. 5-aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barrett’s oesophagus: a randomised trial. Gut. 2004;53:785–790. doi: 10.1136/gut.2003.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P, Wani S, Weston AP, et al. A randomised controlled trial of ablation of Barrett’s oesophagus with multipolar electrocoagulation versus argon plasma coagulation in combination with acid suppression: long term results. Gut. 2006;55:1233–1239. doi: 10.1136/gut.2005.086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Seewald S, Ang TL, Soehendra N. Endoscopic mucosal resection of Barrett’s oesophagus containing dysplasia or intramucosal cancer. Postgrad Med J. 2007;83:367–372. doi: 10.1136/pgmj.2006.054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard N, Velanovich V. Endoscopic endoluminal radiofrequency ablation of Barrett’s esophagus in patients with fundoplications. Surg Endosc. 2007;21:625–628. doi: 10.1007/s00464-007-9199-7. [DOI] [PubMed] [Google Scholar]
- 15.Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 16.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–458. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 17.Hage M, Siersema PD, van Dekken H, et al. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39:1175–1179. doi: 10.1080/00365520410003524. [DOI] [PubMed] [Google Scholar]
- 18.Guardino JM, Khandwala F, Lopez R, et al. Barrett’s esophagus at a tertiary care center: association of age on incidence and prevalence of dysplasia and adenocarcinoma. Am J Gastroenterol. 2006;101:2187–2193. doi: 10.1111/j.1572-0241.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566–572. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava A, Hornick JL, Li X, et al. Extent of low-grade dysplasia is a risk factor for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2007;102:483–493. doi: 10.1111/j.1572-0241.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- 21.Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 22.Provenzale D, Kemp JA, Arora S, et al. A guide for surveillance of patients with Barrett’s esophagus. Am J Gastroenterol. 1994;89:670–680. [PubMed] [Google Scholar]
- 23.Fisher D, Jeffreys A, Bosworth H, et al. Quality of life in patients with Barrett’s esophagus undergoing surveillance. Am J Gastroenterol. 2002;97:2193–2200. doi: 10.1111/j.1572-0241.2002.05972.x. [DOI] [PubMed] [Google Scholar]
- 24.Gerson LB, Ullah N, Hastie T, et al. Patient-derived health state utilities for gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:524–533. doi: 10.1111/j.1572-0241.2005.40588.x. [DOI] [PubMed] [Google Scholar]
- 25.Reid BJ, Haggitt RC, Rubin CE, et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 26.Alikhan M, Rex D, Khan A, et al. Variable pathologic interpretation of columnar lined esophagus by general pathologists in community practice. Gastrointest Endosc. 1999;50:23–26. doi: 10.1016/s0016-5107(99)70339-1. [DOI] [PubMed] [Google Scholar]
- 27.Sagan C, Flejou JF, Diebold MD, et al. Reproducibility of histological criteria of dysplasia in Barrett mucosa. Gastroenterol Clin Biol. 1994;18:D31–D34. [PubMed] [Google Scholar]
- 28.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 29.Reid BJ, Levine DS, Longton G, et al. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery E, Goldblum JR, Greenson JK, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: A follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32:379–388. doi: 10.1053/hupa.2001.23511. [DOI] [PubMed] [Google Scholar]
- 31.Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 32.Weston AP, Sharma P, Topalovski M, et al. Long-term follow-up of Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:1888–1893. doi: 10.1111/j.1572-0241.2000.02234.x. [DOI] [PubMed] [Google Scholar]
- 33.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 34.Rubenstein JH, Sonnenberg A, Davis J, et al. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. gastrointest endosc. 2008;68:849–855. doi: 10.1016/j.gie.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 36.Hage M, Siersema PD, Vissers KJ, et al. Genomic analysis of Barrett’s esophagus after ablative therapy: persistence of genetic alterations at tumor suppressor loci. Int J Cancer. 2006;118:155–160. doi: 10.1002/ijc.21302. [DOI] [PubMed] [Google Scholar]
- 37.Rubenstein JH, Vakil N, Inadomi JM. The cost-effectiveness of biomarkers for predicting the development of oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2005;22:135–146. doi: 10.1111/j.1365-2036.2005.02536.x. [DOI] [PubMed] [Google Scholar]
- 38.Vij R, Triadafilopoulos G, Owens DK, et al. Cost-effectiveness of photodynamic therapy for high-grade dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2004;60:739–756. doi: 10.1016/s0016-5107(04)02167-4. [DOI] [PubMed] [Google Scholar]
- 39.Shaheen NJ, Inadomi JM, Overholt BF, et al. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis Gut. 2004;53:1736–1744. doi: 10.1136/gut.2003.033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of photodynamic therapy for treatment of Barrett’s esophagus with high grade dysplasia. Dig Dis Sci. 2003;48:1273–1283. doi: 10.1023/a:1024146823549. [DOI] [PubMed] [Google Scholar]
- 41.Comay D, Blackhouse G, Goeree R, et al. Photodynamic therapy for Barrett’s esophagus with high-grade dysplasia: a cost-effectiveness analysis. Can J Gastroenterol. 2007;21:217–222. doi: 10.1155/2007/791062. [DOI] [PMC free article] [PubMed] [Google Scholar]