Abstract
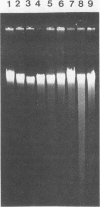
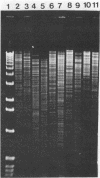
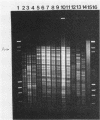
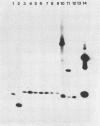
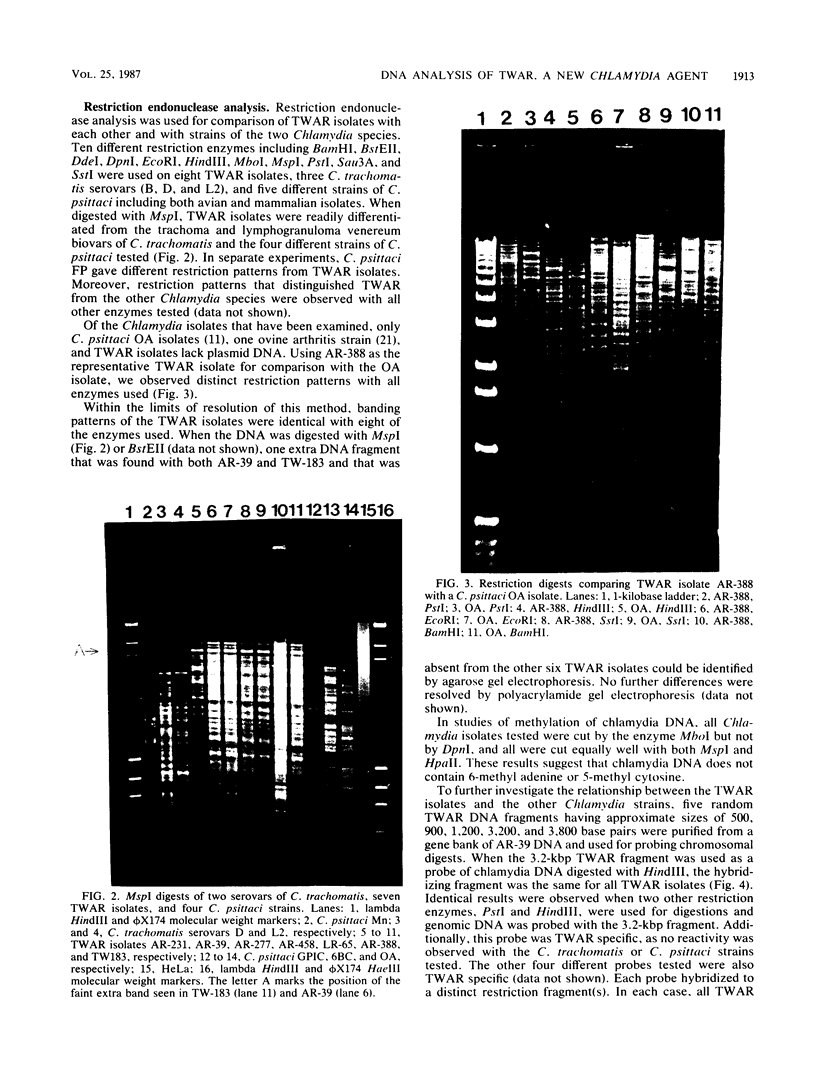
Several molecular techniques were used for comparison of the novel Chlamydia agent, TWAR, with Chlamydia trachomatis and Chlamydia psittaci. Unlike all serotypes of C. trachomatis and most strains of C. psittaci, the eight TWAR isolates examined did not contain extrachromosomal DNA. TWAR was readily distinguished from C. trachomatis or C. psittaci by restriction endonuclease analysis, whereas identical or nearly identical restriction patterns were observed among the TWAR isolates. Southern blot analysis with a gene encoding a portion of the C. trachomatis serovar L2 major outer membrane protein as the probe showed that TWAR, like C. psittaci, contained sequences homologous to this gene. However, while the hybridization patterns were identical for all TWAR isolates, they differed from those of any of the other Chlamydia species tested. A PstI gene bank containing TWAR DNA was constructed in pUC19. Random fragments were purified and used for probing Chlamydia chromosomal digests. All of the five probes tested were TWAR specific, with the TWAR isolates showing identical patterns of homology. Qualitative studies of the DNA homology revealed that TWAR did not have significant homology to any of the Chlamydia strains assayed. Collectively, these results demonstrate that the TWAR isolates represent a single strain or closely allied genotypes and are clearly distinct from any of the other chlamydiae tested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chi E. Y., Kuo C. C., Grayston J. T. Unique ultrastructure in the elementary body of Chlamydia sp. strain TWAR. J Bacteriol. 1987 Aug;169(8):3757–3763. doi: 10.1128/jb.169.8.3757-3763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir S. P., Hakomori S., Kenny G. E., Grayston J. T. Immunochemical studies on chlamydial group antigen (presence of a 2-keto-3-deoxycarbohydrate as immunodominant group). J Immunol. 1972 Jul;109(1):116–122. [PubMed] [Google Scholar]
- Fuchs R., Blakesley R. Guide to the use of type II restriction endonucleases. Methods Enzymol. 1983;100:3–38. doi: 10.1016/0076-6879(83)00043-9. [DOI] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph T., Nano F. E., Garon C. F., Caldwell H. D. Molecular characterization of Chlamydia trachomatis and Chlamydia psittaci plasmids. Infect Immun. 1986 Feb;51(2):699–703. doi: 10.1128/iai.51.2.699-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY E. S. GUINEA PIG INCLUSION CONJUNCTIVITIS VIRUS. I. ISOLATION AND IDENTIFICATION AS A MEMBER OF THE PSITTACOSIS-LYMPHOGRANULOMA-TRACHOMA GROUP. J Infect Dis. 1964 Feb;114:1–12. doi: 10.1093/infdis/114.1.1. [DOI] [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Freeman B. A. Fractionation of Phenol Extracts from Brucella suis: Separation of Multiple Biologically Active Components. Infect Immun. 1970 Sep;2(3):244–249. doi: 10.1128/iai.2.3.244-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martinez J. A., Storz J. Antigenic diversity of Chlamydia psittaci of mammalian origin determined by microimmunofluorescence. Infect Immun. 1985 Dec;50(3):905–910. doi: 10.1128/iai.50.3.905-910.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Characterization of Chlamydia DNA by restriction endonuclease cleavage. Infect Immun. 1983 Aug;41(2):604–608. doi: 10.1128/iai.41.2.604-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Wang S. P., Kleemola M., Brander E., Rusanen E., Grayston J. T. An epidemic of mild pneumonia due to an unusual strain of Chlamydia psittaci. J Infect Dis. 1985 May;151(5):832–839. doi: 10.1093/infdis/151.5.832. [DOI] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Schachter J., Meyer K. F. Lymphogranuloma venereum. II. Characterization of some recently isolated strains. J Bacteriol. 1969 Sep;99(3):636–638. doi: 10.1128/jb.99.3.636-638.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Kuo C. C., Newport G., Agabian N. Molecular cloning and expression of Chlamydia trachomatis major outer membrane protein antigens in Escherichia coli. Infect Immun. 1985 Mar;47(3):713–718. doi: 10.1128/iai.47.3.713-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970 Sep;70(3):367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]