Summary
Leucine is recognized as a nutrient signal, however the long-term in vivo consequences of leucine signaling and the role of branched chain amino acid (BCAA) metabolism in this signaling remains unclear. To investigate these questions, the BCATm gene encoding the enzyme catalyzing the first step in peripheral BCAA metabolism was disrupted. BCATm−/− mice exhibited elevated plasma BCAAs, decreased adiposity and body weight, despite eating more food, along with increased energy expenditure, remarkable improvements in glucose and insulin tolerance, and protection from diet induced obesity. The increased energy expenditure did not seem to be due to altered locomotor activity, uncoupling proteins, sympathetic activity, and thyroid hormones but was strongly associated with food consumption and an active futile cycle of increased protein degradation and synthesis. These observations suggest that either elevated BCAAs and/or loss of BCAA catabolism in peripheral tissues play an important role in regulating insulin sensitivity and energy expenditure.
Introduction
Abundant food supplies and sedentary lifestyle contribute to the epidemic of obesity in Western countries. Obesity results from the positive balance of energy intake and expenditure, i.e., energy intake exceeds energy expenditure. Total energy expenditure consists of obligatory energy expenditure, physical activity, and adaptive thermogenesis (Lowell and Spiegelman, 2000). Adaptive thermogenesis is particularly influenced by environmental temperature and diet, the latter is termed diet-induced thermogenesis (DIT). Despite extensive research, the pathogenesis of human obesity is not fully elucidated; and the prevention and treatment of human obesity has proved difficult. Yet, recent studies in humans suggest that increasing dietary protein may improve body weight control by poorly defined mechanisms that appear to involve both satiety and energy expenditure (Halton and Hu, 2004; Westerterp, 2004). In both short-term and relatively long-term studies, diets with high-protein and low-fat contents were shown to increase energy expenditure, while short-term protein intake induces satiety (Johnston et al., 2002; Leidy et al., 2007; Lejeune et al., 2006). After a fast in humans, whole-body nitrogen turnover and the thermic response to protein diet feeding were found to be significantly greater when compared with a high carbohydrate meal (Robinson et al., 1990). This may be due to the fact that both protein synthesis and proteolysis are energy demanding processes (Reeds, 1985). Thus, protein intake and metabolism positively affects energy expenditure.
The effects of dietary protein are thought to be mediated, at least in part, by the essential amino acid, leucine (Leu), and perhaps the other branched chain amino acids (BCAAs). Leu is recognized as a nutrient signal and is an efficacious regulator of protein turnover through stimulating protein synthesis and inhibiting protein degradation (Buse and Reid, 1975; Fulks et al., 1975). Its stimulation of protein synthesis is linked to activation of a cell signaling pathway involving the mammalian target of rapamycin complex 1 (mTORC1) (Kimball and Jefferson, 2006). An in vitro study has shown that a metabolite(s) of Leu catabolism inhibits proteolysis; while intracellular Leu not Leu metabolites regulates protein synthesis (Tischler et al., 1982). Like dietary protein, Leu has been linked to satiety, body weight control and whole body energy expenditure. For example, Leu has been reported to directly stimulate mTOR signaling in the hypothalamus leading to decreased food intake (Cota et al., 2006). In addition, Leu may influence satiety by stimulating of leptin secretion (Lynch et al., 2006). Dietary supplements of Leu or BCAAs have been shown to decrease fat mass and body weight and to improve glucose metabolism in some cases (Bianchi et al., 2005; Donato et al., 2006; Gordon-Elliott and Margolese, 2006; Layman and Walker, 2006; Mourier et al., 1997; Zhang et al., 2007). These findings suggest that BCAA supplements may be beneficial in controlling obesity.
Paradoxically other findings are not consistent with an anti-obesity role of dietary Leu and Leu signaling. For example, hyperactivation of the TORC1 signaling pathway resulting from over-nutrition, which includes excessive Leu intake, appears to worsen insulin resistance in obesity (Khamzina et al., 2005; Um et al., 2006; Um et al., 2004). In addition, plasma BCAA concentrations are elevated in humans and animal models of obesity (Felig et al., 1969; Rafecas et al., 1991; Wijekoon et al., 2004). Thus, further research is needed to clarify the physiological role of Leu and its potential for protecting or worsening obesity.
In order to examine the effects of persistently elevating plasma Leu resulted from blockage of BCAA metabolism, we have generated and characterized mice in which the gene encoding the mitochondrial branched chain aminotransferase isozyme (BCAT2) has been disrupted. This enzyme catalyzes the first step in BCAA metabolism, which is transfer of the α-amino group of a BCAA to α-ketoglutarate to form glutamate and the three respective branched chain α-keto-acids. BCATm is expressed in most non-neuronal tissues except liver, while the cytosolic isozyme (BCATc) is expressed in the central nervous system (CNS) and in peripheral nerves (Hutson et al., 1992; Suryawan et al., 1998; Sweatt et al., 2004). The expression pattern of BCAA catabolic enzymes in body tissues serves to regulate Leu signaling (Lynch et al., 2003) and to promote interorgan exchange of BCAA metabolites (Suryawan et al., 1998). Because peripheral BCAA catabolism is blocked in the BCATm−/− mice, plasma BCAA concentrations are elevated chronically. These animals consume more food and have increased DIT and are lean when compared with the wild-type mice. In addition, their protein turnover rate is elevated. We propose that increased protein synthesis and degradation directly contribute to increased energy expenditure in mice lacking peripheral BCAA metabolism.
Results
Growth curve, food intake, plasma concentrations of hormones, amino acids and other metabolites
The targeting of the BCATm gene and generation of the conditional and total null alleles using the Cre-loxP system (Figures S1A–S1D) are described in the Supplemental Data available with this article online. As expected, BCATm protein was not detectable in skeletal muscle, kidney, pancreas, brain or adipose tissue of BCATm−/− mice (Figure 1A). Heterozygotes had approximately half as much BCATm protein in measured tissues (data not shown). Liver, which does not express BCATm, showed no detectable bands in both BCATm+/+ and BCATm−/− mice (Figure 1A). BCATm activity was 628 ± 25, 291 ± 18, −0.2 ± 1.5 mU/g tissue (n=2) in gastrocnemius muscle of BCATm+/+, BCATm+/−, and BCATm−/− mice, respectively. Consistent with expression of BCATc exclusively in neurons (Sweatt et al., 2004), BCATc protein amount in brain was unaltered in the BCATm−/− mice (Figure 1A), and immunohistochemistry showed no alteration in the pattern of BCATc expression in brain (data not shown).
Figure 1.
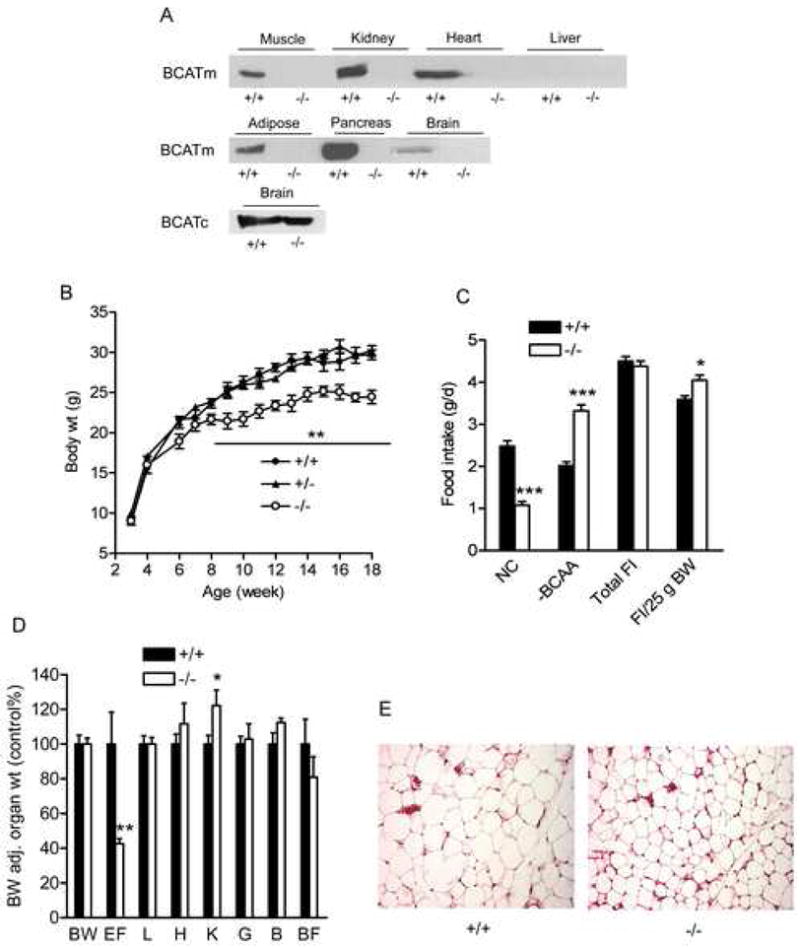
Growth curve, food intake, organ weight, and fat size
A) Immunoblots of BCATm and BCATc in selected organs from wild-type and BCATm null mice. Equal amounts of protein (20μg) were loaded in each lane of tissue samples examined.
B) Growth curve of BCATm null and wild-type mice. Male mice were weighed weekly, ** P < 0.01, n=6–12.
C) Food intake in BCATm−/− and wild-type mice. Mice were fed with a choice of normal chow (NC) and amino acid purified BCAA-free (−BCAA) diets at weaning. Food intake (FI) was measured for 3 weeks at the age of ~10 weeks and calculated as average daily values. It was also adjusted for body weight (25g). * P < 0.05 and *** P < 0.001, n=8–10.
D) Relative organ weights in BCATm null and wild-type mice. Values are expressed as percent of +/+ mice and adjusted for body weight (BW). EF, epididymal fat; L, liver; H, heart; K, kidney; G, gastrocnemius muscle; B, brain, BF, brown fat. * P < 0.05 and ** P < 0.001, n=6, male mice at the age of ~16 weeks.
E) H.E. staining of paraformaldehyde fixed section of epididymal fat.
BCATm−/− mice grew at the same rate as their littermate controls until ~6-weeks of age when the growth curves diverged and the male BCATm−/− mice exhibited a 10–15% lower body weight than control animals (Figure 1B). Because the extreme elevations in plasma BCAAs and their α-keto acids are observed in Maple Syrup Urine Disease have severe neurologic consequences (Chuang and Shih, 2001), we took advantage of the rodent’s ability to discriminate between diets of differing amino acid composition (Harper and Peters, 1989) to prevent a toxic accumulation of BCAAs. Both the BCATm−/− and wild-type mice were provided access to normal chow (NC, Harland 2018) diet and a purified amino acid BCAA-free diet (−BCAA, Dyets 510081). Male BCATm−/− mice preferred the −BCAA diet and consumed 76% of this diet and 24% of the NC diet whereas the wild-type mice ate 45% and 55% respectively of -BCAA and NC diets, (Figure 1C). Total food intake (Figure 1C) and calculated caloric intake (data not shown) did not differ; but when adjusted for body weight, food intake was greater in the BCATm−/− mice (Figure 1C). Male mice had 55% lower epididymal fat pat weight (Figures 1D), and fat cell size was accordingly decreased when compared to BCATm+/+ animals (Figure 1E). Body composition determined by EchoMRI 3-in-1™ (Houston, TX) showed that fat mass expressed as a percent of body weight was lower in the BCATm−/− (11.9 ± 0.3%) compared to the wild-type mice (18.3 ± 2.1%, P < 0.01, n=7), whereas the percent lean body mass was higher in the BCATm−/− (83.5 ± 0.3%) than in wild-type mice (78.6 ± 2.1%, male, P < 0.05, n=7). While the male BCATm−/− mice had somewhat enlarged kidneys, other tissues were normal for their body weight (Figure 1D). The female BCATm−/− mice were also lighter but to a lesser extent than observed in the males (data not shown). Thus, the animals appeared healthy and lean.
Even though BCATm−/− mice consumed far less BCAAs, their fed plasma Leu, Ile and Val were increased 14, 21 and 31 fold, respectively, in the male mice (Table 1) and 25, 33 and 37 fold, respectively, in the female mice (data not shown), consistent with disruption of BCATm, the predominant BCAT isozyme in tissues outside the CNS (Suryawan et al., 1998). Asp and Ala were decreased, whereas Thr, Cit and Arg were elevated in the null mice of both genders (Table 1 and data not shown). We also measured plasma BCAA transamination products, branched chain α-keto acids, KIC, KMV and KIV for Leu, Ile, and Val, respectively. KIC did not differ (data not shown), KMV and KIV concentrations were less in the female BCATm−/− (10.6 ± 0.9 μM for KMV and 6.1 ± 0.7 μM for KIV) than in the wild-type mice (17.6 ± 1.2 μM for KMV and 11.8 ± 0.9 μM for KIV, P < 0.01, n=7). Plasma KMV and KIV also tended to be less in the male BCATm−/− mice. The lower branched chain α-keto acids in the BCATm−/− mice are consistent with the disrupted BCAA metabolism at the BCATm-catalyzed step.
Table 1.
Plasma concentrations of hormones, amino acids, and other metabolitesa
| Nutritional state | +/+ | −/− | |
|---|---|---|---|
|
Hormones |
|||
| Leptin (pg/ml) | 6-h fast | 2309 ± 475 | 277 ± 62*** |
| PAI-1 (pg/ml) | 6-h fast | 805 ± 163 | 597 ± 150 |
| Resistin (pg/ml) | 6-h fast | 1970 ± 186 | 1247 ± 170* |
| Insulin (ng/ml) | 6-h fast | 0.75 ± 0.17 | 0.26 ± 0.04* |
| Adiponectin (ng/ml) | Overnight fast | 10050 ± 1769 | 4500 ± 288** |
| Thyroxine (μg/ml) | Fed | 6.02 ± 0.40 | 6.25 ± 0.21 |
| IGF-1 (ng/ml) | Fed | 429 ± 32 | 398 ± 53 |
| Norepinephrineb (pg/ml) | Fed | 245 ± 52 | 121 ± 17* |
|
| |||
| Amino acidsc (μM) |
|||
| LEU | Fed | 115.4 ± 9.0 | 1621 ± 361*** |
| ILE | Fed | 57.1 ± 4.9 | 1236 ± 301*** |
| VAL | Fed | 139.0 ± 7.7 | 4243 ± 700*** |
| ASP | Fed | 9.6 ± 1.3 | 5.7 ± 0.7* |
| GLY | Fed | 347.5 ± 55.6 | 522.9 ± 53.1* |
| THR | Fed | 189.0 ± 26.0 | 328.4 ± 42.7** |
| CIT | Fed | 44.6 ± 1.9 | 74.7 ± 7.1** |
| ARG | Fed | 118.5 ± 12.4 | 244.6 ± 36.1** |
| B-ALA | Fed | 6.3 ± 0.5 | 3.5 ± 0.5*** |
| ALA | Fed | 436.5 ± 44.7 | 245.4 ± 25.9** |
|
|
|||
| Other metabolites |
|||
| TG (mg/dl) | 6-h fast | 50 ± 5.5 | 41.7 ± 2.0 |
| Cholesterol (mg/dl) | 6-h fast | 116.0 ± 10.4 | 95.8 ± 10.0 |
| FFA (μM) | Overnight fast | 1361 ± 152 | 814 ± 74** |
| BHBA (mg/dl) | Overnight fast | 13.4 ± 1.8 | 6.9 ± 1.2** |
| Albumin (g/dl) | 6-h fast | 2.4 ± 0.0 | 2.5 ± 0.1 |
| BUN (mg/dl) | Fed | 19.1 ± 1.1 | 16.8 ± 1.8 |
| Creatinine (μM) | Fed | 118 ± 20 | 138 ± 25 |
| Lactate (mM) | Fed | 13.9 ± 2.2 | 14.9 ± 1.6 |
| Glucose (mg/dl) | Fed | 202 ± 10.8 | 195 ± 12.5 |
| Glucose (mg/dl) | Overnight fast | 151.1 ± 17.8 | 104.1 ± 10.8 |
Male BCATm−/− and wild-type mice fed a choice of normal chow and amino acid defined BCAA free diet.
Mice fed a choice of amino acid defined BCAA containing and BCAA-free diets.
Data for all other amino acids that were unaltered are not shown.
P < 0.05,
P < 0.01, and
P < 0.001, n=6–10.
Table 1 also shows plasma concentrations of relevant hormones and metabolites. Plasma leptin, adiponectin and resistin were decreased 88%, 55% and 34%, respectively, in the male BCATm−/− mice, whereas IGF-1 and PAI-1 were unaltered compared to the wild-type mice. Plasma adiponectin was decreased by half also in the female mice (data not shown). The lower adiponectin is unexpected considering the lower adiposity of the BCATm−/− mice. Although fed glucose was unaltered, fasting blood glucose and plasma insulin were 31% and 65% lower, respectively in the nulls. Fasting plasma concentrations of FFA and β-hydroxybutyrate were 40% and 50% lower, respectively, in the BCATm−/− than in the BCATm+/+ mice. Plasma concentrations of triglyceride, cholesterol, albumin, creatinine, urea nitrogen and lactate were unaffected by the loss of BCATm−/−.
Plasma hormones and metabolites measured in Table 1 were unaltered in the BCATm+/−mice (data not shown). In addition, body weight (Figure 1B), food intake, body composition, and organ weights (data not shown), as well as glucose tolerance and insulin sensitivity (Figure 2) did not differ between the heterozygotes and wild-types. Thus, a loss of ~50% of the BCATm in heterozygotes is insufficient to cause apparent metabolic alterations.
Figure 2.
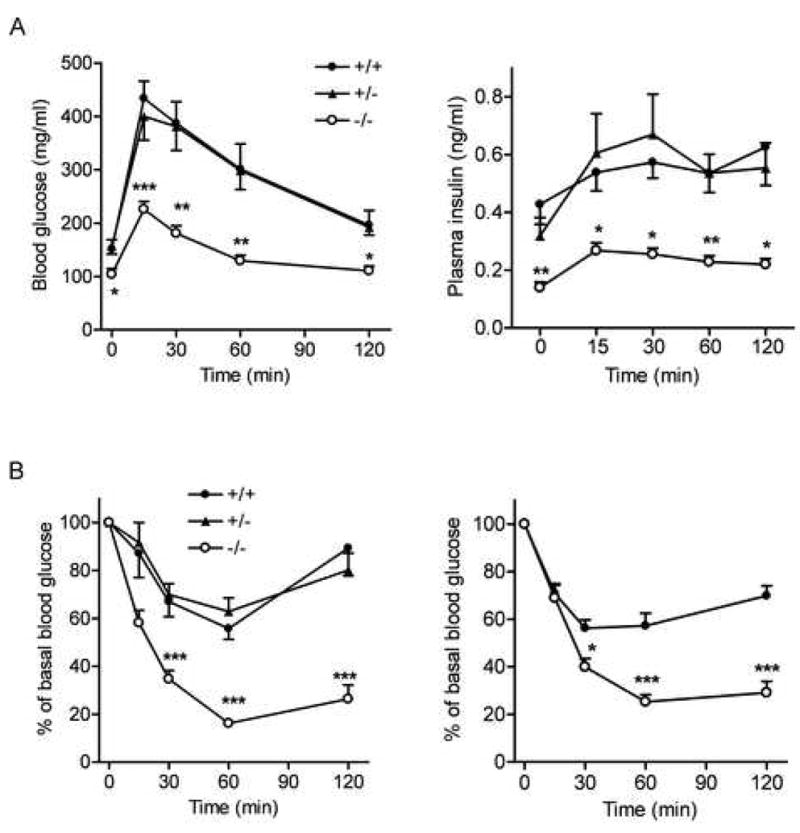
Glucose and insulin tolerance tests in BCATm null and wild-type mice
A) Blood glucose (left panel) and plasma insulin concentrations (right panel) during glucose tolerance test. * P < 0.05, ** P < 0.01 and *** P < 0.001, n=7–8.
B) Blood glucose expressed as a percent of basal level during insulin tolerance test. Left panel: ITT in male mice fed a choice of normal chow and −BCAA diets as described in Figure 1. *** P < 0.001, n=7–9 for each group. Right panel: ITT in male mice fed a 60% fat-containing diet for 10 week as described Figure 3. * P < 0.05 and *** P < 0.001, n=7–8.
Improved insulin sensitivity and glucose tolerance and resistance to high-fat-diet induced obesity in BCATm null mice
The leanness of the BCATm−/− mice and lower fasted plasma glucose and insulin concentrations prompted us to examine glucose metabolism in these animals. After an overnight fast, blood glucose and plasma insulin again were decreased by 33% and 67%, respectively, compared to controls (Figure 2A, left and right panels). Calculated homeostasis model assessment of insulin resistance (HOMA-IR) index was less in the null (0.95 ± 0.0.12) than in the wild-type mice (3.95 ± 0.38, P < 0.001, n=8). Following the glucose challenge in glucose tolerance test (GTT), blood glucose concentrations remained significantly lower (Figure 2A, left panel); and area under the curve during GTT was 51% less in the BCATm−/− mice compared to wild-type mice, suggesting markedly improved glucose tolerance. Their insulin response to glucose was much less (Figure 2A, right panel), suggesting improved insulin sensitivity. Indeed, insulin tolerance test (ITT) showed much greater decreases in blood glucose in response to insulin in both male (Figure 2B, left panel) and female BCATm−/− mice (data not shown) compared to wild-type mice.
We examined the effects on diet-induced obesity by feeding the mice with a 60% fat diet for 15 weeks starting at the age of 6–7 weeks (Figure 3). Whereas wild-type and BCATm+/− mice became obese on the high fat diet, the BCATm−/− mice were totally protected from high fat diet-induced obesity (Figure 3A). MRI showed that both abdominal and subcutaneous fat deposition was much less in the null compared to the wild-type mice (Figure 3C). Importantly, food intake in the null mice was not decreased and actually 30% greater when normalized for body weight (Figure 3B). ITT showed that null mice were protected from worsening insulin sensitivity caused by high fat diet feeding (Figure 2B, right panel).
Figure 3.
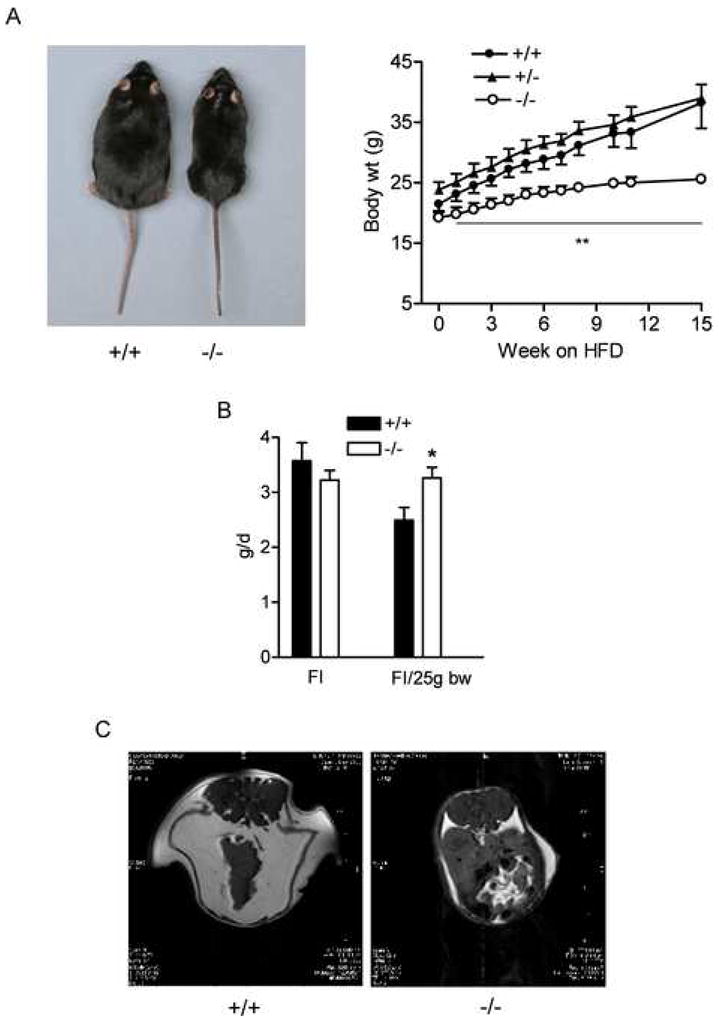
BCATm null mice are protected from high fat diet induced obesity
A) Growth curve (right panel) and representative picture of +/+ and −/− mice after high-fat-diet feeding (left panel). ** P < 0.01, −/− vs. +/+ males at each time point, n=7–8 for each group.
B) Food intake (FI) measured during high fat diet feeding. Food consumption was measured for 1 week; and average food intake was calculated and also normalized to body weigh. * P < 0.05, n=7–9.
C) Representative MRI of mice after high fat diet feeding. Transverse images set at the same distance from the anus were taken for both mice. Abdominal and subcutaneous fat shown as white was separated by the peritoneal membrane.
Enhanced energy expenditure associated with DIT in BCATm null mice
To determine the mechanism by which the BCATm−/− mice are lean and resistant to diet-induced obesity, we measured energy expenditure using indirect calorimetry (Figure 4). Compared to the wild-type mice, VO2 was increased by 18.5% in the BCATm−/− mice fed the NC/–BCAA choice diet (data not shown). To eliminate the possibility that the differences in diet composition and amino acid source (protein versus free amino acids) were influencing food choices, food intake and VO2, 12-week-old mice were switched from feeding the NC/−BCAA diet choice to a choice between a BCAA-containing defined amino acid diet (+BCAA, Dyets 510090) and the −BCAA diet, for 5 weeks. Unlike the NC/−BCAA choices, the +BCAA/−BCAA diets were isonitrogenous and isocaloric. Differences in VO2 measured with mice fed the +BCAA/−BCAA choice diets were greater than in animals on the original NC/−BCAA diet choice. VO2 was 32% higher in the BCATm−/− mice compared to controls based on body weight (Figure 4A, left panel). Commercial calorimeters frequently normalize data to body weight, but it is unclear whether this is valid. However, even VO2 per mouse was 20% higher in the null mice (114.1 ± 2.1 vs. 94.9 ± 1.5 ml/mouse/h in controls, n=8, P < 0.001). Furthermore, when absolute energy expenditure was plotted against fat free mass, different slopes emerged between the genotypes (Fig S2). Taken together these results provide strong evidence for an effect of BCATm disruption on energy expenditure.
Figure 4.
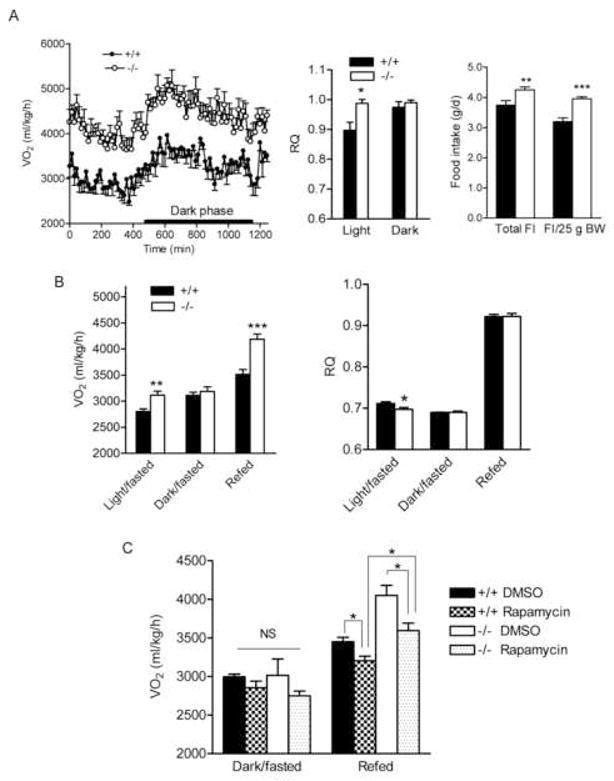
Elevated energy expenditure in BCATm null mice is associated with food consumption and is partially blunted by Rapamycin
A) Oxygen consumption (VO2, left panel), respiratory quotient (RQ, middle panel), and food intake (FI, right panel). Male mice fed a choice of amino acid purified BCAA-containing (+BCAA) and BCAA-free diet for 4 weeks were placed in indirect calorimetry chambers at the age of ~16 weeks. Food intake was measured for 3 weeks and calculated as average daily values. It was also adjusted for body weight (25g). * P < 0.05, ** P < 0.01 and *** P < 0.001, n=8.
B) VO2 (left panel) and RQ (right panel) during fasted-refed. Male mice fed a mix of NC and −BCAA diets were fasted for 21 h and refed with a choice of NC and BCAA diets. VO2 and RQ were measured during fasting (light and dark phases) and a 3-h refeeding period. * P < 0.05, ** P < 0.01 and *** P < 0.001, n=6.
C). VO2 after treated with rapamycin during fasted-refed. 12–15 week old mice were i.p. injected with 0.75 mg/kg of rapamycin at 11 a.m. and fasted for 21 h and injected again with the same dose of rapamycin. Food was provided 1-h after second injection for 3 h. VO2 was measured during the dark phase and refeeding. One way ANOVA was used to compare VO2 among groups under each nutritional condition. No difference was found between groups during fasting. * P < 0.05 between groups during refed, n=8–11.
RQ in the light phase was elevated in the null mice, suggesting that the BCATm−/− mice used more carbohydrate as a fuel (Figure 4A, middle panel). As observed with the NC/−BCAA diet choice, BCATm−/− mice fed the +BCAA/−BCAA diets still preferred the −BCAA diet, while the wild-type mice mainly consumed the +BCAA diet (data not shown). Total food intake and body weight-adjusted food intake were 12% and 22% greater in the BCATm−/− than in the BCATm+/+ mice (Figure 4A, right panel). Body weight difference between two groups of mice were 9.1% after 5 weeks on the +BCAA/−BCAA choice diets. Epididymal fat pad weight was 48% less in the null mice (0.32 ± 0.03 vs. 0.61 ± 0.05 g in the wild-type, P < 0.001, n=8). Thus, on the defined amino acid choice diets, the BCATm null mice still consumed more food and expended more energy and were leaner than wild-type controls.
To investigate further the association between food intake and VO2, we measured VO2 during fasted-refeeding (Figure 4B, left panel). While VO2 in the BCATm−/− mice during initial fasting (light phase) was 11% greater than that in the wild-type mice, it normalized during longer fasting (dark phase). A 3-h refeeding led to a 31% elevation of VO2 from the dark phase level in the null but only a 13% increase in the wild-type mice. RQ during the light phase was lower in the BCATm−/− mice (Figure 4B, right panel), suggesting that the BCATm null can readily use fat as a fuel. These results suggest that increased energy expenditure in the BCATm−/− mice is strongly associated with food consumption.
No major alterations in common factors regulating bioenergetics in BCATm null mice
We sought to determine how energy expenditure is elevated in these null mice. When measured during calorimetry, locomotor activities in BCATm−/− mice were unaltered under most conditions (Figure 5A) or even lower during refeeding (data not shown). At 2 p.m., rectal core temperature was unaltered but was 0.7°C higher in the BCATm−/− mice when measured at 9 p.m. postprandially (Figure 5B), consistent with increased DIT in the null mice. Plasma thyroxin (total T4) did not differ between BCATm−/− and BCATm+/+ mice (Table 1).
Figure 5.
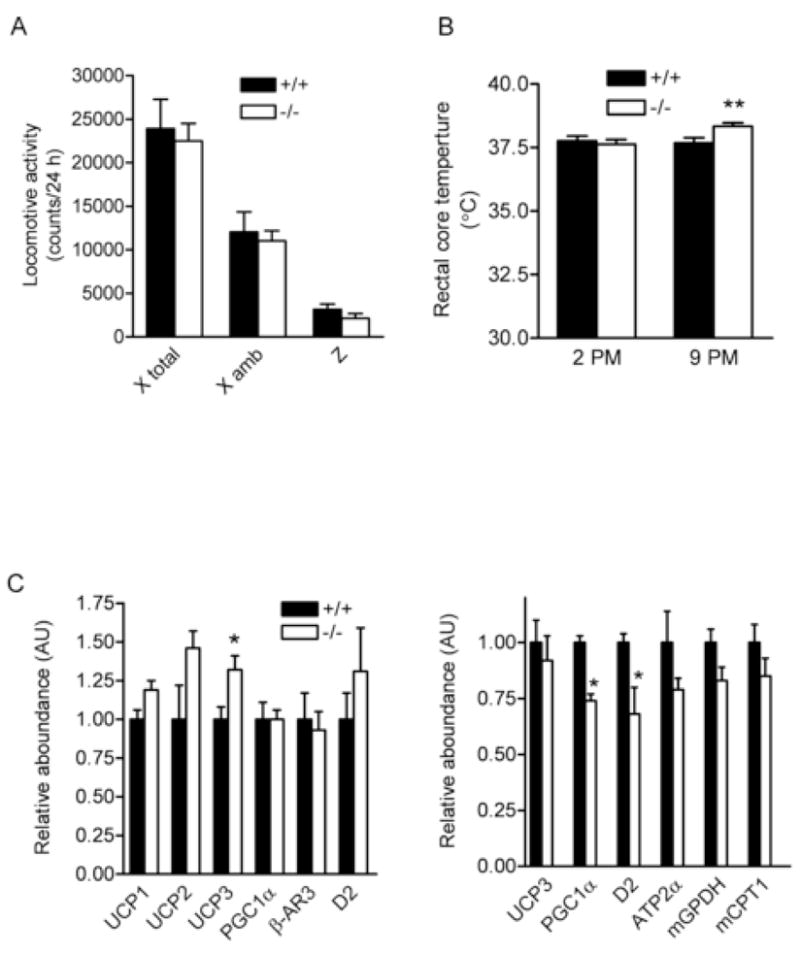
Common factors associated with thermogenesis.
A) Locomotor activity measured in the indirect calorimetry and B) Rectal core temperature in BCATm null and wild-type mice fed with a choice of NC and −BCAA diet. C) mRNA expression of selected genes in brown fat (left panel) and gastrocnemius muscle (right panel) in mice fed with a choice of +BCAA and −BCAA diet.
We measured UCP (uncoupling protein) mRNA and protein levels in various tissues (Figure 5C and S3, and data not shown). UCP1 is mainly expressed in brown fat, and both UCP1 mRNA and protein amounts were unaltered in this tissue of BCATm−/− mice. The UCP1 mRNA in gastrocnemius was 1.00 ± 0.73 vs. 15.83 ± 8.03 (P > 0.05, n=8) for BCATm wild-type and null mice, respectively. Although UCP1 mRNA in gastrocnemius muscle of some null mice was increased, it did not correlate with VO2 (data not shown). Moreover, we could not detect UCP1 protein in gastrocnemius and white fat, suggesting very low level of this protein in these tissues. UCP2 is ubiquitously expressed, and its protein expression was unaltered in gastrocnemius, epididymal fat, kidney and liver of BCATm−/− mice (Figure S2). UCP3 is mainly expressed in skeletal muscle, and its mRNA and protein levels were unaltered in this tissue of the null mice. Since UCP2 and 3 are thought not to promote gross thermogenesis or energetic inefficiency (Brand and Esteves, 2005), a small increase in UCP3 mRNA in brown fat is unlikely the major contributor of elevated energy expenditure in the BCATm−/− mice.
We also measured mRNA for other selective genes involved in the regulation of thermogenesis and mitochondrial biogenesis in BCATm−/− mice compared to the wild-type mice (Figure 5C). PGC1α (PPARγ co-activator 1α) mRNA was unaltered in brown fat and actually 26% lowered in gastrocnemius muscle of BCATm−/− mice. β-AR3 (β-adrenergic receptor 3) mRNA was unaltered in brown fat; and plasma norepinephrine concentrations were 51% lower (Table 1). D2 (type 2 iodothyronine deiodinase) mRNA was unaltered in brown fat and actually 32% decreased in gastrocnemius muscle, suggesting that the conversion of T4 to active T3 is not up-regulated in the null mice. mRNA levels of mGPDH (mitochondrial glycerol-3-phosphate dehydrogenase) (Lee and Lardy, 1965) and mCPT1 (mitochondrial carnitine palmitoyltransferase 1) were unaltered in white fat and skeletal muscle. ATP2α1 (also called SERCA1) (Simonides et al., 2001) mRNA was also unaltered in skeletal muscle. Thus, physical activity, thyroid hormone, uncoupling proteins, adrenergic outflow and futile cycling involving the glycerol phosphate shuttle or calcium release and re-uptake do not appear to contribute to the elevated energy expenditure in the BCATm−/− mice.
Elevated protein turnover and mTOR signaling in BCATm null mice
Leu is known to stimulate protein synthesis through rapamycin sensitive and insensitive mechanisms (Anthony et al., 2000). If protein synthesis was elevated in the BCATm−/− due to the chronically high levels of plasma Leu with a concomitant increase in protein degradation (organs weight showed little change, Figure 2C), the increased energy expenditure observed in the BCATm−/− mice may be related to the energy demand associated with increased protein turnover (protein synthesis and degradation). Compared to wild-type mice, in vivo protein synthesis rates measured in BCATm−/− mice fed a choice of NC/−BCAA diets were elevated by 40%, 39%, 74% and 40% in heart, skeletal muscle, epididymal fat, and kidney, respectively and showed a trend (22%) in liver (Figure 6A). To assess protein degradation, we measured urinary creatinine and 3-methylhistidine, an index of breakdown of myofibrillar proteins (Young and Munro, 1978). While 24-h urine volume was increased 33% in the null mice, total urinary creatinine output was unaltered (Table S2). 24-h 3-methyhistidine and urinary 3-methyhistidine to creatinine ratio were increased by 52% and 78%, respectively, in the BCATm−/− mice, suggesting increased protein breakdown in muscle tissues (Figure 6B). Unaltered skeletal muscle and heart weights as well as plasma and urinary creatinine excretion, indices of muscle mass, agree with the observation that both protein synthesis and degradation are simultaneously elevated in mice lacking peripheral BCAA catabolism.
Figure 6.
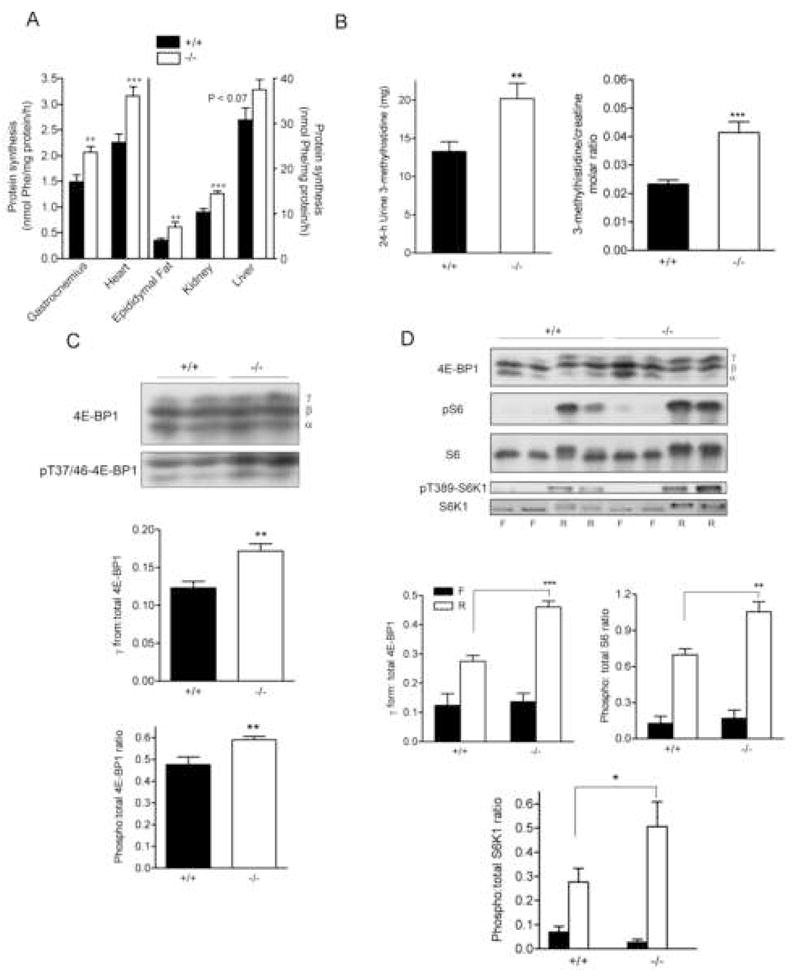
Elevated protein turnover in vivo and mTOR signaling in BCATm null mice
A) In vivo protein synthesis rates measured by flooding does of L-[3H]phenylalanine. ** P < 0.01, n=10–13.
B) Amount of 24-h urinary 3-methyhistidine and molar ratio of urine 3-methyhistidine to creatinine. Eight male mice from each genotype were placed in individual metabolic cages (Nalegene, Rochester, NY) for 2 days. Daily urine was collected for analysis of 3-methyhistidine and creatinine. ** P < 0.01 and *** P < 0.001, n=16.
C) Western blot analysis for 4E-BP1 and pT37/46 4E-BP1 in gastrocnemius of BCATm−/− mice fed a choice of NC/−BCAA diets. ** P < 0.01, n=8.
D) Western blot analysis for 4E-BP1, pS235/236 S6, S6, pT389 S6K1, and S6K1 in gastrocnemius of fasted (F) and fasted-refed (R) BCATm−/− mice. * P<0.05, ** P < 0.01 and *** P < 0.001, n=4 for fasted and 8 for fasted-refed.
To investigate the mechanism by which protein synthesis was elevated in BCATm−/− mice, we examined mTOR signaling and factors involved in protein synthesis. In the randomly fed BCATm−/− mice, total S6K1 protein level and though highly variable, pT389 S6K1, were unaltered measured in gastrocnemius and liver. Both the % of 4E-BP1 in the γ form and pT37/46 4E-BP1 to total 4E-BP1 ratio were significantly elevated in gastrocnemius, compared to the wild-type mice (Figure 6C). No significant changes were observed in the concentration of eIF2Bε, eIF4G, eIf4B or eEF2 in gastrocnemius (data not shown).
In response to refeeding after a 21-h fast, the ratios of pT389 S6K1 to S6K1 and pS 235/236 S6 to S6 (a target of S6K1) as well as 4E-BP1 in γ form were greater in the BCATm−/−mice compared to the wild-type mice (Figure 6D), suggesting greater mTOR activation during refeeding by BCATm disruption. However, no difference was observed in eIF-2α phosphorylation, eIF2Bε and its phosphorylation, as well as mTOR protein in muscle, heart and liver between the null and control mice in the ad libitum fed or fasted-refed state (data not shown). Thus as expected, increased TORC1 activity appeared to be associated with elevated protein synthesis observed in the BCATm−/− mice.
Finally, to evaluate the role of protein synthesis on energy expenditure, we measured VO2 in fasted-refed mice treated with mTOR inhibitor, rapamycin, at a dose reported largely abolished mTOR signaling (Anthony et al., 2000; Lynch et al., 2006) (Figure 4C). While VO2 did not differ among the groups during longer fasting, it was partially blunted by rapamycin in both wild-type and BCATm−/− mice compared with vehicle-treated mice in response to refeeding, suggesting that elevated protein synthesis does contribute to thermogenesis during feeding. However, VO2 during refeeding was still higher in rapamycin-treated BCATm−/− than wild-type mice, suggesting factors other than mTOR may also lead to elevated energy expenditure in the BCATm−/− mice.
Discussion
We have demonstrated in the present study that mice lacking BCATm-catalyzed BCAA metabolism exhibit high levels of plasma BCAAs without elevated branched chain α-keto acids, resulting in a unique phenotype that includes low body fat and increased energy expenditure that is associated with increased food intake, glucose tolerance and insulin sensitivity, and protein turnover. Importantly, we found that VO2 was strongly associated with food consumption in the BCATm−/− mice. During longer fasting, VO2 differences between the null and wild-type mice disappeared but reappeared during refeeding. Stimulation of protein synthesis was also associated with food intake. For instance, fasting inhibits protein synthesis and enhances protein degradation, whereas refeeding immediately stimulates protein synthesis due to elevated insulin and availability of amino acids, especially Leu (Yoshizawa et al., 1997). Cellular metabolic rate is controlled by a number of processes including metabolic demand and substrate supply. It has been thought that substrate metabolism is related to DIT, which is associated with both short-term (i.e. after a meal) and long-term (overeating) feeding (Rolfe and Brown, 1997). Theoretical stoichiometric calculation and in vitro experiments have suggested that the energy cost of pathways of nutrient metabolism greatly varies; and protein synthesis is most sensitive to energy supply (Buttgereit and Brand, 1995). Indeed, protein synthesis accounts for a minimum of 20% of calculated total daily heat production, while fatty acid synthesis accounts for 1% of total heat production in young growing animals (Reeds et al., 1982b). This could partially explain the decreased energy expenditure in leptin deficient ob/ob mice and Zucker fatty rats in which protein synthesis, at least in muscle, is diminished (Reeds et al., 1982a).
On the other hand, we did not observe significant increases in factors frequently associated with altering energy expenditure in the BCATm−/− mice such as UCPs, PGC-1α, β-AR3, SERCA1, thyroid hormone, plasma norepinephrine and locomotor activity. Moreover, leptin and adiponectin, two important fat-derived hormones known to significantly enhance energy expenditure, were greatly decreased in the BCATm−/− mice. PGC1α is known to be master regulator of glucose and lipid metabolism as well as mitochondrial function at the transcription and posttranslational levels (Handschin and Spiegelman, 2006). Moreover, cold exposure causes marked and rapid induction of PGC1α expression in brown fat and skeletal muscle, thereby up-regulating UCP1 and enhancing thermogenesis in these tissues (Lowell and Spiegelman, 2000). The role of UCP1 in maintaining normal body temperature was demonstrated in UCP1−/−mice; however, they do not develop obesity and are paradoxically resistant to diet-induced obesity (Enerback et al., 1997; Liu et al., 2003). Zhang et al reported that Leu supplementation increased energy expenditure and resistance to diet-induced obesity (DIO) due to up regulation of UCP3. However in another recent study, over-expression of UCP3 did not increase energy expenditure in mice (Bezaire et al., 2005). We did not observe increased muscle UCP3 in our mice and have been unable to reproduce the Zhang et al findings on DIO, energy expenditure and ITT, even using a slightly higher concentration of Leu in the drinking water (unpublished data). While PGC1α and uncoupling proteins are important in regulating energy expenditure and weight control, alternative thermogenic mechanisms also exist (Lowell and Spiegelman, 2000; Rolfe and Brown, 1997), especially because little brown fat is present in adult large-size animals and humans living in a thermoneutral environment. Thus, it is highly likely that the elevated protein turnover directly contributes to enhanced energy expenditure in mice lacking BCAA metabolism.
Others have proposed that sympathetic nerve activity through β adrenergic receptor plays a major role in DIT as demonstrated by the β-less mice (lacking all three β adrenergic receptors) which are prone to diet-induced obesity (Lowell and Bachman, 2003). However, we found no difference in β-AR3 mRNA expression in brown fat, and plasma norepinephrine was 50% lower in BCATm−/− mice. Moreover, we found that brain tyrosine was decreased by 87% in male and 66% in female BCATm null mice (unpublished data). Decreases in brain tyrosine could lead to decreased catecholamine concentrations in the nervous system and in the body. Mice lacking the ability to synthesize epinephrine and norepinephrine have elevated energy expenditure and food intake and decreased body weight (Thomas and Palmiter, 1997). The mechanisms for diet selectivity and elevated food intake in the BCATm null mice are unknown. Seeley and coworkers have shown that direct injection of high concentrations of Leu into the feeding center of the hypothalamus resulted in cessation of feeding (Cota et al., 2006). In the BCATm−/− mice chronically high BCAAs do not impair food intake. The lower plasma leptin in the null animals could contribute to increase food intake; however, it remains to be determined whether neurotransmitter pathways affect food intake and energy expenditure in these mice. On the other hand, the lack of apparent neurological consequences of pathologic levels of plasma BCAA in the BCATm−/− mice agree with studies that suggest that the branched chain α-keto acids, rather than BCAAs, are the toxic metabolites in Maple Syrup Urine disease (Jouvet et al., 2000). Because elevations in brain BCAA concentrations were modest (data not shown), the results suggest that BCATc can handle the increased BCAA supply in the CNS of these mice.
Our finding of elevated protein turnover in mice lacking BCAA catabolism raises important questions. What are the mechanisms for elevated protein synthesis and degradation in these mice? We have found that mTOR signaling (i.e. 4E-BP1 and S6K1 activation) were elevated in vivo in randomly fed BCATm−/− mice and/or during fasted-refeeding. eIF4E dissociated from hyperphosphorylated 4E-BP1 binds to eIF4G and hence to form a eIF4F complex, thereby promoting protein synthesis through a cap-dependent translation initiation mechanism. Other unidentified mechanisms could be existed so to increase global protein synthesis in these mice. The mechanisms regulating global protein degradation as occurs in catabolic diseases are not as well understood as protein synthesis. Thus the BCATm−/− mice may provide a useful model to explore such regulation. We hypothesize that lack of BCAA catabolism elevates intracellular Leu concentrations, thereby driving the increase in protein synthesis, while a deficiency of certain metabolites of BCAA catabolism leads to elevated protein degradation in mice lacking BCATm. This is in agreement with a study showing KIC but not Leu infusions significantly lowered the negative N balance and 3-methylhistidine excretion in postoperative patients (Sapir et al., 1983). Similarly, it has been reported that KIC but not Leu decreases the N wasting of starvation (Mitch et al., 1981).
While the mechanisms underlying the marked improved insulin sensitivity and glucose tolerance remains undetermined, increased insulin sensitivity can contribute to elevated protein synthesis in these mice. Because enhanced protein turnover consumes a large amount of ATP, it is conceivable that ATP production from substrate oxidation in mitochondria could be elevated. Indeed, we have found that the mitochondrial membrane potential was significantly increased in cultured primary fibroblasts from BCATm null neonates (unpublished data, also inconsistent with mitochondrial uncoupling). Furthermore, enhanced insulin sensitivity in these mice could lead to increased mitochondrial oxidative capacity. It has been reported that insulin stimulates mitochondrial oxidative phosphorylation in skeletal muscle associated with synthesis of mitochondrial gene transcripts and protein in human subjects (Stump et al., 2003). While it seems counterintuitive that elevated mTOR signaling would be associated with improved insulin signaling, we have found that Leu and KIC, but not insulin stimulated phosphorylation of S6K1 is largely abolished in isolated fat cells, cultured primary fibroblasts, and perfused hearts lacking BCATm (unpublished data). While further studies are needed to determine the mechanism of these changes, the increased insulin sensitivity in BCATm−/− mice is consistent with the S6K1−/−mice (Um et al., 2004).
In summary, we have clearly demonstrated that deletion of BCATm leads to activation of a protein turnover futile cycle that is associated with elevated energy expenditure and improved insulin sensitivity. Since BCAA metabolism is blocked in BCATm−/− mice, the effects of BCATm gene disruption may not be same as those of high protein diet and dietary BCAA supplements. Nevertheless, given that humans and animals can tolerate much higher dose of BCAA supplements (Baker, 2005; Fernstrom, 2005), our study suggests that BCATm may be a suitable peripheral therapeutic target for obesity.
Experimental Procedures
Animals
All animal experiments were approved by the IACUC at the Pennsylvania State University College of Medicine. Animals were given free access to water and were offered a choice of two diets, standard rodent chow (Harland Teklad 2018, Madison, Wisconsin) that has 18% protein as a percent of total weight and a defined amino acid BCAA-free diet (Dyets Inc., Bethlehem, PA that has 17% amino acids as percent of total weight). Subsequently, the rodent chow was replaced with a choice of a defined amino acid diet that had 17% amino acids including BCAA and a defined amino acid BCAA-free diet. The BCAA composition was 43%, 4% and 14% less for Leu, Ile and Val, respectively compared with the standard chow diet. Extra glutamate was added to the −BCAA diet to make it isonitrogenous to the control amino acid defined BCAA containing diet. These amino acid defined diets contained similar amount of carbohydrate, fat, vitamins and minerals compared with those in standard mouse chow. For diet-induced obesity, ~5-week wild-type and knockout mice were fed a 60% high fat diet (D12492, Research Diets, New Brunswick, NJ) for 15 weeks.
Insulin sensitivity and glucose tolerance tests
ITT and GTT were performed in 6-h and overnight food-deprived mice, respectively. Mice were injected intraperitoneally with insulin (human insulin from Eli Lilly) at 0.75 mU/g body weight or 20% glucose at 2.0 mg/g body weight; and blood glucose was measured at 15, 30, 60 and 120 min after injection. About 20 μl of blood was collected from the tail at each time point during GTT for measuring plasma insulin.
Energy expenditure, activity, and core temperature
Energy expenditure was assessed using indirect calorimetry (Oxymax, Columbus Instruments, Columbus, OH). Constant airflow (0.6 liters/min) was drawn through the chamber and monitored by a mass-sensitive flowmeter. The concentrations of oxygen and carbon dioxide were monitored at the inlet and outlet of the sealed chambers to calculate oxygen consumption and respiratory quotient. Each chamber was measured for one min at 15 min intervals. Physical activity was measured using infrared technology (OPT-M3, Columbus Instruments). The counts of three dimensional beam breaking (X total, X ambulatory, and Z) were measured. Rectal core temperature was measured using Fluke 51II thermometer with a mouse thermocouple probe (Harvard Apparatus).
Body composition
MRI scans were taken starting at the lungs and ending at the hips in mice using a 7T (300 MHz) MRI magnet with a 20cm bore (Biospec 70/20as, Bruker Instruments, Ettlingen, Germany). The pulse sequence used had a TR/TE = 500/12, 2 averages, 1 echo, 256×256 matrix, 4.3 cm2 FOV, 1mm slice thickness, 1mm slice distance, with a total scan time of 4 minutes for each mouse. Body fat and lean body mass was also measured using a QNMR system (Echo Medical Systems, LLC, Huston, TX).
Protein synthesis and degradation
Rates of protein synthesis in ad libitum fed mice were measured using the flooding-dose method to measure the incorporation of radioactive phenylalanine into protein as previously described (Lynch et al., 2002). Briefly, the mice were injected intraperitoneally with L- [3H] phenylalanine (150 mM, 30 μCi/ml, 1m/100g body wt). Fifteen minutes after injection of the radioisotope, mice were decapitated, and blood and tissue samples were collected. Plasma phenylalanine concentrations were determined by HPLC analysis of supernatants from TCA extracts of plasma.
The radioactivity in the phenylalanine peak was measured to calculate plasma specific activity of [3H] phenylalanine. Frozen powdered tissue was homogenized in ice-cold 3.6% PCA and centrifuged. The supernatant was decanted; and the pellets were dissolved in 0.1 M NaOH after washing with 3.6% PCA, acetone, a mixture of chloroform-methanol and water. Aliquots were used for assays of protein and radioactivity. Urine 3-methylhistidine was measured by Scientific Research Consortium, Inc. (St. Paul, MN) using the method of Moore et al. (Moore et al., 1958) which employs post-column derivitization by ninhydrin.
Real-time quantitative RT-PCR
Tissue total RNA was isolated using combined reagents of Trizol (Invitrogen) and RNeasy kit (Qiagen). First strand cDNA was synthesized from 1.0 μg of total RNA the SuperScript III reverse transcription kit (Invitrogen). Quantitative RT-PCR was performed on the ABI 7900HT Sequence Detection System using needed primers and probes and TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). The primers for individual genes were ordered from Applied Biosystems. ABI SDS 2.2.2 software and the 2−ΔΔCt analysis method were used to quantify relative amounts of product using β-actin as an endogenous control.
Western blot analysis
Standard procedures were used as described previously (Lynch et al., 2002). Briefly, aliquots of frozen powdered tissues were homogenized on ice in 7-3 volumes of a phospho-preserving homogenization buffer. Equal amounts of protein were loaded for electrophoresis and transferred to PVDF membranes. The membranes were then probed with antibodies against S6K1, 4E-BP1, (Bethyl Laboratories, Inc), pT389-S6K1, S6, pS235/236, or pT37/46 4E-BP1 (Cell Signaling). For detection of BCATm, affinity purified BCATm antibodies were used as described (Suryawan et al., 1998).
Analytical procedures
Plasma concentrations of glucose, triglyeride, cholesterol, urea, albumin, creatinine, and lactate were measured using a Vitros Chemistry Analyzer (Ortho-Clinical Diagnosis, New York, NY). Plasma insulin (Linco Research, Inc, St. Louise, MO) and thyroxine (Alpha Diagnostic International, TX) were measured using ELISA kits. Plasma adiponection was measured using a RIA kit (Linco Research, Inc, St. Louis, MO). Plasma concentrations of FFA (Waco Pure Chemical Industries, Osaka, Japan) and β-hydroxybutyrate (Stanbio Laboratory, Boerne, TX) were measured using commercial kits. Plasma leptin, PAI1 and resistin were measured using a LINCOplex panel (Linco Research, Inc, St. Louis, MO). Plasma norepinephrine was measured by HPLC with electrochemical detection (CoulArray system, ESA, Chelmsford, MA). A onestep ultra filtration method was used as described previously (Ueyama et al., 2003). Samples (10 μl) were injected into a 15-cm column with 3 mm bore, 3 μm C-18 packing (ESA MD-150). Plasma amino acids and branched chain α-keto acids were measured using fluorometric HPLC methods. Separation of the o-phthaldialdehyde amino acid derivatives was made by gradient elution from a Supelcosil™ LC-18 column (15cm × 4.6 mm, 3μm) (Sigma) (Wu and Knabe, 1994). Plasma α-ketoacids were derivitized with o-phenylenediamine and separation was made by gradient elution from a Spherisorb ™ ODS2 column (250mm × 4.6 mm, 5μm; Waters) (Pailla et al., 2000). Total plasma BCAA concentrations were also measured by an enzymatic method (Beckett, 2000). BCAT activity was measured as described previously (Hutson et al., 1988).
Statistical analysis
Two tailed non-paired t-test was used to assess the difference between the BCATm−/− and wild-type mice. Values are means ± SE, and P < 0.05 was considered significantly different.
Supplementary Material
Acknowledgments
We thank Dr Charles Lang, Beth Halle, Heng Liu and Michelle Bryan for technical assistance. We also thank Dr. Thomas Gettys (Pennington Biomedical Research Center, Baton Rouge, LA) for generously providing UCP standards and antibodies. This study was supported by NIH DK053843 (CJL), NIHDK062880 (CJL), NIH GM 39722 (TCV), NIH AA-12814 (TCV), NIH DK34738 (SMH), and NIH NS 038641 (SMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Baker DH. Tolerance for branched-chain amino acids in experimental animals and humans. J Nutr. 2005;135:1585S–1590S. doi: 10.1093/jn/135.6.1585S. [DOI] [PubMed] [Google Scholar]
- Beckett PR. Spectrophotometric assay for measuring branched-chain amino acids. In: Harris RA, Sokatch JR, editors. Methods in Enzymology. Branched-chain amino acids, Part B. Academic Press; 2000. pp. 40–47. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Spriet LL, Campbell S, Sabet N, Gerrits M, Bonen A, Harper ME. Constitutive UCP3 overexpression at physiological levels increases mouse skeletal muscle capacity for fatty acid transport and oxidation. Faseb J. 2005;19:977–979. doi: 10.1096/fj.04-2765fje. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Marzocchi R, Agostini F, Marchesini G. Update on nutritional supplementation with branched-chain amino acids. Curr Opin Clin Nutr Metab Care. 2005;8:83–87. doi: 10.1097/00075197-200501000-00013. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995;312:163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DI, Shih VE. Disorders of branched chain amno acid and keto acid metabolism. In: Shriver CR, editor. The Metabolic Basis of Inherited Diseas. New Nork: McGraw; 2001. pp. 1239–1277. [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Donato JJ, Pedrosa RG, Cruzat VF, Pires ISdO, Tirapegui J. Effects of leucine supplementation on the body composition and protein status of rats submitted to food restriction. Nutrition. 2006;22:520–527. doi: 10.1016/j.nut.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135:1539S–1546S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- Fulks RM, Li JB, Goldberg AL. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975;250:290–298. [PubMed] [Google Scholar]
- Gordon-Elliott JS, Margolese HC. Weight loss during prolonged branched-chain amino acid treatment for tardive dyskinesia in a patient with schizophrenia. Aust N Z J Psychiatry. 2006;40:195. doi: 10.1080/j.1440-1614.2006.01774_4.x. [DOI] [PubMed] [Google Scholar]
- Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Harper AE, Peters JC. Protein intake, brain amino acid and serotonin concentrations and protein self-selection. J Nutr. 1989;119:677–689. doi: 10.1093/jn/119.5.677. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Fenstermacher D, Mahar C. Role of mitochondrial transamination in branched chain amino acid metabolism. J Biol Chem. 1988;263:3618–3625. [PubMed] [Google Scholar]
- Hutson SM, Wallin R, Hall TR. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues. J Biol Chem. 1992;267:15681–15686. [PubMed] [Google Scholar]
- Johnston CS, Day CS, Swan PD. Postprandial thermogenesis is increased 100% on a high-protein, low-fat diet versus a high-carbohydrate, low-fat diet in healthy, young women. J Am Coll Nutr. 2002;21:55–61. doi: 10.1080/07315724.2002.10719194. [DOI] [PubMed] [Google Scholar]
- Jouvet P, Rustin P, Taylor DL, Pocock JM, Felderhoff-Mueser U, Mazarakis ND, Sarraf C, Joashi U, Kozma M, Greenwood K, Edwards AD, Mehmet H. Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: implications for neurological impairment associated with maple syrup urine disease. Mol Biol Cell. 2000;11:1919–1932. doi: 10.1091/mbc.11.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:319S–323S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- Lee YP, Lardy HA. Influence of Thyroid Hormones on L-Alpha-Glycerophosphate Dehydrogenases and Other Dehydrogenases in Various Organs of the Rat. J Biol Chem. 1965;240:1427–1436. [PubMed] [Google Scholar]
- Leidy HJ, Mattes RD, Campbell WW. Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity (Silver Spring) 2007;15:1215–1225. doi: 10.1038/oby.2007.143. [DOI] [PubMed] [Google Scholar]
- Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94. doi: 10.1093/ajcn/83.1.89. [DOI] [PubMed] [Google Scholar]
- Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Bachman ES. Beta-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, Vary TC. Leucine In Food Mediates Some Of The Postprandial Rise In Plasma Leptin Concentrations. Am J Physiol Endocrinol Metab. 2006;291:E621–630. doi: 10.1152/ajpendo.00462.2005. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Halle B, Fujii H, Vary TC, Wallin R, Damuni Z, Hutson SM. Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab. 2003;285:E854–863. doi: 10.1152/ajpendo.00153.2003. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab. 2002;283:E824–835. doi: 10.1152/ajpendo.00085.2002. [DOI] [PubMed] [Google Scholar]
- Mitch WE, Walser M, Sapir DG. Nitrogen sparing induced by leucine compared with that induced by its keto analogue, alpha-ketoisocaproate, in fasting obese man. J Clin Invest. 1981;67:553–562. doi: 10.1172/JCI110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Spackman DH, Stein WH. Chromatography of amino acids on sulfonated polystyrene resins: an inproved system. Analytical Chemistry. 1958;30:1158–1190. [Google Scholar]
- Mourier A, Bigard AX, de Kerviler E, Roger B, Legrand H, Guezennec CY. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int J Sports Med. 1997;18:47–55. doi: 10.1055/s-2007-972594. [DOI] [PubMed] [Google Scholar]
- Pailla K, Blonde-Cynober F, Aussel C, De Bandt JP, Cynober L. Branched-chain keto-acids and pyruvate in blood: measurement by HPLC with fluorimetric detection and changes in older subjects. Clin Chem. 2000;46:848–853. [PubMed] [Google Scholar]
- Rafecas I, Esteve M, Remesar X, Alemany M. Plasma amino acids of lean and obese Zucker rats subjected to a cafeteria diet after weaning. Biochem Int. 1991;25:797–806. [PubMed] [Google Scholar]
- Reeds pj, Fuller MF, Nicholson A. Metabolic basis of energy expenditure with particular reference to protein. In: Garrow JSH, editor. Substrate and Energy Meabolim. London: John Libbey; 1985. pp. 46–57. [Google Scholar]
- Reeds PJ, Haggarty P, Wahle KW, Fletcher JM. Tissue and whole-body protein synthesis in immature Zucker rats and their relationship to protein deposition. Biochem J. 1982a;204:393–398. doi: 10.1042/bj2040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds PJ, Wahle KW, Haggarty P. Energy costs of protein and fatty acid synthesis. Proc Nutr Soc. 1982b;41:155–159. doi: 10.1079/pns19820025. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Jaccard C, Persaud C, Jackson AA, Jequier E, Schutz Y. Protein turnover and thermogenesis in response to high-protein and high-carbohydrate feeding in men. Am J Clin Nutr. 1990;52:72–80. doi: 10.1093/ajcn/52.1.72. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Sapir DG, Stewart PM, Walser M, Moreadith C, Moyer ED, Imbembo AL, Rosenshein NB, Munoz S. Effects of alpha-ketoisocaproate and of leucine on nitrogen metabolism in postoperative patients. Lancet. 1983;1:1010–1014. doi: 10.1016/s0140-6736(83)92643-0. [DOI] [PubMed] [Google Scholar]
- Simonides WS, Thelen MH, van der Linden CG, Muller A, van Hardeveld C. Mechanism of thyroid-hormone regulated expression of the SERCA genes in skeletal muscle: implications for thermogenesis. Biosci Rep. 2001;21:139–154. doi: 10.1023/a:1013692023449. [DOI] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab. 2004;286:E64–76. doi: 10.1152/ajpendo.00276.2003. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature. 1997;387:94–97. doi: 10.1038/387094a0. [DOI] [PubMed] [Google Scholar]
- Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982;257:1613–1621. [PubMed] [Google Scholar]
- Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond) 2004;1:5. doi: 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijekoon EP, Skinner C, Brosnan ME, Brosnan JT. Amino acid metabolism in the Zucker diabetic fatty rat: effects of insulin resistance and of type 2 diabetes. Can J Physiol Pharmacol. 2004;82:506–514. doi: 10.1139/y04-067. [DOI] [PubMed] [Google Scholar]
- Wu G, Knabe DA. Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr. 1994;124:415–424. doi: 10.1093/jn/124.3.415. [DOI] [PubMed] [Google Scholar]
- Yoshizawa F, Nagasawa T, Nishizawa N, Funabiki R. Protein synthesis and degradation change rapidly in response to food intake in muscle of food-deprived mice. J Nutr. 1997;127:1156–1159. doi: 10.1093/jn/127.6.1156. [DOI] [PubMed] [Google Scholar]
- Young VR, Munro HN. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc. 1978;37:2291–2300. [PubMed] [Google Scholar]
- Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


