Abstract
Purpose
To determine the effect of keratocyte-derived MT1-MMP on calf pulmonary artery endothelial cell (CPAE) proliferation and migration.
Methods
Keratocyte lines were generated from MT1-MMP knockout (KO) and wild type (WT) mice. WT keratocytes were transfected with WT or mutant MT1-MMP DNAs (ΔTC or E240A). The effect of keratocyte-conditioned media on CPAE proliferation and migration was assayed.
Results
KO keratocyte conditioned media resulted in the greatest increase of CPAE cell proliferation (190.5±6.0%; P<0.01). WT keratocyte conditioned media showed higher CPAE proliferation (155.4±3.6%) than WT/MT1-MMP-transfected keratocytes (119.7±2.2%; P<0.001). Migration assays confirmed these findings.
Conclusions
Keratocyte-derived MT1-MMP has anti-angiogenic effects in CPAE cells.
Keywords: keratocyte, angiogenesis, gene/expression, metalloproteinases, MT1-MMP, keratocyte-derived MT1-MMP
INTRODUCTION
Matrix metalloproteinases (MMPs) are zinc- and calcium-dependent enzymes capable of degrading a variety of extracellular matrix components.1–3 MMPs are divided into two structurally distinct subgroups, the secreted MMPs and membrane-type MMPs (MT-MMPs).4 Membrane-type 1 MMP (MT1-MMP or MMP-14) is expressed during angiogenesis at the surface of invading vascular endothelial cells and has pro-angiogenic properties that are mediated, in part, by MMP-2 and regulated by tissue inhibitor of MMP (TIMP-2).5 Implantation of MT1-MMP knockout (KO) mouse corneas with basic fibroblast growth factor (b-FGF)-containing pellets fails to elicit corneal neovascularization, confirming the pro-angiogenic role of MT1-MMP in vivo.6
We have previously shown that MT1-MMP is expressed in stromal keratocytes after keratectomy wounding in vivo.7,8 Immunohistochemical analysis of corneas with bFGF pellet-induced vascularization showed co-localization of MT1-MMP with CD31, a vascular endothelial cell marker. We used VEGF promoter-regulated LacZ mice to demonstrate that intrastromal injection of naked DNA (MT1-MMP) combined with hemilimbal injury enhances β-galactosidase activity resulting in transcriptional upregulation of VEGF. 8 We have also observed that keratocyte-derived MT1-MMP has anti-angiogenic effects as well as pro-angiogenic effects in the cornea.8 The pro-angiogenic effects of this metalloproteinase are associated with increased VEGF production in vivo, while its anti-angiogenic effects are associated with the generation of neostatin-14, an endostatin-like anti-angiogenic degradation product of collagen XVIII.2
In this study, we investigated the direct effects of keratocyte-derived MT1-MMP on vascular endothelial cell migration and proliferation in vitro. We demonstrate that calf pulmonary artery endothelial (CPAE) cell proliferation and migration are stimulated by conditioned media from MT1-MMP KO keratocytes and inhibited by media from MT1-MMP-overexpressing keratocytes.
MATERIALS AND METHODS
Immortalized cell lines
Immortalized mouse corneal cell lines from wild type (WT) mice were generated as described previously.9 Briefly, the entire mouse corneal stroma was excised and incubated with Dulbecco’s modification of Eagle’s medium (DMEM, HyClone Laboratories, Logan, UT) containing 3.3 mg/ml collagenase type II (Sigma-Aldrich, St. Louis, MO) at 37°C with shaking for 90 min. Isolated keratocytes were grown in DMEM supplemented with 10% fetal calf serum (FCS, HyClone Laboratories) at 37°C in a 5% CO2 humidified atmosphere. Subconfluent stromal fibroblasts were supplemented with a mixture containing polybrene (4 ng/ml) and an equal volume of pZIPTEX virus (containing SV40T antigen).
Immortalized corneal cell lines from MT1-MMP KO mice were generated in a similar manner. Mice deficient in MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, or MMP-12 are viable,10–13 but MT1-MMP-deficient mice have severe defects in skeletal development and angiogenesis.6, 14 MT1-MMP KO mouse corneal cells were obtained from donor eyes that were provided courtesy of Dr. Zhongjun Zhou. Corneal cells were obtained and immortalized as described above for WT cell lines.
Characterization of corneal keratocytes
Immortalized corneal cells from WT or MT1-MMP KO mice were subcloned to generate corneal keratocyte cell lines. These cell lines were characterized immunohistochemically using antibodies against vimentin, α smooth muscle actin (α SMA), and keratin AE1/AE3. Cultured keratocytes were fixed for 15 min in 1 ml of cold (−20°C) methanol and rinsed three times in phosphate-buffered saline (PBS). Nonspecific binding was blocked by incubating fixed cells in PBS containing 1% bovine serum albumin (Sigma-Aldrich) and 0.2% Triton X-100 (Sigma-Aldrich) for 30 min at room temperature (RT). Cells were then incubated with primary antibody diluted in 1% BSA-PBS for 1 h at RT. Monoclonal mouse anti-keratin (AE1/AE3) antibody (ICN, San Francisco, CA) was diluted 1:100, and monoclonal mouse anti-α SMA antibody (ICN) was diluted 1:200. The secondary antibody, fluorescein-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), was diluted 1:200 in 1% BSA-PBS and was incubated with fixed cultured cells for 1 h at RT. Slides were washed three times in PBS and mounted with medium containing propidium iodide (Vector Laboratories, Burlingame, CA) to permit visualization of the nuclei. Specimens were viewed using a fluorescence microscope (Eclipse E800; Nikon, Tokyo, Japan). As a negative control, preimmune mouse IgG (Jackson ImmunoResearch Laboratories) was substituted for the primary antibody.
Cell culture
The protocols used in this study were approved by the Schepens Eye Research Institute Animal Care and Use Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. CPAE cells obtained from VEC Technologies (Rensselaer, NY) were routinely grown in specific media with FCS and antibiotics (MCDB-131 complete; VEC Technologies, Inc.). Stably transfected cell lines and corneal keratocytes from WT and MT1-MMP KO mice were routinely grown in a 5% CO2 atmosphere at 37°C in DMEM (Cellgro by Mediatech Inc., Herndon, VA) supplemented with 10% heat-inactivated FCS (Sigma-Aldrich), 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B (Cellgro). All experiments were initiated with cells in the log phase of growth and designed to be completed at around 80% confluence. Cells were incubated with DMEM containing 0.5% FCS to induce starvation. For western blot analysis, the conditioned media were removed, and the cells were washed three times with PBS (Cellgro). Cells were then incubated with fresh serum-free medium for at least 24 h. The serum-free conditioned medium was collected, and the cells were lysed.
Retroviral vector construction
MT1-MMP cDNA fragments were generated using primers based on the mouse MT1-MMP cDNA sequence. These cDNA fragments were subcloned into pFB plasmid (Invitrogen, Carlsbad, CA). pFB constructs were created using full-length WT mouse MT1-MMP, a MT1-MMP mutant with a transmembrane and cytoplasmic domain deletion (MT1-MMP-ΔTC)15, or an enzymatically inactive mutant (MT1-MMP-E240A). All constructs were sequenced to confirm that unwanted mutations had not been introduced during the mutagenesis process.
Transfection of 293T cells for generation and amplification of recombinant virus
High-titer stocks of recombinant virus were generated with 293T cells, which amplify viruses. Recombinant viruses were generated by transfection of 293T cells with enhanced GFP (EGFP)-expressing pFB vector encoding MT1-MMP or its mutant forms, ΔTC or E240A. A subset of cells was transfected with empty vector. One microgram of virus envelope and 1 μg of gcg-pol were mixed with 16 μl of Enhancer and 300 μl buffer for efficient DNA condensation. Effectene transfection reagent (Qiagen) was used to achieve a high transfection efficiency.
Infection of WT keratocytes with recombinant retrovirus encoding MT1-MMP
The corneal keratocyte cell lines from WT mice were stably transfected with MT1-MMP, empty vector, MT1-MMP-ΔTC, or MT1-MMP-E240A. The resulting recombinant cell lines are summarized in Table 1. For infection, keratocyte cell lines were grown to 80% confluence and incubated with 293T media containing a high-titer of recombinant virus. Infection was enhanced by supplementing media with 4 μg/μl polybrene. Cells were incubated at 37°C for 1 – 3 days and monitored daily for the presence of EGFP expression using an Epi-fluorescence microscope (Eclipse E800) with an FITC filter. Keratocytes exposed to media collected from non-transfected and empty vector-transfected 293T cells served as controls.
Table 1.
Keratocyte cell lines used in the study
| I. Non-Transfected WT Keratocyte Cell Lines | |
| WT-Ker | WT keratocytes (non-transfected) |
| KO-Ker | MT1-MMP KO keratocytes (non-transfected) |
| II. MT1-MMP Transfected Keratocyte Cell Lines | |
| WT-Ker / pFB | WT keratocytes transfected with empty vector |
| WT-Ker / MT1-MMP | WT keratocytes transfected with MT1-MMP |
| WT-Ker / MT1-MMP-ΔTC | WT keratocytes transfected with MT1-MMP-ΔTC (mutant) |
| WT-Ker / MT1-MMP-E240A | WT keratocytes transfected with MT1-MMP-E240A (mutant) |
WT: wild type; pFB: empty vector; MT1-MMP: membrane type 1 metalloproteinase; KO: knockout.
Sorting of GFP-expressing keratocytes
Keratocytes were sorted based on the expression of EGFP by the Flow Cytometry Core Facility at the Schepens Eye Research Institute, Harvard Medical School. Single-cell suspensions were prepared, washed with cold PBS, trypsinized, centrifuged at 1,000 rpm for 5 min, and fixed in PBS containing 1% paraformaldehyde. Flow cytometric analysis was performed using a Coulter EPICS XL-MCL flow cytometer (Coulter Electronics Inc., Miami, FL), and the stained cells were sorted into GFP-positive and -negative populations using a Coulter ELITE cell sorter (Miami, FL). Viable cells were gated by their forward- and side-scatter characteristics, and gates were set to sort positive and negative cell populations. The percentage of GFP-positive cells was determined after compensating for autofluorescence, which was determined from uninfected cells. The GFP-positive population was then cultured and sorted again, when needed. The stability of EGFP expression was monitored by flow cytometric analysis, and MT1-MMP expression was confirmed by Western blotting.
Collection of cell conditioned media
EGFP-positive cells were sorted and then plated at a concentration of 10,000 cells/ml in DMEM supplemented with 10% heat-inactivated FCS. Cells were grown to 80% confluence at 37°C in 5% CO2. The cells were then washed three times with PBS and incubated in fresh serum-free medium. After 24 h, the cell-conditioned media were collected and filtered through a 0.45-μm Millipore filter (Millipore, Bedford, MA).
Western blotting
Filtered conditioned media were aliquoted and stored at −80°C until analysis by Western blotting. Sodium docecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out using 4 – 20% Tris-glycine gradient gels (Invitrogen). Proteins were electrophoretically transferred to a hydrophobic polyvinylidene difluoride membrane (Immobilon-P from Millipore Co.). The membranes were prehybridized at RT for 1 h in TBST containing 3% BSA (Sigma-Aldrich) and hybridized at RT for 1 h in TBST containing the primary antibodies, rabbit anti-MT1-MMP (1:2,000; Ab 815 from Chemicon, Temecula, CA), goat anti-VEGFB, or C (R & D Systems, Minneapolis, MN), or rabbit anti-endostatin antibodies.16 The membranes were then incubated at RT for 30 min in TBST containing horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG (1:20,000; Amersham Biosciences, England) or HRP-conjugated donkey anti-goat IgG. Each step was followed by extensive washing in TBST. After the final incubation with secondary antibody, the membranes were rinsed and washed three times with TBST for 10 min at RT. Antigens were detected by incubating the membranes for 1 min at RT with ECL detection solution (Pierce Biotechnology Inc., Rockford, IL). Membranes were then exposed to X-Omat Blue XB-1 Film (Kodak Inc., Rochester, NY) for 10 s and, if necessary, up to 30 min.
CPAE cell proliferation
Cells were harvested with 0.05% trypsin-EDTA solution (Cellgro), incubated with 0.4% trypan blue (Sigma-Aldrich), and mounted on a hemocytometer. Cell viability was then determined by counting dye-excluding cells under a phase-contrast microscope. CPAE cells in cultures with high viability (90 – 95%) were seeded in 96-well plates at a concentration of 15,000 cells/well. Cells were starved for 24 h in DMEM (100 μl/well) containing 0.5% FCS. The starvation media was replaced by keratocyte-conditioned media, which had previously been filtered and stored at −80°C. Control experiments were performed using 0.5% FCS in DMEM as a negative control and 10% FCS in DMEM as a positive control. Forty-eight hours after incubation in conditioned media, the colored formazan product assay was performed to calculate the number of living CPAE cells. In this assay, CPAE cells were incubated with MTS tetrazolium solution (20 μl/well; Promega Inc., Madison, WI), which is converted into a water-soluble formazan. Formazan absorbance at 490 nm was measured using an ELISA reader (EAR 400AT; SLT Labinstruments, Austria).
CPAE cell migration assay
Migration assays were performed using a modified 10-well Boyden chamber (Neuro Probe, Inc., Gaithersburg, MD), which consists of upper and lower wells separated by a membrane filter through which cells can migrate to reach a chemoattractant (i.e., keratocyte-conditioned media). Polyvinylpyrrolidone-free polycarbonate filters (8-μm pore size) (Neuro Probe) were coated with fibronectin (10 ng/μl; BD Biosciences, Bedford, MA) to induce chemotaxis. Keratocyte lines were preincubated for 24 h in starvation medium (0.5% FCS) then changed to serum free media for additional 24 h to produce conditioned media to be used as chemoattractant. The bottom-plate wells were filled with 400 μl of conditioned media. CPAE cells (2 × 105 per well) were seeded onto the filters in the upper chamber and incubated for 6 h in DMEM with 0.1% BSA. The non-migrating cells remaining on the upper surface of the filter were removed. The filter was then rinsed in PBS, incubated for 15 min at 37°C with 100 μM/ml Cell Tracker Green CMFDA (5-chloromethylfluorescein diacetate; Molecular Probes, Inc., Eugene, OR) diluted in DMEM, incubated in DMEM for 30 min, and fixed with 3.7% formaldehyde. The cells that actively migrated to the under surface of the filter were qualitatively analyzed under an Epifluorescence microscope (Eclipse E800), and images were acquired using RT Color and SPOT software (Diagnostic Instruments, Inc., Sterling Heights, MI). Control experiments were performed using 0.5% FCS in DMEM as negative control, and 10% FCS in DMEM as positive control. Semi-quantitative analysis of CPAE migration was performed using the Image-J program. Two blinded observers were instructed to adjust the threshold for accurate representation of the borders of fluorescent CPAE cells traversing the filter. The area fractions were calculated for each micrograph.
Data analysis
Data are expressed as mean ± SEM, and statistical analysis was performed using a Student’s t-test (Microsoft Excel). Values of p <0.05 were considered significant.
RESULTS
Characterization of immortalized corneal keratocytes
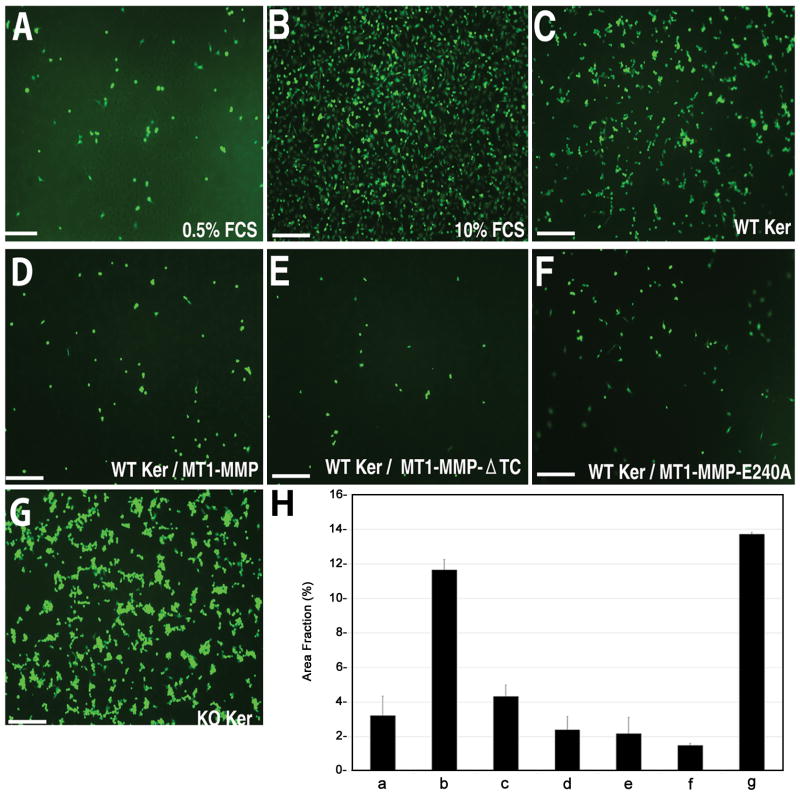
Immortalized corneal cells from WT and MT1-MMP KO mice were subcloned to generate corneal keratocyte cell lines. The resulting corneal keratocytes expressed aSMA and vimentin (Fig. 1A–D), but not keratin AE1/AE3 (Fig. 1E–F), showing that the keratocytes were free of corneal epithelial cell contamination.
Figure 1.
Effect of deficient keratocyte MT1-MMP expression on CPAE proliferation. (A–F): Immunohistochemical characterization of corneal keratocyte cell lines. Corneal cell lines derived from WT and MT1-MMP KO mice were stained for aSMA (A,B), vimentin (C,D), and keratin AE1/AE3 (E,F). Nuclei were counterstained with propidium iodide. Staining with preimmune mouse serum served as a negative control (G,H). (I): CPAE proliferation assay. CPAE cells were serum-starved for 24 h and incubated with conditioned media obtained from WT or MT1-MMP KO keratocytes. Forty-eight hours later, the colored formazan product assay was performed to calculate the number of living CPAE cells. Incubation of cells in 0.5% and 10% FCS served as a negative and positive control, respectively. Optical densities (OD) were normalized relative to the 0.5% FCS OD, which was set at 100%. (J–L): Western blot analyses. Conditioned media from serum-starved WT and KO cells were analyzed for VEGF-B (J), VEGF-C (K), and endostatin (L). Lane 1: Serum-free medium (negative control); Lane 2: Conditioned media from WT keratocytes; Lane 3: Conditioned media from KO keratocytes.
Effect of keratocyte-derived MT1-MMP on CPAE proliferation
The effect of keratocyte-conditioned media on the proliferation of serum-starved CPAE cells was analyzed using the colored formazan product assay (Fig. 1I). After a 48-h incubation, CPAE proliferation (expressed as a percent of proliferation under control conditions) was similar between the 10% FCS-exposed group (187.97 ± 4.56%) and the group exposed to conditioned media from MT1-MMP KO keratocytes (190.55 ± 5.99%, p = 0.732, two-tailed t-test). However, the addition of conditioned media from WT keratocytes significantly lowered CPAE cell proliferation compared to conditioned media from MT1-MMP KO keratocytes (155.35 ± 3.65%, p < 0.001). Western blot analysis did not reveal any major differences in the levels of endostatin or VEGF B or C between the conditioned media of WT and MT1-MMP KO cell lines (Fig. 1J–L).
Transfection of MT1-MMP, MT1-MMP-ΔTC, and MT1-MMP-E240A in WT keratocytes
WT keratocyte cell lines were infected using media from 293T cells, which were used to amplify recombinant virus encoding WT MT1-MMP or either of the mutant forms of MT1-MMP, ΔTC or E240A (Fig. 2). Fluorescence-activated sorting was performed to isolate EGFP-positive cells (Fig. 3A, B). Immunofluorescent analysis of these GFP-positive WT keratocyte cell lines confirmed that they expressed MT1-MMP (Fig. 3C, D), MT1-MMP-ΔTC (Fig. 3E, F), or MT1-MMP-E240A (Fig. 3G, H).
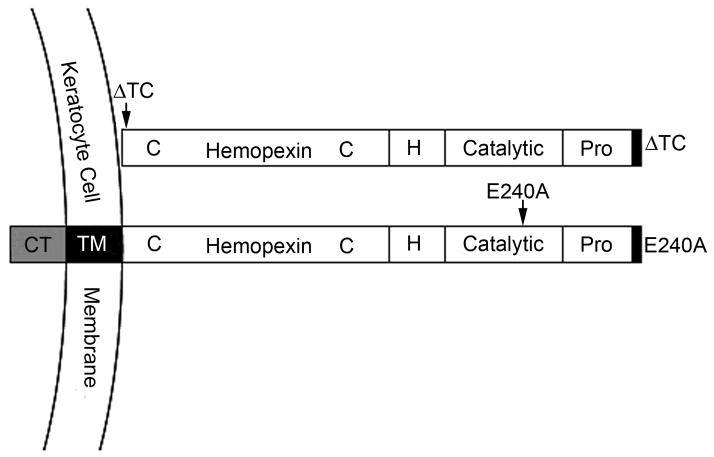
Figure 2.
Domain structure of the mutant MT1-MMPs expressed in wild type stromal keratocytes. MT1-MMP-E240A is a catalytically inactive mutant of soluble MT1-MMP and contains an Alanine in place of the active-site Glutamic acid 240. MT1-MMP-ΔTC lacks both the transmembrane domain and the cytoplasmic tail. Pro, prodomain; H, hinge region; TM, transmembrane domain; CT, cytoplasmic tail.
Figure 3.
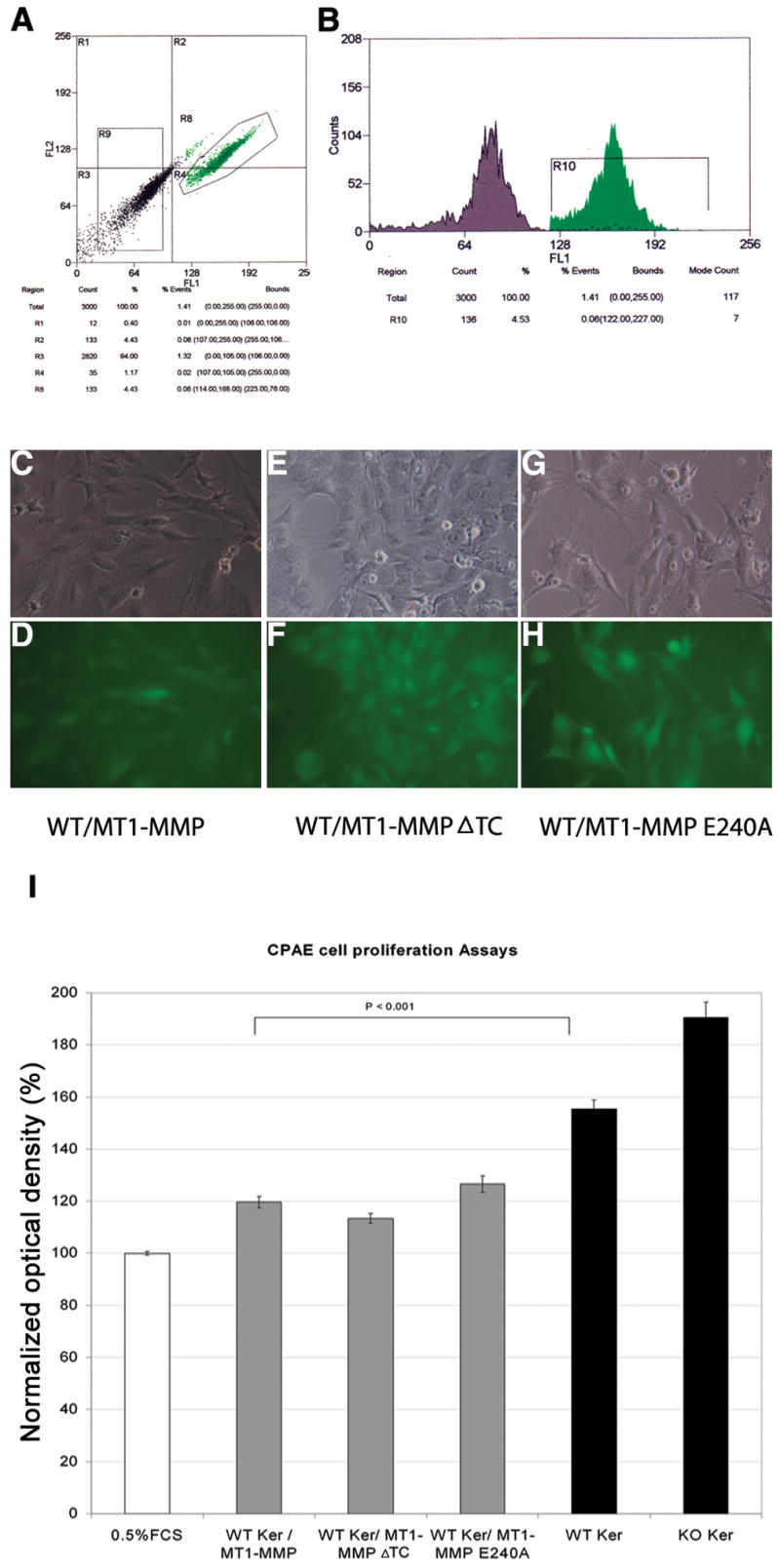
Effect of keratocyte MT1-MMP overexpression on CPAE proliferation. WT keratocytes transfected with MT1-MMP were sorted by flow cytometry based on GFP expression (A, B). Flow cytometry was also used to monitor the stability of GFP expression. Fluorescence microscopy was used to confirm expression of MT1-MMP (C, D), MT1-MMP-ΔTC (E, F), and MT1-MMP-E240A (G, H). CPAE cells were incubated for 48 h with conditioned media from WT keratocytes stably transfected with WT or mutant MT1-MMP. The colored formazan product assay was then performed to measure CPAE cell proliferation (I). Experiments were performed using 0.5% FCS as a negative control and 10% FCS as a positive control. Optical densities (OD) were normalized relative to the 0.5% FCS OD, which was set at 100%. Proliferation values obtained in the presence of WT and KO conditioned media from Fig. 1 are included for comparison (solid black bars).
Effect of keratocyte-derived MT1-MMP, MT1-MMP ΔTC, and MT1-MMP E240A on CPAE Proliferation
Conditioned media obtained from each MTI-MMP-expressing keratocyte line were added to CPAE cell cultures, and CPAE proliferation was assayed in vitro. Media from MT1-MMP-overexpressing WT keratocytes decreased CPAE proliferation compared to media from WT keratocytes (119.7 ± 2.2% vs.155.35 ± 3.65%; p < 0.001) (Fig. 3I). Media from WT cells overexpressing MT1-MMP-ΔTC (113.41 ± 1.96%) or MT1-MMP-E240A (126.58 ± 3.11%) also reduced CPAE proliferation compared to media from WT keratocytes.
Effects of keratocyte-derived MT1-MMP, MT1-MMP-ΔTC, and MT1-MMP-E240A on CPAE Migration
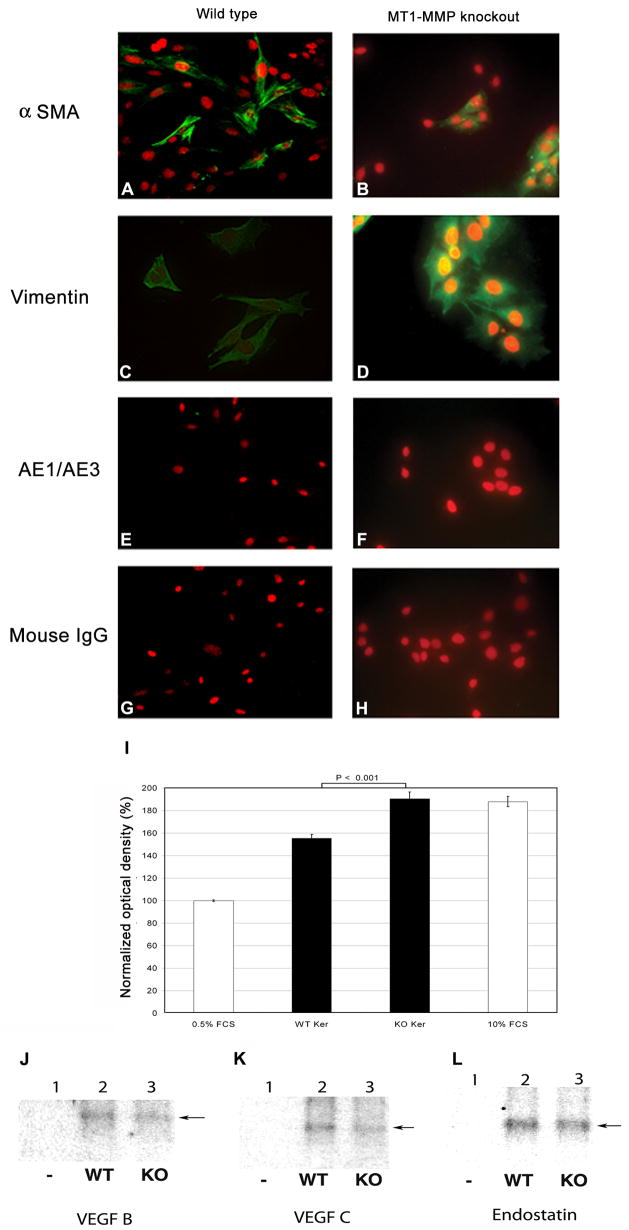
CPAE migration into fibronectin-coated filters was analyzed using Boyden chambers. CPAE cells showed minimal migration in 0.5% FCS medium and maximal migration in the 10% FCS medium. Compared to migration in the 10% FCS, migration in the presence of media from WT keratocytes was decreased (Fig. 4C). This decrease in migration was even greater in the presence of media from keratocytes overexpressing MT1-MMP (Fig. 4D), MT1-MMP-ΔTC (Fig. 4E), or MT1-MMP-E240A (Fig. 4F). In contrast, CPAE migration in the presence of media from MT1-MMP KO cell lines was increased compared to migration in media from WT keratocytes (Fig. 4G).
Figure 4.
Effect of keratocyte MT1-MMP overexpression on CPAE migration. CPAE cell migration into fibronectin-coated membranes was evaluated using the Boyden chamber method. (A–G): Representative epifluorescent micrographs of CPAE cells traversing the filters in the presence of 0.5% FCS (A), 10% FCS (B), WT keratocyte media (C), media from keratocytes overexpressing WT (D) or mutant MT1-MMP (E, F), and MT1-MMP KO-conditioned media (G). Scale bars, 200 μm. (H): Semi-quantitative analysis of CPAE migration, as determined by Image-J. Columns a–g correspond to the groups shown in images A–G.
DISCUSSION
In these experiments, conditioned media from MT1-MMP KO keratocytes resulted in greater stimulation of CPAE proliferation and migration than media from WT keratocyte cultures. Furthermore, conditioned media derived from MT1-MMP-overexpressing keratocytes reduced CPAE proliferation and migration, with mutant forms of MT1-MMP (MT1-MMP-ΔTC or MT1-MMP-E240A) failing to alter this outcome. These results suggest that keratocyte-derived MT1-MMP has an anti-angiogenic role in vitro. This is in contrast to the previously reported pro-angiogenic role of MT1-MMP in vivo.8 One possible explanation of the anti-angiogenic effect of MT1-MMP is the production of neostatin-14, an endostatin-like proteolytic product resulting from MT1-MMP degradation of collagen XVIII.2 This is unlikely in our setup where the cells were not cultured on a matrix containing collagen XVIII. Media from MT1-MMP-overexpressing keratocytes may also inhibit CPAE proliferation as a result of reduced VEGF. That is, secreted MT1-MMP may cleave ambient angiogenic substances in conditioned media, such as VEGF and MMPs. However, it is not clear whether secreted MT1-MMP can retain its functionality. Furthermore, we did not observe a major difference in the levels of neostatin-14 or VEGFs between conditioned media from WT and KO keratocytes.
We have previously shown that MT1-MMP is localized to the corneal basal epithelium and stromal keratocytes in unwounded corneas and that MT1-MMP expression is upregulated in stromal keratocytes after wounding.7 We have also observed that upregulation of keratocyte-derived MT1-MMP induces VEGF expression as well as extracellular matrix cleavage and remodeling in vivo.8 This is consistent with the hypothesis that keratocyte-derived MT1-MMP exhibits pro- and anti-angiogenic properties and is involved in tipping the balance toward or against angiogenesis in the cornea.17
The extracellular components of MT-MMPs include a signal peptide, a prodomain, a catalytic domain, a hinge region, and a hemopexin domain.18 MT1-MMP is anchored onto the cell membrane via a type I integral transmembrane domain, which is followed by a short cytosolic tail. MT1-MMP forms a complex with pro-MMP2 through TIMP-2. This triple complex then functions as a receptor for soluble pro-MMP2, allowing another TIMP2-free MT1-MMP to cleave pro-MMP2.19 Different domains of MT1-MMP have been shown to play distinct roles in pro-MMP2 activation.20 The cytoplasmic tail of MT1-MMP may negatively regulate pro-MMP2 activation by mediating its internalization from the cell surface.21–23
Conditioned media from keratocytes overexpressing MT1-MMP-ΔTC or MT1-MMP-E240A did not significantly alter CPAE proliferation or migration compared to media from WT keratocytes. Thus, a MT1-MMP domain other than the transmembrane and cytoplasmic domain may be critical for the inhibition of CPAE cell proliferation. The change in levels of active MMP2 may be involved in this process. MMP-2 has been reported to show autocrine actions activating intracellular signaling of Erk MAPK in cultured keratinocytes.24 It is thus possible that the results of this study could be ascribed to the MT1-MMP-generated MMP-2.
A limitation of this paper is that we have examined angiogenic effects using CPAE cells only. Different cell lines may behave differently under altered test conditions. Additional studies are needed to identify the specific factors that are stimulated by, or derived from, MT1-MMP-expressing keratocytes. A better understanding of the conditions that tip the balance of keratocyte-derived MT1-MMP activity against angiogenesis may lead to new strategies for the treatment of corneal neovascularization and other angiogenic disorders.
Acknowledgments
Grant support: An unrestricted Departmental grant from Research to Prevent Blindness, New York (NY) and National Institutes of Health Grants EY10101 (DTA), EY001792 (DTA), and EY14048 (JHC).
Abbreviations
- CPAE
calf pulmonary artery endothelial
- MMP
matrix metalloproteinases
- TIMPs
tissue inhibitors of metalloproteinases
Footnotes
Commercial Relationships: None
References
- 1.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 2.Chang JH, Javier JA, Chang GY, Oliveira HB, Azar DT. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Kojima T, Azar DT, Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582:2515–2520. doi: 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 5.Williamson RA, Hutton M, Vogt G, Rapti M, Knauper V, Carr MD, Murphy G. Tyrosine 36 plays a critical role in the interaction of the AB loop of tissue inhibitor of metalloproteinases-2 with matrix metalloproteinase-14. J Biol Chem. 2001;276:32966–32970. doi: 10.1074/jbc.M101843200. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye HQ, Maeda M, Yu FS, Azar DT. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol Vis Sci. 2000;41:2894–2899. [PubMed] [Google Scholar]
- 8.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 9.Kojima T, Chung TY, Chang JH, Sayegh R, Casanova FH, Azar DT. Comparison of EphA receptor tyrosine kinases and ephrinA ligand expression to EphB-ephrinB in vascularized corneas. Cornea. 2007;26:569–578. doi: 10.1097/ICO.0b013e3180335526. [DOI] [PubMed] [Google Scholar]
- 10.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 11.Mudgett JS, Hutchinson NI, Chartrain NA, Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D, Shen CF, Stevens K, Chen H, Trumbauer M, Visco DM. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci U S A. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 15.Lehti K, Valtanen H, Wickstrom SA, Lohi J, Keski-Oja J. Regulation of membrane-type-1 matrix metalloproteinase activity by its cytoplasmic domain. J Biol Chem. 2000;275:15006–15013. doi: 10.1074/jbc.M910220199. [DOI] [PubMed] [Google Scholar]
- 16.Lin HC, Chang JH, Jain S, Gabison EE, Kure T, Kato T, Fukai N, Azar DT. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- 17.Raza SL, Cornelius LA. Matrix metalloproteinases: pro- and anti-angiogenic activities. J Investig Dermatol Symp Proc. 2000;5:47–54. doi: 10.1046/j.1087-0024.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 18.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 19.Lafleur MA, Tester AM, Thompson EW. Selective involvement of TIMP-2 in the second activational cleavage of pro-MMP-2: refinement of the pro-MMP-2 activation mechanism. FEBS Lett. 2003;553:457–463. doi: 10.1016/s0014-5793(03)01094-9. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Cho YS, Park JM, Yoon SO, Kim KW, Chung AS. Pro-MMP-2 activation by the PPARgamma agonist, ciglitazone, induces cell invasion through the generation of ROS and the activation of ERK. FEBS Lett. 2007;581:3303–3310. doi: 10.1016/j.febslet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Rozanov DV, Savinov AY, Williams R, Liu K, Golubkov VS, Krajewski S, Strongin AY. Molecular signature of MT1-MMP: transactivation of the downstream universal gene network in cancer. Cancer Res. 2008;68:4086–4096. doi: 10.1158/0008-5472.CAN-07-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozanov DV, Ghebrehiwet B, Ratnikov B, Monosov EZ, Deryugina EI, Strongin AY. The cytoplasmic tail peptide sequence of membrane type-1 matrix metalloproteinase (MT1-MMP) directly binds to gC1qR, a compartment-specific chaperone-like regulatory protein. FEBS Lett. 2002;527:51–57. doi: 10.1016/s0014-5793(02)03153-8. [DOI] [PubMed] [Google Scholar]
- 23.Gingras D, Michaud M, Di Tomasso G, Beliveau E, Nyalendo C, Beliveau R. Sphingosine-1-phosphate induces the association of membrane-type 1 matrix metalloproteinase with p130Cas in endothelial cells. FEBS Lett. 2008;582:399–404. doi: 10.1016/j.febslet.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Xue M, Jackson CJ. Autocrine Actions of Matrix Metalloproteinase (MMP)-2 Counter the Effects of MMP-9 to Promote Survival and Prevent Terminal Differentiation of Cultured Human Keratinocytes. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.136. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]