Abstract
The Ser/Thr-specific phosphatase PHLPP (pleckstrin homology domain leucine-rich repeat protein phosphatase) regulates the amplitude and duration of agonist-evoked Akt signaling by dephosphorylating the hydrophobic motif (Ser473) of Akt, therefore inactivating Akt. We recently reported that gene transfer of adenylyl cyclase type 6 (AC6) into neonatal rat cardiac myocytes was associated with increased Akt phosphorylation and activity. To determine the underlying mechanisms for AC6-associated increase in Akt activation, we determined how AC6 gene transfer regulated the activity of PHLPP2 (one of the three PHLPP family phosphatases) in neonatal rat cardiac myocytes. We found that increased Akt activity was associated with inhibition of PHLPP2 activity by AC6. AC6 was physically associated with PHLPP2, which prevents PHLPP2 mediated Akt dephosphorylation. However, isoproterenol or forskolin stimulation immediately activated PHLPP2, which resulted in markedly dephosphorylation of Akt at Ser473. Activation of PHLPP2 by isoproterenol and forskolin was cAMP-independent, but required an intact cytoplasmic domain of AC6. Mutation in the cytoplasmic domain of AC6 abolished agonist-induced PHLPP2 activation. This novel bidirectional regulation of Akt activity may contribute to the unexpected favorable effects of AC6 on the failing heart.
Introduction
The Akt (PKB) signaling plays important roles in the heart by balancing cell survival and programmed cell death, and thereby influences cardiac function [1,2]. Akt is activated by stress and extracellular signals that activate phosphoinositide 3-kinase (PI3K) through interaction with tyrosine kinase receptors. Activated PI3K catalyzes phosphatidylinositol bisphosphate (PIP2) to generate phosphoinositide 3 (PIP3) [3,4]. PIP3 binds to the PH domain on Akt and recruits Akt to the inner surface of the plasma membrane to be phosphorylated on Akt activation loop (Thr308 in Akt1) by PDK-1 [5] and hydrophobic motif (Ser473 in Akt1) by a functional mTOR2 complex (SIN1-mLST8-rictor-mTOR) [6,7]. Akt activity is negatively regulated by phosphatase and tensin homolog (PTEN) through blocking the formation of PIP3 [8] and by PH domain leucine-rich repeat protein phosphatase (PHLPP), a phosphatase that directly dephosphorylates Akt at Ser473 [9–11].
PHLPP belongs to protein phosphatase 2C (PP2C) family and comprises three isoforms: PHLPP1α, PHLPP1β and PHLPP2. The PHLPP protein contains a PH domain followed by a leucine rich region, a PP2C catalytic domain and a PDZ binding motif at the C-terminus. In addition, PHLPP1β and PHLPP2 contain a Ras-association domain (RA domain) preceding the PH domain [9–11]. The homologue of PHLPP in yeast termed “CYR1” retains PHLPP domain structure. In addition, it contains an adenylate cyclase (AC) domain at the C-terminus of the CYR1 protein [12], which indicates a close link between PHLPP and AC. Recent studies by Newton’s group showed that PHLPP controls the amplitude and duration of agonist-evoked Akt signaling by dephosphorylation of Akt at Ser473, therefore inactivating Akt [9–11]. Increased expression of PHLPP in cancer cells rapidly dephosphorylates Akt at Ser473, showing the high efficiency of the phosphatase, while knockdown of PHLPP is associated with increased Akt phosphorylation, confirming the specificity of PHLPP as an Akt phosphatase [9–11]. However, how endogenous PHLPP is activated remains unknown.
The function of PHLPP in cardiac myocytes has not been determined. When studying how gene transfer of adenylate cyclase type 6 (AC6) increased phosphorylation of Akt in cardiac myocytes [13], we discovered that Akt phosphorylation at Ser473 was rapidly disappeared upon agonist stimulation. The current study is focused upon determining how the PHLPP2 phosphatase was activated by agonist stimulation, therefore, provide mechanisms for the bidirectional regulation of Akt activity in cardiac myocytes expressing AC6.
Methods and materials
Antibodies to Akt, phospho-Akt, and PI3K subunit p85 were purchased from Cell Signaling. Anti-PHLPP2 antibody and PHLPP2 blocking peptide were purchased from Bethyl Laboratories. PHLPP2 siRNAs were obtained from Dharmacon RNA Technologies. Anti-AC5/6 and anti-SCOP (PHLPP1) were obtained from Santa Cruz Biotechnology. Anti-AU1 antibody and its blocking peptide were purchased from Convance. Fugene HD and X-treme GENE SiRNA transfection reagents were obtained from Roche. Kinase inhibitors PKI and H89 were obtained from Calbiochem and Rp-8-CPT-cAMP was purchased from Biolog Life Science Institute. Isoproterenol and forskolin were purchased from Sigma. NKH477, a water-soluble forskolin derivative was obtained from Nippon Kayaku, Japan. Quick-change II kit was obtained from Stratagene.
Generation of recombinant adenovirus encoding AC6 mutant: The mutant of AC6 was generated by mutating aspartic acid (D) at position 426 to alanine (A) in the C1 domain of AC6 using the Quick-change kit. The primers are: m6C1F: 5′CAAGATCTTAGGAGCCTGTTACTACTGCGTG and m6C1R: 5′CACGCAGTAGTAACAGGCTCCTAAGATCTTG. The PCR fragment containing D426A was ligated to rest of AC6 fragments and the full length AC6 mutant was tagged with an AU1 epitop (DTYRYI) at the C-terminus. The recombinant adenovirus expressing AC6 mutant (Ad.AC6mut) was generated through co-transfection of pACCMV- AC6mut with pJM17 into H293 cells.
Cardiac myocyte culture and gene transfer: Neonatal rat cardiac myocytes were isolated and cultured as previously described [13].
Transfection of cardiac myocytes with plasmid DNA or siRNA: Isolated cardiac myocytes, one day after plating, were transfected with PHLPP2 DNA (2 μg/9.6 cm2) using Fugene HD (10 μl/9.6 cm2), or with siRNAs (100 nM) using X-treme GENE SiRNA reagent (4 μl/9.6 cm2) followed the instructions from manufacture.
Cyclic AMP measurement: Adenylyl cyclase activity was determined by measuring the amount of cAMP using cAMP Biotrak Enzymeimmunoassay System (GE Healthcare) following the instruction from manufacture.
Immunoblotting analysis and Immunofluorescence staining were performed as previously described [13].
Co-Immunoprecipitation was performed as previously described [14].
Results
Regulation of PHLPP2 activity
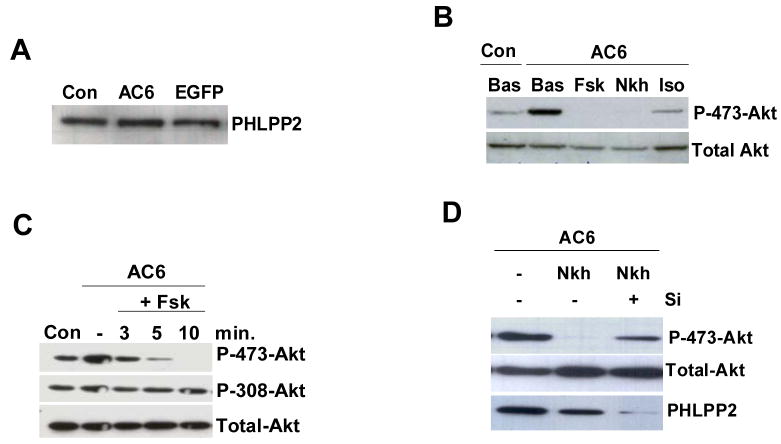
Gene transfer of AC6 did not alter the PHLPP2 expression (Fig. 1A), but greatly increased phosphorylation of Akt at both Ser473 and Thr308 as we previously reported [13]. During agonist stimulation, AC6-induced Akt phosphorylation at Ser473 was rapidly diminished (Fig. 1B and 1C). For example, when stimulated with forskolin or NKH477, Akt phosphorylation at Ser473 rapidly was reduced within 2 min and completely abolished within 10 min (Fig. 1B), but Thr308 phosphorylation was unaffected (Fig. 1C). Isoproterenol stimulation also reduced Akt phosphorylation at Ser473, a less robust but similar effect to that of forskolin (Fig. 1B). Isoproterenol and forskolin did not affect Akt phosphorylation in uninfected cardiac myocytes or in Ad.Null- infected cardiac myocytes (data not shown). Total Akt protein amount was unchanged in all conditions. These data suggest that isoproterenol and forskolin stimulation rapidly activated a high efficiency Akt phosphatase that only dephosphorylates Ser473, but not Thr308, therefore, ruling out the possibility of PTEN being the candidate phosphatase in this event.
Figure 1.
Expression and activity of PHLPP2. (A) Endogenous PHLPP2 protein was detected using anti-PHLPP2 antibody in immunoblotting and was unchanged by AC6 or EGFP expression. (B) Immunoblot analysis of Akt and phosphorylated Akt in cell lysates of Ad.AC6 infected and uninfected (Con) cells without (Bas) or with forskolin (Fsk), or NKH477 (Nkh) or isoproterenol (Iso) (10 μM, 10 min). (C) Ad.AC6 infected cardiac myocytes were stimulated with forskolin for different time and immunoblots analysis was performed. Phospho-Thr308 and total Akt were unchanged. (D) Ad.AC6 infected cardiac myocytes were transfected with PHLPP2-specific siRNA (Si, 100 nM, 48 hr) followed by NKH477 stimulation (10 μM, 10 min). Phospho-Akt Ser473, total Akt and PHLPP2 were detected by immunoblot analysis. Immunoblots are a representative of three or more independent experiments with similar results.
Since PHLPP could terminate Akt phosphorylation at Ser473 with high efficiency [9–11] and PHLPP2 was highly expressed in cardiac myocytes (Fig. 1A), but PHLPP1 expression was low (data not shown) as in other cells [15], we asked whether PHLPP2 was responsible for agonist-induced Akt dephosphorylation. We knocked down PHLPP2 using PHLPP2-specific siRNA and then stimulated cardiac myocytes with NKH477. Reduction of PHLPP2 by siRNA prevented NKH477-induced dephosphorylation of Akt resulted in a 14-fold increase in phosphorylated Akt at Ser473 (Fig. 1D), which confirmed that PHLPP2 was the phosphatase activated by NKH477. These experiments indicate that PHLPP2 plays a key role in regulating Akt phosphorylation in cardiac myocytes with increased levels of AC6.
Interaction of AC6 with PHLPP2
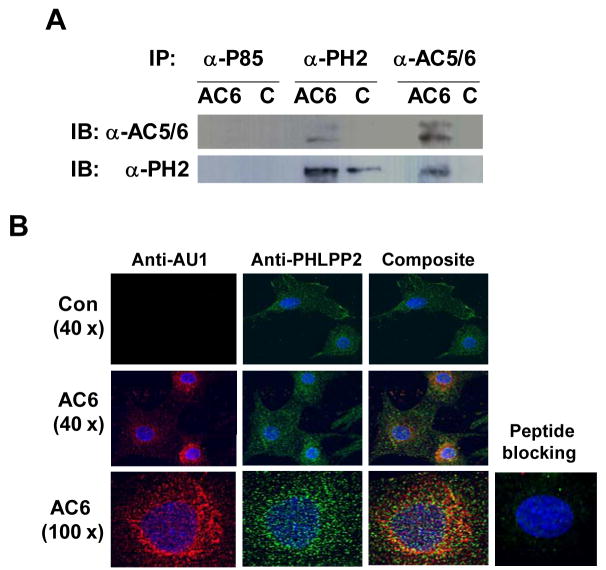
To determine mechanisms for activation of PHLPP2 by isoproterenol and forskolin stimulation, we asked whether AC6 and PHLPP2 proteins were associated within the cell. Using immunoprecipitation, we found that an anti-PHLPP2 antibody co-immunoprecipitated PHLPP2 and AC6, and an anti-AC6 antibody co-immunoprecipitated AC6 and PHLPP2 (Fig. 2A). Epitope matched anti-p85 antibody did not immunoprecipitate PHLPP2 or AC6 (Fig. 2A). These data suggest that transgene AC6 and endogenous PHLPP2 exhibit substantial physical association in cardiac myocytes.
Figure 2. Association and intracellular co-localization of AC6 with PHLPP2.
(A) AC6 or PHLPP2 were co-immunoprecipitated (IP) from the cell lysates with anti-AC5/6, or anti-PHLPP2 or anti-p85 antibody as control followed by immunoblotting (IB). Anti-AC5/6 antibody co-immunoprecipitated PHLPP2 and anti-PHLPP2 antibody co-immunoprecipitated AC6. Anti-p85 antibody did not immunoprecipitate AC6 or PHLPP2. These data demonstrate that AC6 is specifically associated with PHLPP2. (B) Immunofluorescence staining of fixed cells with anti-AU1 (detecting AC6), or anti-PHLPP2 or both antibodies for 24 hr at 4°C followed by incubation with secondary antibodies conjugated with Alexa Fluo 488 (green) or 647 (red). Nuclei were stained with Hoechst dye (blue). The images were obtained with 40x and 100x lens using a DeltaVision system and were subjected to deconvolution analysis. Image showed that increased AC6 and endogenous PHLPP2 are localized in multiple subcellular domains. Co-localization of AC6 with PHLPP2 is evident (yellow). Specific signals were undetectable after pre-incubating antibodies with AU1-specific and PHLPP2-specific blocking peptides, confirm the specificity of the immunofluorescence staining.
Additional evidence for an AC6:PHLPP2 association was provided using anti-AU1 (for transgene AC6) and anti-PHLPP2 antibodies in immunofluorescence staining followed by deconvolution analysis. Transgene AC6 was widely distributed in the cytoplasm with increased signal in the sarcoplasmic reticulum and perinuclear regions. PHLPP2 was distributed throughout the cell. Co-localization of AC6 and PHLPP2 were visualized by immunofluorescence staining using both anti-AU1 and anti-PHLPP2 antibodies (Fig. 2B). A similar co-localization pattern was detected in the presence of forskolin, suggests that forskolin did not affect PHLPP2:AC6 interaction (data not shown). The specificity of immunofluorescence staining was confirmed using blocking peptides.
PHLPP activation: independent of cAMP and PKA
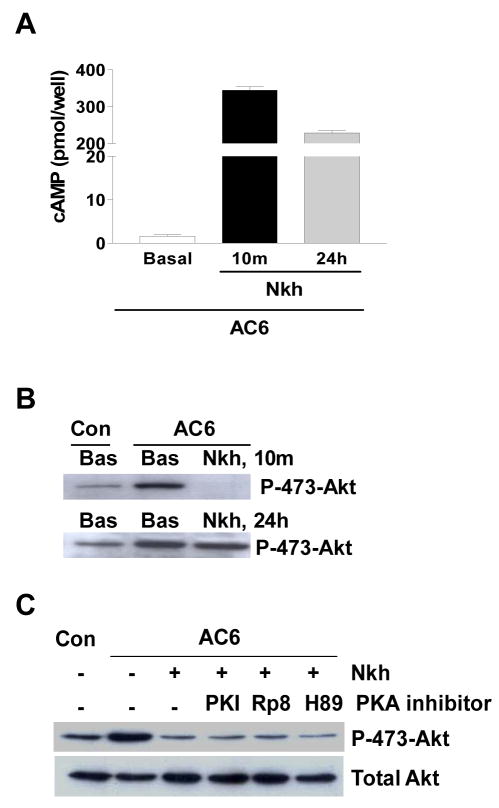
We next asked whether cAMP was required for activation of PHLPP2. Stimulation of Ad.AC6 infected cardiac myocytes with NKH477 (10 μM, 10 min) led to anticipated increase in cAMP. Incubation of NKH477 for 24 hr was also associated with high level of cAMP (Fig. 3A). However, phospho-Akt at Ser473 was diminished at 10 min, and then returned to a high level at 24 hr (Fig. 3B), indicating that cAMP was not involved in reduced Akt phosphorylation. PKA inhibitors did not prevent NKH477-induced dephosphorylation of Akt (Fig. 3C). These data indicate that the mechanism by which AC stimulation rapidly dephosphorylates Akt at Ser473 is unrelated to cAMP or to PKA.
Figure 3.
NKH477 stimulated reduction of Akt phosphorylation is not dependent on cAMP or PKA. (A) Cyclic AMP in Ad.AC6 infected cardiac myocytes was measured. NKH477 (Nkh) stimulation for 10 min (10 m) or 24 hr (24 h) is associated with high level of cAMP when compared with unstimulated cells (basal). (B) Immunoblot analysis of phospho-Akt at ser473. Phospho-Akt is diminished in 10 min (top row) and return to high level in 24 hr (bottom row), even the level of cAMP is high at 24 hr. (C) PKA is not involved in forskolin-induced reduction of phospho-Akt. PKA inhibitors [PKI (20 μM), Rp-8-CPT-cAMP (20 μM) and H89 (10 μM)] were added to cardiac myocytes 2 hr post Ad.AC6 virus infection and incubated with myocytes for 24 hr followed by NKH477 stimulation (5 μM, 10 min). PKA inhibitors did not prevent NKH477-induced reduction of phospho-Akt. Each experiment was repeated three times with reproducible results.
PHLPP2 activation requires conformational change of AC6
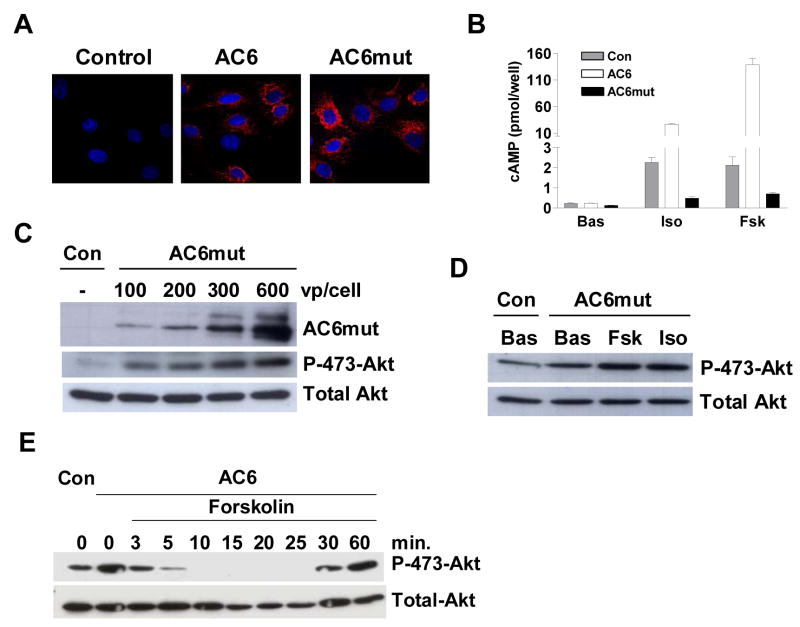
Agonist stimulation induces conformational change of AC protein through binding of Gαs or Gαi or forskolin to the AC molecule [16–19]. Having determined that cAMP was not required for PHLPP2-mediated Akt dephosphorylation, we next asked whether an AC6 conformational change induced by agonist stimulation influenced PHLPP2 activation. We generated an AC6 mutant by substitution of aspartic acid with alanine at position 426 in the C1 domain. This choice for mutation was based on the findings that mutation of an analogous site in AC5 affected AC conformation and Gsα and forskolin-stimulated AC activity [18,19]. We generated an adenovirus encoding the AC6 mutant (AC6mut) and infected cardiac myocytes. The protein level and intracellular distribution of Ad.AC6mut were similar to that observed after Ad.AC6 treatment (Figs. 4A and 4C, top row). As expected, AC6mut was no longer response to isoproterenol and forskolin stimulation to generate cAMP (Fig. 4B). AC6mut was still able to induce Akt phosphorylation in a dose-dependent manner (Fig. 4C, middle row), indicate that this mutant was able to inhibit PHLPP2 activity. However, isoproterenol and forskolin could no longer activate PHLPP2 to dephosphorylated Akt at Ser473 (Fig. 4D). Since cAMP was not required for activation of PHLPP2, these results suggest that a correct conformation in the C1 domain of AC6 was critical for PHLPP2 activation during agonist stimulation.
Figure 4.
The C1 domain of AC6 is required for PHLPP2 activation by agonist stimulation. (A) Expression and intracellular distribution of AC6 and AC6mut in cardiac myocytes, detected by using anti-AU1 antibody (red) in immunofluorescence staining. Nuclei were stained with Hoechst dye (blue). The images were obtained with 10x lens using a DeltaVision system and were subjected to deconvolution analysis. (B) AC6mut did not increase cAMP generation after isoproterenol or forskolin stimulation. Cyclic AMP was measured in uninfected (Con) and in Ad.AC6 and Ad.AC6mut infected cardiac myocytes before and after stimulation with isoproterenol (Iso), or forskolin (Fsk, 10 μM, 10 min). (C) AC6mut induced Akt phosphorylation at Ser473 in a dose-dependent manner. AC6mut protein, Akt and phospho-Akt were detected using antibodies in immunoblotting. (D) AC6mut did not induce Akt dephosphorylation in response to agonist stimulation. Ad.AC6mut infected cardiac myocytes were unstimulated or stimulated with forskolin or isoproterenol (10 μm, 10 min). Akt and phospho-Akt were detected using antibodies in immunoblotting analysis. (E) Rapid and reversible dephosphorylation of Akt. Ad.AC6 infected cardiac myocytes were incubated with forskolin (10 μM) for indicated time, cells then were lysed and western blot analysis was performed to detect Akt and phosphorylated Akt at Ser473. Experiments were repeated three times with similar results.
PHLPP mediated Akt dephosphorylation: rapid and reversible
Conformational change-induced alteration in protein:protein interaction are often rapid and reversible [20,21]. It is the case in AC6 and PHLPP2 interaction. Akt phosphorylation at Ser473 disappeared within 2 min and then returned to pre-stimulation levels within 30 min (Fig. 4E), a fully rapid and reversible process. Both dephosphorylation and re-phosphorylation of Akt at Ser473 occurred in the presence of high levels of cAMP, which confirmed that both events were cAMP-independent. These results support our hypothesis that the conformational change of AC6 occurred during isoproterenol or forskolin stimulation is critical for PHLPP2 activation.
Discussion
The present study demonstrates that PHLPP2 activity was suppressed by increased AC6 in cardiac myocytes through their protein-protein interaction, which resulted in high levels of Akt phosphorylation. However, AC6-inhibited PHLPP2 was rapidly activated by isoproterenol and forskolin stimulation, which led to marked dephosphorylation of Akt at Ser473, but not Thr308. Knocking down of PHLPP2 prevented agonist-induced PHLPP2 activation. Activation of PHLPP2 was cAMP and PKA-independent but required an intact catalytic domain of AC6 for the conformational change of AC6 during agonist stimulation. This novel regulation of PHLPP2 activity by AC6 provides a bidirectional regulation of Akt activity, which may contribute to the unexpected favorable effects of AC6 on heart [22–23].
PHLPP2 and AC6 interaction
PHLPP, members of PP2C family phosphatases, have been showed that dephosphorylate Akt at Ser473 and attenuates Akt signaling [9–11]. In vitro studies showed that only 10% Akt activity came from single phosphorylation event at Thr308 and maximal Akt activation required phosphorylation at both Ser473 and Thr308 sites [24,25]. Thus, phosphorylation of Ser473 controls the activity of Akt. AC6 gene transfer greatly increased phosphorylated Akt at Ser473 and Thr308 as we previously reported [13]. However, phosphorylated Akt at Ser473 appears to be more accessible to dephosphorylation associated with isoproterenol and forskolin stimulation (Fig. 1B–1D). Dephosphorylation of Akt at Ser473, but not Thr308, indicates that PTEN is not activated by agonist stimulation. Knockdown of PHLPP2 using PHLPP2-specific siRNA prevents agonist-induced dephosphorylation of Akt at Ser473 (Fig. 1D), confirmed that PHLPP2 is activated by agonist stimulation. These data indicate that, in the absence of agonists, inhibition of PHLPP2 activity by AC6 contributed to increased Akt phosphorylation.
Inhibition of PHLPP2 activity requires the interaction of AC6 with PHLPP2. Endogenous AC is localized predominantly in the plasma membrane, but is also found in multiple intracellular subdomains in cardiac myocytes [26]. Gene transfer of AC6 results in increased intracellular distribution of AC6 in these subdomains [27]. PHLPP2 is widely distributed throughout the cell [9], providing ample opportunity for an AC6: PHLPP2 interaction. Indeed, AC6 and PHLPP2 were co-immunoprecipitated by anti-AC6 and anti-PHLPP2 antibodies, confirming a physical association between these two molecules. Although the association of AC6 and PHLPP2 has not been reported previously, it is noteworthy that AC and PP2C are translated into a single protein in yeast [12], so there is a biological precedent for this otherwise unexpected association. It appears that association of AC6 with PHLPP2 suppresses PHLPP2 activity. However, forskolin and isoproterenol stimulation releases PHLPP2 from AC6 inhibition so that consequent dephosphorylation of Akt occurs, a process that is rapid and reversible.
Conformational dynamics of AC
Conformational changes of AC at the interface of the C1 and C2 catalytic domains during agonist stimulation are the key to regulate AC activity. Many regulatory proteins and small molecules can alter the conformation of AC. For example, Gαs binds to the C2 domain and increases the affinity of C2 with C1, with consequent catalysis and generation of cAMP. In contrast, Gαi binds to the C1 domain, reduces the affinity of C1 for C2 and reduces AC activity. Forskolin binding to the C1C2 domains stabilizes the interaction between these domains, therefore alters the conformation of the active site enabling enzyme activation [16–19].
The conformational changes of the full-length AC6 during agonist stimulation have not been precisely determined. However, the crystal structure of a soluble active form of C1C2 fusion protein showed binding sites for many regulatory elements to the catalytic core [19]. For example, an aspartic acid in the C1 domain of AC5 was intimately involved in bridging the Mg++ binding pocket that was critical for active site structure and catalysis. Mutation of this site markedly reduced Gsα and forskolin-stimulated AC activity [18,19]. Since AC5 and AC6 shared 89% homologous in C1 and C2 domains, we elected to mutate the analogous site Asp426 in AC6. To our surprise, this mutant not only markedly reduced agonist-stimulated cAMP generation capacity (Fig. 4B), but also abolished PHLPP2 activation (Fig. 4D), indicated the importance of the Asp426 in the C1 domain of AC6 in PHLPP2 activation. Since cAMP is not required for the activation of PHLPP2, altered conformation of AC6mut might be responsible for inactivation of PHLPP2. Data on rapid and reversible dephosphorylation of Akt at Ser473 (Fig. 4E) support this speculation. The precise mechanisms by which AC6 mutant affects AC6 and PHLPP2 activities will require additional study. Out data indicate that AC6 has functions through its interaction with other proteins, independently of its function to generate cAMP.
Acknowledgments
This work was supported by grants from NIH (5P01HL066941, HL081741, HL088426-01), the Department of Veterans Affairs and the American Heart Association (0765064Y and 0865147F). The authors are grateful to Dr. K. Hammond for his generous support, suggestions and reviewing manuscript. We thank Dr. Alexandra Newton for comments and providing reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shiojima I, Walsh K. Regulation of cardiac growth and coronary aniogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 2.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Brazil DP, Hemmings BA. Ten years of protein kinase B signaling: A hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 6.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye MB, Yuan XJ, Wood J, Ross C, Sawyers CL, Whang YE. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci USA. 2000;97:4233–4238. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab. 2008;19:223–230. doi: 10.1016/j.tem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao T, Furnari F, Newton AC. A phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Shima F, Okada T, Kido M, Sen H, Tanaka Y, Tamada M, Hu CD, Yamawaki-Kataoka Y, Kariya K, Kataoka T. Association of yeast adenylyl cyclase with cyclase-associated protein CAP forms a second Ras-binding site which mediates its Ras-dependent activation. Mol Cell Biol. 2000;20:26–33. doi: 10.1128/mcb.20.1.26-33.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao MH, Tang T, Guo T, Miyanohara A, Yajima T, Pestonjamasp K, Feramisco JR, Hammond HK. Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. J Biol Chem. 2008;283:33527–33535. doi: 10.1074/jbc.M805825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang T, Gao MH, Miyanohara A, Hammond HK. Galphaq reduces cAMP production by decreasing Galphas protein abundance. Biochem Biophys Res Commun. 2008;377:679–684. doi: 10.1016/j.bbrc.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu K, Okada M, Takano A, Nagai K. SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 1999;458:363–369. doi: 10.1016/s0014-5793(99)01190-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen-Goodspeed M, Lukan AN, Dessauer CW. Modeling of Galpha(s) and Galpha(i) regulation of human type V and VI adenylyl cyclase. J Biol Chem. 2005;280:1808–1816. doi: 10.1074/jbc.M409172200. [DOI] [PubMed] [Google Scholar]
- 17.Sunahara RK, Dessauer CW, Whisnant RE, Kleuss C, Gilman AG. Interaction of Gsalpha with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem. 1997;272:22265–22271. doi: 10.1074/jbc.272.35.22265. [DOI] [PubMed] [Google Scholar]
- 18.Tang WJ, Stanzel M, Gilman AG. Truncation and alanine-scanning mutants of type I adenylyl cyclase. Biochemistry. 1995;34:14563–14572. doi: 10.1021/bi00044a035. [DOI] [PubMed] [Google Scholar]
- 19.Tesmer JJG, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 20.McIntire WE. Structural determinants involved in the formation and activation of G protein betagamma dimers. Neurosignals. 2009;17:82–99. doi: 10.1159/000186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, Scott JD. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth DM, Bayat H, Drumm J, Gao MH, Swaney JS, Ander A, Hammond HK. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Tang T, Lai NC, Roth DM, Rebolledo B, Saito M, Lew WY, Clopton P, Hammond HK. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation. 2006;114:388–396. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- 24.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Kawamura K, James TN. Intracellular distribution of adenylate cyclase in human cardiocytes determined by electron microscopic cytochemistry. Microsc Res Tech. 1998;40:479–487. doi: 10.1002/(SICI)1097-0029(19980301)40:6<479::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Phan HM, Gao MH, Lai NC, Tang T, Hammond HK. New signaling pathways associated with increased cardiac adenylyl cyclase 6 expression: implications for possible congestive heart failure therapy. Trends Cardiovasc Med. 2007;17:215–221. doi: 10.1016/j.tcm.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh KJ. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- 30.Feldman AM. Adenylyl cyclase: a new target for heart failure therapeutics. Circulation. 2002;105:1876–1878. doi: 10.1161/01.cir.0000016965.24080.12. [DOI] [PubMed] [Google Scholar]
- 31.Sesti C, Kloner RA. Gene therapy in congestive heart failure. Circulation. 2004;110:242–243. doi: 10.1161/01.CIR.0000137593.62669.67. [DOI] [PubMed] [Google Scholar]