Abstract
Background
The diagnostic and prognostic importance of brachial artery flow-mediated dilation (BFMD) for cardiovascular disease (CVD) is not certain and associations between BFMD and recognized measures of atherosclerosis have not been well established.
Methods
We investigated cross-sectional and longitudinal correlations between repeated measures of BFMD and quantitative coronary artery angiographic (QCA) measurements of average percent diameter stenosis, number of lesions and minimum luminal diameter (MLD), and ultrasonographic measurement of carotid artery intima-media thickness (CIMT) in an ethnically diverse cohort of postmenopausal women (n = 132) with coronary artery disease (CAD). Subjects were participants in a 3-year randomized, placebo-controlled clinical trial, testing the efficacy of hormone therapy on atherosclerosis progression. Associations between BFMD and QCA measures, and between BFMD and CIMT were examined using measurements from the same study visit.
Results
BFMD was significantly inversely correlated with coronary artery stenosis at baseline (β = −1.21% [S.E.(β) = 0.38], p = 0.002). BFMD levels significantly predicted rate of change in CIMT over the trial period (β = −0.76 μm/year [S.E.(β) = 0.29], p = 0.008).
Conclusions
Physiological and anatomical measures of atherosclerosis are correlated among postmenopausal women with CAD, which provides some validation of BFMD as a measure of atherosclerosis in high-risk populations.
Keywords: Brachial artery flow-mediated dilation, Endothelial function, Cardiovascular disease, Atherosclerosis, Coronary artery, Angiography, Post-menopausal women, Longitudinal
1. Introduction
Atherosclerosis is the most common pathologic process underlying cardiovascular disease (CVD) [1–3]. The atherosclerotic process occurs on a continuum, beginning with damage to the endothelium and progressing to plaque formation and stenosis [2]. Assessment of the presence and extent of atherosclerosis may be directly measured along this continuum. Quantitative coronary angiographic (QCA) measurements of coronary artery stenoses and ultrasonographic measurements of carotid artery intima-media thickness (CIMT) have been the primary anatomical imaging methods used to assess atherosclerosis and extent of disease in interventional trials and observational studies [2].
At one end of the continuum, endothelial dysfunction represents the earliest physiological stage of atherosclerosis [4,5]. Measurement of brachial artery flow-mediated (endothelium-dependent) dilation (BFMD) is used to assess endothelial function. While QCA and CIMT are associated with prevalent CVD and have strong prognostic value for incident CVD independent of risk profiles [1,3,6–9], the diagnostic and prognostic importance of BFMD for CVD is less certain [10]. Although some studies have shown BFMD to be predictive of future coronary events [11,12], only a handful of studies have reported an inverse association between BFMD and CIMT [13–18]. The majority of these studies were small, cross-sectional evaluations of men with pre-existing coronary artery disease (CAD) [13,15–17]. Furthermore, associations between BFMD and other recognized measures of atherosclerosis have not been well established. More importantly, longitudinal relationships between BFMD and anatomic atherosclerosis measures have not been examined. Questions remain as to the validity of BFMD as a clinical measure of subclinical atherosclerosis [19] as well as to whether the association between BFMD and cardiovascular disease risk extends beyond low-risk populations [20]. To date, there is a dearth of information specifically concerning the relationship between BFMD and QCA and between BFMD and CIMT among postmenopausal women.
We investigated cross-sectional and longitudinal correlations between BFMD and QCA and CIMT in an ethnically diverse cohort of postmenopausal women with angiographically established CAD enrolled in The Women’s Estrogen-Progestin Lipid Lowering Hormone Atherosclerosis Regression Trial (WELL-HART). Given the difference in CVD risk profiles among diabetics and non-diabetics, and since almost half of the WELL-HART cohort was diabetic, we also investigated whether correlations between the measures varied by diabetes status. Furthermore, since the WELL-HART cohort was comprised of women with CAD that ranged from mild to moderate, we were able to evaluate whether BFMD associations with QCA and CIMT were independent of disease severity.
2. Methods
2.1. Study design
WELL-HART was a randomized, double-blind, placebo-controlled, serial coronary angiographic trial with a median follow-up of 3.3 years. The trial design and primary results have been described in detail elsewhere [21]. Briefly, 226 postmenopausal women aged 48–75 years with angiographically established CAD were randomized to one of three treatment arms: daily micronized 17β-estradiol 1 mg/day plus medroxyprogesterone acetate (MPA) placebo for 12 days each month (“estrogen”), daily micronized 17β-estradiol 1 mg/day plus active MPA 5 mg/day for 12 days each month (“estrogen plus progestin”), and daily 17β-estradiol placebo plus MPA placebo for 12 days each month (“control”). Participants received dietary counseling and had LDL-cholesterol (LDL-C) levels reduced below 130 mg/dL with dietary interventions and/or lipid-lowering therapy (primarily using an HMG-CoA reductase inhibitor). Extensive data on demographic characteristics, biochemical variables and behavioral risk factors were collected.
2.2. Study participants
A detailed description of inclusion and exclusion criteria has been presented elsewhere [21]. Briefly, women with or without a uterus were eligible if they were post-menopausal (serum estradiol <20 pg/ml), ≤75 years old, had LDL-C levels of 100–250 mg/dL, and total triglyceride levels <400 mg/dL, and had at least one ≥30% diameter stenosis coronary artery lesion as measured by QCA. All participants signed an informed consent approved by the Institutional Review Board at the University of Southern California.
2.3. Measurement of atherosclerosis
2.3.1. Coronary angiography—QCA analyses
Coronary angiography was performed with the percutaneous femoral technique; right and left anterior oblique views were obtained to show all lesions [21–23]. Follow-up angiography scheduled 3 years after the baseline angiogram used the baseline angiogram as a visual guide and was performed according to the same protocol. A clinically indicated coronary angiogram obtained within 6 months before the scheduled final angiography or before a revascularization procedure was used as the final angiogram if one was not available.
QCA analyses were performed according to validated methods by a single technician who was unaware of treatment group assignment [21–23]. Arterial segments were defined as extending from branch to branch. All identified lesions in native arteries were evaluated; in our endpoint analyses, we included only lesions ≥20% (at baseline and/or on the final angiogram). Computer software developed to track the edges of coronary segments was used to obtain a series of measurements for each pair of arterial segments from baseline and final angiograms using identical views of the coronary arteries for the baseline and end-of-trial measures [21–23]. Measurements were predominantly derived from diameter measurements and were averaged over three sequential frames [24]. These measurements were made at the site of lesions previously identified by a panel of angiographers, the imaging analyst, or both. Lesions proximal to grafts were not analyzed. For the present analyses, three variables were derived from the QCA data: (1) “number of lesions”—calculated by counting the total number of lesions in all native coronary arteries (measured at baseline only); (2) “average percent diameter stenosis”—calculated by taking the average of the percent diameter stenoses of lesions measured over all native coronary arteries (measured at baseline and end-of-trial); (3) “minimum luminal diameter” (MLD)—calculated by taking the average of the minimum diameters of the lumen at lesion sites measured over all native coronary arteries (measured at baseline and end-of-trial). QCA data for graft arteries of seven study participants with grafts were not used in the analyses.
2.3.2. Carotid ultrasound—CIMT
Carotid artery ultrasonography to measure far wall CIMT was performed at baseline and at 6-month intervals during the trial. Participants were placed in a supine position with their head rotated to the left using a 45° head block. The jugular vein and carotid artery were located in the transverse view, with the jugular vein stacked above the carotid artery [25]. The transducer was then rotated 90° around the central line of the transverse image to obtain a longitudinal image while the stacked position of the vessels was maintained. All images contained anatomic landmarks for reproducing probe angulation; a hard copy of each participant’s baseline image was used as a guide for follow-up examinations. For each participant, the depth of field, gain, monitor intensity setting, and other instrumentation settings used at baseline examination were used at all follow-up examinations. These techniques significantly reduce measurement variability [26,27]. An image analyst blinded to treatment assignment measured the intima-media thickness of the distal common carotid artery far wall with automated computerized edge detection using in-house software (Pro-win, 2005, 2006), as described elsewhere [25–27]. CIMT was the average of approximately 70–100 individual measurements between the intima–lumen and media–adventitia interfaces along a 1-cm length just distal to the carotid artery bulb. This method standardized the location and distance over which CIMT was measured and ensured that the same portion of the arterial wall was measured in each image and compared within and across all participants.
2.3.3. Brachial artery vasoreactivity—BFMD
Brachial artery vasoreactivity was measured at baseline and at 6-month intervals during the trial. Baseline images from participants who did not agree to follow-up measurements were not entered into the study database. Participants fasted for 8 hours were asked to withhold agents that could affect vasoreactivity 8 h prior to the studies, including analgesics, lipid-lowering medications, antihypertensives, anticoagulants, nitrates, caffeine, aspirin, and non-steroidal anti-inflammatory drugs. Participants were placed in a supine position with their right arm fully extended with elbow support; a blood pressure cuff was placed on the upper arm. The transducer was placed along the brachial crease so the right brachial artery was visualized transversely using B-mode. The scan head was then rotated around the central image line 90° to the longitudinal position so the far wall lumen–intima interface and the near wall adventitia–media interface were clearly visualized and could serve as landmarks for future examinations. This image was recorded for 15 s to serve as the baseline measurement (P0). The probe position and angle were stabilized and held in place with a mechanical arm throughout the procedure.
To assess BFMD, the blood pressure cuff was inflated to 50 mmHg above the systolic blood pressure (SPB) for 5 min and Doppler ultrasound was used to ensure that blood flow had ceased. The period of blood pressure cuff inflation was recorded with a time coder. The same degree and period of blood pressure cuff inflation was used at subsequent examinations. Imaging of the right brachial artery was begun approximately 30 s before deflating the cuff and continued 5 min after deflation. Calculations of the brachial artery diameter were made using P0, and 1 min after cuff deflation (P1). BFMD was calculated as the percentage change of brachial arterial diameter 1 min after cuff deflation relative to the baseline measurement [BFMD = (P1−P0)/P0 × 100; P0 = brachial artery diameter at baseline, P1 = brachial artery diameter 1 min after cuff deflation].
2.4. Statistical analysis
2.4.1. Cross-sectional analysis
Baseline mean values of BFMD, CIMT, and the QCA measurements (average percent diameter stenosis, number of lesions and MLD) were calculated for participants. Independent sample t-tests were used to examine whether there were differences in (1) CVD risk factors measured at baseline between subjects for whom brachial artery vasoreactivity measurements were available and for whom they were not and (2) baseline measures of BFMD, CIMT, and QCA measurements between diabetics and non-diabetics. Associations between BFMD and QCA measurements, and between BFMD and CIMT were examined using pairs of the measurements for participants taken at the same baseline visit. Linear regression methods were used to evaluate the baseline association between BFMD (independent variable) and CIMT, number of coronary artery lesions, average percent diameter stenosis, and MLD measured by QCA (dependent variables). β coefficients from regression models indicated the direction and magnitude of association. For analytic purposes, participants were divided into two groups of disease severity based on the mean CIMT measured at baseline (“less severe disease” (<843 μm) versus “more severe disease” (≥843 μm)) or by mean average percent diameter stenosis measured by QCA at baseline (“less severe disease” (<36% average stenosis) versus “more severe disease” (≥36% average stenosis)). To assess whether cross-sectional associations between BFMD and CIMT or QCA measures differed by disease severity (more versus less severe disease) or diabetes status (diabetic versus non-diabetic), interaction terms for disease severity or diabetes status with the independent atherosclerosis variable were included in regression models. A p-value of <0.05 for the interaction term was used to determine whether the interaction was significant. If there was significant effect modification, additional models were run with stratification by the modifying factor.
2.4.2. Longitudinal analysis
We calculated coefficients of variation (CV) to assess the variability of each of the atherosclerosis measures within subjects over study visits. Multivariable mixed-effects models were used to evaluate change in anatomic atherosclerosis measures (CIMT, average percent diameter stenosis and MLD) (dependent variables) in relation to BFMD (independent variable) measured over study visits. Separate models were used to evaluate the association between each anatomic atherosclerosis measure and BFMD. For QCA measures, the follow-up angiogram was identified as the angiogram performed within 6 months of the final end-of-trial follow-up BFMD. Regression of the atherosclerosis measures on follow-up time (years since randomization) was used to estimate the average rate of change in the measures. Repeated measures of BFMD obtained during the study were included as independent variables. Treatment groups (indicator variables for estrogen only and estrogen plus progestin) were included as covariates. An interaction term for BFMD × follow-up time tested whether BFMD at a given visit influenced the subsequent rate of change in each atherosclerosis measure. To determine whether on-trial use of lipid-lowering medications modified the association between BFMD and rate of change in CIMT, a three-way interaction term for BFMD × follow-up time × medication use was tested. To assess whether associations between BFMD and CIMT differed by disease severity or diabetes status, three-way interaction terms for BFMD × follow-up time × disease severity or diabetes status were tested. Additional models stratified by on-trial lipid-lowering medication use (yes/no), disease severity (more versus less severe disease), or diabetes status (diabetic versus non-diabetic) were run if the p-value associated with the interaction term was <0.05. BFMD was centered on its baseline mean so that results were interpreted for a woman of average BFMD. All analyses used SAS version 9.0 (SAS Institute Inc., Cary, NC, USA.).
3. Results
Of the 226 randomized subjects, 57 (25.2%) withdrew from the study prior to final angiography and 37 (16.4%) did not have vasoreactivity measurements. The present study is based on 132 (58.4%) subjects for whom vasoreactivity measures were available.
Demographic characteristics, cardiovascular disease risk factors at baseline and mean baseline values of atherosclerosis measures (BFMD, CIMT, and number, average percent diameter stenosis and MLD of coronary artery lesions) of the study population are summarized in Table 1. Most participants were obese with a mean body mass index (BMI) = 29.7 kg/m2; 43.8% had diabetes, and more than half (53.0%) reported having smoked regularly.
Table 1.
Baseline demographic characteristics, cardiovascular disease risk factors, medication histories, and atherosclerosis measures for 132 female WELL-HART participants
| Variable | Mean ± S.D. or Number (%) |
|---|---|
| Age (years) | 63.2 ± 6.4 |
| Ethnicity | |
| Non-hispanic white | 39(29.6%) |
| Non-hispanic black | 22(16.7%) |
| Hispanic | 56(42.4%) |
| Asian/other | 15(11.4%) |
| Marital status | |
| Single, never married | 11(8.3%) |
| Married | 72(54.6%) |
| Separated, widowed or divorced | 49(37.1%) |
| Educational level | |
| High school or less | 77(58.3%) |
| More than high school | 55(41.7%) |
| Annual family income | |
| <20,000 | 53(45.3%) |
| 20,000–39,999 | 33(28.2%) |
| 40,000–79,999 | 31(26.5%) |
| Diabetic | 49(43.8%) |
| Ever smoked | 70(53.0%) |
| Body-mass index (kg/m2) | 29.7 ± 4.9 |
| Blood pressure (mmHg) | |
| Systolic | 136.0 ± 5.8 |
| Diastolic | 75.5 ± 8.7 |
| Total cholesterol (mg/dL) | 230.3 ± 46.1 |
| LDL cholesterol (mg/dL) | 144.9 ± 41.0 |
| HDL cholesterol (mg/dL) | 48.6 ± 8.5 |
| Triglycerides (mg/dL) | 198.4 ± 133.0 |
| Blood pressure medications | 118(89.4%) |
| Lipid-lowering medications | 8(6.1%) |
| BFMD (%) | 5.07 ± 1.93 |
| CIMT (μm) | 818.6 ± 197.0 |
| Average percent diameter stenosis (%)a | 35.70 ± 8.21 |
| Number of coronary artery lesions (n)a | 8.5 ± 3.9 |
| Average minimum lumen diameter (mm)a | 1.85 ± 0.46 |
n = 111 subjects; percent diameter stenosis and minimum lumen diameter averaged over coronary artery lesions.
3.1. Cross-sectional analysis of baseline atherosclerosis measures
Compared to study participants who had vasoreactivity measurements available, participants who did not have vasoreactivity measures (n = 94, 41.6%) had significantly greater mean BMI (31.9 kg/m2 versus 29.7 kg/m2, p = 0.015) and SBP (150.5 mmHg versus 136.0 mmHg, p < 0.001) at baseline. There were no other differences in demographic factors or CVD risk factors between subjects with and without vasoreactivity measures.
At baseline, women with diabetes had significantly more coronary artery lesions compared with non-diabetics (9.47 ± 4.16 versus 7.79 ± 3.64, respectively, p = 0.025) and a higher mean CIMT (854.5 ± 249.4 μm versus 785.9 ± 125.7 μm, respectively, p = 0.052). Diabetics and non-diabetics did not differ in baseline BFMD (5.19 ± 1.89% versus 4.68 ± 1.92%, p = 0.16), mean average percent diameter stenosis of coronary artery lesions (35.55 ± 7.62% versus 36.59 ± 9.01%, p = 0.55), or average MLD (1.83 ± 0.50 mm versus 1.83 ± 0.41 mm, p = 0.99).
β coefficients with corresponding p-values from linear regression models estimating the cross-sectional associations between baseline vasoreactivity and angiographic or ultrasound measures are summarized in Table 2. BFMD was non-significantly inversely associated with CIMT (mean CIMT increased 12.90 μm per 1% decrease in BFMD). The p-value associated with the interaction term for BFMD by diabetes was 0.068, and there was a significant main effect of diabetes status on CIMT (p = 0.01). Stratified models showed that diabetics had greater increases in CIMT thickness than non-diabetics as vasoreactivity decreased (β = −34.53 [S.E.(β) = 17.89], p = 0.059 in diabetics compared to β = −1.26 [S.E.(β) = 9.03], p = 0.89 in non-diabetics). After excluding one diabetic who had very high CIMT, results were β = −24.81 [S.E.(β) = 12.44], p = 0.05 among diabetics. The BFMD–CIMT association did not vary by disease severity (CIMT-based definition of severity) (p-value for interaction = 0.59).
Table 2.
β coefficients from linear regression models of cross-sectional associations between brachial artery flow-mediated dilation and atherosclerosis measures at baseline visit for 132 female WELL-HART participants
| Anatomical measure | Unit change per 1% difference in BFMD (S.E.) | p-value |
|---|---|---|
| CIMT (μm) | −12.90 (9.86) | 0.19 |
| Number of lesions in coronary arteries (n)a | −0.28 (0.19) | 0.14 |
| Average percent diameter stenosis (%)a | −1.21 (0.38) | 0.002 |
| Average minimum lumen diameter (mm)a | 0.04 (0.02) | 0.098 |
n = 111.
BFMD was significantly inversely correlated with coronary artery stenosis at baseline (Table 2) (average percent diameter stenosis increased 1.21% per 1% decrease in BFMD). The p-value associated with the interaction term for BFMD by diabetes was 0.08, and there was no significant independent main effect of diabetes status on average percent diameter stenosis (p = 0.08). There was no evidence that the BFMD-average percent diameter stenosis association varied by disease severity (QCA-based definition of severity) (p-value for interaction = 0.78).
Number of coronary artery lesions was inversely correlated with BFMD (Table 2), but the association did not approach significance (mean number of lesions increased 0.28 per 1% decrease in BFMD). There was no evidence for effect modification by diabetes status (p-value for interaction = 0.72) or disease severity (p-value for interaction = 0.54) using the QCA-based definition of severity. BFMD was non-significantly positively associated with average MLD of the coronary arteries (Table 2) (mean MLD decreased 0.04 mm per 1% decrease in BFMD). There was also no evidence for effect modification by diabetes status (p-value for interaction = 0.34), or disease severity (p-value for interaction = 0.50).
3.2. Longitudinal analysis
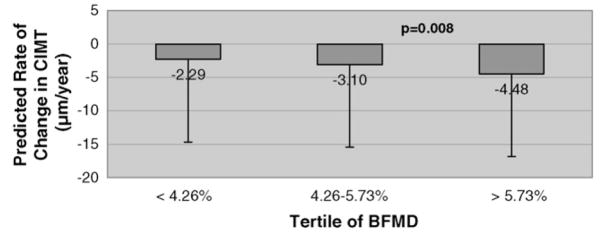
The coefficients of variation were 22.7% for BFMD, 2.2% for CIMT, 11.0% for average percent diameter stenosis, and 10.7% for MLD, indicating a range of variability in the atherosclerosis measures within subjects over follow-up visits. β coefficients with corresponding p-values estimating the longitudinal associations between vasoreactivity and angiographic or ultrasound measures are summarized in Table 3. Over follow-up time, BFMD was significantly inversely associated with rate of change in CIMT. For each 1% decrease in BFMD, the CIMT annual rate increased by 0.76 μm/year. Predicted CIMT change rates by tertiles of BFMD from the fitted mixed-effects model are presented in Fig. 1. BFMD was non-significantly positively correlated with change in average percent diameter stenosis over the trial period (Table 3) (average percent diameter stenosis decreased 0.33% per year for each 1% decrease in BFMD), and non-significantly inversely correlated with change in average MLD over the trial period (Table 3) (MLD increased 0.01 mm/year for each 1% decrease in BFMD).
Table 3.
Parameter estimates from mixed-effects regression modelsa of longitudinal associations between brachial artery flow-mediated dilation and atherosclerosis measures
| Effect | Atherosclerosis measure (unit change) attributable to each effect |
|||||
|---|---|---|---|---|---|---|
| CIMT (μm/year) |
Average percent diameter stenosis (%/year) |
Average minimum lumen diameter (mm/year) |
||||
| β coefficient [S.E.(β)] | p-value | β coefficient [S.E.(β)] | p-value | β coefficient [S.E.(β)] | p-value | |
| Follow-up time (years)b | −3.13 (12.3) | 0.80 | 0.55 (0.35) | 0.12 | −0.03 (0.02) | 0.07 |
| BFMD (%)c | 1.02 (0.45) | 0.02 | −1.00 (0.36) | 0.22 | 0.02 (0.02) | 0.54 |
| BFMD × follow-up time (%/year)d | −0.76 (0.29) | 0.008 | 0.33 (0.16) | 0.29 | −0.01 (0.01) | 0.37 |
Adjusted for treatment group.
Average change in atherosclerosis measure per year in a woman with mean BFMD (5.07%).
Cross-sectional association; average change in atherosclerosis measure per unit BFMD averaged over follow-up visits.
Longitudinal association; average rate of change per year in atherosclerosis measure for each 1% difference in BFMD.
Fig. 1.
Predicted rate of change in CIMT by level of BFMD from mixed effects regression model.
Per trial protocol, the majority of patients (n = 110, 83.3%) started taking lipid-lowering medications during the trial or continued pre-trial use. There was evidence that statin use modified the association between BFMD and CIMT rate of change (p-value for three-way interaction term = 0.02). The association between BFMD and CIMT change was stronger among women who did not use lipid-lowering medications during the trial period (n = 22, 16.7%) (β = −3.7 [S.E.(β) = 1.18], p = 0.002) compared to women who did use the medications (β = −0.64 [S.E.(β) = 0.30], p = 0.03). However, it should be noted that patients who did not take lipid-lowering medications during the trial had higher mean CIMT (856.2 μm versus 842.5 μm) and BFMD (5.34% versus 5.02%) at baseline than patients who did take the medications, thus had the potential for greater change in the measurements over follow-up time. Neither disease severity nor diabetes status modified the association between BFMD and CIMT rate of change (p-value for three-way interaction term = 0.24 and 0.46, respectively).
4. Discussion
In summary, we found that BFMD was correlated with anatomical measures of atherosclerosis both cross-sectionally and longitudinally in postmenopausal women with angiographically demonstrated CAD. At baseline, BFMD was significantly inversely correlated with average percent diameter stenosis and positively associated with MLD of coronary arteries. BFMD was non-significantly inversely associated with CIMT and number of lesions in coronary arteries. Higher levels of BFMD over follow-up time were significantly inversely correlated with annualized rates of change in atherosclerosis measured by CIMT. Our findings are consistent with a biological model for atherosclerosis progression, such that as arteries become increasingly hardened and narrowed, the ability of arteries to dilate is diminished. Decreased vasoreactivity and carotid artery wall thickening occur earlier in the atherosclerotic process than anatomical changes measured by QCA; thus it is not surprising that BFMD and CIMT are more strongly correlated longitudinally than BFMD and QCA measures. This is the first report of longitudinal associations between BFMD and changes in anatomic atherosclerosis measures.
This study had 80% power to detect an effect size of 0.25 in standardized β coefficients. Despite the significance of the findings, the magnitude of the β coefficients between vasoreactivity and CIMT and some of the angiographic measures observed were small. The weak correlations may be due to the fact that each method measures atherosclerosis at different vascular locations and at different stages of disease.
Explanations for differences in the magnitude of associations observed between BFMD and CIMT and QCA measures of atherosclerosis estimated in cross-sectional and longitudinal analyses include the therapy tested in the trial and use of lipid-lowering or other medications during the trial differentially affecting the atherosclerosis measures. A greater alteration in atherosclerosis at one vascular site compared to another due to experimental treatment or lipid-lowering therapy could account for differences in correlations between measures of atherosclerosis at the different vascular sites observed prior to and after initiating therapy. The hypothesis that on-trial treatment may have differentially affected atherosclerosis measurements is supported by the observation that the longitudinal correlation between BFMD and coronary artery stenosis differed in direction and magnitude between treatment groups (β = 3.50, p = 0.03 for estrogen and β = −0.61, p = 0.73 for estrogen plus progestin). The observation that statin use modified the longitudinal association between BFMD and CIMT provides additional support for the hypothesis that the effect of lipid-lowering therapy may have served to attenuate the association between the two measures over the trial period.
Theoretically, it is possible that at some stage in the progression of atherosclerosis, pathological changes of the endothelium reach a maximum beyond which further decreases in dilation are unlikely to be observed. In other words, a threshold may exist in the disease process beyond which correlations between further atherosclerosis progressions measured anatomically and minimum dilatory ability will be attenuated. Thus, the inverse relationship between vasoreactivity and anatomic measures of atherosclerosis (CIMT and QCA) may be limited to some definable range of disease severity that may explain the weak associations observed. This would also suggest that vasoreactivity may be limited in usefulness to measure atherosclerosis severity in individuals with advanced cardiovascular disease. We did not, however, observe differences in associations between vasoreactivity and anatomical measures of atherosclerosis by disease severity. This suggests that the applicability of vasoreactivity as a measure of atherosclerosis is not restricted by level of disease severity at least within the range of disease severity evident in our sample. Nevertheless, our study is limited by the fact that the average percent diameter stenosis of study participants was 35%, placing them at mild to moderate levels of disease, and thus limiting the generalizability of the findings.
Consistent with the literature [28], we found that diabetics had more extensive atherosclerotic disease at baseline than non-diabetics, but contrary to the literature [29], did not differ in baseline vasoreactivity measurements. However, cross-sectional associations between BFMD and CIMT by diabetes status suggest that for a given anatomical level of atherosclerosis, diabetics have greater endothelial dysfunction compared to non-diabetics.
Previous studies have shown that a gender difference exists in endothelial function, with women tending to have greater BFMD than men of the same age [30,31]. The majority of studies to date that have examined the relationship between BFMD and anatomical measures of atherosclerosis have tended to focus on men or populations without clinical CVD. Correlations between BFMD and IMT reported by these studies ranged from r = −0.21 to −0.41 [13,15–17], which are of greater magnitude than we observed (r = −0.12). Our findings in women with mild to moderate CAD provide support that the relationship between BFMD and anatomical measures of atherosclerosis holds true in women and among those with CAD, albeit at a lower magnitude than previously reported.
Our longitudinal analyses used measurements of BFMD and anatomical measures of atherosclerosis taken at multiple visits for participants over the study duration, thus reducing the influence of single data points on estimates of association. In addition, QCA variables used in regression models were an average of multiple lesions, thus minimizing the effect of an extreme value on the overall measure. With longitudinal data, we were also able to evaluate whether levels of BFMD were associated with changes in anatomical measures of atherosclerosis over the trial period.
The finding that participants who did not have follow-up vasoreactivity assessments had higher BMI and SBP than those who had follow-up assessments suggests that vasoreactivity may not be the optimal tool to assess atherosclerosis in women who fit these criteria perhaps due to greater discomfort from the measurement technique. However, BMI and SBP were not individually correlated with BFMD. It is important to note that the women who refused follow-up vasoreactivity measurements did agree to follow-up angiography. Angiography is a standard clinical tool; it is possible that participants were more willing to undergo angiography for its recognized clinical value. Regardless, vasoreactivity may not be an appropriate tool for large-scale longitudinal studies since selective dropout may occur.
In conclusion, this study provides evidence that physiological and anatomical measures of atherosclerosis are correlated both cross-sectionally and longitudinally among postmenopausal women with CAD, and thus provides some validation of BFMD as a measure of atherosclerosis in high-risk populations.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute and by the Office of Research on Minority Health (U01-HL-49298) and from the National Institute on Aging, #5-T32-AG00037, Multidisciplinary Research Training in Gerontology.
Footnotes
This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author’s institution,sharing with colleagues and providing to institution administration.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Azen SP, Mack WJ, Cashin-Hemphill L, et al. Progression of coronary artery disease predicts clinical coronary events. Long-term follow-up from the Cholesterol Lowering Atherosclerosis Study. Circulation. 1996;93:34–41. doi: 10.1161/01.cir.93.1.34. [DOI] [PubMed] [Google Scholar]
- 2.Blankenhorn DH, Hodis HN. George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler Thromb. 1994;14:177–92. doi: 10.1161/01.atv.14.2.177. [DOI] [PubMed] [Google Scholar]
- 3.Vigen C, Hodis HN, Selzer RH, Mahrer PR, Mack WJ. Relation of progression of coronary artery atherosclerosis to risk of cardiovascular events (from the Monitored Atherosclerosis Regression Study) Am J Cardiol. 2005;95:1277–82. doi: 10.1016/j.amjcard.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–8. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 6.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 7.Crouse JR, 3rd, Craven TE, Hagaman AP, Bond MG. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995;92:1141–7. doi: 10.1161/01.cir.92.5.1141. [DOI] [PubMed] [Google Scholar]
- 8.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23:1752–60. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 10.Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–63. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 11.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 12.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 13.Furumoto T, Fujii S, Saito N, Mikami T, Kitabatake A. Relationships between brachial artery flow-mediated dilation and carotid artery intima-media thickness in patients with suspected coronary artery disease. Jpn Heart J. 2002;43:117–25. doi: 10.1536/jhj.43.117. [DOI] [PubMed] [Google Scholar]
- 14.Enderle MD, Schroeder S, Ossen R, et al. Comparison of peripheral endothelial dysfunction and intimal media thickness in patients with suspected coronary artery disease. Heart. 1998;80:349–54. doi: 10.1136/hrt.80.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haraki T, Takegoshi T, Kitoh C, et al. Carotid artery intima-media thickness and brachial artery flow-mediated vasodilation in asymptomatic Japanese male subjects amongst apolipoprotein E phenotypes. J Intern Med. 2002;252:114–20. doi: 10.1046/j.1365-2796.2002.01011.x. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto M, Eto M, Akishita M, et al. Correlation between flow-mediated vasodilatation of the brachial artery and intima-media thickness in the carotid artery in men. Arterioscler Thromb Vasc Biol. 1999;19:2795–800. doi: 10.1161/01.atv.19.11.2795. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Akishita M, Yu W, et al. Interrelationship between non-invasive measurements of atherosclerosis: flow-mediated dilation of brachial artery, carotid intima-media thickness and pulse wave velocity. Atherosclerosis. 2004;173:13–8. doi: 10.1016/j.atherosclerosis.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Juonala M, Viikari JS, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–23. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 19.Yan RT, Anderson TJ, Charbonneau F, et al. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol. 2005;45:1980–6. doi: 10.1016/j.jacc.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 20.Witte DR, Westerink J, de Koning EJ, et al. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol. 2005;45:1987–93. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 21.Hodis HN, Mack WJ, Azen SP, et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535–45. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- 22.Blankenhorn DH, Azen SP, Kramsch DM, et al. Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS) Ann Intern Med. 1993;119:969–76. doi: 10.7326/0003-4819-119-10-199311150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Blankenhorn DH, Selzer RH, Mack WJ, et al. Evaluation of colestipol/niacin therapy with computer-derived coronary end point measures. A comparison of different measures of treatment effect. Circulation. 1992;86:1701–9. doi: 10.1161/01.cir.86.6.1701. [DOI] [PubMed] [Google Scholar]
- 24.Selzer RH, Hagerty C, Azen SP, et al. Precision and reproducibility of quantitative coronary angiography with applications to controlled clinical trials. A sampling study. J Clin Invest. 1989;83:520–6. doi: 10.1172/JCI113913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Selzer RH, Hodis HN, Kwong-Fu H, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 27.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–93. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 28.Toto RD. Heart disease in diabetic patients. Semin Nephrol. 2005;25:372–8. doi: 10.1016/j.semnephrol.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Bots ML, Westerink J, Rabelink TJ, de Koning EJ. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J. 2005;26:363–8. doi: 10.1093/eurheartj/ehi017. [DOI] [PubMed] [Google Scholar]
- 30.Herrington DM, Fan L, Drum M, et al. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8:319–28. doi: 10.1177/174182670100800512. [DOI] [PubMed] [Google Scholar]
- 31.Celermajer DS, Sorensen KE, Spiegelhalter DJ, et al. Aging is associated with endothelial dysfunction in healthy men, years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–6. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]