Abstract
Polyamines are required for optimal growth and function of cells. Regulation of their cellular homeostasis is therefore tightly controlled. The key regulatory enzyme for polyamine catabolism is the spermidine/spermine N1-acetyltransferase (SSAT). Depletion of cellular polyamines has been associated with inhibition of growth and programmed cell death. To investigate the physiological function SSAT, we generated a transgenic rat line overexpressing the SSAT gene under the control of the inducible mouse metallothionein I promoter. Administration of zinc resulted in a marked induction of pancreatic SSAT, overaccumulation of putrescine, and appearance of N1-acetylspermidine with extensive depletion of spermidine and spermine in transgenic animals. The activation of pancreatic polyamine catabolism resulted in acute pancreatitis. In nontransgenic animals, an equal dose of zinc did not affect pancreatic polyamine pools, nor did it induce pancreatitis. Acetylated polyamines, products of the SSAT-catalyzed reaction, are metabolized further by the polyamine oxidase (PAO) generating hydrogen peroxide, which might cause or contribute to the pancreatic inflammatory process. Administration of specific PAO inhibitor, MDL72527 [N1,N2-bis(2,3-butadienyl)-1,4-butanediamine], however, did not affect the histological score of the pancreatitis. Induction of SSAT by the polyamine analogue N1,N11-diethylnorspermine reduced pancreatic polyamines levels only moderately and without signs of organ inflammation. In contrast, the combination of N1,N11-diethylnorspermine with MDL72527 dramatically activated SSAT, causing profound depletion of pancreatic polyamines and acute pancreatitis. These results demonstrate that acute induction of SSAT leads to pancreatic inflammation, suggesting that sufficient pools of higher polyamine levels are essential to maintain pancreatic integrity. This inflammatory process is independent of the production of hydrogen peroxide by PAO.
Intracellular polyamine levels are tightly maintained by a number of mechanisms, suggesting their importance for cell function. This polyamine homeostasis is controlled by their biosynthesis, interconversion, catabolism, secretion, and uptake. Polyamine biosynthesis is regulated by the activities of ornithine and S-adenosylmethionine decarboxylases. Overexpression of ornithine decarboxylase in transgenic rodents affected spermatogenesis (1, 2), rendered animals more resistant to chemically or electrically induced seizure activity (3) and to ischemic insults (4), and enhanced skin papilloma formation (5). However, the activation of polyamine biosynthesis caused by overexpression of ornithine decarboxylase did not result in enhanced accumulation of the higher polyamines spermidine and spermine. Polyamine catabolism is controlled by the activity of spermidine/spermine N1-acetyltransferase (SSAT; refs. 6 and 7). The acetylated products are oxidized further by the polyamine oxidase (PAO) to spermidine and putrescine. We recently generated transgenic mouse lines with activated polyamine catabolism caused by an overexpression of SSAT under its own promoter (7) or governed by mouse heavy metal-inducible metallothionein I promoter (8). These animals showed a striking distortion of polyamine pools in liver, spleen, brain, and testis characterized by massive accumulation of putrescine, appearance of N1-acetylspermidine, and decreases in tissue spermidine and/or spermine pools. Changes in the SSAT activity in kidney and heart were less pronounced (8). Induction of SSAT by zinc resulted in a 2- to 3-fold increase in enzyme activity in the liver, with a 50% depletion of the spermidine and a 85% depletion of the spermine pool (8). However, metallothionein is expressed, not only in the liver, but also in the pancreas of the rat in a heavy metal-inducible fashion (9). In regard to polyamines, the pancreas occupies a very special position among mammalian organs and tissues. The pancreas seems to contain the highest concentration of spermidine in the mammalian body (10). Therefore, the aim of our study was to generate a transgenic rat line expressing the mouse SSAT gene under the control of the metallothionein I promoter to elucidate the roles of the higher polyamines in the pancreas. We chose to overexpress the gene in rats in particular, because transgenic rats are more easily subjected to surgical manipulation (of, for instance, gastrointestinal tract and brain) and preparation of tissues. Moreover, the substantial body of literature in the field in physiology makes the rat the animal of choice. In regard to transgene technology applied to polyamine metabolism, the present approach, i.e., an inducible activation of polyamine catabolism, offers a unique and powerful tool to investigate the consequences of disturbed tissue polyamine homeostasis.
Materials and Methods
Generation of Transgenic Rats.
The production of transgenic Wistar rats has been described (11). Briefly, the donor females were superovulated and mated with mature males, and the zygotes were collected and microinjected with the metallothionein-SSAT gene. To maximize the number of the recipient females, the phase of the estrous cycle was determined with the aid of vaginal smear. The females in proestrus/early estrus were made pseudopregnant by mating them with vasectomized males. The zygotes were transferred into oviducts of the recipient females immediately after microinjection. The metallothionein-SSAT transgene construct was the same one that was used recently to produce transgenic mice (8).
Chemicals.
Zinc was administered i.p. as zinc sulfate dissolved in distilled water. The PAO inhibitor MDL72527 [N1,N2-bis(2,3-butadienyl)-1,4-butanediamine] was a generous gift from Hoechst–Roussel. N1,N11-Diethylnorspermine (DENSPM) was synthesized with a modification of the method described in ref. 12. Both drugs were administered i.p. Apoptosis was analyzed by using the in situ cell death detection system (terminal deoxynucleotidyltransferase-mediated UTP end labeling) obtained from Roche Molecular Biochemicals.
Analytical Methods.
Polyamines and their acetylated derivatives were determined with the aid of high-performance liquid chromatography as described by Hyvönen et al. (13). The activity of SSAT was assayed according to Bernacki et al. (14).
Histological Analyses of the Pancreatic Specimens.
Formalin-fixed pancreatic specimens were embedded in paraffin, cut into 5-μm-thick slices, and stained with hematoxylin/eosin. The stained sections were coded and blindly scored by the participating pathologist (J.J.P.) according to the method of Niederau et al. (15). The details of the histological scoring are presented in the legend for Table 2. Apoptotic staining was performed according to the protocol provided by the manufacturer. The apoptotic index was calculated as percentage of apoptotic cells per high-power field (×400) defined by morphology and staining.
Table 2.
Effect of inhibition of PAO by MDL72527 on the pancreatic SSAT activity and polyamine concentrations in metallothionein-SSAT rats treated with DENSPM
| Group | SSAT, pmol/mg/10 min | Putrescine | N1-Acetylspermidine pmol/mg tissue | Spermidine | N1-Acetylspermine | Spermine |
|---|---|---|---|---|---|---|
| Sg | 11.9 ± 9.4 | 21 ± 36 | 55 ± 3 | 4,850 ± 620 | ND | 870 ± 50 |
| Sg + MDL | 9.6 ± 2.9 | ND | 70 ± 2** | 4,440 ± 710 | ND | 1,020 ± 50* |
| Sg + DENSPM | 14.7 ± 4.6 | 120 ± 80 | 56 ± 9 | 4,510 ± 750 | ND | 500 ± 90** |
| Sg + MDL + DENSPM | 43.4 ± 22.2 | 67 ± 52 | 300 ± 200 | 3,320 ± 460* | ND | 990 ± 130 |
| Tg | 34.4 ± 3.3 | 2,680 ± 500 | 69 ± 10 | 5,010 ± 880 | ND | 670 ± 110 |
| Tg + MDL | 27.3 ± 38.0 | 840 ± 370** | 3,420 ± 90*** | 3,230 ± 650* | ND | 620 ± 130 |
| Tg + DENSPM | 820 ± 690 | 3,800 ± 650 | 133 ± 42 | 2,140 ± 340** | ND | 300 ± 35** |
| Tg + MDL + DENSPM | 59,600 ± 14,600*** | 540 ± 260*** | 4,960 ± 420*** | 440 ± 70*** | 61 ± 2 | 49 ± 22*** |
Combined treatment: rats were injected i.p. with 30 mg/kg of MDL72527 and 1 h later with 100 mg/kg of DENSPM on 2 days. Animals were killed 24 h after the latter DENSPM injection. *, P < 0.05; **, P < 0.01; ***, P < 0.001 as compared with the untreated animals. Sg, syngenic; Tg, transgenic; ND, not detected.
Statistical Analyses.
Data are expressed as means ± SD and analyzed by two-tailed Student's t test.
Results
Induction of Pancreatic SSAT Activity by Zinc.
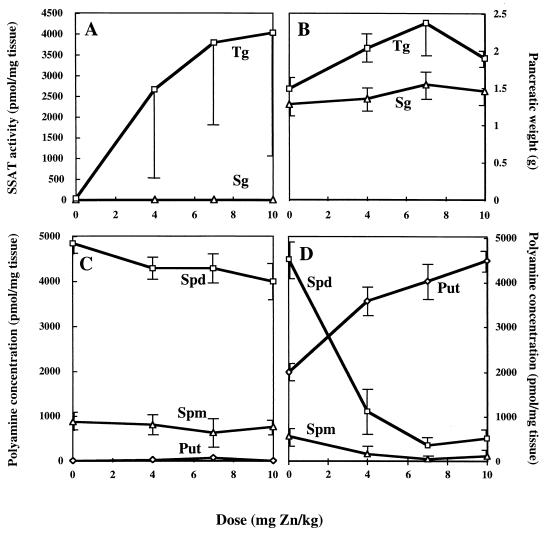
Zinc dose-dependently induced SSAT in transgenic pancreas (Fig. 1A). In the syngenic littermates, the same zinc dose did not affect polyamine levels. The treatments likewise significantly (P < 0.05) increased the weight of transgenic pancreas, whereas the changes in syngenic pancreas were marginal (Fig. 1B). Apart from a slight reduction of spermidine pool, increasing concentrations of zinc had no effect on polyamine pools in syngenic pancreas (Fig. 1C), whereas spermidine and spermine pools in transgenic pancreas decreased strikingly in response to the treatments (Fig. 1D). The depletion of pancreatic spermidine was especially dramatic as shown in the figure (Fig. 1D). The disappearance of the higher polyamines was associated with a steady increase in the pancreatic putrescine pool in the transgenic animals.
Figure 1.
Effect of increasing doses of zinc on pancreatic SSAT activity in nontransgenic and transgenic rats (A) and on pancreatic weight (B). Polyamine concentrations in nontransgenic (C) and transgenic (D) rats. The analyses were carried out 24 h after zinc treatment with three animals in each group. Error bars represent SEM. Tg, transgenic; Sg, syngenic; Put, putrescine; Spd, spermidine; Spm, spermine.
Effect of Inhibition of PAO.
The oxidative degradation of spermidine and spermine is accomplished by the concerted action of inducible SSAT acetylating spermidine and spermine and of the constitutively expressed PAO. The latter enzyme is a peroxisomal flavoprotein that only feebly catalyzes the oxidation of spermidine and spermine but strongly prefers their acetylated derivatives as substrates (16). Hence, the rate-controlling enzyme in polyamine catabolism or interconversion is SSAT. The ultimate oxidation of spermidine to putrescine and spermine to spermidine is accompanied by the formation of hydrogen peroxide and acetaminopropanal, substances potentially able to create oxidative stress. MDL72527 is a highly specific and potent inhibitor of PAO (17). In nontransgenic animals, any of the treatments, i.e., zinc or MDL72527 alone or in combination with each other, had only marginal effects on pancreatic SSAT activity or polyamine pools (Table 1). In fact, only the combination of zinc and MDL72527 increased the content of N1-acetylspermidine over the detection level. In transgenic animals, MDL72527 had little effect on the pancreatic levels of the higher polyamines, but the drug produced a massive accumulation of N1-acetylspermidine, indicative of a blockage of the PAO reaction. Although zinc distinctly induced pancreatic SSAT, the treatment likewise resulted in a more than 80% decrease in the concentrations of spermidine and spermine (Table 1). The pools of the higher polyamines remained at a low level: putrescine content clearly decreased, and there was a striking accumulation of N1-acetylspermidine as well as the appearance of N1-acetylspermine in response to the combined treatment with zinc and MDL72527.
Table 1.
Effect of inhibition of PAO by MDL72527 on pancreatic SSAT activity and polyamine concentrations in metallothionein-SSAT rats treated with zinc
| Group | SSAT, pmol/mg/10 min | Putrescine | N1-Acetylspermidine, pmol/mg tissue | Spermidine | N1-Acetylspermine | Spermine |
|---|---|---|---|---|---|---|
| Sg | 5.5 ± 2.6 | 23 ± 20 | ND | 3,990 ± 330 | ND | 560 ± 50 |
| Sg + MDL | 7.2 ± 1.0 | 22 ± 19 | ND | 4,080 ± 580 | ND | 800 ± 110 |
| Sg + Zn | 3.7 ± 0.6 | 36 ± 27 | ND | 3,620 ± 210 | ND | 480 ± 50 |
| Sg + MDL + Zn | 3.1 ± 0.6 | 29 ± 20 | 61 ± 18*** | 3,740 ± 640 | ND | 700 ± 130 |
| Tg | 37.5 ± 4.8 | 2,950 ± 1,620 | 33 ± 10 | 2,630 ± 190 | ND | 500 ± 40 |
| Tg + MDL | 48.0 ± 13.0 | 1,340 ± 220 | 2,090 ± 390*** | 2,740 ± 220 | ND | 420 ± 40 |
| Tg + Zn | 636 ± 347* | 2,830 ± 760 | 41 ± 9 | 370 ± 150*** | ND | 64 ± 36*** |
| Tg + MDL + Zn | 310 ± 50*** | 780 ± 140* | 1,340 ± 100*** | 320 ± 100*** | 37 ± 5* | 120 ± 50*** |
Combined treatment: rats were injected i.p. with 30 mg/kg of MDL72527 and 1 h later with 10 mg/kg of zinc. Animals were killed 24 h after the zinc injection. There were three to four animals in each group. *, P < 0.05; ***, P < 0.001 as compared with untreated animals. Sg, syngenic; Tg, transgenic; ND, not detected.
Induction of Pancreatic SSAT by the Polyamine Analogue DENSPM.
Polyamine analogues, such as DENSPM, are extremely powerful inducers of SSAT transgene (8, 18). The results of experiments with syngenic and transgenic rats treated with DENSPM or MDL72527 alone or in combination are summarized in Table 2. DENSPM alone had very little effect on SSAT activity or polyamine pools, with the possible exception of a significant decrease in pancreatic spermine content in nontransgenic rats. Apart from the significant increase in N1-acetylspermidine and spermine, MDL72527 alone exerted only marginal effects in nontransgenic animals. The combination of both drugs moderately decreased the pancreatic spermidine pool with a concomitant increase in the content of N1-acetylspermidine (Table 2). The changes caused by the two drugs were much more pronounced in the pancreas of transgenic animals. Typically, MDL72527 caused a massive accumulation of N1-acetylspermidine and decreases in both putrescine and spermidine (Table 2). DENSPM powerfully induced SSAT activity and moderately (less than 60%) decreased the pancreatic concentrations of spermidine and spermine. The combination, however, exerted the most striking effects on the pancreas of the transgenic animals. SSAT activity was enhanced further by 70-fold over that achieved with the analogue alone; N1-acetylspermidine was increased to a very high level, whereas pancreatic spermidine and spermine pools were depleted by more than 90%. The synergistic induction of SSAT by the combinations of DENSPM and MDL72527 could have resulted from an enhanced accumulation of the analogue in the pancreas in response to the PAO inhibitor or from the fact that some of the products of PAO are powerful endogenous inhibitors of SSAT. Macroscopically, only the combination of the two drugs, but not any of the other treatments, resulted in acute pancreatitis.
Extent of Pancreatic Inflammation by Acute SSAT Induction.
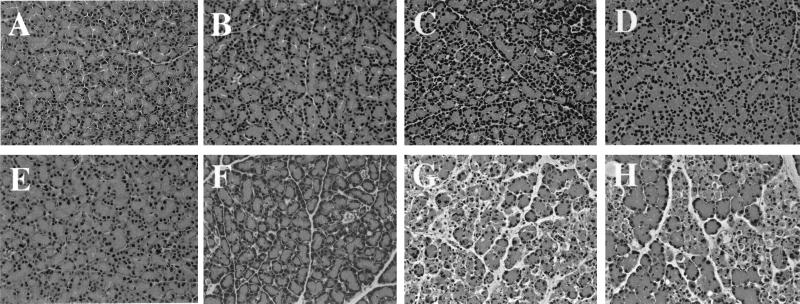
The pancreatic histologies of syngenic and transgenic animals receiving zinc, the polyamine analogue DENSPM, or the PAO inhibitor alone or in combination are depicted in Figs. 2 and 3. Syngenic animals, irrespective of the treatments, displayed normal pancreatic histology both in the exocrine and endocrine part of the organ (Fig. 2 A–D). Similarly, untreated transgenic rats and transgenic animals receiving MDL72527 had a normal pancreatic histology (Fig. 2 E and F). In contrast, transgenic animals receiving zinc (Fig. 2G) showed distinct signs of acute pancreatitis characterized by expansion of interlobular and intralobular spaces caused by moderate to strong interstitial edema, disorganization of the acinar structure, infiltration of inflammatory cells, necrosis, and apoptosis. Fat necrosis was also a distinct feature in these animals. The endocrine pancreas appeared normal. The histopathology of the exocrine pancreas of the transgenic rats treated with the combination of zinc and MDL72527 (Fig. 2H) resembled that of the group receiving zinc alone with clear signs of acute pancreatitis.
Figure 2.
Histology of pancreas after MDL72527 and/or zinc treatment. Syngenic animals (A–D) and transgenic animals (E–H). Both control animals (A and E) showed normal pancreatic histology. Similar results were found in syngenic animals exposed to MDL72527, zinc, or both (B–D, respectively). Transgenic rats treated with MDL72527 (F) did not show marked histological changes, whereas zinc induced pancreatitis (G). The combination of MDL72527 and zinc treatment in transgenic animals induced a more severe pancreatitis (H).
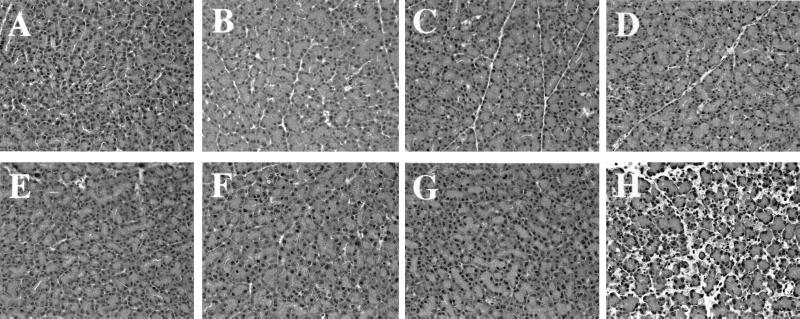
Figure 3.
Histology of pancreas after MDL72527 and/or DENSPM treatment. Syngenic animals (A–D) and transgenic animals (E–H). Control animals (A and E), MDL72527-treated animals (B and F), and DENSPM-treated animals (C and G) showed normal pancreatic histology. Syngenic rats exposed to MDL72527 and DENSPM (D) did not show any histological changes, but the combination of the two drugs in transgenic animals induced acute pancreatitis (H).
The pancreatic histologies of syngenic and transgenic animals receiving the polyamine analogue DENSPM or PAO inhibitor alone or in combination are depicted in Fig. 3. None of the treatments caused histopathological changes in the pancreas of syngenic animals (Fig. 3 A–D). There was a normal pancreatic histology in untreated transgenic animals (Fig. 3E) and transgenic animals receiving either MDL72527 or DENSPM alone (Fig. 3 F and G). However, transgenic animals treated with the combination of the drugs revealed distinct signs of acute pancreatitis (Fig. 3H). These animals were also the only ones that had a profound depletion of their pancreatic spermidine and spermine levels (Table 2).
A grading of the pancreatic histopathological changes in response to the different treatment regimes of syngenic and transgenic animals is presented in Table 3. Treatment of the transgenic rats with zinc alone or in combination with the PAO inhibitor MDL72527 resulted in the most severe scores.
Table 3.
Scoring of histological alterations
| Animals and treatment | Histological scoring (0 to 4)
|
|||
|---|---|---|---|---|
| Edema | Vacuolization | Inflammation | Necrosis | |
| Sg + none (n = 3) | 0 | 0 | 0 | 0 |
| Sg + MDL72527 (n = 3) | 0 | 0 | 0 | 0 |
| Sg + Zn (n = 7) | 1 | 0 | 1 | 0 |
| Sg + MDL72527 + Zn (n = 7) | 1 | 0 | 1 | 0 |
| Sg + DENSPM (n = 3) | 0 | 0 | 0 | 0 |
| Sg + DENSPM + MDL (n = 3) | 0 | 0 | 0 | 0 |
| Tg + none (n = 3) | 0 | 0 | 0 | 0 |
| Tg + MDL72527 (n = 3) | 0 | 2 | 0 | 0 |
| Tg + Zn (n = 7) | 2 | 1* | 2–3 | 3–4 |
| Tg + MDL72527 + Zn (n = 7) | 3 | 0* | 3 | 4 |
| Tg + DENSPM (n = 3) | 0 | 0 | 0 | 0 |
| Tg + DENSPM + MDL (n = 3) | 2 | 2 | 2 | 2 |
The histological alterations were scored blindly by a pathologist (J.J.P.). The scores range from 0 (absent) and 1 (minimal) to 4 (maximal). For necrosis and vacuolization, the scores refer to an approximate percentage of cells involved. 0, 0–5%; 1, 5–15%; 2, 15–35%; 3, 35–50%; 4, >50%. Sg, syngenic; Tg, transgenic.
Difficult to assess because of extensive necrosis in most samples.
Using the terminal deoxynucleotidyltransferase-mediated UTP end labeling methodology to visualize apoptotic cells, we calculated the apoptotic index as percentage of apoptotic cells per high-power field. In syngenic animals, the index was below 0.03%; addition of the PAO antagonist MDL72527 did not change the index, whereas zinc and/or DENSPM increased the index up to 0.7 ± 0.36%. In transgenic animals, the index was the same as in syngenic animals; addition of MDL72527 increased the index to 0.22 ± 0.11%. Zinc treatment resulted in a marked raise of the index to 2.9 ± 1.38%, which was increased further to 13.8 ± 2.6% by MDL72527 treatment. The index for the DENSPM alone was 0.25 ± 0.07%; in combination with MDL72527, the index increased to 5.8 ± 0.28%.
Discussion
The pancreas is the richest source of the polyamine spermidine in the mammalian body (10). Various hormones, such as cholecystokinin and its analogue caerulein, enhance pancreatic polyamine biosynthesis (10, 19). On the other hand, specific inhibition of ornithine decarboxylase by α-difluoromethylornithine not only prevented the accumulation of putrescine and spermidine but also retarded pancreatic growth (20). Stimulation of ornithine decarboxylase leads to an accumulation of putrescine associated with the initiation of pancreatic growth, whereas intracellular spermidine accumulation apparently is needed for the maintenance of the growth (10, 19–23). However, determinations of the exact functions of the polyamines in the pancreas or in any mammalian tissues are only tentative. The pancreas has a very high molar ratio of spermidine/spermine, according to the present results, of around 7 (Tables 1 and 2). High spermidine/spermine ratio is often associated with active proliferation of tissues, especially in young animals (24). Our results underline the important role of the polyamines in pancreatic function and integrity. In regard to transgene technology applied to polyamine metabolism, the present approach, i.e., an inducible activation of polyamine catabolism, offers a powerful tool to disturb tissue polyamine homeostasis. The induction of pancreatic SSAT activity by zinc in the transgenic animals resulted in a rapid depletion of spermidine and spermine pools with the development of acute pancreatitis. Depletion of spermidine and/or spermine as the cause of the disease is supported by several pieces of experimental evidence. (i) Administration of zinc to nontransgenic animals did not induce SSAT, nor did it deplete polyamines. There were no macroscopic or microscopic signs of acute pancreatitis. (ii) Administration of zinc in normal rats, at doses relevant to those used in this study, rather alleviated signs of caerulein-induced pancreatitis by substantial elevation of pancreatic metallothionein and glutathione levels (9). (iii) Induction of SSAT by the polyamine analogue DENSPM in combination with the PAO inhibitor MDL72527 equally depleted spermidine and spermine levels resulting in pancreatitis. (iv) The possibility that striking accumulation of acetylspermidine in response to MDL72527 treatments would be toxic to pancreas is rendered somewhat unlikely by the fact that the drug alone resulted in a marked accumulation of acetylspermidine without signs of pancreatitis (Tables 1 and 2). (v) Finally, inhibition of PAO by MDL72527 did not alleviate pancreatitis-associated changes, indicating that possible oxidative stress does not contribute significantly to the inflammatory process in this model. The latter finding is in agreement with our earlier studies with fibroblasts obtained from SSAT overexpressing transgenic fetuses, in which cytotoxicity associated with SSAT induction could not be alleviated by an inhibition of PAO, but the inhibitor merely enhanced the cytotoxicity (18). However, under certain conditions, it has been shown that the oxidation process catalyzed by PAO contributes to the cytotoxicity (25).
This investigation is, to our knowledge, the first demonstration that depletion of higher polyamines leads to acute pancreatitis. The mechanism of the organ inflammation induced by polyamine depletion is unknown. Stabilization of DNA by polyamines has been long recognized (26). Profound depletion might therefore destabilize DNA, interfere with transcription, and result in cell death. In addition, spermidine is essential for hypusine synthesis, an indispensable component of the eukaryotic translation initiation factor 5A (27). The eukaryotic translation initiation factor 5A has been found in all eukaryotic cells and has been shown to be required for cell proliferation. Depletion of spermidine resulted in growth arrest in the G1/S phase of the cell cycle. Furthermore, disruption of the S-adenosylmethionine decarboxylase gene resulted in a depletion of spermidine and spermine in yeast (28). The cells were characterized by an increase in size, accumulation of vesicle-like bodies, and an abnormal distribution of actin-like material. Recently, we demonstrated that cholecystokinin-stimulated activation of p125 focal adhesion kinase (p125FAK) is associated with actin cytoskeleton rearrangement and changes in cellular morphology in pancreatic acinar cells (29).
Pancreatitis results from acute or chronic acinar injury with extracellular release of digestive enzymes and subsequent inflammation. In animal models of acute pancreatitis, the extent of organ destruction is determined by acinar cell necrosis and seems to be inversely correlated to apoptosis (30). Neutrophil infiltration augments the severity of acute pancreatitis probably by converting acinar cell apoptosis to necrosis (31). In addition, induction of apoptosis might even protect against acinar cell injury and reduce severity of caerulein-induced pancreatitis (32). However, the authors of the latter report did not calculate the apoptotic index in the pancreas of the treated groups. In our experimental groups, the apoptotic index did not change significantly from controls with the exception of the application of zinc and/or DENSPM. In transgenic animals, an application of zinc or the analogue especially in combination with MDL72527 resulted in a marked increase in the apoptotic index without affecting the degree of necrosis. Interestingly, MDL72527 has been reported recently to induce apoptosis of transformed hematopoietic cells (33).
After the initial event of acinar injury with active pancreatic enzyme extraversion, cytokine release and activation of the complement system will follow immediately (34). Spermine has been proposed to act as a natural counterregulator of acute inflammatory responses inhibiting the synthesis of proinflammatory cytokines in human mononuclear cells stimulated with lipopolysaccharide (35). Furthermore, local application of spermine inhibited the development of acute footpad inflammation induced by carrageenin (35). The administration of an inhibitor of polyamine transport system worsened the inflammation (36), indicating that spermine released from injured cells is taken up actively by cells of the immune system (37). The inhibition of the counterregulatory mechanisms by spermine depletion by acute overexpression of the rate-limiting catabolic enzyme SSAT might contribute to the observed necrosis.
In summary, profound depletion of pancreatic spermidine and spermine by zinc-induced activation polyamine catabolism results in the development of pancreatitis independently of the generation of hydrogen peroxide by PAO. Sufficient pools of higher polyamine levels are therefore essential to maintain pancreatic integrity. The depletion of the higher polyamines might attenuate natural antiinflammatory responses.
Acknowledgments
We thank Ms. Tuula Reponen and Ms. Sisko Juutinen for skillful technical assistance. This work was supported in part by a grant from the Academy of Finland and from National Institutes of Health Grant CA-76428. K.-H.H. was supported by the Werner-Creutzfeldt-Stipend of the German Gastroenterological Association and a grant from the B. Braun–Foundation, Melsungen, Germany.
Abbreviations
- SSAT
spermidine/spermine N1-acetyltransferase
- PAO
polyamine oxidase
- DENSPM
N1,N11-diethylnorspermine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140122097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140122097
References
- 1.Halmekytö M, Hyttinen J-M, Sinervirta R, Utriainen M, Myöhänen S, Voipio H-M, Wahlfors J, Syrjänen S, Syrjänen K, Alhonen L, Jänne J. J Biol Chem. 1991;266:19746–19751. [PubMed] [Google Scholar]
- 2.Hakovirta H, Keiski A, Toppari J, Halmekytö M, Alhonen L, Jänne J, Parvinen M. Mol Endocrinol. 1993;7:1430–1436. doi: 10.1210/mend.7.11.8114757. [DOI] [PubMed] [Google Scholar]
- 3.Halonen T, Sivenius J, Miettinen R, Halmekytö M, Kauppinen R, Sinervirta R, Alakuijala L, Alhonen L, MacDonald E, Jänne J, et al. Eur J Neurosci. 1993;5:1233–1239. doi: 10.1111/j.1460-9568.1993.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 4.Lukkarinen J A, Kauppinen R A, Gröhn O H J, Oja J M E, Sinervirta R, Järvinen A, Alhonen L I, Jänne J. Eur J Neurosci. 1998;10:2046–2055. doi: 10.1046/j.1460-9568.1998.00216.x. [DOI] [PubMed] [Google Scholar]
- 5.Halmekytö M, Syrjänen K, Jänne J, Alhonen L. Biochem Biophys Res Commun. 1992;187:493–497. doi: 10.1016/s0006-291x(05)81521-9. [DOI] [PubMed] [Google Scholar]
- 6.Casero R A, Pegg A E. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 7.Pietilä M, Alhonen L, Halmekytö M, Kanter P, Jänne J, Porter C W. J Biol Chem. 1997;272:18746–18751. doi: 10.1074/jbc.272.30.18746. [DOI] [PubMed] [Google Scholar]
- 8.Suppola S, Pietilä M, Parkkinen J J, Korhonen V P, Alhonen L, Halmekytö M, Porter C W, Jänne J. Biochem J. 1999;338:311–316. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z H, Iguchi H, Ohshio G, Imamura T, Okada N, Tanaka T, Imamura M. Pancreas. 1996;13:173–183. doi: 10.1097/00006676-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Löser C, Fölsch U R, Cleffmann U, Nustede R, Creuzfeldt W. Digestion. 1989;43:98–112. doi: 10.1159/000199867. [DOI] [PubMed] [Google Scholar]
- 11.Lukkarinen J, Gröhn O, Sinervirta R, Järvinen A, Kauppinen R A, Jänne J, Alhonen L. Stroke. 1997;28:639–645. doi: 10.1161/01.str.28.3.639. [DOI] [PubMed] [Google Scholar]
- 12.Rehse K, Puchert E, Leissring S. Arch Pharm (Weinheim) 1990;323:287–294. doi: 10.1002/ardp.19903230507. [DOI] [PubMed] [Google Scholar]
- 13.Hyvönen T, Keinänen T A, Khomutov A R, Khomutov R M, Eloranta T O. J Chromatogr. 1992;574:17–21. doi: 10.1016/0378-4347(92)80093-6. [DOI] [PubMed] [Google Scholar]
- 14.Bernacki R J, Oberman E J, Seweryniak K E, Atwood A, Bergeron R J, Porter C W. Clin Cancer Res. 1995;1:847–857. [PubMed] [Google Scholar]
- 15.Niederau C, Liddle R A, Ferrell L D, Grendell J H. J Clin Invest. 1986;78:1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hölttä E. Biochemistry. 1977;16:91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- 17.Bey P, Bolkenius F N, Seiler N, Casara P. J Med Chem. 1985;28:1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- 18.Alhonen L, Karppinen A, Uusi-Oukari M, Vujcic S, Korhonen V-P, Halmekytö M, Kramer D L, Hines R, Jänne J, Porter C W. J Biol Chem. 1998;273:1964–1969. doi: 10.1074/jbc.273.4.1964. [DOI] [PubMed] [Google Scholar]
- 19.Löser C, Fölsch U R, Sahelijo-Krohn P, Creutzfeldt W. Eur J Clin Invest. 1989;19:448–458. doi: 10.1111/j.1365-2362.1989.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 20.Morisset J, Grondin G. Pancreas. 1987;2:303–311. doi: 10.1097/00006676-198705000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Jurkowska G, Rydzewska G, Andrzejewska A. J Physiol Pharmacol. 1997;48:789–804. [PubMed] [Google Scholar]
- 22.Lin C-H, Lu R-B, Lebenthal E, Luk G D, Lee P-C. Biochim Biophys Acta. 1991;1093:65–71. doi: 10.1016/0167-4889(91)90139-o. [DOI] [PubMed] [Google Scholar]
- 23.Löser C, Fölsch U R. Digestion. 1993;54:213–223. doi: 10.1159/000201040. [DOI] [PubMed] [Google Scholar]
- 24.Jänne J, Raina A, Siimes M. Acta Physiol Scand. 1964;62:352–358. doi: 10.1111/j.1748-1716.1964.tb10433.x. [DOI] [PubMed] [Google Scholar]
- 25.Ha H C, Woster P M, Yager J D, Casero R A. Proc Natl Acad Sci USA. 1997;94:11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu H S, Schwietert H C A, Feuerstein B G, Marton L J. Biochem J. 1990;269:329–334. doi: 10.1042/bj2690329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park M H, Wolff E C, Folk J E. BioFactors. 1993;4:95–104. [PubMed] [Google Scholar]
- 28.Balasundaram D, Tabor C W, Tabor H. Proc Natl Acad Sci USA. 1991;88:5872–5876. doi: 10.1073/pnas.88.13.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiehne K, Herzig K H, Fölsch U R. Digestion. 1999;60:153–160. doi: 10.1159/000007641. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser A M, Saluja A K, Sengupta A, Saluja M, Steer M L. Am J Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- 31.Sandoval D, Gukovskaya A, Reavey P, Gukovsky S, Sisk A, Braquet P, Pandol S J, Poucell-Hatton S. Gastroenterology. 1996;111:1081–1091. doi: 10.1016/s0016-5085(96)70077-x. [DOI] [PubMed] [Google Scholar]
- 32.Saluja A, Hofbauer B, Yamaguchi Y, Yamanaka K, Steer M. Biochem Biophys Res Commun. 1996;220:875–878. doi: 10.1006/bbrc.1996.0498. [DOI] [PubMed] [Google Scholar]
- 33.Dai H Q, Kramer D L, Yang C Y, Murti K G, Porter C W, Cleveland J L. Cancer Res. 1999;59:4944–4954. [PubMed] [Google Scholar]
- 34.Karne S, Gorelick F S. Surg Clin North Am. 1999;79:699–710. doi: 10.1016/s0039-6109(05)70036-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Caragine T, Wang H, Cohen P S, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, et al. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Borovikova L V, Wang H, Metz C, Tracey K J. Mol Med. 1999;5:595–605. [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan D M L. Essays Biochem. 1987;23:82–115. [PubMed] [Google Scholar]