Abstract
Itch evoked by cowhage or histamine is reduced or blocked by capsaicin desensitization, suggesting that pruriceptive neurons are capsaicin-sensitive. Topical capsaicin can evoke both nociceptive sensations and itch, whereas intradermal injection of capsaicin evokes only burning pain. To dissociate the pruritic and nociceptive sensory effects caused by the chemical activation of sensory neurons, chemicals were applied in a punctiform manner to the skin of the forearm using individual, heat-inactivated cowhage spicules treated with various concentrations of capsaicin (1–200 mg/ml) or histamine (0.01–100 mg/ml). Perceived intensities of itch, pricking/stinging and burning were obtained every 30s using the general version of the Labeled Magnitude Scale and compared with ratings evoked by individual native cowhage spicules. Similar to cowhage, capsaicin and histamine spicules reliably evoked sensations of itch in a dose-dependent manner that were most often accompanied by pricking/stinging and to a lesser extent burning. Spicules containing 200 mg/ml capsaicin or 10 mg/ml histamine yielded peak magnitudes and durations of sensations comparable to those elicited by cowhage. Each type of spicule also produced comparable areas of dysesthesias (enhanced mechanically evoked itch or pain) and/or skin reactions (wheal and/or flare) in surrounding skin, though inconsistently. The incidence of flare was greater in response to histamine than to capsaicin or cowhage. These results suggest the possibility that capsaicin, histamine and cowhage activate common peripheral or central neural mechanisms that mediate pruritic sensations and associated dysesthesias.
1. Introduction
Chemical substances that evoke itch can also elicit qualities of pain and vice versa. For example, histamine is known for its pruritic effects but can evoke an initial sensation of pain upon injection into the skin [1,4]. Cowhage spicules (trichomes) from the tropical legume, Mucuna pruriens, elicit a histamine-independent itch [9] that is accompanied by nociceptive sensations of pricking/stinging and burning [10,12,19]. Conversely, capsaicin, the algesic chemical in red peppers, elicits pain and not itch when injected intradermally [11,22,24, unpublished observations]. But when applied topically to the forearm, capsaicin can elicit an itch that dominates over lesser sensations of pricking/stinging and burning [6]. With repeated topical applications, capsaicin not only reduces the magnitude of chemically evoked itch and nociceptive sensations [6] but also attenuates the itch mediated by an intradermal injection of histamine and abolishes the itch elicited by cowhage [9]. Thus, pruriceptive neurons responsive to cowhage or to histamine may also respond to, and become desensitized by, capsaicin. In addition, nociceptive neurons responsive to capsaicin may act centrally to block itch.
It is not known to what extent algesic and pruritic chemicals can elicit similar sensations or activate the same types of pruriceptive or nociceptive sensory neurons. There have been no direct comparisons of the quality, magnitude and time course of sensations evoked by histamine, cowhage and capsaicin. In addition, the studies to date have differed in the method of chemical delivery which, in turn, may have contributed to differences in neuronal activation. For example, the sensory nerve endings activated by the tip of a cowhage spicule or by the topical application of a chemical like capsaicin might be more superficially located than those responsive to chemicals delivered by a hypodermic needle inserted into the skin. The volume and distribution of the chemical also differ for the different methods of application. Thus, psychophysical comparisons of the sensory effects of capsaicin, histamine and cowhage require that each chemical agent should be delivered in the same manner.
The cowhage spicule provides a unique way of delivering a chemical to a punctate region of skin. The native cowhage spicule delivers to the skin a cysteine protease, called mucunain, that has been shown capable of activating the protease activating receptors PAR2 and PAR4 [26]. Autoclaving the cowhage spicules inactivates the heat-labile protease. These inert spicules produce no sensations when inserted superficially into the skin of the volar forearm of human volunteers. Thus, by soaking them in various solutions, the spicules can be used to study the response to the punctate application of other chemicals.
We adopted this strategy to learn whether the punctate application of capsaicin or histamine selectively elicits itch and/or nociceptive sensations. The quality and magnitudes of sensations, cutaneous dysesthesias and skin reactions (wheal and flare) produced by the insertion of a single, native cowhage spicule or chemically-soaked (capsaicin and histamine) inactivated spicule in the volar forearm of human subjects were examined. Preliminary data have been reported [22].
2. Material and Methods
2.1. Subjects
All protocols were approved by Yale University Human Investigation Committee (HIC). 21 healthy subjects: 14 females and 7 males, volunteered to participate in the study. The subjects were required to refrain from antihistamines and pain medications at least 24 hrs prior to an experiment. Subjects presenting a history of dermatological, neurological, cardiac or immunological disorders were excluded. All subjects were monetarily compensated for their time.
2.2. Preparation of chemically-filled spicules
Spicules/trichomes on the pod of the cowhage plant (Mucuna pruriens) were autoclaved to inactivate the active pruritic ingredient, mucunain [26], thereby rendering the spicules chemically inert. The autoclaved spicules were soaked in solutions of histamine (Sigma-Aldrich, St. Louis, MO) made in double-distilled water or capsaicin (Sigma-Aldrich, St. Louis, MO) dissolved in 80% ethanol. This method of chemical application has a distinct advantage over certain others in that there is no delivery to the skin of a vehicle that itself may elicit a sensation. That is, histamine was mixed in de-ionized water instead of the pH adjusted salt solution required for injection; capsaicin was prepared in alcohol that was subsequently evaporated. Thus, the histamine or capsaicin that remained in dried spicules could be delivered without the confounding effects of vehicle irritants such as salts and alcohol.
The different concentrations of chemical were: 0.01, 0.1, 1, 10, 100 mg/ml for histamine and 1, 10, 50, 200 mg/ml for capsaicin. The spicules were observed under a stereomicroscope as they filled with the chemical solution. Filling was determined by the displacement of air by the solution within the spicule. The filled spicules were taken out of the solution and allowed to dry on a filter paper placed in a Petri dish. The tip of a single spicule, held by forceps, was inserted into the superficial skin of the forearm laterally at an angle of approximately 30 degree from the surface of the skin.
It is likely that the chemical contained only in the tip of the spicule can enter the skin. As the soaked spicule dries, the fluid within the spicule disappears but does not gravitate towards the tip while evaporating. Thus, we assume that chemical in the entire spicule does not concentrate in the tip. Rather, only the chemical in the fluid that is held within the tip contributes to the stimulus. The spicules were 2–4 mm in length and each had a diameter of about 1–3 μm at the tip. The approximate length of the spicule entering the skin was 0.2 mm (200 μm). The radius, r, of the spicule, 0.2 mm from the tip, was 0.012 mm. The volume (1/3πr2h) of a conically shaped cowhage spicule tip with a radius of 0.012 mm and a height, h, of 0.2 mm was 0.00003 mm3, or 30 picoliters. A 200 mg/ml solution contained 200 pg per 1 pL. The theoretical amount of chemical contained in the 30 pL tip was 30 × 200 = 6000 pg or 6 ng. Thus, the amount of capsaicin in the tip of a spicule soaked in solutions of 1 to 200 mg/ml was 30 pg up to 6 ng, respectively. The amount of histamine contained in the tip of a spicule, soaked in concentrations of 0.01–100 mg/ml was 0.3 pg to 3 ng.
It is likely that the actual amount of chemical that diffused away from the tip of a spicule was less than the total amount contained within the tip. After drying, the spicule did not appear, by microscopic inspection, to have crystals of chemical on the outside and, upon removal from the skin appeared to contain no fluid in the tip. Thus, it is possible that the chemical actually delivered to the skin may have been contained within the walls of the tip. However, an experimental analysis of the mechanism of chemical delivery and the precise amount delivered are beyond the scope of the present study.
2.3. Measurements of the quality and perceived intensity of sensation
Each subject on different occasions received a native cowhage spicule, or a heat-inactivated spicule soaked in one of different concentrations of histamine (or the vehicle alone) or capsaicin (or its vehicle). The different types of spicules were applied in a random manner with each subject being tested twice in a session, once on each arm. The frequency of sessions varied from 24 hrs to a week to accommodate the availability of the subjects and experimenters. In response to a given spicule, subjects made periodic judgments of the perceived intensity of itch and nociceptive sensations of pricking/stinging and burning. Itch was defined as a sensation that provoked a desire to scratch. Pricking/stinging was described as a sharp, localized sensation having the quality of needle stick or insect bite that was either transient (pricking) or prolonged (stinging). Burning was described as having the quality of sensation that might be produced by rubbing the skin abrasions or by extreme temperature stimuli. The subjects were instructed that following the insertion of a spicule they may or may not experience the sensations of itch, pricking/stinging and burning. They were asked to rate only sensations that they experienced. Additionally, they were instructed that the sensation of pricking/stinging and burning may or may not be painful.
The subjects rated the perceived intensity of itch and nociceptive sensations using the general version of the Labeled Magnitude Scale (gLMS) [2,5]. This scale presented subjects with intensity markers including “no sensation”, “barely detectable”, “weak”, “moderate”, “strong”, “very strong” positioned at appropriate locations along the scale in relation to the “strongest imaginable sensation of any kind”, which was placed at the top. Ratings of each of the three sensory qualities were obtained every 30 sec from the moment of spicule insertion until 20 minutes elapsed or until each quality was judged as zero three times in a row. The subject judged the magnitude of each quality by using a computer mouse to move a cursor along the scale as presented on a videoscreen. Each subject received prior training during which they used the gLMS to judge the magnitude of familiar sensory experiences such as the “sting of a bee” and the “itch from a mosquito bite.” Any sensation rated below barely detectable on the scale was considered as “zero”.
The subjects were trained in the practice session to use the gLMS by rating responses to a single cowhage spicule inserted in their forearm. The results of this test were discarded and the cowhage spicule was tested again in a random manner in all the subjects. In the event, that a spicule evoked less than barely detectable or no sensations, it was removed and a new spicule was inserted a few millimeters away from the previous site of insertion. Up to three such insertions were tried before it was determined that a particular concentration of capsaicin or histamine or a native cowhage spicule did not produce a response. The results presented include each subject’s ratings of the first single trial that was rated higher than barely detectable for each concentration of capsaicin or histamine or the native cowhage spicule tested.
2.4. Measurements of cutaneous dysesthesias
Once all sensations had disappeared, the presence or absence of each of the following dysesthesia was determined, and when present, the borders of the dysesthetic areas were mapped and marked on the skin. a) Alloknesis: A cotton swab mounted on a coping-saw blade that delivered approximately 100 mN of compressional force [21] was applied in a series of short strokes, each about 40 mm/s. The stroking began on normal skin and proceeded towards the spicule insertion site until the subject reported a sensation of itch during the stroking or until the strokes came within 1 cm of the application site. The area of alloknesis was mapped by repeating this procedure along 8 to 12 radial paths. b) Hyperalgesia: A Von Frey filament fitted with a tip diameter of 200 μm and exerting a bending force of 100 mN was used to produce brief indentations of skin starting from normal skin and proceeding toward the spicule site along a series of radial lines as described. The subjects were instructed to attend to the magnitude of pricking pain evoked and indicate if and when the magnitude of this sensation was noticeably enhanced over that elicited in normal skin and in a consistent manner as the filament was advanced further toward the application site. c) Hyperknesis: The protocol was the same as described in b) except that the von Frey filament had a tip of 50 μm diameter and exerted a bending force of 20 mN. The subjects were instructed to ignore the initial pricking sensation and only judge the magnitude of any itch that occurred. An area of hyperknesis was marked out within which the subject reported noticeably enhanced itch over that elicited in normal conditions.
Once mapping of the dysesthetic regions was completed, the borders of any skin reaction consisting of a wheal, a raised edematous region, or an area of redness (neurogenic flare), were drawn on the skin. Occasionally, an inactive cowhage spicule produced a minute region of redness or edema of the order of 1 – 2 mm in diameter. A skin reaction was required to have a minimum diameter of 0.5 cm for a wheal and 1 cm for flare to be included in the analyses. A digital camera was used to photograph the areas of dysesthesias and skin reactions associated with a spicule after each test.
2.5. Data Analyses
Magnitudes of sensory ratings were logarithmically transformed to achieve normality and a value of 1.0 was added to each score to avoid the presence of any zeros. From the ratings of each sensory quality for each subject, the logarithmic values of the following parameters were obtained: The latency of onset of sensation (onset of nonzero ratings), the peak magnitude of the ratings, the time from the onset of sensation to the peak, the duration of sensation (time between onset of sensation and the first of the three consecutive ratings of zero or until the 20 min period allotted for ratings had elapsed) and the area under the rating curve (AUC). For each parameter pertaining to the ratings of each sensory quality, a mean log value was obtained. Except for the peak magnitude, data for the other parameters were only included for sensory qualities that were rated as barely detectable or greater.
For each sensory quality, a linear regression was computed between the log concentration of capsaicin (or histamine) and the mean value of each parameter. The parameter was considered to be dependent on concentration of a chemical if the slope of the regression line was significantly different from zero. Correlations between data sets were calculated using the Pearson coefficient of correlation.
For each sensory quality and response parameter, the differences between means obtained with native cowhage, 200 mg/ml capsaicin and 10 mg/ml histamine spicule were compared using one-way ANOVAs. Bonferroni post hoc tests for multiple comparisons were performed to determine the significance of differences between means of individual groups.
The significance of the relationship between the incidence of a given dysesthesia, a wheal, or a flare, and the concentration of capsaicin or histamine was determined using a logistic regression analysis. Fisher’s exact test was used to determine the significance of the associations of the incidence of a type of dysesthesia or type of skin reaction and the type of chemical delivered, i.e. capsaicin, histamine and cowhage.
Image J software (obtained from the National Institute of Health) was used to measure the areas of each type of dysesthesia and skin reaction outlined in the digitized pictures of the skin surrounding each spicule.
For each statistical test, the criterion for significance was a probability value of less than 0.05. GraphPad Prism 4.03 software (GraphPad Software, San Diego, CA) was used to perform all calculations. Data are presented as means ± S.E.Ms.
3. Results
3.1 Effects of the concentration of capsaicin on the incidence, magnitude and time course of itch and nociceptive sensations
No subject reported any sensation in response to an inactive spicule soaked in vehicle (80% ethanol). In contrast, spicules treated with the concentrations of 200 and 50 mg/ml of capsaicin evoked one or more sensations in all subjects tested. Spicules soaked in 10 and 1 mg/ml of capsaicin failed to produce any sensations in 2 and 4 subjects, respectively. These observations appear to reflect local variations in the availability or the sensitivity of capsaicin-sensitive nerve fibers in the skin. A high concentration of capsaicin is likely to diffuse over a larger area than a lower concentration and increase the probability of activating one or more fibers.
All concentrations of capsaicin elicited itch accompanied by pricking/stinging and burning (Fig. 1A–C). The temporal profile of the perceived intensity was similar for each quality, each reaching peak magnitude within the first minute and then decreasing more slowly over time. Of the 21 subjects tested with capsaicin, the number of subjects reporting itch (and pricking/stinging) in order of increasing concentration from 1 to 200 mg/ml was 15 (and 17), 19 (19), 19 (21), and 20 (21). Three of the subjects experienced nociceptive sensations (pricking/stinging or burning) without itch. Two of these subjects reported only nociceptive sensations with a spicule containing 50 mg/ml capsaicin, but rated both itch and nociceptive sensations at other concentrations. The third subject reported only nociceptive sensations with the highest concentration of capsaicin (200 mg/ml). The sensation of burning was usually rated as lesser in magnitude than the other two sensory qualities. The number of subjects reporting burning in response to increasing concentrations of capsaicin from 1 to 200 mg/ml, respectively, were 9, 10, 9 and 17.
Fig. 1.
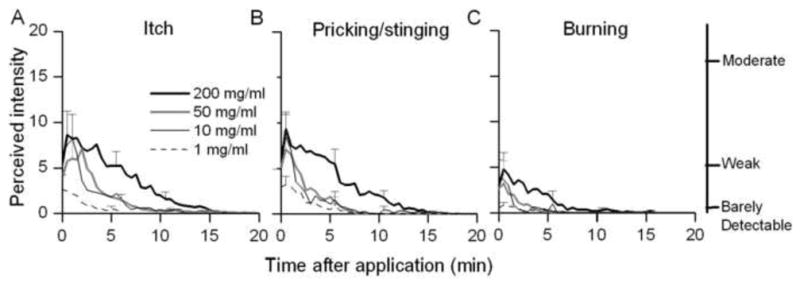
Time course of the perceived intensity of itch and nociceptive sensations evoked by different concentrations of capsaicin. Mean ratings of the perceived intensity of itch (A), pricking/stinging (B), and burning (C) were obtained at successive intervals of 30 sec in response to the indicated concentrations of capsaicin.
Linear regressions were used to study the relationships between the concentration of capsaicin and the log peak magnitude, log duration and the log area under the rating curve (AUC). The slopes of the regression lines were significantly different from zero for each measurement of itch and pricking/stinging but not for any measurements of burning. Thus, the magnitude and duration of itch and pricking/stinging significantly increased with the concentration of capsaicin.
There were no significant differences, due to concentration, in the latency of onset of sensation or the latency to the peak magnitude of itch, pricking/stinging or burning.
3.2 Effects of the concentration of histamine on the incidence, magnitude and time course of itch and nociceptive sensations
Subjects reported no sensations in response to an inactive spicule soaked in the vehicle of double-distilled water. Each concentration of histamine elicited qualities of sensations similar to those evoked by capsaicin (Fig 3A–C). The mean perceived intensities of itch and pricking/stinging were virtually identical and each greater than the perceived intensity of burning. Sensations were evoked in all subjects tested with 10 and 100 mg/ml histamine spicule. However, for spicules treated with 1, 0.1 and 0.01 mg/ml of histamine, no sensations were produced in 3, 5 and 10 subjects, respectively. One subject reported experiencing the sensation of itch but no pricking/stinging or burning with each histamine spicule. In ascending order of concentration delivered, the number of subjects reporting itch (and pricking/stinging) were 11 (and 8), 16 (15), 18 (17), 21 (19) and 21 (17). The corresponding numbers of subjects reporting burning were 4, 8, 7, 10 and 12. Thus, the number of subjects reporting one or more qualities of sensation increased with the concentration of histamine. In addition, the results indicate the possibility of regional variations in the sensitivity of histamine-sensitive fibers in the skin or in their proximity to the histamine spicule.
Fig. 3.
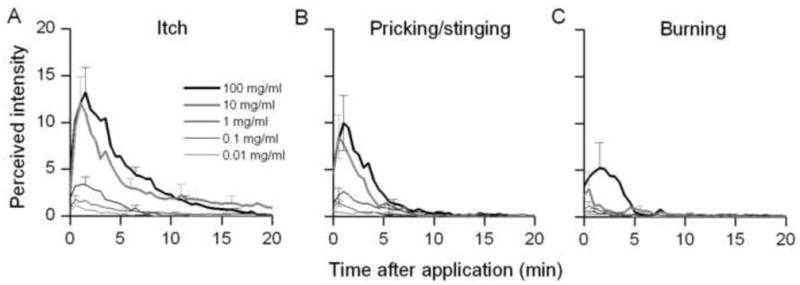
Time course of the perceived intensity of itch and nociceptive sensations evoked by a spicule containing different concentrations of histamine. Mean ratings of the perceived intensity of itch (A), pricking/stinging, (B) and burning (C) were obtained at successive intervals of 30 sec in response to each indicated dose of histamine.
Linear regression lines were plotted for log histamine concentration and the following response variables: log peak magnitude, log duration and log AUC (Fig. 4). For both itch (Fig. 4A – C) and pricking/stinging (Fig. 4D – F), there was a significant increase in the peak magnitude, duration and AUC with increases in the concentration of histamine. Similarly, there was a corresponding significant increase in the peak magnitude and AUC for burning. In contrast, there was no significant change in duration of burning or in the latency of onset, or the latency from onset to the peak magnitude, of itch, pricking/stinging or burning as a function of the concentration of histamine.
Fig. 4.
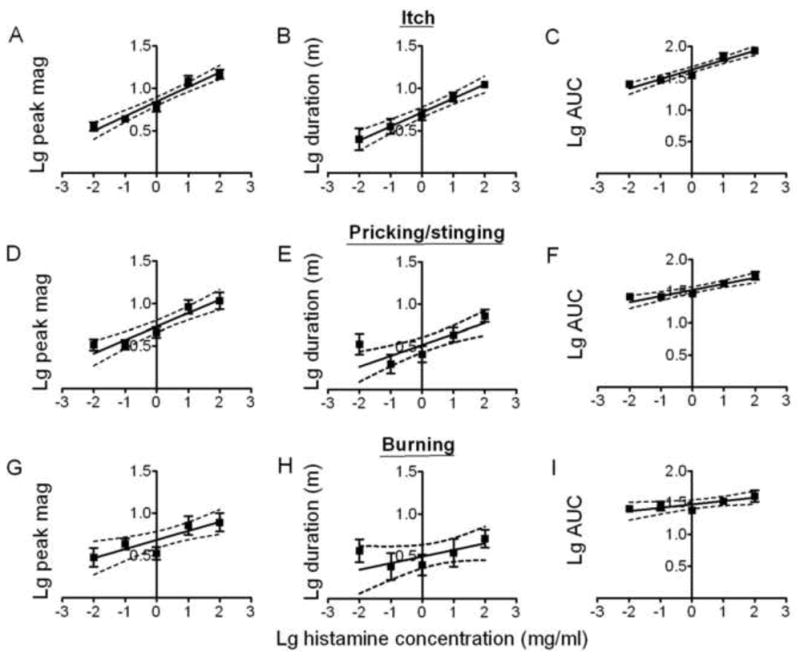
Effects of the concentration of histamine on the magnitude and duration of itch, pricking/stinging, and burning. Linear regressions for logarithmic (Lg) histamine concentrations and logarithmic (Lg) values of the mean peak magnitude (“Lg peak mag”), duration (“Lg duration” in minutes, m), and AUC (Lg AUC) for itch (A, B, and C, respectively), pricking/stinging (D, E, and F), and burning. With the exception of the duration of burning, the slope of each line was significantly different from zero (see main text).
3.3 Comparison of sensations evoked by capsaicin and histamine with those elicited by native cowhage
Similar to capsaicin and histamine spicules, native cowhage spicules evoked a predominant sensation of itch in confirmation results obtained from a separate group of subjects tested with single or multiple, native cowhage spicules [10]. One subject reported only itch and no pricking/stinging or burning sensation. This same subject also reported only itch in response to all concentrations of histamine but reported nociceptive sensations (pricking/stinging and burning) in addition to itch in response to capsaicin. 21, 20 and 12 subjects reported itch, pricking/stinging and burning respectively. The mean log peak magnitude of sensation for all subjects tested with cowhage was not significantly different for itch, pricking/stinging, and burning (one-way ANOVA). The mean log duration and mean log AUC were each significantly greater for itch than for burning though not significantly different between itch and pricking/stinging (one-way ANOVA and Bonferroni posthoc tests).
The rating curves obtained in response to each concentration of histamine and capsaicin were examined to determine the lowest concentration of each chemical that evoked sensations that were similar to those elicited by cowhage. The sensations evoked by cowhage were found to be similar in magnitude and time course to sensations elicited by spicules soaked either in 200 mg/ml of capsaicin or 10 mg/ml of histamine (Fig. 5). For each of the three sensory qualities, the mean log values of peak magnitude, duration, and AUC were not significantly different for the three chemicals (p> 0.05, one way ANOVA). Thus, capsaicin and histamine at concentrations equipotent to a native cowhage spicule produced sensory qualities of similar magnitude and duration.
Fig. 5.
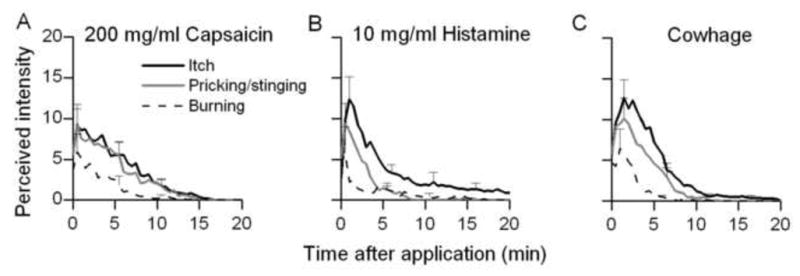
Comparison of itch and nociceptive sensations evoked by capsaicin, histamine and native cowhage. The doses of capsaicin (A) and histamine (B) (200 and 10 mg/ml, respectively) were selected as the lowest concentrations required to produce sensations comparable in overall magnitude to those evoked by cowhage (C).
The present findings suggest an order of magnitude greater potency of histamine over capsaicin. Yet, in studies using electrophysiology and calcium imaging techniques, it has been shown that when applied directly to cells, capsaicin is more potent than histamine in eliciting membrane depolarization [18,21,27]. Perhaps this is explained by the higher water solubility of histamine compared with capsaicin when each is applied by spicule to the skin. Its higher water solubility may facilitate the transport of histamine from the spicule to the target site and allow a greater volume of tissue to be stimulated.
3.4 Distribution of categories assigned to the peak magnitude of sensations evoked by capsaicin, histamine and cowhage
The peak magnitudes of sensations evoked by spicules containing 200 mg/ml of capsaicin, 10 mg/ml of histamine, and native cowhage were grouped according to the corresponding category labels of perceived intensity presented on the gLMS scale. Each category included values below and equal to those assigned for perceived intensity labels marked on the gLMS scale. For example, Barely detectable (BD, ≤1) included values between zero and 1: Weak (WK, >1≤6) included values greater than 1 but less than or equal to 6. The sensations of itch and pricking/stinging presented similar distributions of categories for the three spicules and peaked at “moderate”. The category most often chosen by subjects to describe burning was either “barely detectable” for histamine and cowhage (Fig 6B–C) or “weak” to “moderate” for capsaicin spicule (Fig 6A). Thus, burning was a less reliable and less frequently used quality compared with itch or pricking/stinging to describe sensations evoked by each of the three chemical stimuli.
Fig. 6.
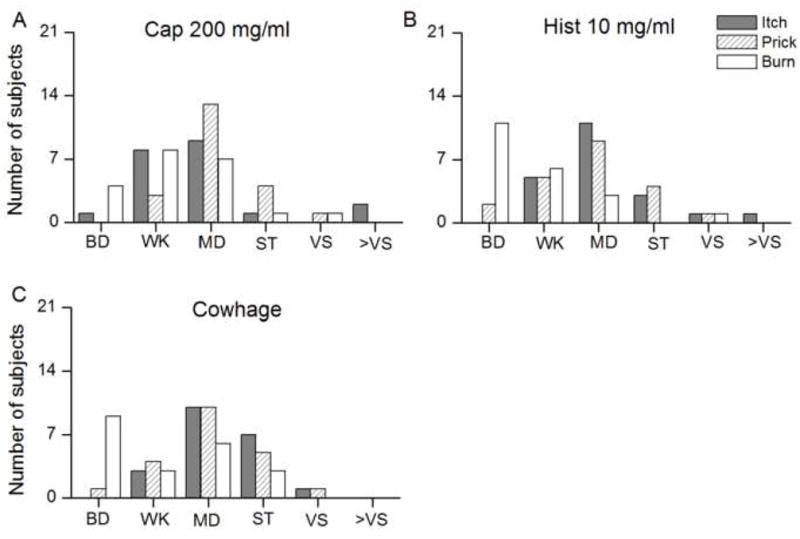
Distributions of the categories on the gLMS scale chosen by 21 subjects to represent the sensations of itch, pricking/stinging and burning in response to capsaicin (200 mg/ml), histamine (10 mg/ml), and native cowhage (A, B, and C, respectively). Data presented are same as those used to calculate average responses in Fig. 5. The labels correspond to the following categories and numerical values on the gLMS scale: barely detectable (BD, ≤ 1), weak (WK, >1≤6), moderate (MD, >6≤17), strong (ST, >17≤35), very strong (VS, >35≤53) and greater than very strong (>VS, >53).
The present finding that similar nociceptive sensations were evoked by a histamine spicule and a cowhage spicule differs from the anecdotal reports in previous studies in which histamine was applied by iontophoresis. These have reported an “almost pure” itch sensation (Handwerker et al. 1991) or a “purer” itch than that evoked by cowhage spicules (Namer et al. 2008). However, in those studies, subjects were not instructed to make direct comparisons of different qualities of sensation nor is it clear how sensory ratings may have differed according to the method of chemical delivery (iontopohoresis vs. spicule). These technical difficulties were overcome in the present study in which different chemical agents were applied in the same way, via the tip of a single spicule, and the subjects were instructed to judge the magnitude and time course of itch and nociceptive sensations using the same labeled magnitude scale
3.5 Cutaneous dysesthesias
One or more types of dysesthesias sometimes accompanied the sensations evoked by the single chemically-soaked spicules as illustrated for one subject in Fig. 7a. Not all subjects reporting itch and nociceptive sensations exhibited dysesthesias. However, one or more dysesthesias were only observed when a spicule produced a sensation of itch regardless of whether there was also a nociceptive sensation. Subjects tested with an inactive cowhage spicule soaked in vehicle reported neither sensations nor dysesthesias.
Fig. 7.
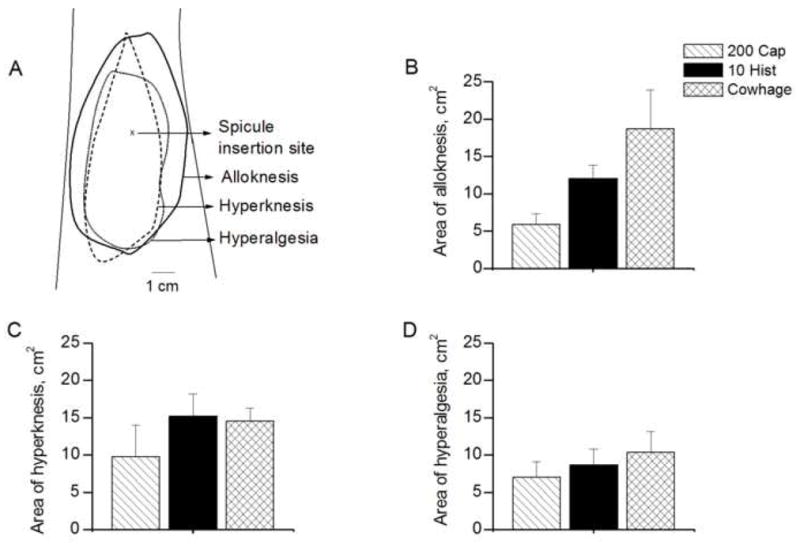
The areas of dysesthesia evoked by a spicule containing native cowhage, capsaicin or histamine. A: Areas of each type of dysesthesia evoked in a subject given a single spicule of 100 mg/ml histamine. The mean areas of dysesthesia obtained in response to capsaicin (200 mg/ml), histamine (10 mg/ml) and native cowhage spicule are indicated for alloknesis (B), hyperknesis (C) and hyperalgesia (D).
3.5.1 Effects of the concentration of capsaicin on the incidences and the areas of dysesthesias
For each type of dysesthesia, the incidence of its occurrence (Table 1) was analyzed as a function of the concentration of capsaicin using logistic regression analysis. The incidence of alloknesis, hyperknesis, and hyperalgesia each increased significantly with the concentration of capsaicin. For example, alloknesis was reported by only 9.5% of the total subjects in response to 1 mg/ml of capsaicin but by 42.9% in response to 200 mg/ml (Table 1).
Table 1.
Percentage of the 21 subjects tested that exhibited each type of dysesthesia and skin reaction in response to each concentration of capsaicin and histamine and also to native cowhage spicule.
| Dysesthesias | Skin reactions | ||||
|---|---|---|---|---|---|
| Alloknesis | Hyperknesis | Hyperalgesia | Wheal | Flare | |
| Capsaicin (conc, mg/ml) | |||||
| 1 | 9.5 | 14.3 | 33.3 | 0 | 4.8 |
| 10 | 38.1 | 33.3 | 38.1 | 0 | 9.5 |
| 50 | 23.8 | 47.6 | 47.6 | 0 | 19 |
| 200 | 42.9 | 47.6 | 57.1 | 0 | 28.6 |
| Histamine (conc, mg/ml) | |||||
| 0.01 | 4.8 | 19 | 28.6 | 0 | 0 |
| 0.1 | 14.3 | 23.8 | 23.8 | 0 | 9.5 |
| 1 | 28.6 | 52.4 | 52.4 | 4.8 | 14.3 |
| 10 | 52.4 | 71.4 | 61.9 | 47.6 | 71.4 |
| 100 | 57.1 | 81 | 66.6 | 61.9 | 85.7 |
| Cowhage | 42.9 | 38.1 | 52.4 | 0 | 14.3 |
There was no significant effect of the concentration of capsaicin on the area of each type of dysesthesia. Regression lines plotted for log capsaicin concentration and log areas of alloknesis, hyperknesis and hyperalgesia had slopes that did not significantly differ from zero. For example, the areas (cm2) of hyperalgesia measured with 1, 10, 50 and 200 mg/ml capsaicin spicule were 4.6 ± 1.1, 10.7 ± 4.4, 3.5 ± 1.2 and 7 ± 2.1, respectively. Thus, the incidences of dysesthesias but not their areas were dependent on capsaicin concentration. Additionally, no significant relationship existed between the peak magnitudes of itch, pricking/stinging or burning evoked by the various concentrations of capsaicin and the areas of alloknesis, hyperknesis or hyperalgesia in correlation analyses.
3.5.2 Effects of the concentration of histamine on the incidences and areas of dysesthesias
Logistic regression analyses were used to determine the significance of the relation between the incidence of alloknesis, hyperknesis and hyperalgesia (Table 1) and the log concentration of histamine. There was a significant relation between the concentration of histamine and the incidence of alloknesis, hyperknesis, and hyperalgesia. Thus, higher concentrations of histamine were more likely than a lower concentration to produce a dysesthesia. For example, alloknesis was exhibited by only 4.8% of the total subjects in response to 0.01 mg/ml of histamine and by 57.1% in response to 100 mg/ml (Table 1).
The log mean area of each type of dysesthesia was obtained in response to each concentration of histamine. The slope of the regression lines (log mean area vs. log concentration) was significantly different from zero for alloknesis and for hyperknesis but not for hyperalgesia. For example; the areas of hyperknesis (cm2) evoked by 0.01, 0.1, 1, 10 and 100 mg/ml histamine were: 3.8 ± 1.1, 4.7 ± 2.5, 12.2 ± 4.5, 15.2 ± 3 and 13.1 ± 2.6, respectively. Additionally, a significant correlation was observed between the peak magnitude of itch obtained for the various concentrations of histamine and both the area of alloknesis (Pearson r =0.21, p< 0.05) and the area of hyperknesis (Pearson r =0.25, p< 0.05). Thus, the incidences and the areas of alloknesis and hyperknesis but not hyperalgesia were dependent on the concentration of histamine.
3.5.3 The incidences and areas of dysesthesia produced by native cowhage were similar to those produced by certain concentrations of capsaicin and histamine
For each type of dysesthesia, the incidence and the mean area (with one exception) obtained in response to native cowhage did not significantly differ from those evoked by 200 mg/ml of capsaicin and 10 mg/ml of histamine (incidence: Fisher’s exact test, Table 1; area: ANOVA, Fig 7B–D). The exception was that the mean area of alloknesis (Fig. 7B) was significantly greater for cowhage than for capsaicin although not significantly different between histamine and cowhage or between histamine and capsaicin (one-way ANOVA and Bonferroni’s Multiple Comparison Test).
No dysesthesias were reported in the absence of itch and/or nociceptive sensations. A sensation of itch, which was typically accompanied by pricking/stinging and/or burning, was required to produce a dysesthesia. But a dysesthesia did not invariably accompany an intense magnitude of itch, pricking/stinging or burning sensation (s). For example, 3 subjects that rated itch as moderate to very strong (7 ≤ 53) presented no areas of dysesthesias with a 200 mg/ml capsaicin spicule. In contrast, one subject that rated itch to a 200 mg/ml capsaicin spicule as weak exhibited areas of hyperknesis and hyperalgesia in the range of 25–39 cm2. Hence, large areas of dysesthesias could exist for spicules evoking barely detectable to weak sensations. Alternatively, sensations rated as moderate to very strong may be accompanied by small areas of dysesthesias or none at all.
3.6.1 Skin reactions produced by capsaicin, histamine and cowhage
Cowhage and capsaicin never evoked a wheal. Only histamine, in concentrations of 1 mg/ml or greater, was capable of eliciting a wheal (Fig. 8B). For concentrations of 1, 10 and 100 mg/ml, the mean areas of the wheal were calculated (range 0.22–0.67 cm2). Linear regression lines for log area vs. log concentration had slopes that did not significantly differ from zero suggesting no effect of concentration.
Fig. 8.
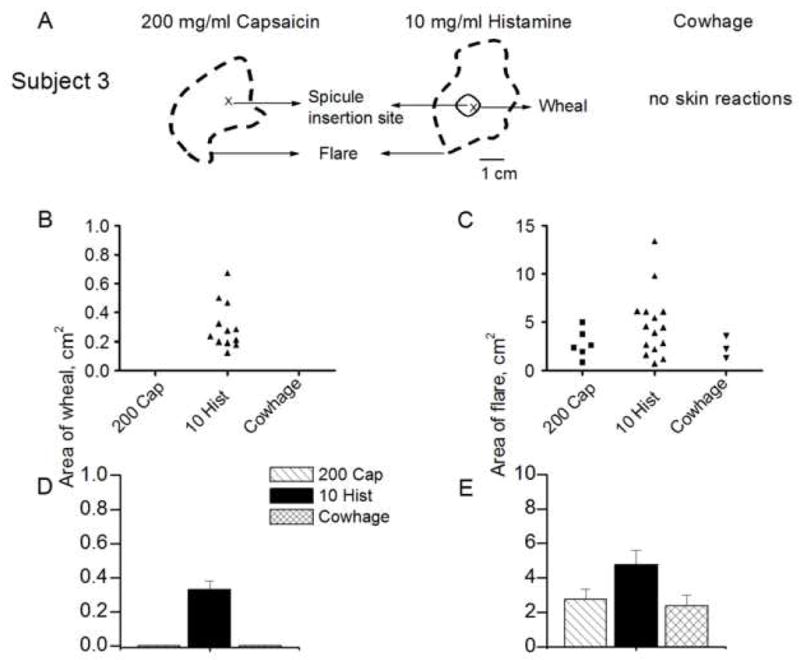
The areas of skin reactions evoked by histamine, capsaicin and cowhage. A: Skin reactions produced in a subject by capsaicin (200 mg/ml) and histamine (10 mg/ml) but not by cowhage. Areas of wheal (B) and flare (C) reported in individual subjects tested with a capsaicin (200 mg/ml), histamine (10 mg/ml) and cowhage. Cowhage and capsaicin never evoked a wheal. D–E: Mean areas of wheal and flare obtained in response to each type of spicule.
Both capsaicin and histamine were capable of eliciting a flare (Fig. 8C). For both chemicals, there was a significant effect of the concentration on the incidence of the flare (Table 1 and logistic regression analyses). In contrast there was no significant effect of the concentration of either chemical on the area of the flare (which exhibited a range of 0.85 to 5.2 cm2 for capsaicin and 1 to17.1 cm2 for histamine). Regression lines for log area vs. concentration had slopes that were not significantly different from zero.
For cowhage and for the different concentrations of capsaicin, there were no significant correlations between the area of flare and the peak magnitude of itch, pricking/stinging or burning. In contrast, for the various concentrations of histamine delivered, the area of flare significantly correlated not only with the peak magnitude of itch (Pearson r = 0.26, p< 0.01) but also with the peak magnitude of pricking/stinging (Pearson r = 0.25, p< 0.01) and burning (Pearson r = 0.3, p< 0.01). Nevertheless, not only capsaicin and cowhage but also histamine was capable of eliciting a weak-to-strong itch in the absence of a flare. One subject exhibited no visible flare in response to histamine despite perceiving a strong itch at the highest concentration. Thus, the sensations were likely to be mediated by neural mechanisms independent from those controlling the flare.
3.6.2 Histamine produced skin reactions with higher incidences than capsaicin or cowhage
The incidence of a flare was compared for the concentrations of capsaicin and histamine (200 and 10 mg/ml, respectively) that produced sensations comparable to those evoked by a native cowhage spicule. For cowhage and these concentrations of capsaicin and histamine, the percentage of subjects exhibiting a flare were significantly different at 14.3, 28.6, and 71.4, respectively (Fisher’s exact test, and Table 1). In contrast, the areas of flare produced by cowhage and these concentrations of capsaicin and histamine were not significantly different (one-way ANOVA, Fig 8E).
4. Discussion
The central finding of the study is that a single spicule containing capsaicin or histamine can elicit the same pruritic and nociceptive sensations and dysesthesias as native cowhage. In addition, for both histamine and capsaicin, the magnitude and duration of itch and pricking/stinging sensations (but not the dysesthesias) were concentration-dependent. There were differences in the skin reactions produced by histamine and capsaicin or cowhage. A wheal occurred only in response to histamine, indicating that this chemical reached the vascularized dermis to produce protein extravasation. And, the incidence of a neurogenic flare was higher for histamine than for capsaicin or native cowhage. But despite such differences in skin reaction, the similar pruritic and sensory effects produced by the punctate application of each chemical by means of a spicule suggest the possible activation of a common subset of peripheral sensory fibers or common central mechanisms that produce similar qualities of sensation and types of dysesthesias.
In humans and monkeys, the nociceptive afferent peripheral nerve fibers responsive to pruritic chemicals (pruriceptive afferents) can be broadly differentiated into two classes: those that are mechanosensitive (MSAs), and those that are mechanically-insensitive (MIAs) [7,8,13,16,17]. In general, the MIAs are more responsive to histamine than to cowhage whereas the reverse is true for MSAs [8, 13, 16]. For example, the responses of certain MIAs to iontophoresis of histamine are stronger, and provide a better match to the time course of itch, than the responses of the polymodal nociceptor MSAs [16]. Under such conditions, histamine induced itch would not appear to require the activation of these MSAs. Similarly, an injection of capsaicin activates certain MIAs more vigorously than MSAs with polymodal nociceptors [3,11,15].
In the present study, in those instances where a flare was produced, either by histamine, capsaicin, or cowhage, MIAs were likely to have been activated. MIAs are thought to mediate the flare [14]. In contrast, a spicule of histamine, capsaicin or cowhage was capable of eliciting itch and nociceptive sensations in the absence of a visible flare. The absence of a visible flare does not exclude the possibility that MIAs were activated. Conversely, a correlation between the flare area and itch for histamine spicules does not exclude the involvement of MSAs. Although in a recent study histamine soaked spicules failed to stimulate MSAs, the negative finding was based on tests of only 5 MSAs with polymodal nociceptors [13]. Nevertheless, it is reasonable to hypothesize that MIAs account for the itch and nociceptive sensations evoked by spicules containing histamine and possibly even capsaicin whereas MSAs mediate the same sensations elicited by spicules containing cowhage regardless of whether a flare occurs. If this is the case, it is interesting that a predominant activation of MIAs by histamine or by capsaicin and of MSAs by cowhage produce comparable itch and nociceptive sensations. Alternatively, it is conceivable that all three pruritic agents, when delivered by spicule, elicit itch and nociceptive sensations primarily by activating pruriceptive MSAs, sometimes with parallel activation of MIAs. An additional hypothesis is that sensations such as itch and pricking/stinging and the types of dysesthesia elicited by the activation of MSAs or MIAs depend on the types of second order neurons to which they project [10].
In the present study, capsaicin elicited itch accompanied by pricking/stinging and burning as did histamine and native cowhage. The findings with cowhage are consistent with recent observations that there was a positive rather than an inverse correlation between the magnitude and duration of itch and the magnitude and duration of nociceptive sensations evoked by single or multiple spicules [10]. Similarly, itch has been reported to co-exist with nonpainful nociceptive sensations produced when capsaicin was topically applied to the skin of the forearm [6]. Yet when hypodermically injected into the skin, capsaicin produces pain and not itch [11,24]. It is possible that the punctate delivery of capsaicin by means of a spicule or by topical application may activate pruriceptive afferents, whereas its intradermal injection may activate MIAs, such as the C-heat nociceptive afferents [3,15] that may be “nociceptive specific” (unresponsive to pruritic chemicals) and act centrally to inhibit itch. Additionally, capsaicin delivered by intradermal injection may desensitize the same types of MSAs [3] that it activates when delivered in much smaller amounts via spicules. The mechanisms underlying pruritic vs. nociceptive sensations produced by capsaicin require further investigation.
The responses of MSAs to cowhage and possibly to other pruritic chemicals applied by spicule pose a dilemma for the coding of itch because such nociceptors respond to mechanical or heat noxious stimuli that can elicit pain in the absence of itch and, indeed, may act to suppress an ongoing itch as evidenced by the effects of scratching. Again, one possibility to pursue in future investigations is that such noxious stimuli activate additional nociceptive specific afferents that mediate pain and act centrally to suppress itch.
Three types of mechanically induced dysesthesias are evoked by an intradermal injection of histamine [1,23], or by the application of cowhage spicules [10,12,20]: Alloknesis, defined as itch to light stroking of the skin, hyperknesis, and hyperalgesia, defined respectively as enhanced itch and pain to punctate mechanical indentation of the skin. The present study extends these findings with the observations that all three types of dysesthesias can be evoked by a punctate (spicule) chemical application not only of histamine but also of capsaicin. In contrast, when injected intradermally, capsaicin evokes allodynia, not alloknesis, to light stroking and hyperalgesia, but not hyperknesis, to punctate mechanical stimulation. Thus, alloknesis and hyperknesis may be evoked by a chemical irritant that evokes itch, even if accompanied by nociceptive sensations, whereas allodynia and hyperalgesia, in the absence of alloknesis and hyperknesis, may be elicited by an irritant that evokes pain in the absence of itch. Available evidence suggests that a central, rather than peripheral, neuronal sensitization contributes to the allodynia and hyperalgesia produced by an intradermal injection of capsaicin [11,24]. But further study is required to determine whether a similar mechanism might apply to the dysesthesias induced by pruritic chemical stimulation.
Neither the areas of dysesthesia nor the area of flare increased with the concentration of capsaicin in a spicule. In contrast, when capsaicin was hypodermically injected into the skin, a flare and an area of allodynia were evoked and the areas of each increased with the dose of capsaicin [24]. But the areas evoked by injection were significantly larger than those elicited by a capsaicin spicule. This may be explained by the amount of capsaicin used in the two studies, namely, micrograms with the injections and picograms to lower nanograms with spicules. It is possible that an effect of concentration on the area of flare might have occurred if the spicules could have been made to deliver higher concentrations of capsaicin (> 200 mg/ml) (but see methods).
The area of flare and the areas of alloknesis and hyperknesis each increased with the concentration of histamine applied by spicule. Similarly, the areas of flare and alloknesis increased with the dose of histamine hypodermically injected into the skin [23,25]. Similar to the effects of capsaicin, the areas of alloknesis and flare applied by a spicule were considerably smaller than those evoked when histamine was injected, possibly due to differences in the amount of chemical delivered. The areas of flare, and also of the wheal, produced in the present study were comparable to those reported when histamine was pricked into the skin [4]. A pricking of the skin might be expected to deliver an amount of chemical more comparable to that delivered by a spicule than by injection.
There was a correlation between the areas of alloknesis or hyperalgesia evoked by a histamine spicule and the peak magnitude of itch. In contrast, no such correlation was found between the areas of any type of dysesthesia and the peak magnitudes of itch or nociceptive sensations in response to capsaicin or native cowhage. In addition, there were occasions when the peak itch evoked by histamine, capsaicin or cowhage was rated as strong or very strong without the occurrence of any type of dysesthesia. Conversely, although no dysesthesias were reported in the absence of a spicule evoked itch, the areas of dysesthesia evoked by one or another type of spicule were occasionally substantial despite the presence of only a barely detectable or weak itch. These results suggest that the neural mechanisms responsible for dysesthesias may be different from those mediating the sensations [10].
In conclusion, a single spicule is an effective means of delivering an exceptionally small amount of chemical to a punctate region of skin. A spicule containing capsaicin or histamine produced similar qualities and magnitudes of sensations and associated dysesthesias as native cowhage, with or without a flare that is known to be mediated by mechanically insensitive peripheral nerve fibers. The similar pruritic and sensory effects produced by the punctate application of each chemical by means of a spicule suggest the possible activation of a common subset of peripheral nerve fibers or common central mechanisms that result in similar qualities of sensation and types of dysesthesias.
Fig. 2.
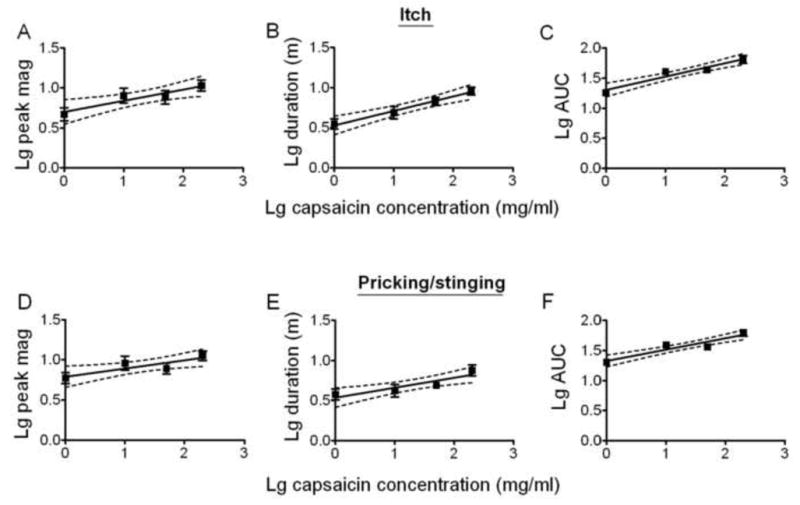
Effects of the concentration of capsaicin on the magnitude and duration of itch and pricking/stinging. Linear regressions for logarithmic (Lg) values of concentration and the peak magnitude (“Lg peak mag”), duration (Lg duration in minutes, m), and area under the rating curve (Lg AUC) for itch (A, B, and C, respectively) and pricking/stinging (D, E, and F). Dashed lines provide 95% confidence limits. The slope of each line was significantly different from zero (see main text). Significant slopes were not obtained for burning (data not shown).
Acknowledgments
The authors thank Ronik Bhangoo, Catherine Haar, Brett Pantera, Kavan Reddy and Adrianne Smits for technical assistance and Daniel Zelterman for his help with statistical analyses. This study was supported by the NINDS grant P01 NS 047399. The authors report no conflict of interest between the funding source and aims of their experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atanassoff PG, Brull SJ, Zhang J, Greenquist K, Silverman DG, LaMotte RH. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens Mot Res. 1999;16:291–8. doi: 10.1080/08990229970357. [DOI] [PubMed] [Google Scholar]
- 2.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–14. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: The search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 4.Darsow U, Ring J, Scharein E, Bromm B. Correlations between histamine-induced wheal, flare and itch. Arch Dermatol Res. 1996;288:436–41. doi: 10.1007/BF02505231. [DOI] [PubMed] [Google Scholar]
- 5.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–34. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 6.Green BG, Shaffer GS. The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain. 1993;53:323–34. doi: 10.1016/0304-3959(93)90228-H. [DOI] [PubMed] [Google Scholar]
- 7.Handwerker HO, Forster C, Kirchhoff C. Discharge patterns of human C-fibers induced by itching and burning stimuli. J Neurophysiol. 1991;66:307–15. doi: 10.1152/jn.1991.66.1.307. [DOI] [PubMed] [Google Scholar]
- 8.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–69. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol. 2009 Jan 14; doi: 10.1152/jn.91268.2008. [Epub ahead of print] PMID: 19144738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaMotte RH, Shain CN, Simone DA, Tsai E. Neurogenic hyperalgesia: Psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 12.Masood K, Green BG, LaMotte RH. Washington, DC: Society for Neuroscience; 2005. Psychophysical measurements of pruritic and nociceptive sensations and dysesthetic states evoked by cutaneous application of cowhage spicules in human. Program No. 50.9, 2005. Abstract viewer/itinerary planner. Available from: ( http://web.sfn.org/) [Google Scholar]
- 13.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–9. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmelz M, Michael K, Weidner C, Schmidt R, Torebjörk HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport. 2000;11:645–8. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- 15.Schmelz M, Schmid R, Handwerker HO, Torebjörk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;3:560–71. doi: 10.1093/brain/123.3.560. [DOI] [PubMed] [Google Scholar]
- 16.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schepers RJ, Johanek LM, Hartke TV, Shim B, Borzan J, Meyer RA, Ringkamp M. Washington, DC: Society for Neuroscience; 2008. 2008. A subpopulation of Aδ nociceptors in monkey is vigorously activated by cowhage spicules. Program No. 170.3, 2008. Abstract viewer/itinerary planner. Available from: ( http://web.sfn.org/) [Google Scholar]
- 18.Shim WS, Tak MH, Lee MH, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–7. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada SG, Green BG, LaMotte RH. San Diego, CA: Society for Neuroscience; 2007. Effects of a conduction block in myelinated nerve fibers on pruritic and nociceptive sensations evoked by application of cowhage spicules in human. Program No. 70.12, 2007. Abstract viewer/itinerary planner. Available from: ( http://web.sfn.org/) [Google Scholar]
- 20.Shimada SG, Green BG, Zelterman D, LaMotte RH. Atlanta, GA: Society for Neuroscience; 2006. Itch exhibits spatial summation. Neurosci. Abstr. Program No. 552.2, 2006. Abstract viewer/itinerary planner. Available from: ( http://web.sfn.org/) [Google Scholar]
- 21.Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol. 2007;581:631–47. doi: 10.1113/jphysiol.2006.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sikand P, Shimada SG, Green BG, LaMotte RH. Washington, DC: Society for Neuroscience; 2008. Capsaicin and histamine produce itch when delivered via inactivated-cowhage spicules. Program No. 2216, 2008. Abstract viewer/itinerary planner. Available from: ( http://web.sfn.org/) [Google Scholar]
- 23.Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res. 1991;8:271–9. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- 24.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 25.Simone DA, Ngeow JYF, Whitehouse J, Becerra-Cabal L, Puterman GJ, LaMotte RH. The magnitude and duration of itch produced by intracutaneous injections of histamine. Somatosens Mot Res. 1987;5:81–92. doi: 10.3109/07367228709144620. [DOI] [PubMed] [Google Scholar]
- 26.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–5. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]


