Abstract
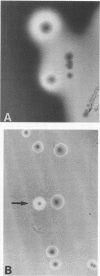
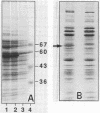
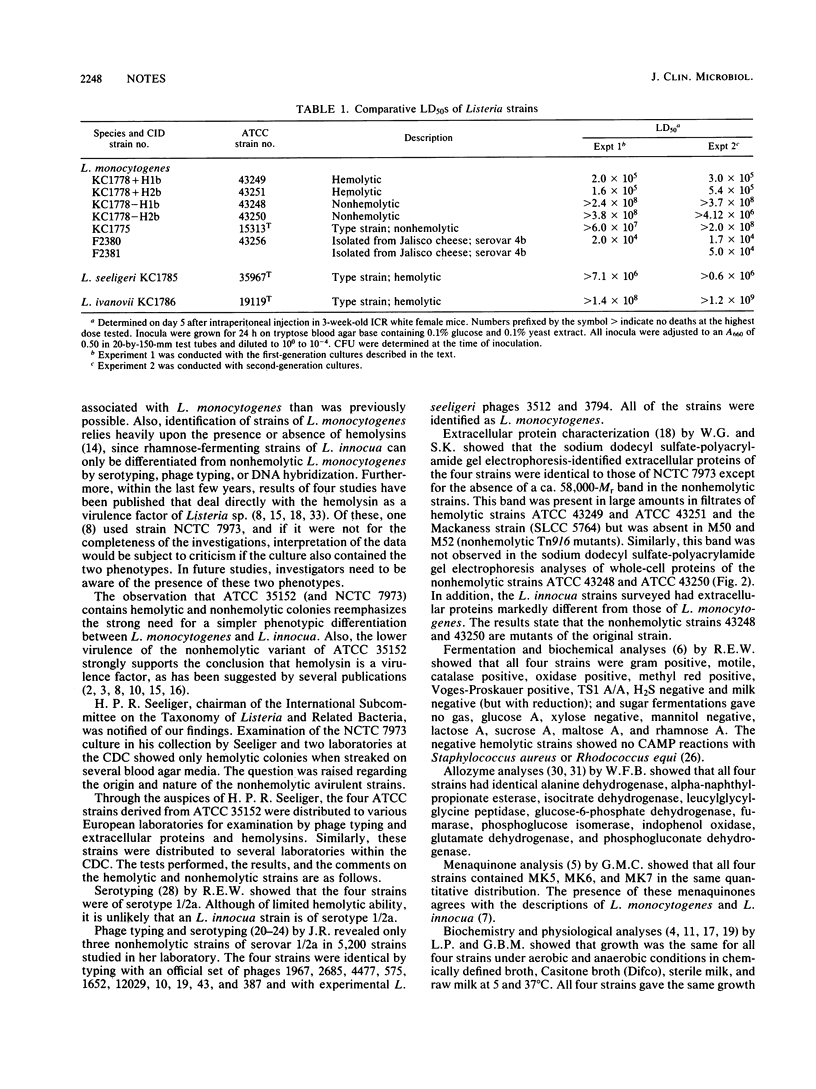
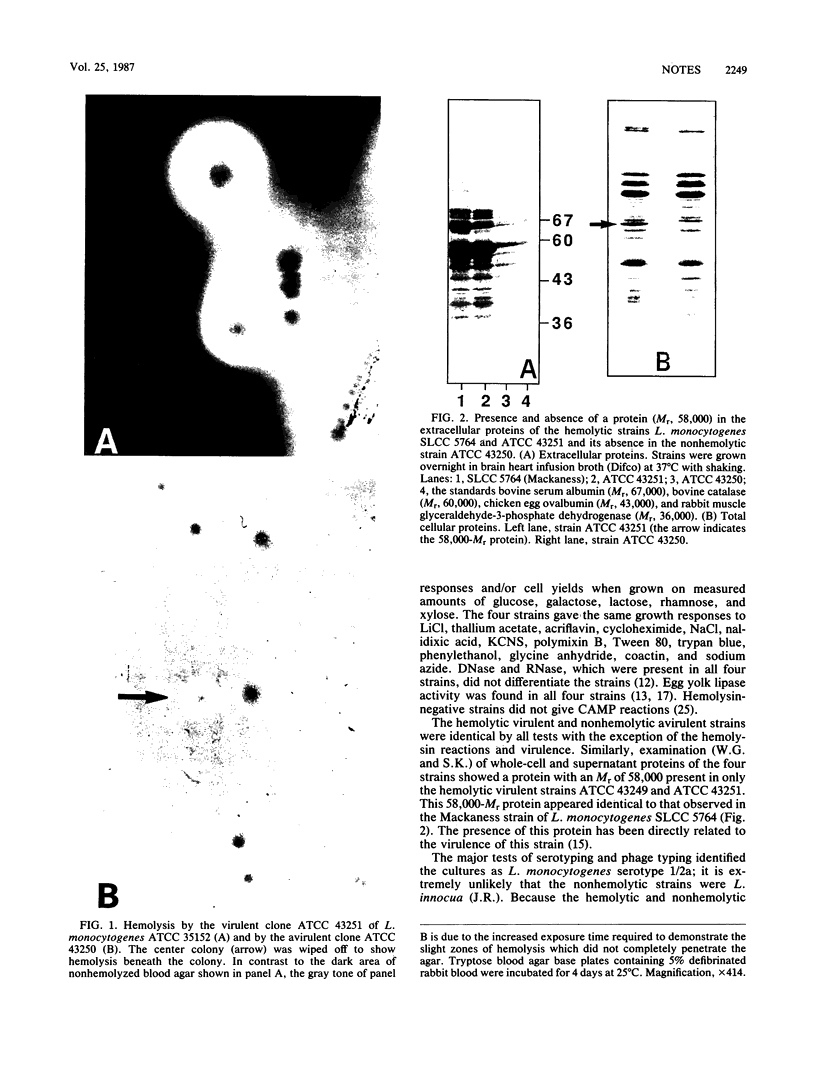
Listeria monocytogenes NCTC 7973 and this same strain deposited as ATCC 35152 contain two phenotypes: hemolytic virulent colonies and nonvirulent colonies that show no zones of hemolysis when streaked on heart infusion agar containing 5% rabbit blood. Results of examinations of these virulent and nonvirulent strains by investigators at the Centers for Disease Control, Atlanta, Ga., the Pasteur Institute, Paris, France, and the University of Würzburg, Federal Republic of Germany, support the conclusion that the avirulent strain is a nonhemolytic mutant of the virulent strain and that hemolysin is a virulence factor for L. monocytogenes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARROW G. I., PUGH R. J. Listeria (Erysipelothrix) monocytogenes meningitis in the newborn. J Pathol Bacteriol. 1958 Jan;75(1):9–16. [PubMed] [Google Scholar]
- Basher H. A., Fowler D. R., Rodgers F. G., Seaman A., Woodbine M. Role of haemolysin and temperature in the pathogenesis of Listeria monocytogenes in fertile hens' eggs. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(2-3):223–231. doi: 10.1016/s0176-6724(84)80040-1. [DOI] [PubMed] [Google Scholar]
- Berger U. Einige Eigenschaften der Keimträgerstämme von Listeria monocytogenes. Med Microbiol Immunol. 1975 Sep 19;161(4):215–229. doi: 10.1007/BF02122709. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Pine L. Path of glucose breakdown and cell yields of a facultative anaerobe, Actinomyces naeslundii. J Gen Microbiol. 1967 Feb;46(2):225–236. doi: 10.1099/00221287-46-2-225. [DOI] [PubMed] [Google Scholar]
- Carlone G. M., Anet F. A. Detection of menaquinone-6 and a novel methyl-substituted menaquinone-6 in Campylobacter jejuni and Campylobacter fetus subsp. fetus. J Gen Microbiol. 1983 Nov;129(11):3385–3393. doi: 10.1099/00221287-129-11-3385. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981 Jun;45(2):316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL A., Jr, PINE L. Studies on the growth of species of Actinomyces. I. Cultivation in a synthetic medium with starch. J Bacteriol. 1956 Jan;71(1):47–53. doi: 10.1128/jb.71.1.47-53.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof H. Virulence of different strains of Listeria monocytogenes serovar 1/2a. Med Microbiol Immunol. 1984;173(4):207–218. doi: 10.1007/BF02122112. [DOI] [PubMed] [Google Scholar]
- JEFFRIES C. D., HOLTMAN D. F., GUSE D. G. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol. 1957 Apr;73(4):590–591. doi: 10.1128/jb.73.4.590-591.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E. M., Watson B. B. Extracellular Antigens from Listeria monocytogenes I. Purification and Resolution of Hemolytic and Lipolytic Antigens from Culture Filtrates of Listeria monocytogenes. Infect Immun. 1971 Apr;3(4):589–594. doi: 10.1128/iai.3.4.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorz W., Hof H. Zur Pathogenität von Listerien. Immun Infekt. 1986 Apr;14(2):76–80. [PubMed] [Google Scholar]
- O'LEARY W. M., WELD J. T. LIPOLYTIC ACTIVITIES OF STAPHYLOCOCCUS AUREUS. I. NATURE OF THE ENZYME PRODUCING FREE FATTY ACIDS FROM PLASMA LIPIDS. J Bacteriol. 1964 Nov;88:1356–1363. doi: 10.1128/jb.88.5.1356-1363.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrisius J., Bhakdi S., Roth M., Tranum-Jensen J., Goebel W., Seeliger H. P. Production of listeriolysin by beta-hemolytic strains of Listeria monocytogenes. Infect Immun. 1986 Jan;51(1):314–319. doi: 10.1128/iai.51.1.314-319.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., George J. R., Reeves M. W., Harrell W. K. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1979 May;9(5):615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocourt J., Catimel B. Caractérisation biochimique des espèces du genre Listeria. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Oct;260(2):221–231. [PubMed] [Google Scholar]
- Rocourt J., Catimel B., Schrettenbrunner A. Isolement de bactériophages de Listeria seeligeri et L. welshimeri. Lysotypie de L. monocytogenes, L. ivanovii, L. innocua, L. seeligeri et L. welshimeri. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 May;259(3):341–350. doi: 10.1016/s0176-6724(85)80036-5. [DOI] [PubMed] [Google Scholar]
- Rocourt J., Schrettenbrunner A., Seeliger H. P. Différenciation biochimique des groupes génomiques de Listeria monocytogenes (sensu lato). Ann Microbiol (Paris) 1983 Jan-Feb;134A(1):65–71. [PubMed] [Google Scholar]
- Seeliger H. P. Apathogene listerien: L. innocua sp. n. (Seeliger et Schoofs, 1977). Zentralbl Bakteriol Mikrobiol Hyg A. 1981;249(4):487–493. [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., McKinney R. M., Whittam T. S., Bibb W. F., Brenner D. J., Nolte F. S., Pattison P. E. Genetic structure of populations of Legionella pneumophila. J Bacteriol. 1985 Sep;163(3):1021–1037. doi: 10.1128/jb.163.3.1021-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]