Abstract
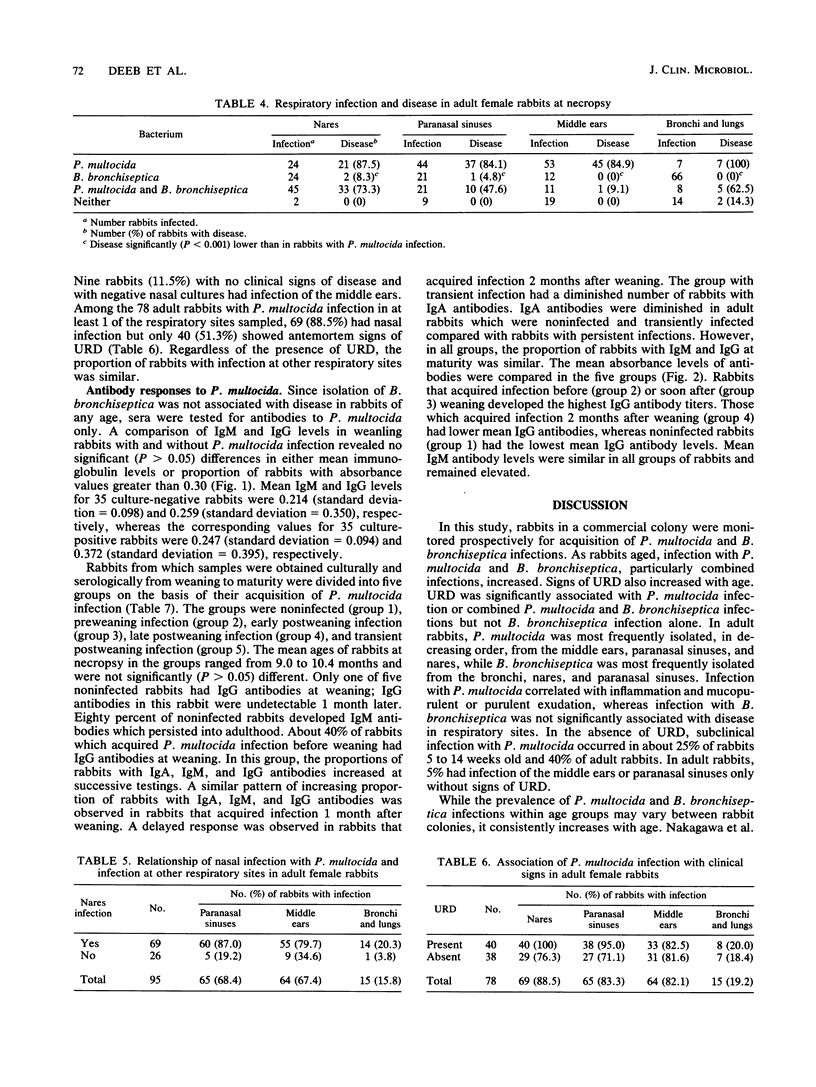
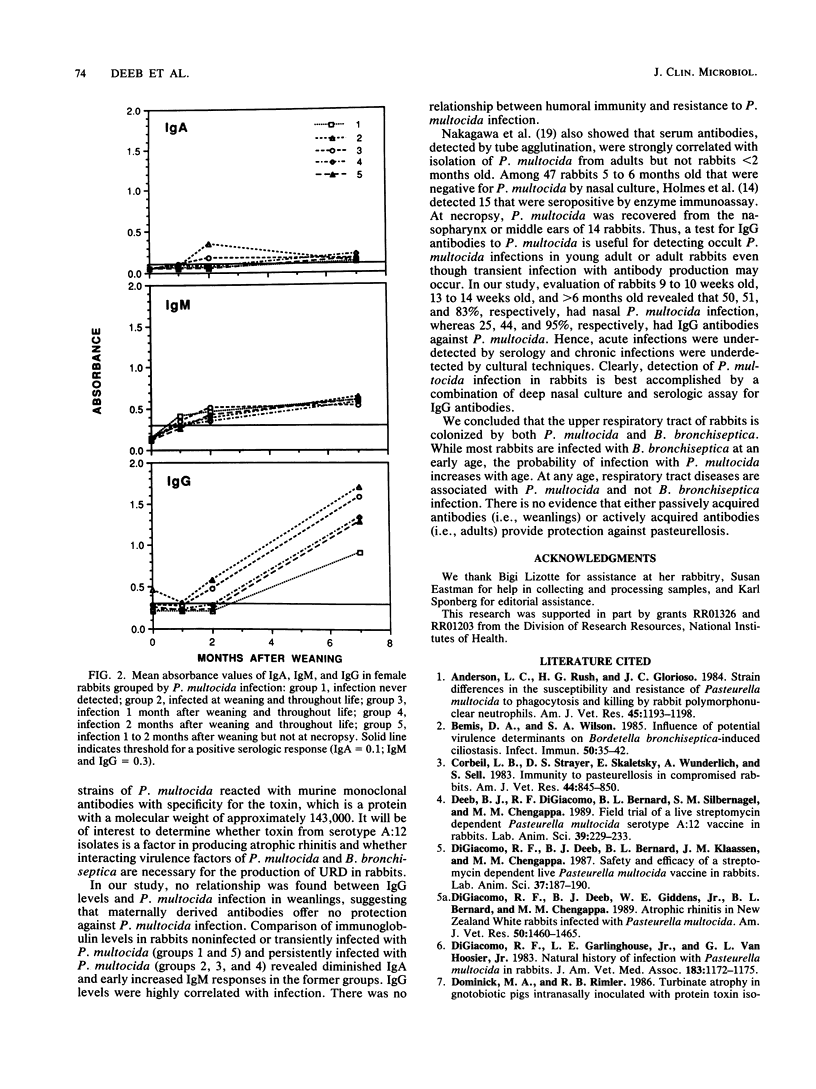
The natural history of infection with Pasteurella multocida and Bordetella bronchiseptica in domestic rabbits was studied prospectively at a commercial rabbitry. At weaning, about 25% of rabbits had nasal infections with P. multocida and 75% had infections with B. bronchiseptica. Infection of weanling rabbits paralleled nasal infections of their dams. The proportion of rabbits with both infections increased with age. At 2 to 4 months old, about 50% of rabbits with P. multocida or P. multocida and B. bronchiseptica infections had upper respiratory disease (URD), whereas rabbits with B. bronchiseptica infection had no disease. In rabbits about 10 months old, 75% with P. multocida or P. multocida and B. bronchiseptica infections had URD, whereas virtually none with B. bronchiseptica infection had disease. Disease of the nares, paranasal sinuses, middle ears, and lungs was associated with P. multocida and not B. bronchiseptica infection. In adult rabbits with nasal P. multocida infection, with or without signs of URD, about 80% had concurrent infection of the paranasal sinuses and middle ears and 20% had infection of the bronchi and lungs. In rabbits without nasal P. multocida infection, 20 to 35% had P. multocida infection of the paranasal sinuses and middle ears. Weanling rabbits with and without P. multocida infection had similar immunoglobulin G (IgG) levels. In rabbits observed prospectively, the only antibody differences between those transiently and persistently infected with P. multocida were a diminished IgA response in nasal lavages and an earlier IgM response in sera of transiently infected rabbits. IgG levels increased with the duration of infection. There was no relationship between immunoglobulin levels and freedom from P. multocida infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. C., Rush H. G., Glorioso J. C. Strain differences in the susceptibility and resistance of Pasteurella multocida to phagocytosis and killing by rabbit polymorphonuclear neutrophils. Am J Vet Res. 1984 Jun;45(6):1193–1198. [PubMed] [Google Scholar]
- Bemis D. A., Wilson S. A. Influence of potential virulence determinants on Bordetella bronchiseptica-induced ciliostasis. Infect Immun. 1985 Oct;50(1):35–42. doi: 10.1128/iai.50.1.35-42.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Strayer D. S., Skaletsky E., Wunderlich A., Sell S. Immunity to pasteurellosis in compromised rabbits. Am J Vet Res. 1983 May;44(5):845–850. [PubMed] [Google Scholar]
- Deeb B. J., DiGiacomo R. F., Bernard B. L., Silbernagel S. M., Chengappa M. M. Field trial of a live streptomycin dependent Pasteurella multocida serotype A:12 vaccine in rabbits. Lab Anim Sci. 1989 May;39(3):229–233. [PubMed] [Google Scholar]
- DiGiacomo R. F., Deeb B. J., Bernard B. L., Klaassen J. M., Chengappa M. M. Safety and efficacy of a streptomycin dependent live Pasteurella multocida vaccine in rabbits. Lab Anim Sci. 1987 Apr;37(2):187–190. [PubMed] [Google Scholar]
- DiGiacomo R. F., Deeb B. J., Giddens W. E., Jr, Bernard B. L., Chengappa M. M. Atrophic rhinitis in New Zealand white rabbits infected with Pasteurella multocida. Am J Vet Res. 1989 Sep;50(9):1460–1465. [PubMed] [Google Scholar]
- DiGiacomo R. F., Garlinghouse L. E., Jr, Van Hoosier G. L., Jr Natural history of infection with Pasteurella multocida in rabbits. J Am Vet Med Assoc. 1983 Dec 1;183(11):1172–1175. [PubMed] [Google Scholar]
- Dominick M. A., Rimler R. B. Turbinate atrophy in gnotobiotic pigs intranasally inoculated with protein toxin isolated from type D Pasteurella multocida. Am J Vet Res. 1986 Jul;47(7):1532–1536. [PubMed] [Google Scholar]
- Duclos P., Caillet J., Javelot P. Flore bactérienne aérobie des cavités nasales du lapin d'élevage. Ann Rech Vet. 1986;17(2):185–190. [PubMed] [Google Scholar]
- Elling F., Pedersen K. B. The pathogenesis of persistent turbinate atrophy induced by toxigenic Pasteurella multocida in pigs. Vet Pathol. 1985 Sep;22(5):469–474. doi: 10.1177/030098588502200506. [DOI] [PubMed] [Google Scholar]
- Foged N. T., Nielsen J. P., Pedersen K. B. Differentiation of toxigenic from nontoxigenic isolates of Pasteurella multocida by enzyme-linked immunosorbent assay. J Clin Microbiol. 1988 Jul;26(7):1419–1420. doi: 10.1128/jcm.26.7.1419-1420.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Jones G. W., Rush H. G., Pentler L. J., Darif C. A., Coward J. E. Adhesion of type A Pasteurella mulocida to rabbit pharyngeal cells and its possible role in rabbit respiratory tract infections. Infect Immun. 1982 Mar;35(3):1103–1109. doi: 10.1128/iai.35.3.1103-1109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofing G. L., Rush H. G., Petkus A. R., Glorioso J. C. In vitro killing of Pasteurella multocida: the effect of rabbit granulocyte and specific antibody source. Am J Vet Res. 1979 May;40(5):679–683. [PubMed] [Google Scholar]
- Holmes H. T., Matsumoto M., Patton N. M., Zehfus B. R. Serologic methods for detection of Pasteurella multocida infections in nasal culture negative rabbits. Lab Anim Sci. 1986 Dec;36(6):640–645. [PubMed] [Google Scholar]
- Klaassen J. M., Bernard B. L., DiGiacomo R. F. Enzyme-linked immunosorbent assay for immunoglobulin G antibody to Pasteurella multocida in rabbits. J Clin Microbiol. 1985 Apr;21(4):617–621. doi: 10.1128/jcm.21.4.617-621.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. S., Ringler D. H., Park J. S. Characterization of Pasteurella multocida isolates from the nares of healthy rabbits with pneumonia. Lab Anim Sci. 1978 Dec;28(6):691–697. [PubMed] [Google Scholar]
- Maheswaran S. K., Thies E. S. Influence of encapsulation on phagocytosis of Pasteurella multocida by bovine neutrophils. Infect Immun. 1979 Oct;26(1):76–81. doi: 10.1128/iai.26.1.76-81.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Takino T. Scanning electronmicroscopic studies of Bordetella bronchiseptica on the rabbit tracheal mucosa. J Med Microbiol. 1980 Feb;13(1):159–161. doi: 10.1099/00222615-13-1-159. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Nakayama K., Saito M., Takayama S., Watarai S. Bacteriological and serological studies on Pasteurella multocida infection in rabbits. Jikken Dobutsu. 1986 Oct;35(4):463–469. doi: 10.1538/expanim1978.35.4_463. [DOI] [PubMed] [Google Scholar]
- Rutter J. M., Mackenzie A. Pathogenesis of atrophic rhinitis in pigs: a new perspective. Vet Rec. 1984 Jan 28;114(4):89–90. doi: 10.1136/vr.114.4.89. [DOI] [PubMed] [Google Scholar]
- Skaletsky E., Corbeil L. B., Wunderlich A., Sell S., Strayer D. S. Proliferative responses of rabbit lymphocytes to Pasteurella multocida decrease with prolonged immunization. Infect Immun. 1982 Oct;38(1):383–385. doi: 10.1128/iai.38.1.383-385.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. I., Nedelman J., Hendley J. O., Hewlett E. L. Species specificity of Bordetella adherence to human and animal ciliated respiratory epithelial cells. Infect Immun. 1983 Nov;42(2):692–695. doi: 10.1128/iai.42.2.692-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. T., Goldsboro J. A., Williams F. P., Sueur R. Experimental respiratory infection with Pasteurella multocida and Bordetella bronchiseptica in rabbits. Lab Anim Sci. 1975 Aug;25(4):459–464. [PubMed] [Google Scholar]
- Zeligs B. J., Zeligs J. D., Bellanti J. A. Functional and ultrastructural changes in alveolar macrophages from rabbits colonized with Bordetella bronchiseptica. Infect Immun. 1986 Sep;53(3):702–706. doi: 10.1128/iai.53.3.702-706.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


