SUMMARY
How TCR specificity evolves in vivo following protein vaccination is central to the development of T helper cell function. Most models of clonal selection in the T helper cell compartment favor TCR affinity-based thresholds. Here, we demonstrate that depot-forming vaccine adjuvants do not require TLR agonists to induce clonal dominance in antigen-specific T helper cell responses. However, readily dispersible adjuvants using TLR-9 and TLR-4 agonists skew TCR repertoire usage by increasing TCR selection thresholds and enhancing antigen-specific clonal expansion. In this manner, vaccine adjuvants control the local accumulation of T helper cells expressing TCR with the highest peptide MHC class II binding. Clonal composition was altered by mechanisms that blocked the local propagation of clonotypes independent of antigen dose and not as a consequence of inter-clonal competition. This capacity of adjuvants to modify antigen-specific Th cell clonal composition has fundamental implications for the design of future protein sub-unit vaccines.
INTRODUCTION
Vaccines prime the adaptive immune system to anticipate future pathogens. Most vaccines in use today rely heavily on antigen-specific B cell memory and the sustained production of high-affinity antibodies for effective long-term protection (Kaufmann, 2007; Pulendran and Ahmed, 2006). This high-affinity antigen-specific B cell memory is dependent on the cognate regulation of antigen-specific effector T helper cells that emerge rapidly upon initial vaccine challenge (McHeyzer-Williams and McHeyzer-Williams, 2005). The mechanisms that control clonal selection within the antigen-specific T helper cell compartment are central to the development of appropriate effector T helper cells, but the details of these processes remain poorly resolved.
Initial T cell receptor (TCR) recognition of foreign peptide MHC class II (pMHCII) complexes on antigen presenting cells (APC) defines the first major checkpoint in antigen-specific T helper cell development (Fazilleau et al., 2007b; McHeyzer-Williams and McHeyzer-Williams, 2005). Unlike CD8 T cells, T helper cells require persistent TCR-pMHCII for maximal clonal expansion (Obst et al., 2005) that may arise from multiple contacts with pMHCII-expressing APC (Celli et al., 2005). Individual TCR-pMHCII interactions are characteristically low affinity involving conformational co-operativity (Rudolph et al., 2006) with TCR junctional region plasticity upon binding (Reiser et al., 2002) and multimer formation at the T helper-APC cellular interface (Davis et al., 2007). Nevertheless, most models still favor TCR affinity thresholds or functional avidity for pMHC binding as controlling ‘best fit’ for antigen-specific TCR selection and evidence for peptide binding kinetics determining clonal dominance (Lazarski et al., 2005). Specifically in the peripheral T helper compartment, there is evidence for preservation of clonotypes with slower TCR off rates (Savage et al., 1999), indications of competition for antigen among monoclonal T helper cells (Falta et al., 2005; Fasso et al., 2000; Garcia et al., 2007; Rees et al., 1999) and overall TCR affinity-based selection (Malherbe et al., 2000; Malherbe et al., 2004; Skokos et al., 2007; Weber et al., 2005). Importantly, the duration of T helper-APC contact (Iezzi et al., 1998; Miller et al., 2004) and the strength of TCR-pMHCII binding (Gett et al., 2003; Lee et al., 2003) can impact clonal expansion (Celli et al., 2007) and the differential acquisition of effector T helper cell function (Chang et al., 2007; Lanzavecchia and Sallusto, 2002; Reiner et al., 2007). Hence, the dynamics of clonal evolution among antigen-specific T helper cells can fundamentally impact all subsequent phases of T helper cell regulated adaptive immunity.
The I-Ek restricted murine response to pigeon cytochrome c (PCC) (Jorgensen et al., 1992) provides access to antigen-specific T helper cells as they develop in vivo (Bikah et al., 2000; McHeyzer-Williams et al., 1999; McHeyzer-Williams and Davis, 1995; Panus et al., 2000). Local vaccination with whole protein into B10.BR mice induces Vα11Vβ3-expressing T helper cells with a limited set of TCR junctional regions that confer specificity to one dominant PCC epitope (McHeyzer-Williams et al., 1999). Appearance of these PCC-specific TCR helps to define the dynamics of clonal evolution and the extent of clonal diversity within the antigen-specific T helper cell response (Malherbe et al., 2004; McHeyzer-Williams and McHeyzer-Williams, 2004; McHeyzer-Williams et al., 1999). In this model of protein vaccination, clonal selection occurs rapidly over a threshold TCR affinity for pMHCII without further bias towards higher affinity TCR above this threshold (Malherbe et al., 2004). Surprisingly, lowering antigen dose until clonal expansion was compromised has no effect on clonal diversity. Overall, the selection mechanism for T helper cells appears to be ‘programmed’ early during the immune response, ‘intrinsic’ to the affinity of the expressed TCR and not dependent on clonal competition (Fazilleau et al., 2007b). Whether this clonal selection mechanism can be regulated by secondary microenvironmental cues that alter T helper clonal composition remains unknown.
All vaccines deliver antigens within an inflammatory context to prime antigen-specific immune responses (Medzhitov and Janeway, 1997). In particular, protein sub-unit vaccine formulations all require an exogenous vaccine adjuvant to provide this inflammatory context and promote effective immunity (Guy, 2007; Pashine et al., 2005; Pulendran and Ahmed, 2006). Aluminium-containing adjuvants (Alum) and Incomplete Freunds Adjuvant (IFA) contain no known toll-like receptor (TLR) agonist activity but promote depots of antigen at the injection site and support protective immunity in humans and mice (Lindblad, 2004). In contrast, Complete Freund’s Adjuvant (CFA) includes a complex mixture of inflammatory mediators through the addition of heat-killed mycobacterium into the mineral oil of IFA (Billiau and Matthys, 2001; Garcon et al., 2007). Aqueous formulations of CpG oligodeoxynucleotide and antigen are TLR-9 agonist driven adjuvants with no capacity to promote antigen depots (Klinman, 2006). Similarly, an oil-in-water monophosphoryl lipid A (MPL) based adjuvant provides a TLR-4 driven immune stimulant (Baldridge et al., 2004; Garcon et al., 2007) with antigen in metabolizable squalene oil (O’Hagan, 2007). These adjuvants are used across many protein vaccination models, however their impact on clonal selection within the helper T cell compartment is not known.
In the current studies, varying vaccine adjuvants revealed the dynamics of a unique mechanism of antigen-specific T helper cell selection. While all adjuvants induced clonal dominance in the helper T cell compartment, the antigen-specific TCR repertoire was differentially skewed across the different formulations. Independent of TLR activity, all depot adjuvants (Alum, IFA and CFA) promoted antigen-specific clonotypes expressing different sets of TCR with lower pMHCII binding. In contrast, the dispersible adjuvants (CpG and MPL) re-set the TCR-based selection threshold to recruit and propagate clonotypes with higher pMHCII binding. In this manner and independent of antigen dose, vaccine adjuvants control the local penetrance of T helper cells expressing TCR with the highest peptide MHC class II binding. Thus, altering TCR-based selection thresholds with different vaccine adjuvant formulations provides a fundamentally new mechanism for regulating adaptive immunity.
RESULTS
Local accumulation of antigen-specific Th cells across different adjuvants
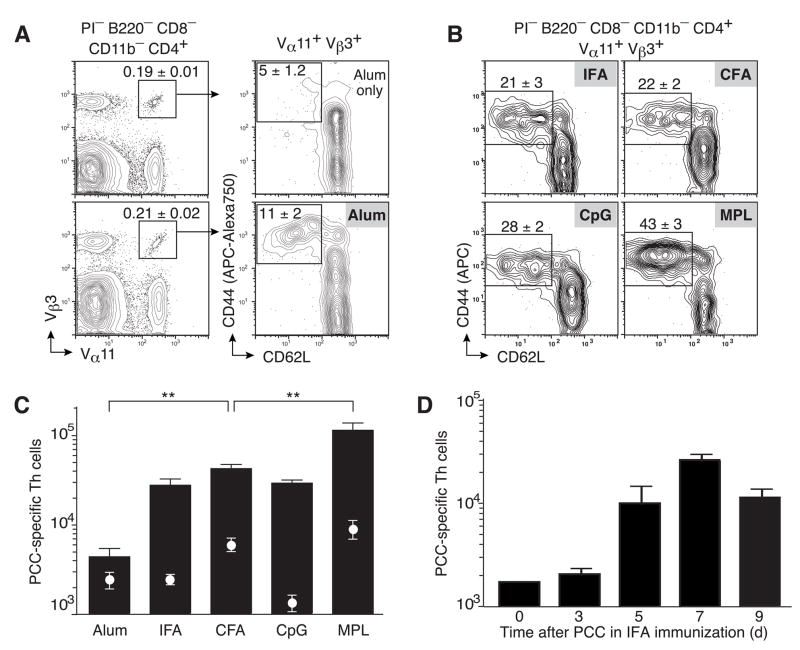
An MPL-based oil-in-water adjuvant induces maximal clonal accumulation in draining lymph nodes (LN) of B10.BR mice at day 7 after subcutaneous priming with 400μg whole protein antigen PCC (McHeyzer-Williams et al., 1999). Here, we compare the LN accumulation of PCC-specific T helper cells (Vα11+Vβ3+ CD44hiCD62Llo) across five different vaccine adjuvants commonly used in murine immune response models (Fig. 1a,b). Precipitating protein antigen with Alum induces the lowest number of PCC-specific Th cells (Fig. 1a,c). In contrast, IFA induces significantly higher levels of PCC-specific Th cells (Fig. 1b,c) with peak numbers reached at day 7 (Fig. 1d). CFA without antigen induces higher levels of background than Alum or IFA alone (Fig 1c white circles) but surprisingly, induces similar levels of PCC-specific T helper cells as IFA (Fig. 1b,c). Dispersible aqueous formulations of the TLR-9 agonist CpG and PCC also induce similar amounts of clonal accumulation as the IFA and approximately 2-fold fewer cells than CFA. Finally, the MPL-based adjuvant induces significantly higher local accumulation of PCC-specific T helper cells (p<0.001) compared to all other adjuvants. These data reveal a hierarchy of antigen-specific T helper cell responsiveness across five different adjuvants that appear independent of TLR agonist activity and the capacity to form antigen depots at the injection site.
Figure 1. Local accumulation of antigen-specific Th cells across different adjuvants.
(a,b) PCC-specific T helper cells (Vα11+Vβ3+CD44hiCD62Llo) at day 7 in lymph nodes from B10.BR mice immunized with (a) Alum with (lower panels) or without PCC (upper panels) or (b) with PCC and the indicated adjuvant. Profiles gated on propidium iodide (PI) negative cells that are CD4+B220−CD8−CD11b− and Vα11+Vβ3+ as indicated with mean ±SEM (n≥3) percent of cells within insert box (c) Total number of PCC-specific T helper cells 7 days after immunization with Alum (n=7), IFA (n=6), CFA (n=15), CpG (n=6) and MPL (n=25) with (bars) or without PCC (circles); means ±SEM n≥3 p ≤ 0.01 (**) (two-tailed t-test) comparing Alum or MPL to any other adjuvant. (d) Total number of PCC-specific T helper cells 3, 5, 7 or 9 days after immunization with IFA and PCC. Mean ±SEM for at least three animals.
While there is activity within the CD44hiCD62Lhi compartment of Vα11+Vβ3+ cells at day 7 of the primary response across all adjuvants (Supp Fig 1a), their total number (Supp Fig 1b) parallels what is found in the CD44hiCD62Llo compartment. Furthermore, the dynamics of the PCC-specific T cell response was similar for IFA, CFA, CpG and MPL with all reaching peak levels at day 7, while the Alum response increased to day 9 but still reached levels significantly lower than seen for all other adjuvant formulations (supp Fig 2).
Clonal dominance without TLR agonists or antigen depots
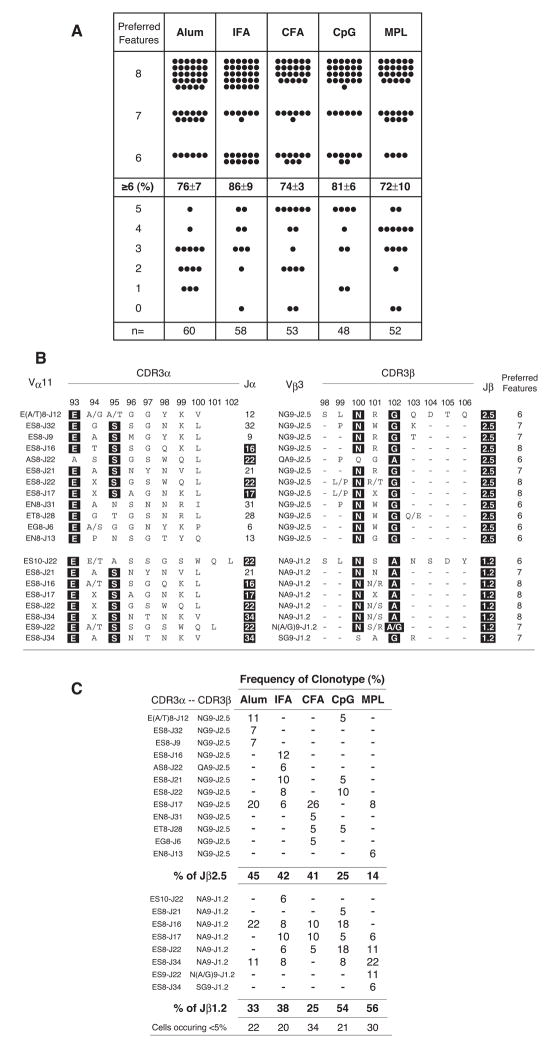
To assess the clonal composition of the PCC-specific Th cell compartment that accumulates using the different adjuvants, we sorted single antigen-specific Th cells for TCR repertoire analysis. Based on early hybridoma analyses (Jorgensen et al., 1992) and single cell studies using the MPL-based adjuvant (Fazilleau et al., 2007a; Malherbe et al., 2004; McHeyzer-Williams et al., 1999; McHeyzer-Williams and Davis, 1995), we have previously defined 8 preferred features within the CDR3 of the expressed TCRαβ in B10.BR mice that assort with PCC-specificity (CDR3α: Eα93, Sα95, Jα16/22/34/17 and length of 8aa; CDR3β: Nβ100, A/Gβ102, Jβ1.2/2.5 and length of 9aa). Single cells that express TCR with ≥ 6 of these preferred features across both chains of the expressed TCR from individual PCC-specific T helper cells defines clonal dominance in this polyclonal immune response (Malherbe et al., 2004; McHeyzer-Williams et al., 1999; McHeyzer-Williams and Davis, 1995). Using this initial level of resolution to the repertoire studies, it is clear that all five adjuvants induced high levels of clonal dominance (>70% of responders for all conditions), regardless of the extent of clonal accumulation (Fig. 2a). Thus, TLR agonists or antigen depots are not required to induce clonal dominance in the antigen-specific Th cell compartment.
Figure 2. Clonal dominance without TLR agonists or antigen depots.
Single-cell repertoire analysis of individual PCC-specific T helper cells (Vα11+Vβ3+CD44hi CD62Llo) sorted from mice immunized with PCC and indicated adjuvant. (a) Each filled circle represents sequence from single cells representing the number of preferred CDR3 features known to be selected in the PCC response (TCR-α: E at α93; S at α 95; CDR3α length of 8aa; and TCRJα 16, 17, 22 and 34. TCR-β: N at β100; A/G at β102; CDR3β length of 9aa; and TCRJβ 1.2 and 2.5). Cells with ≥ 6 preferred features express restricted TCR of the dominant clonotype and the percentage ±SEM; across 3 individual animals with n = number of single cells used in the analysis displayed with individual animals contributing different numbers of sequences, Alum (n=21,23,16); IFA (n=22,18,18); CFA (n=18,17,18); CpG (n=15,16,17) MPL (n=16,19,17) (b) TCR sequences from the dominant clonotypes (≥6 preferred CDR3 features). Columns (left to right): individual CDR3α chain designation (based on amino acids at α93 and α95, CDR3α length and Jα usage); CDR3α, with positions α93E and α95S ‘highlighted’ in black as canonical, motif length depicted; Jα gene usage; individual CDR3β designation (based on amino acids at β100 and β102, CDR3β length and Jβ usage); CDR3β, with positions β100N and β102A or β102G ‘highlighted’ in black as canonical, motif length depicted; Jβ gene segment usage; total number of ‘preferred’ features in both CDR3 regions combined. (c) Penetrance of dominant clonotypes after priming with PCC and the indicated adjuvant as a percent of dominant clonotypes and organized in the same order as (b) with summaries for each adjuvant across Jβ2.5 and Jβ1.2 usage as displayed.
Vaccine adjuvants skew antigen-specific TCR repertoire usage
We have previously shown that antigen-specific TCRβ transgenic T helper cells expressing different Jβ regions confer broadly distinct pMHCII binding properties regardless of the paired TCRα chain (Malherbe et al., 2004). In particular, PCC-specific TCRβ expressing Jβ2.5 (2B4β chain) display slower off rates but overall lower affinity for pMHCII binding than Jβ1.2 (5CC7β chain) (Malherbe et al., 2004). Further, different TCRα chains pairing with the same TCRβ also confer different binding kinetics that broadly assort with Jα region expression. Thus, it is important and informative to scrutinize the clonotypic distribution within the dominant compartment of PCC-specific responders.
Next, for more in depth clonal analysis, we broadly divide the responding set of dominant clones (≥ 6 features) across all adjuvants according to Jβ usage and further designate individual dominant TCRαβ clonotypes based on preferred CDR3 features (Fig. 2b & 2c). Clonotypes expressing Jβ2.5 were more prevalent using the three depot forming adjuvants (Alum:45%, IFA:42% and CFA:41%)(top panel in each section) than the non-depot forming adjuvant (CpG:25% and MPL:14%). The Jβ2.5 expressing clonotypes display restricted TCRα chains (based on preferred features) but many use non-canonical Jα segments expressed (Fig. 2b top panel). Interestingly, among the depot adjuvants (Alum, IFA and CFA), Jβ2.5 expressing clonotypes are largely non-overlapping (Fig. 2c top panel). In contrast, clonotypes expressing Jβ1.2 display highly restricted TCRα chains with predominantly canonical Jα regions (Fig. 2b bottom panel) and emerge to dominant levels using the two non-depot adjuvant formulations (CpG:54% and MPL: 56%)(Fig 2c, bottom panel). These data indicate that different adjuvants promote the local accumulation of antigen-specific T helper cells with broadly differing TCR repertoires.
Skewed J region usage among dominant clonotypes
Due to the substantial clonal heterogeneity in non-transgenic animals it is reasonable to consider the variation of individual CDR3 features as a means of evaluating the impact of adjuvant on clonal variation (Supp Fig. 3 & 4). These analyses again highlight TCRα chain variation largely based on Jα region usage (Supp Fig. 3a). Across the depot adjuvants, IFA promotes the highest level of the dominant clonotypes (71 ± 3%) many expressing Jα22 (44%). Alum and CFA promote lower levels of the dominant clonotypes (55 ± 15% and 49 ± 6% respectively) with far fewer Jα22 clonotypes (11% and 12%) but many clones expressing Jα16 (32% and 23%) & Jα17 (32% and 54%). Both non-depot adjuvants favored dominant clonotypes expressing Jα22 (CpG:46% and MPL:43%). All other CDR3α features were highly restricted across all adjuvant formulations (Supp Fig 3b–d). As suggested by the clonal analysis, the Jβ region usage was the most consistent and systematic change encountered (Supp Fig 4a) and will be pursued in greater detail throughout these studies. The CDR3β feature of G or A at β102 also assorts with the Jβ region differences (Supp Fig. 4b) contributed by the Dβ2 or Dβ1 segment respectively. The CDR3β length of 9aa (Supp Fig. 4c) and the selection for N at β100 (Supp Fig. 4d) is similarly restricted across all adjuvants. These data further support the capacity of vaccine adjuvants to skew J region gene usage among the TCR repertoire of the dominant antigen-specific T helper cell compartment.
Vaccine adjuvants impact local clonal composition
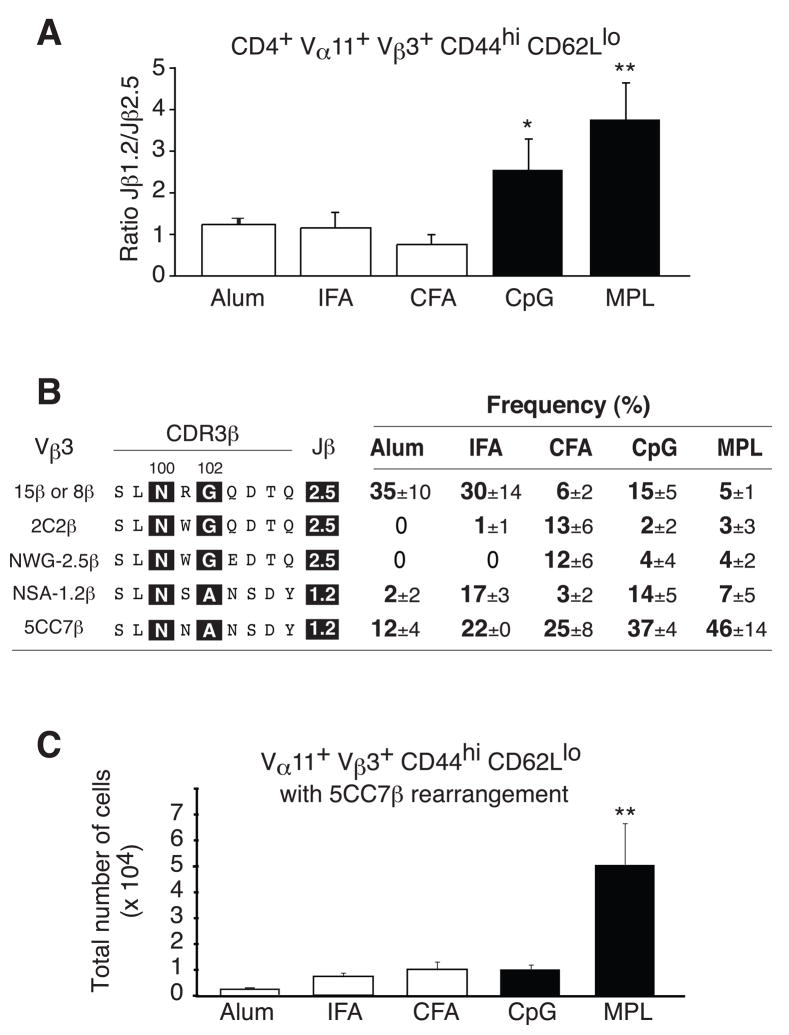
Next, we extended clonal analysis across the different adjuvants focused on Jβ region usage including an alternate RT-PCR based assay with single cell resolution for Jβ1.2/2.5 usage (see methods for details). As indicated from the previous clonal analysis, CpG and MPL based adjuvant significantly skewed the responding T helper cell compartment towards clones expressing Jβ1.2 (Fig. 3a). To monitor the impact of adjuvant on CDR3β chain, we considered the distribution of five dominant TCRβ chains regardless of TCRα chain assortment (Fig. 3b). Jβ2.5 expressing clones similar to the TCRβ from PCC-specific hybridoma 15 or 8 emerge to high frequencies with Alum and IFA but only to low levels from animals immunized with CFA. In contrast, CFA promotes two different CDR3β chains, one similar to the hybridoma 2C2β and the other designated NWG-1.2β. As predicted, Jβ1.2 usage is present with the depot adjuvants at variable penetrance for some clonotypes as evidenced by NSA-1.2β but increasing frequencies found after CpG and MPL-based adjuvant as exemplified by the penetrance of TCR with 5CC7β chain (Fig 3b). Finally, clonal expansion also plays a role in the propagation of particular selected clonotypes as demonstrated by the substantial exaggeration of the 5CC7β chain expressing clonotype after MPL adjuvant that is not evident with CpG (Fig. 3c). Thus, adjuvant formulation can substantially alter clonal composition and the MPL-based adjuvant can preferentially promote antigen-specific T helper cells with broadly higher pMHCII binding.
Figure 3. Adjuvants skew TCR β chain usage.
(a) Relative abundance of PCC-specific Th cells (Vα11+Vβ3+CD44hiCD62Llo) expressing Jβ1.2 or Jβ2.5 gene segments for the indicated adjuvant (mean ±SEM.; n≥3). (b) Predicted amino acid sequences of CDR3β regions mean ±SEM of five predominant clonotypes (n≥3) for PCC-specific Th cells isolated from mice immunized with indicated adjuvant (c) Total number of PCC-specific Th cells expressing the 5C.C7β (SLNNANSDY) rearrangement mean ±SEM n≥3.
Adjuvants re-set TCR selection thresholds
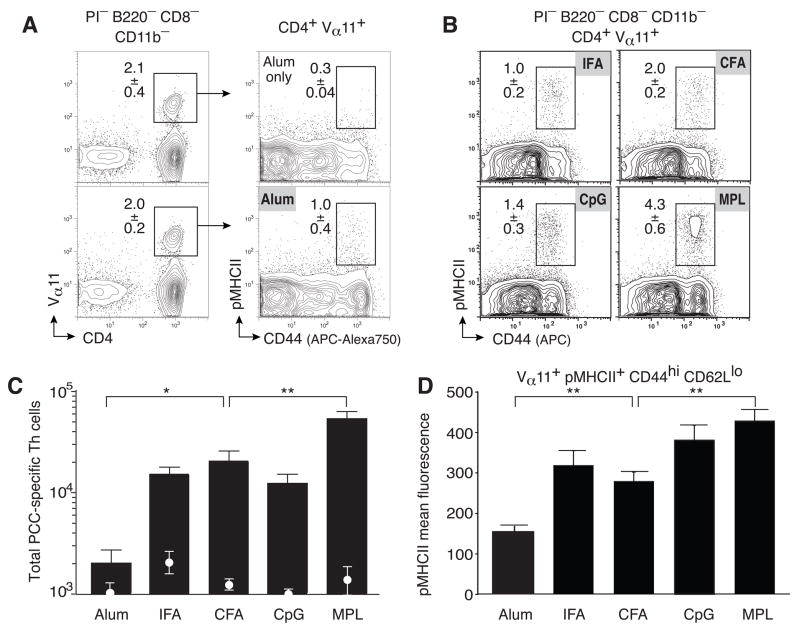
Labeling with pMHCII tetramers provides a direct means to visualize TCR ligand binding by antigen-specific T helper cells. The mean fluorescence intensity (MFI) for pMHCII binding at optimal concentrations of pMHCII tetramer provides an index for the level and distribution of TCR binding strength within polyclonal antigen-specific T helper cell compartments. Importantly, in the current labeling strategy (Fig. 4a,b) Vα11 MFI also estimates total TCR levels per cellular population and were similar across all adjuvant conditions. The pMHCII-based assay for monitoring T helper cell responses has lower background for all adjuvant only controls (Fig 4c white circles) but detects ~2-fold fewer pMHCII+Vα11+ antigen-specific T helper cells than using the V region based strategy (that doesn’t require pMHCII binding in Fig 1). Nevertheless, similar relative differences of clonal accumulation across different adjuvants (Fig 4a–c) parallel that found using the TCR V region based assay (in Fig. 1a).
Figure 4. Adjuvants re-set the TCR selection threshold.
PCC-specific T helper cells as PI−CD8−B220−CD11b− cells expressing CD4 and Vα11, binding pMHCII tetramers and expressing high level of CD44 displayed within the inserted box; percent of cells mean±SEM n≥3 at day 7 in lymph nodes from B10.BR mice immunized with (a) Alum with (lower panels) or without PCC (upper panels) or (b) with PCC and the indicated adjuvant. (c) Total numbers of CD4+Vα11+pMHCII+CD44hiCD62Llo T helper cells after immunization with (bars) or without (circles) PCC and the indicated adjuvant; means ±SEM n≥3 p ≤ 0.01 (**) (two-tailed t-test) comparing Alum or MPL to any other adjuvant. (d) Mean fluorescence intensity of pMHCII tetramer staining for PCC-specific T helper cells after immunization with PCC and the indicated adjuvant (mean ±SEM; n≥3).
Even though the cellular response was low, Alum induces a broad range of pMHCII tetramer binding (Fig. 4a,c) but the population expressed the lowest pMHCII MFI compared to all other adjuvants (Fig. 4d). A similar range of pMHCII binding was seen within IFA and CFA responses (Fig. 4b,c) but resulted in higher mean fluorescence intensity due to increased presence of high pMHCII binding cells (Fig. 4b,d). The shift towards higher pMHCII binding cells was more pronounced with CpG and significantly higher using the MPL-based adjuvant (p<0.01)(Fig. 4b,d). These trends in MFI are consistent with the TCR repertoire studies and suggest that the MPL-based adjuvant substantially alters clonal composition by increasing the proportions of high pMHCII binding T helper cells within the antigen-specific population in the LN.
To test more directly whether polyclonal T helper cells that expressed Jβ1.2 bound higher level of pMHCII, we sorted single antigen-specific T helper cells with high versus low levels of pMHCII binding (Supp Fig. 5a). As predicted, TCR with Jβ1.2 were present to high levels within the pMHCII high binders using CFA or MPL-based adjuvants. More importantly, Jβ2.5-expressing clonotypes were only found within the low pMHCII binding compartment (Supp Fig 5b). As a consequence of these studies, we noted that the proportion of Jβ2.5 expressing pMHCII+ T helper cells was lower for the CFA condition than would have been predicted by the repertoire studies. To evaluate this more closely, we sorted antigen-specific T helper cells after CFA immunization using both labeling strategies from individual animals to evaluate TCR representation (Supp Fig 5c). These data indicate that the pMHCII labeling underestimates the presence of most Jβ2.5 expressing clonotypes as they most likely fall below the level of detection for this particular reagent. Appearance of these TCR in vivo revealed by the V region based strategy (Fig 2) and a table of PCC-specific hybridomas (Supp Fig 6) suggests that lack of binding reveals the detection limit of the pMHCII reagent and not the specificity of the T helper cells. Nevertheless, the trends of pMHCII binding support the overall notion that polyclonal antigen-specific TCR that express Jβ2.5 have a lower capacity for pMHCII binding and that adjuvants can re-set the threshold for clonal selection within the T helper cell compartment.
Blocking the selection of low affinity clonotypes
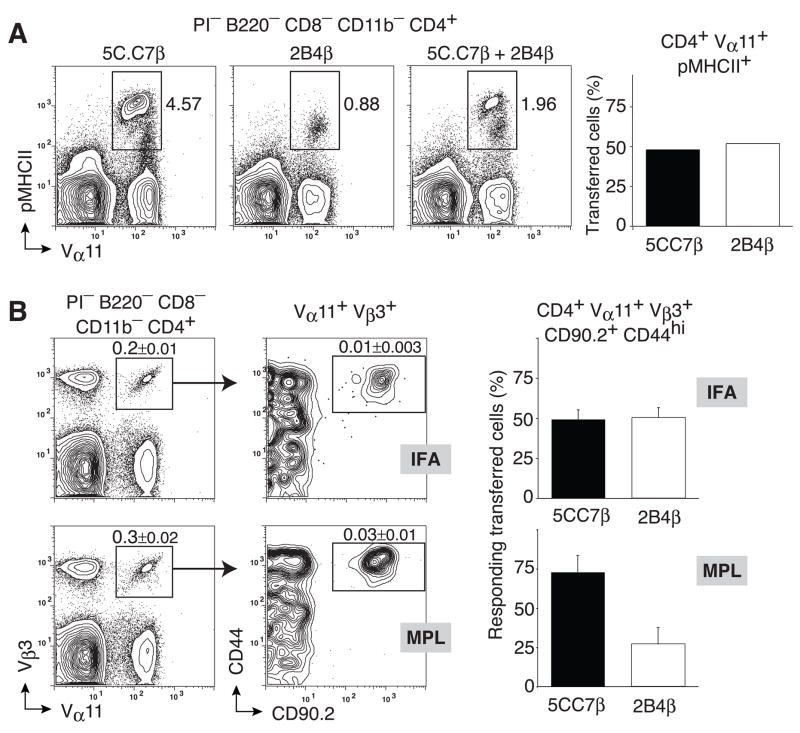
As demonstrated in these analyses, clonal diversity in non-transgenic immune responses is substantial. Furthermore, antigen-specific clonal expansion and effector cell differentiation may itself skew the evaluation of TCR binding strength using pMHCII tetramers and makes direct comparisons between developmentally-distinct Th cell types equivocal. To overcome these issues, we mixed equal numbers of higher affinity oligoclonal 5CC7β (NA9-Jβ1.2: SLNNANSDY) and lower affinity 2B4β (NS9Jβ2.5: NWSQDTQ) TCRβ transgenic Th cells and co-transferred them into B10.BR recipients (Fig. 5a). Following PCC immunization with IFA and extensive clonal expansion of transferred cells the balance of antigen-specific responder cells from these two TCRβ transgenic sources remained equivalent (Fig. 5b). In contrast, PCC in MPL substantially skewed the responder population towards the higher affinity 5CC7β clonotype (Fig. 5b). As demonstrated in detail in our previous studies (Malherbe et al., 2004), the TCR repertoire skewing in the presence of the MPL-based adjuvant was due to a proportionate loss of the lower affinity 2B4β clonotypes and not exaggerated over-expansion of the higher affinity subtypes. Taken together, these data indicate that adjuvants regulate clonal composition using a mechanism that alters initial TCR-based selection thresholds and relies most heavily on blocking the propagation of antigen-specific clonotypes expressing low affinity TCR.
Figure 5. Blocking the selection of low affinity clonotypes.
0.33×105 5C.C7β and 1.2×105 2B4β splenocytes were mixed and (a) stained with pMHCII tetramers for pre-immune repertoire analysis or (b) transferred into Thy1.1 syngeneic hosts. Transferred mice were immunized with 400μg PCC in IFA or MPL-based adjuvant. (a) Representative probability contours of pMHCII tetramer staining versus Vα11 for 5C.C7β (left), 2B4β (middle) and the 5C.C7β/2B4β cell mixture as a profile of cells (right) and evaluation of cell origin after cell sorting (Vα11+pMHCII+) and RT-PCR for Jβ1.2/2.5 expression representing 5CC7β and 2B4β respectively (bar graph) (b) Representative probability contours of CD44 and CD90.2 expressions (right panel) by Vα11+ Vβ3+ (left panel) cells from draining lymph nodes of recipient mice 7 days after immunization with IFA (upper panels) or MPL-based adjuvant (lower panels); single antigen-experienced PCC-specific T helper cells (Vα11+Vβ3+CD90.2+CD44hi) were sorted and the relative abundance of 5C.C7β and 2B4β T helper cells was evaluated by single-cell RT-PCR using Jβ1.2 and Jβ2.5 specific primers, respectively. Means ±SEM n=3 for each condition.
TCR-independent enhancement of clonal accumulation
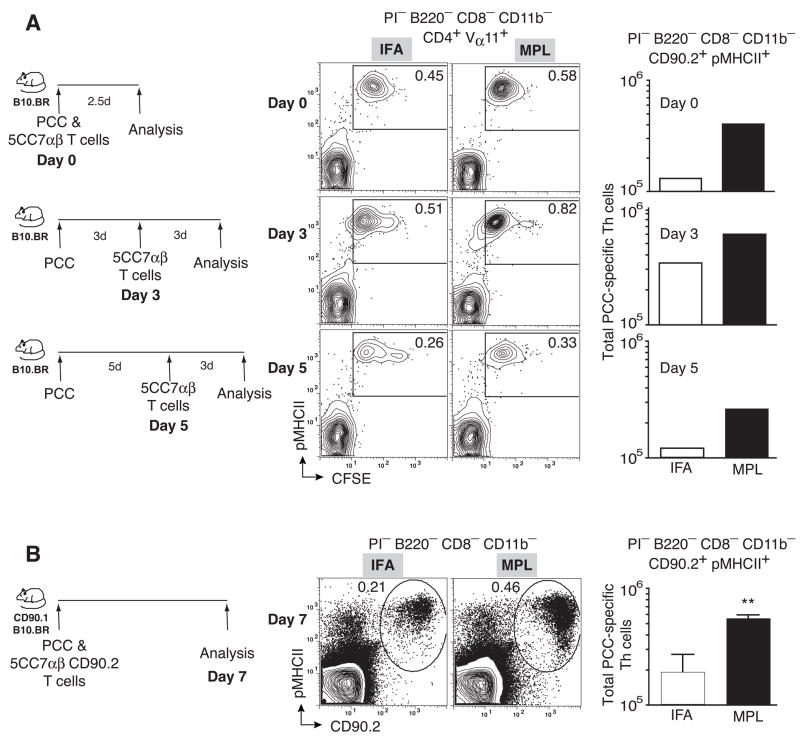
While different adjuvant formulations are expected to create depots at the injection site, it is more difficult to ascertain the effective level of antigen presentation in the draining lymphoid tissue. In a preliminary series of studies, we transferred naive (Vα11+Vβ3+CD4+CD44loCD62Lhi) 5CC7αβ transgenic Th cells into B10BR recipients that had been immunized with PCC in either IFA or the MPL-based adjuvant, day 0, 3 and 5 days after immunization. We then revealed clonal expansion using a congenic marker (CD90.2), pMHCII tetramer binding and CFSE dilution 3 days after the transfer. There was evidence for activating antigen in the draining LNs at each of the three time-points tested with the MPL inducing higher levels of expansion in each case (Fig. 6a). Hence, the MPL-based adjuvant provided as consistent a local antigen stimulant as the depot forming IFA for at least 5 days in draining LNs. Importantly, differences in clonal accumulation were significantly different between IFA and MPL over 7 days in vivo (p<0.001) even for Th cells expressing the same monoclonal TCRαβ (Fig. 6b). Hence, we suspect that the exaggerated clonal accumulation seen with the MPL-based adjuvant in the polyclonal model is independent of the TCR-based selection threshold and exerts itself independent of the initial TCR-pMHCII binding strength. Nevertheless, TCR independent influences of adjuvant can further exaggerate differences in clonal composition established at the early selection checkpoints in Th cell recruitment.
Figure 6. TCR-independent enhancement of clonal accumulation.
(a) 2×105 CFSE-labeled 5C.C7αβ splenocytes were transferred into syngeneic B10.BR hosts at the time of immunization (upper panels), 3 days (middle panels) or 5 days (lower panels) after immunization with PCC and IFA or MPL-based adjuvant. Representative probability contours of pMHCII tetramer staining versus CFSE for IFA (left) and MPL (middle) immunized recipients. Total number of Vα11+CFSElopMHCIITet+ cells in draining lymph nodes of recipients immunized with IFA or MPL-based adjuvant in bar graphs (far right). (b) 2×105 5C.C7αβ splenocytes were transferred into CD90.1+ syngeneic hosts and recipients were immunized with PCC and IFA or MPL-based adjuvant. Representative probability contours of pMHCII tetramer staining versus CD90.2 for IFA(left) and MPL (middle) immunized recipients. Total number of Vα11+CD90.2+pMHCII+ cells in draining lymph nodes of recipients immunized with IFA or MPL-based adjuvant (far right), mean ±SEM n=3 p< 0.01 (**)(two-tailed Student’s t-test).
Adjuvant action on selection is independent of antigen dose
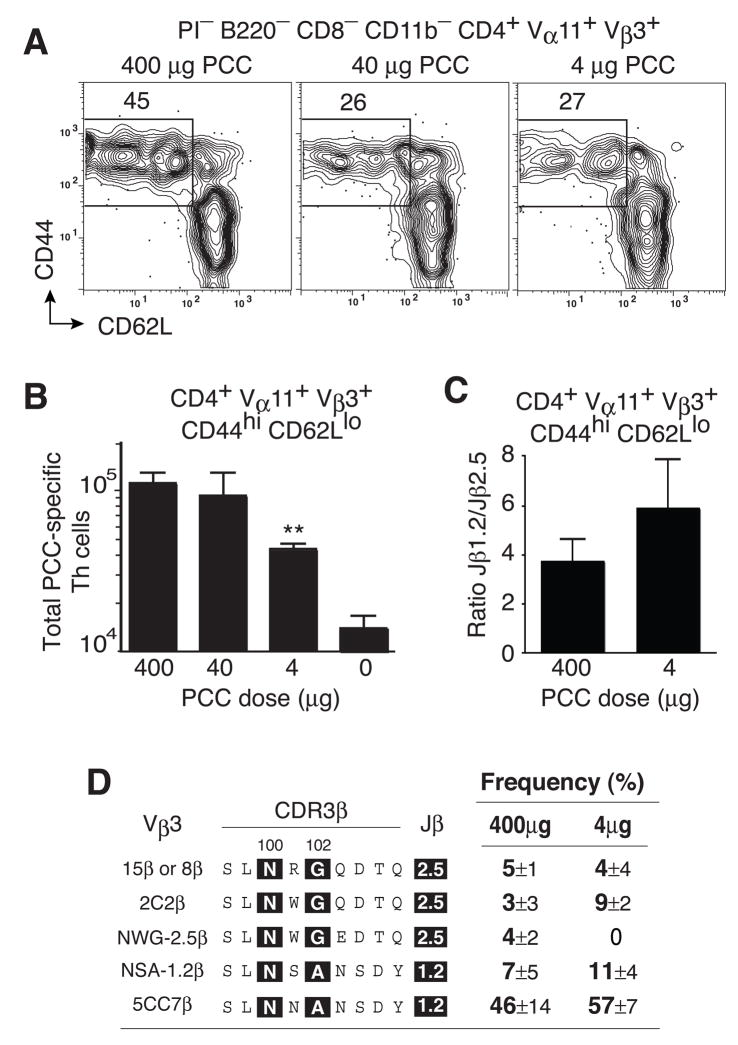
In the TCRβ transgenic transfer model, we had previously demonstrated that TCR-based selection was independent of antigen dose (Malherbe et al., 2004). Due to the concern of exaggerated precursor frequencies, it was important to readdress this issue in the context of the polyclonal B10.BR mice (Fig. 7a). Using the MPL-based adjuvant, 400μg and 40μg of whole protein antigen induced similar levels of local T helper cell accumulation at the peak of the PCC-specific response in B10.BR mice (Fig. 7b). In contrast, local antigen-specific Th cell numbers were significantly truncated using 4μg of protein antigen. However, there was no significant increase in the Jβ1.2/2.5 ratio (Fig. 7c) or the penetrance of five different sets of TCRβ clonotypes as seen using the optimal priming dose of antigen (Fig. 7d). Thus, in the polyclonal model the MPL-based adjuvant differentially regulates clonal composition in ways that appear independent of clonal expansion and the initial priming dose of antigen.
Figure 7. Selection threshold is re-set independent of the antigen dose.
(a) PCC-specific T helper cells (Vα11+Vβ3+CD4 4hiCD62Llo) at day 7 in lymph nodes from mice immunized with MPL-based adjuvant containing 400μg (left), 40μg (middle) or 4μg (right) of PCC. (b) Total number of PCC-specific T helper cells after immunization with 400, 40, 4 and 0 μg PCC. mean ±SEM n≥3. p < 0.05 (*) two-tail Student’s t-test, comparing to any other antigen dose. (c) Relative abundance of PCC-specific Th cells expressing Jβ1.2 or Jβ2.5 gene segments for indicated PCC dose, mean ±SEM n≥3 (d) Prevalence of 5 dominant TCRβ chain expressing clonotypes comparing 400ug dose (data from Fig 3c) to 4ug dose, mean ±SEM n=3.
DISCUSSION
Overall, these studies using classic vaccine adjuvants reveal the dynamics of clonal selection mechanisms in the T helper cell compartment. Alum supported negligible local clonal accumulation compared to all other adjuvants, but still promoted clonal dominance (Fig 1, 2 & 3). Hence, TLR agonist activity was not required to promote clonal dominance with either Alum or IFA. Surprisingly, CFA promoted no further local increase in antigen-specific T helper cells than IFA alone, but did alter clonal composition within selected clonotypes. The antigen depot characteristic of these classical adjuvants was also not required to induce clonal dominance by the dispersible TLR-9 and TLR-4 based adjuvants. Most importantly, the different adjuvants promoted the accumulation of dominant clonotypes with different TCR repertoires (Fig 2, 3 & 4). Alum, IFA and CFA promoted antigen-specific T helper cells expressing the Jβ2.5 gene segment and lower pMHCII binding. In contrast, the lower binding TCR were decreased using CpG and MPL, thereby substantially altering the local presence of T helper cells expressing Jβ1.2 and high pMHCII binding T helper cells (Fig 5 & 6). Thus, adjuvant formulation can modify the TCR-based selection threshold that regulates T helper clonal composition in response to protein vaccination.
Clonal selection in the T helper compartment is not altered by antigen dose. This was a surprising result in our previous studies using a TCRβ chain adoptive transfer model (Malherbe et al., 2004) and is reproduced here within the polyclonal immune system (Fig. 7). Hence, antigen-specific T helper clonal selection is not driven by inter-clonal competition for antigen. Rather, an intrinsic attribute of the TCR-pMHCII binding event governs T helper cell fate. While slower TCR off rates may be selected during thymic development (Savage et al., 1999) and the memory T helper response (Savage et al., 1999), it is the overall pMHCII binding capacity of the TCR that is selected at these early checkpoints in antigen-specific T helper cell development. There are reports of clonal competition for antigen (Falta et al., 2005; Fasso et al., 2000; Garcia et al., 2007; Rees et al., 1999) that may be a consequence of the adoptive transfer model and not found in oligoclonal or polyclonal systems. (Malherbe et al., 2004; McHeyzer-Williams et al., 1999; McHeyzer-Williams and Davis, 1995). Thus, with limiting numbers of antigen-specific precursors, the strength of pMHCII binding is the dominant TCR attribute used to shape the antigen-specific T helper cell compartment.
We have proposed that the overall affinity of the TCR-pMHCII interaction sets the threshold for T helper clonal selection (Malherbe et al., 2004). Using the MPL-based adjuvant and oligoclonal transfers, the lowest affinity PCC-specific clonotypes bind pMHCII but were never recruited into the immune response. The next lowest affinity clonotypes emerged to day 3 of the response, but only above a higher affinity threshold were clonotypes fully propagated to the peak of the local response at day 7. In the polyclonal system (McHeyzer-Williams et al., 1999), the most diverse antigen-specific clonotypes were also lost between days 3 to 5 after priming and were not present at the peak of the response. In this context, we propose that different adjuvant formulations influence antigen-specific clonal composition by altering initial TCR-based selection thresholds.
The oligoclonal co-transfer experiments (Fig. 5) also supports an affinity threshold mechanism that promotes the full propagation of higher affinity T helper cell responses. It is also possible that different adjuvants promote differing functional outcomes sequestering particular clonotypes into separate microenvironmental niches in vivo (Reinhardt et al., 2001). Clonal analysis of the CD44hiCD62Lhi PCC-responsive compartment may begin to examine these issues. Further, the differential export or migration patterns of Th cell subsets may also be alter local clonal composition and be under the control of differing adjuvants. Studies of developing Th responses under the lymphocyte sequestering action of drugs such as FTY-720 (Mandala et al., 2002) may help to resolve these issues.
The initial stable contact between pMHCII-expressing APC and naive T helper cells may be the defining event in this clonal selection mechanism. It is plausible that the strength of first contact indelibly impacts the initial T helper cell developmental program (Chang et al., 2007; Reiner et al., 2007). The program may then take days to express in vivo resulting in differential clonal expansion and effector function (Celli et al., 2007; Gett et al., 2003; Lanzavecchia and Sallusto, 2002; Lee et al., 2003). In contrast, multiple TCR-pMHCII contacts are required for maximal T helper cell propagation (Obst et al., 2005) and can occur between multiple dendritic cells (DC) (Celli et al., 2005) or between effector T helper cells and pMHCII-expressing B cells (Okada et al., 2005). The rapid selection dynamics in the current model (McHeyzer-Williams et al., 1999) favors the very earliest checkpoints in T helper cell development but does not exclude secondary influences on cell fate or development due to secondary TCR-pMHCII interactions (Celli et al., 2005). Our recent studies correlating strength of TCR-pMHCII binding and the acquisition of follicular B helper T cell (TFH) function (Fazilleau et al., 2007a) also implicated early clonal selection mechanisms in the imprinting of subsequent T helper cell function. Thus, altering TCR-based selection threshold provides a fundamentally new mechanism to control T helper cell fate and regulate multiple facets of adaptive immunity in vivo.
How vaccine adjuvants alter TCR-based selection thresholds remains an open and important question. It is clear that adjuvants can directly or indirectly influence DC maturation (Banchereau and Steinman, 1998; Medzhitov and Janeway, 1997). Targeting various DC subsets either at the site of vaccination or within draining lymphoid tissues can be influenced by the adjuvant formulation (Garcon et al., 2007; Guy, 2007; Kaufmann, 2007). The local antigen concentration is most readily influenced by depot or non-depot formulations, however the ‘downstream’ consequences for DC maturation or T helper cell priming in vivo remain poorly characterized. The capacity of a DC to uptake, process and present protein antigen can be altered by the inflammatory context of the priming event (Mellman and Steinman, 2001; Trombetta and Mellman, 2005). In this manner, vaccine adjuvants can influence the level of pMHCII expressed by mature DC. Further, the DC maturation program driven by different innate signals can impact a multitude of cell surface and secreted molecules that vary the cellular and molecular context of pMHCII expression (Pulendran and Ahmed, 2006). These changes in antigen presentation can either positively or negatively impact TCR-pMHCII interactions to regulate the clonal selection threshold or its impact on antigen-specific T helper cell fate.
While Alum is the most widely used adjuvant for human vaccines, its action in vivo remains poorly understood (Lindblad, 2004). Alum precipitated protein provides an antigen depot as well as a local irritant effect to the innate immune system. IFA is also not metabolized in vivo and sets up local inflammation with no known TLR agonist activity (Billiau and Matthys, 2001; Garcon et al., 2007). However, the mixture of TLR agonists in heat-killed mycobacterium tuberculosis is complex and the cellular targets of adjuvant action must be varied but remain poorly characterized (Billiau and Matthys, 2001; Garcon et al., 2007). While all three depot-forming formulations promoted lower pMHCII binding clonotypes there were still differences in TCR repertoire that may explain a differential impact on adaptive immunity (Pulendran and Ahmed, 2006). In contrast, the aqueous CpG adjuvant is a simple dispersible TLR-9 agonist with soluble antigen that independently reaches target cell populations (Klinman, 2006). Intracellular TLR-9 is expressed by DC subsets and CpG is known to drive DC maturation even in the absence of antigen (Klinman, 2006). The MPL-based adjuvant can drive DC maturation by TLR-4 agonist activity of the MPL itself and has been added to Alum-based Hepatitis B vaccine formulations for use in humans (Baldridge et al., 2004; Garcon et al., 2007). This adjuvant formulation also contains squalene (Glenn and O’Hagan, 2007) and Trehalose dimycolate (TDM) that may promote clonal expansion independent of TLR expression (Yamagami et al., 2001). Hence, it remains important to discriminate the action of different adjuvants on the maturation of pMHCII-expressing DC populations that in turn initiate clonal selection in the antigen-specific T helper cell compartment.
Understanding the mechanisms of immune protection following vaccination is central to the rational design of all future vaccines. Protein sub-unit vaccines are considered a safer alternative to attenuated microorganisms and primarily depend on identifying the appropriate antigen on the target pathogen. Effective protein vaccination then relies heavily on the vaccine adjuvant formulation to promote the development of long-term antigen-specific immune protection (Guy, 2007; Kaufmann, 2007; Pashine et al., 2005; Pulendran and Ahmed, 2006). Importantly, the strength of pMHCII complex recognition by the TCR initiates the progression of antigen-specific T helper cell development (Gett et al., 2003; Iezzi et al., 1998; Lee et al., 2003; Miller et al., 2004) with consequences for T helper cell expansion and functional differentiation (Celli et al., 2007; Chang et al., 2007; Lanzavecchia and Sallusto, 2002; Reiner et al., 2007). Our current studies reveal that adjuvants can regulate these initial TCR-based selection events and alter the clonal composition of antigen-specific T helper cell compartment. We propose that this fundamental attribute of adjuvants can be used to design effective new vaccine formulations that also enhance long-term antigen-specific adaptive immunity.
EXPERIMENTAL PROCEDURES
Mice
B10.BR, B10.BR-Thy1.1 congenic, 5C.C7αβ, 5C.C7β and 2B4β transgenic mice were maintained under pathogen-free conditions at The Scripps Research Institute. The Scripps Research Institute and the Institutional Animal Care and Use Committee reviewed and approved all experiments.
Protein vaccination
Mice were immunized subcutaneously at the base of the tail with 400μg, 40μg or 4μg of whole PCC (Sigma) either precipitated in Alum (Jordan et al., 2004) or in combination with CFA (Sigma), IFA (Sigma), 100μg CpG ODN 1826 (Coley) or monophosphoryl lipid A-based adjuvant (Ribi from Corixa and lab formulation based on procedures in (Baldridge and Crane, 1999)). For the Alum and IFA preparations, PCC was detoxified using the Detoxi-gel endotoxin removing gel (Pierce).
Adoptive transfer
For co-transfer experiments, splenocytes were transferred intravenously into B10.BR-Thy1.1 congenic mice. The recipients were then immunized with 400μg whole PCC in IFA or MPL-based adjuvant. For transfer experiments, 2×105 5C.C7αβ splenocytes labeled or not with 5μM CFSE (carboxyfluorescein diacetate succinimidyl ester, Molecular Probes) for 10min at 37°C were transferred intravenously into B10.BR mice or B10.BR-Thy1.1 congenic mice at the time of immunization or 3 and 5 days after immunization with PCC in IFA or MPL-based adjuvant. Mice were sacrificed at various times after transfer for analysis of cells by flow cytometry.
Flow Cytometry
As described (McHeyzer-Williams et al., 1999), single-cell suspensions from lymphoid tissues in PBS with 5% FCS were labeled for 45 min at 4°C at a density of 2.0×108 cells/ml with predetermined optimal concentrations of the following fluorophore-labeled monoclonal antibodies; fluorescein isothiocyanate-conjugated anti-Vα11 (RR8.1), phycoerythrin (PE)-conjugated anti-Vβ3 (KJ25), Allophycocyanin (APC)-conjugated anti-Vβ3 (KJ25) and anti-CD44 (Pgp1; all produced in the lab); PE-conjugated anti-CD90.2 (30-H12), Cy5-PE-conjugated anti-B220 (6B2) and anti-CD8α (53–6.7; all from BD biosciences); Cy5-PE-conjugated anti-CD8α (53–6.7) and anti-CD11b (M1/70), Cy7-PE conjugated anti-CD62L (MEL14), Cy7-APC conjugated anti-CD4 (GK1.5; all from Biolegend); PE- and Cy7-PE-conjugated anti-CD4 (GK1.5), APC-Alexa Fluor® 750 conjugated anti-CD44 (IM7), Cy7-APC conjugated anti-CD62L (MEL-14; all from eBioscience). PE-MCC/I-Ek tetramers (pMHCII tetramer) prepared as previously described (Malherbe et al., 2004) were incubated for 2 hours at room temperature at a final concentration of 230nM. After staining with tetramer, cells were washed twice and then labeled separately on ice for 45mins with the labeled monoclonal antibodies to other cell surface antigens as described above. The cells we then suspended in 2μg/ml propidium iodide (PI) (for dead cells exclusion) for analysis. Data were collected on a FACS Vantage SE (BD Biosciences) and were analyzed with FlowJo software (TreeStar). Profiles are presented as 5% probability contours with outliers.
Single-Cell Repertoire Analysis
Single cells with the appropriate surface phenotype were sorted for repertoire analysis using a FACS Vantage SE and CloneCyt software (BD Biosciences). The synthesis of cDNA and amplification of TCR Vα11 and Vβ3 regions were carried out as previously described (McHeyzer-Williams et al., 1999). Direct sequencing of the purified PCR products was carried out with a Vα11-specific or Vβ3-specific primer using the BigDye Terminator cycle sequencing kit, and sequences were analyzed on an ABI 373A DNA Sequencer (Applied Biosystems).
Jβ-specific single-cell PCR
2μl of cDNA from single-cell cDNA reactions were first used for amplification reactions of the TCRVβ3, using primers specific for the Vβ3 region (Vβ3.L2) and constant region (Cβ.2) as previously described (McHeyzer-Williams et al., 1999). 1μl of the first PCR product was used for Jβ-specific amplifications using nested primer specific for Vβ3 region [5′-TATCTGGTG AAAGGGCAAGG-3′] and primer specific for Jβ1.2 gene segment [5′-CCTGAGCCGAAGGTGTA GTC-3′] or primer specific for Jβ2.5 gene segment [5′-GCCCAAAGTACTGGGTGTCT-3′]. Second round PCR reactions begins with 95°C for 5 min; then 35 cycles of 95°C for 15s, 55°C for 45s, and 72°C for 90s; and ends with 72°C for 5min. 5μl of second round PCR product was run on 1.5% agarose gel to screen for Jβ1.2 and Jβ2.5 expressing cells.
Statistical analysis
Data are shown as means ± standard error means (SEM) and were compared with the one- or two-tailed Student’s t-test using GraphPad Prism (GraphPad Software).
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI071182, AI040215 and AI059475 (MMW), and fellowships from the European Molecular Biology Organization (L. Malherbe) and Wenner-Grenn Fellowship (L. Mark). This is TSRI manuscript #19329
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–107. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, Persing D. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4:1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bikah G, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Regulating T helper cell immunity through antigen responsiveness and calcium entry. Nat Immunol. 2000;1:402–412. doi: 10.1038/80841. [DOI] [PubMed] [Google Scholar]
- Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–860. [PubMed] [Google Scholar]
- Celli S, Garcia Z, Bousso P. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J Exp Med. 2005;202:1271–1278. doi: 10.1084/jem.20051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Davis MM, Krogsgaard M, Huse M, Huppa J, Lillemeier BF, Li QJ. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- Falta MT, Fontenot AP, Rosloniec EF, Crawford F, Roark CL, Bill J, Marrack P, Kappler J, Kotzin BL. Class II major histocompatibility complex-peptide tetramer staining in relation to functional avidity and T cell receptor diversity in the mouse CD4(+) T cell response to a rheumatoid arthritis-associated antigen. Arthritis Rheum. 2005;52:1885–1896. doi: 10.1002/art.21098. [DOI] [PubMed] [Google Scholar]
- Fasso M, Anandasabapathy N, Crawford F, Kappler J, Fathman CG, Ridgway WM. T cell receptor (TCR)-mediated repertoire selection and loss of TCR vbeta diversity during the initiation of a CD4(+) T cell response in vivo. J Exp Med. 2000;192:1719–1730. doi: 10.1084/jem.192.12.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007a;8:753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Local development of effector and memory T helper cells. Curr Opin Immunol. 2007b;19:259–267. doi: 10.1016/j.coi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Garcia Z, Pradelli E, Celli S, Beuneu H, Simon A, Bousso P. Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion. Proc Natl Acad Sci U S A. 2007;104:4553–4558. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–739. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- Glenn GM, O’Hagan DT. Adjuvants: progress, regress and pandemic preparedness. Expert Rev Vaccines. 2007;6:651–652. doi: 10.1586/14760584.6.5.651. [DOI] [PubMed] [Google Scholar]
- Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- Jorgensen JL, Reay PA, Ehrich EW, Davis MM. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. The contribution of immunology to the rational design of novel antibacterial vaccines. Nat Rev Microbiol. 2007;5:491–504. doi: 10.1038/nrmicro1688. [DOI] [PubMed] [Google Scholar]
- Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:135–154. doi: 10.1080/08830180600743057. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Lazarski CA, Chaves FA, Jenks SA, Wu S, Richards KA, Weaver JM, Sant AJ. The kinetic stability of MHC class II: peptide complexes is a key parameter that dictates immunodominance. Immunity. 2005;23:29–40. doi: 10.1016/j.immuni.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- Lindblad EB. Aluminium adjuvants--in retrospect and prospect. Vaccine. 2004;22:3658–3668. doi: 10.1016/j.vaccine.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Malherbe L, Filippi C, Julia V, Foucras G, Moro M, Appel H, Wucherpfennig K, Guery JC, Glaichenhaus N. Selective activation and expansion of high-affinity CD4+ T cells in resistant mice upon infection with Leishmania major. Immunity. 2000;13:771–782. doi: 10.1016/s1074-7613(00)00075-3. [DOI] [PubMed] [Google Scholar]
- Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Developmentally distinct Th cells control plasma cell production in vivo. Immunity. 2004;20:231–242. doi: 10.1016/s1074-7613(04)00028-7. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, Panus JF, Mikszta JA, McHeyzer-Williams MG. Evolution of antigen-specific T cell receptors in vivo: preimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J Exp Med. 1999;189:1823–1838. doi: 10.1084/jem.189.11.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6:699–710. doi: 10.1586/14760584.6.5.699. [DOI] [PubMed] [Google Scholar]
- Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panus JF, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific T helper cell function: differential cytokine expression in primary and memory responses. J Exp Med. 2000;192:1301–1316. doi: 10.1084/jem.192.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci U S A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Reiser JB, Gregoire C, Darnault C, Mosser T, Guimezanes A, Schmitt-Verhulst AM, Fontecilla-Camps JC, Mazza G, Malissen B, Housset D. A T cell receptor CDR3beta loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity. 2002;16:345–354. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, Schwickert T, Nussenzweig MC, Dustin ML. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Weber KS, Donermeyer DL, Allen PM, Kranz DM. Class II-restricted T cell receptor engineered in vitro for higher affinity retains peptide specificity and function. Proc Natl Acad Sci U S A. 2005;102:19033–19038. doi: 10.1073/pnas.0507554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami H, Matsumoto T, Fujiwara N, Arakawa T, Kaneda K, Yano I, Kobayashi K. Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces foreign-body- and hypersensitivity-type granulomas in mice. Infect Immun. 2001;69:810–815. doi: 10.1128/IAI.69.2.810-815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.