Abstract
There is an increase in the numbers of neural precursors in the SVZ (subventricular zone) after moderate ischaemic injuries, but the extent of stem cell expansion and the resultant cell regeneration is modest. Therefore our studies have focused on understanding the signals that regulate these processes towards achieving a more robust amplification of the stem/progenitor cell pool. The goal of the present study was to evaluate the role of the EGFR [EGF (epidermal growth factor) receptor] in the regenerative response of the neonatal SVZ to hypoxic/ischaemic injury. We show that injury recruits quiescent cells in the SVZ to proliferate, that they divide more rapidly and that there is increased EGFR expression on both putative stem cells and progenitors. With the amplification of the precursors in the SVZ after injury there is enhanced sensitivity to EGF, but not to FGF (fibroblast growth factor)-2. EGF-dependent SVZ precursor expansion, as measured using the neurosphere assay, is lost when the EGFR is pharmacologically inhibited, and forced expression of a constitutively active EGFR is sufficient to recapitulate the exaggerated proliferation of the neural stem/progenitors that is induced by hypoxic/ischaemic brain injury. Cumulatively, our results reveal that increased EGFR signalling precedes that increase in the abundance of the putative neural stem cells and our studies implicate the EGFR as a key regulator of the expansion of SVZ precursors in response to brain injury. Thus modulating EGFR signalling represents a potential target for therapies to enhance brain repair from endogenous neural precursors following hypoxic/ischaemic and other brain injuries.
Keywords: brain injury, epidermal growth factor receptor (EGFR), neural stem cell, neurosphere, subventricular zone
Abbreviations: a.u., arbitrary units; BrdU, bromodeoxyuridine; CCA, common carotid artery; CNS, central nervous system; DAPI, 4′,6-diamidino-2-phenylindole; DIV, days in vitro; EGF, epidermal growth factor; EGFP, enhanced green fluorescent protein; EGFR, EGF receptor; FGF, fibroblast growth factor; FGFR, FGF receptor; GFP, green fluorescent protein; H/I, hypoxia/ischaemia; HRP, horseradish peroxidase; IRES, internal ribosome entry site; NG2, neuro-glial protein 2; NSP, neural stem progenitor; P6 etc. postnatal day 6 etc, ; PSA-NCAM, poly-sialated neural cell adhesion molecule; SSEA-1, stage-specific embryonic antigen-1; SVZ, subventricular zone; TCA, trichloroacetic acid; TGF-α, tranforming growth factor-α
INTRODUCTION
A number of studies have demonstrated that the brain possesses a limited regenerative capacity following injury (Magavi et al., 2000; Arvidsson et al., 2002; Parent et al., 2002; Goings et al., 2004; Ohab et al., 2006). These investigations have predominantly evaluated neuronal production in the adult brain following injury (Jin et al., 2001; Zhang et al., 2001; Plane et al., 2004). Although encouraging, the extent of cell replacement is limited. However, by understanding the adaptive mechanisms that occur in the SVZ (subventricular zone) subsequent to injury, therapeutic strategies can be designed to achieve a more significant level of regeneration after CNS (central nervous system) injury.
Brain injury, as a consequence of cardio-respiratory insufficiency, is the most common cause of morbidity in premature and term infants. In the US alone, it has been estimated that one in every eight babies is born premature, and that over 80 000 infants per year are born very pre-term. Up to 40% of the infants born pre-term and weighing less than 1500 g will develop neurological and psychological problems ranging from classic cerebral palsy to mild learning deficits that may not appear until later in life (Volpe, 2001). There is an even larger cohort of infants that acquire developmental brain injuries as a consequence of insults such as intrauterine infection or asphyxia at birth. Presently, there are limited treatments available to prevent brain damage from injuries sustained in the perinatal period and there are no approved therapeutic regimens to stimulate CNS regeneration.
EGFRs [EGF (epidermal growth factor) receptors] are expressed in the infant, juvenile, young adult and adult human SVZs (Weickert et al., 2000), as well as on actively dividing cells in the postnatal rodent SVZ (Seroogy et al., 1995; Weickert and Blum, 1995). EGFR and its ligands maintain embryonic and adult neural stem cells (Doetsch et al., 1999; Junier, 2000; Yarden, 2001; Doetsch et al., 2002) and promote the diversification of neural precursors during embryonic cortical development (Burrows et al., 1997; Kornblum et al., 1997; Burrows et al., 2000; Caric et al., 2001). Postnatally, EGFR confers a motile phenotype to non-migratory cortical neuronal progenitors (Aguirre et al., 2005). Surprisingly, there are very few studies on the role of EGFR in the regenerative responses of the neural stem/progenitor cells following brain damage (Justicia and Planas, 1999; Teramoto et al., 2003).
Using a rodent model of neonatal brain injury, in the present study we demonstrate that there is a robust increase in EGFR signalling in both neural stem cells and progenitors. We also show that the regenerative response can be recapitulated in vitro by transfecting NSPs (neural stem progenitors) with a constitutively active EGFR, and that pharmacologically inhibiting EGFR in vitro abrogates the expansion of the NSPs observed subsequent to injury. The results of the present study suggest that enhanced EGFR expression is critical for NSP expansion.
MATERIALS AND METHODS
Neonatal H/I (hypoxia/ischaemia) model
All animal work was approved by the institutional animal care and use committee guidelines of the New Jersey Medical School. Timed pregnant Wistar rats (Charles River Laboratories) were housed on a 12 h light/dark cycle at 25°C with Purina rodent chow #5001. Following a normal delivery, the litter size was culled to 12 pups per litter. Neonatal H/I was induced on postnatal day 6 (P6) rat pups (P0 as day of birth) by a permanent right CCA (common carotid artery) cauterization followed by systemic hypoxia. Briefly, P6 rat pups were anaesthetized with isofluorane (5% induction, 3% maintenance) following which a midline neck incision was made. The right CCA was separated from the vagus nerve and coagulated using a bipolar cauterizer (Malis bipolar cauterize and bipolar cutter; Codman) at 10 V. The skin incision was sutured using 4-0 silk and the pups were returned to the dam for 2 h. For mouse neonatal H/I, the litter size was culled to 12 pups per litter and permanent CCA cauterization was performed on P10 pups as explained above for rats. Prior to hypoxia, the pups were pre-warmed in jars partially submerged in a 37°C water bath for 20 min after which rat pups were subjected to systemic hypoxia in jars at 8% O2/92% N2 for 75 min and mouse pups at 10% O2/92% N2 for 65 min. Sham-operated control animals had their right CCA separated from the vagus nerve, but it was not coagulated. They were subjected to hypoxia. Following hypoxia, the animals were left in the jars for 15 min at normoxia, after which they were returned to their cages.
Primary neurosphere assay
After 3 days of recovery from H/I, P9 rat pups were decapitated and their brains removed. Under aseptic conditions, a cut was made 2 mm from the anterior pole of the brain. A second cut was made approx. 3 mm posterior to the first cut. The hippocampus, corpus callosum and the meninges were removed under the microscope. Using forceps, 12 o'clock and 3 o'clock incisions were made and the region enclosed between the cortex and the ventricle containing the SVZ was removed and placed in fresh PGM [1 mM MgCl2 and 0.6% dextrose in PBS (pH 7.3)]. The tissue was mechanically minced using forceps and then enzymatically dissociated using 0.05% trypsin/EDTA at 37°C for 7 min. The trypsin was inactivated by the addition of an equal volume of newborn calf serum and the tissue was resuspended in ProN media [DMEM (Dulbecco's modified Eagle's medium)/F12 (1:1) media containing 10 ng/ml d-biotin, 25 μg/ml insulin, 20 nM progesterone, 20 mM Hepes, 100 μM putrescine, 5 ng/ml selenium, 50 μg/ml apo-transferrin and 50 μg/ml gentamycin] and triturated in ProN. The triturated suspension was passed through a 40 μM Nitex screen and the cells were collected by centrifugation at 200 g for 2 min and washed with ProN. The cells were counted with 0.1% Trypan Blue dye under a haemocytometer and plated at 5×104 cells/ml in ProN medium supplemented with 2 ng/ml EGF or 1 ng/ml FGF (fibroblast growth factor)-2 or as stated. The cells were cultured at 37°C in 5% CO2 incubators and fed every 2 days by removing approx. half of the medium and replenishing with fresh medium.
Neurosphere quantification
A neurosphere was defined as a free-floating cluster of at least 25 μm diameter. Prior to counting the spheres, the plates were shaken to ensure uniform distribution of the spheres in the well. The number of neurospheres in five random fields under 4×magnification was determined for each well. The total number of neurosphere-producing cells, the neural stem/progenitor cells, in the population was extrapolated from the average number of spheres per field, the area of the field and the area of the well. For neurosphere volumetric measurements, digital photographs were captured from at least ten neurospheres from each condition (n = 6 SVZ per condition) and volumes were calculated using IP Lab 3.6 software.
Immunofluorescence
Cryostat brain sections were collected from animals killed at 48 h of recovery. Sections were stained using a rabbit anti-EGFR antibody (Santa Cruz Biotechnology, catalogue number 1005; used at a 1:100 dilution). Secondary antibodies were LRSC (lissamine rhodamine sulfonyl chloride)-conjugated goat anti-rabbit IgG (Jackson Immunoresearch). Sections were thawed in ice-cold PBS and blocked with PGB superblock (10% BSA and 10% normal goat serum in PBS) for 1 h at room temperature (20°C). The superblock was aspirated and primary antibodies were applied in a 1:5 dilution of PGB superblock and incubated overnight at 4°C. The slides were rinsed extensively with PBS and then incubated in secondary antibodies for 1 h at 37°C. The sections were washed, counterstained with DAPI (4′,6-diamidino-2-phenylindole), and coverslipped with Gel Mount (Biomeda). To control for non-specific staining, the primary antibody was replaced with a 1:200 dilution of normal rabbit serum. Images were obtained using a Zeiss LSM410 confocal laser-scanning microscope using a 40× objective. The exposure time for capturing the images was identical. Images were cropped and assembled into a montage using Photoshop 9.0.
Flow cytometry
The SVZ was dissected out of 3 day H/I recovery and sham-operated control P9 Wistar rat pups and dissociated as described above. Approx. 2×105 cells were used for each analysis. The cells were incubated with a rabbit polyclonal anti-EGFR antibody (Ab 15669, Abcam; used at a 1:50 dilution) together with either a mouse monoclonal anti-NG2 (neuro-glial protein 2; Millipore; used at a 1:100 dilution) or a mouse monoclonal IgM anti-PSA-NCAM (poly-sialated neural cell adhesion molecule; Millipore; used at a 1:500 dilution) for 1 h on ice. After several washes, the cells were incubated with appropriate secondary antibodies conjugated with fluorochromes for 1 h on ice protected from light. To analyse EGFR-expressing cells in mice, subsequent to neonatal H/I, approx. 2×105 cells were stained using a mouse IgM monoclonal anti-SSEA-1 (stage-specific embryonic antigen-1)/LeX antibody (Zymed Laboratories). Following several washes, the cells were incubated with biotin-conjugated anti-rabbit antibody and then streptavidin-conjugated Alexa Fluor® 488 for EGFR detection, and RedX-conjugated anti-mouse IgM for LeX detection for 1 h each on ice and protected from direct light. The cells were analysed using a BD FACS Calibur™. A population of unstained rat SVZ cells was used to calibrate the BD FACS Calibur™ machine prior to analysis. Negative controls used included replacing the primary antibody with normal rabbit serum (Jackson ImmunoResearch; used at a 1:3000 dilution) and normal mouse serum (Jackson ImmunoResearch; used at a 1:3000 dilution) followed by secondary and tertiary antibodies as described above. The data were analysed using FlowJo version 4.4.4 software.
Western blot analysis
SVZ tissue dissected from H/I and sham-operated P9 rat pups was homogenized with a tissue/lysis buffer ratio of 0.5 g of tissue/2 ml of lysis buffer in a glass homogenizer (Kontes glass homogenizer with Pestle ‘A'). The HES lysis buffer used contained 10 mM Hepes, 1 mM EDTA and 250 mM sucrose (pH 7.4) with 2 mM sodium orthovanadate and one tablet of protease inhibitor (Complete Mini PI cocktail tablets; Roche diagnostics) for 10 ml of buffer, added just prior to use. The homogenate was spun at 1000 g for 10 min in a table top Beckman centrifuge to pellet nuclei. The supernatant was then spun at 150 000 g in a Beckman Ultracentrifuge with the titanium SW55Ti swinging bucket rotor. This membrane-enriched fraction was resuspended in HES buffer and the protein concentration was estimated using the BCA (bicinchoninic acid) assay (Pierce Biotech). Total protein (30 μg) was mixed with 4× NuPage LDS sample buffer and 10× NuPage reducing agent and heated for 10 min at 90°C and loaded on to 4–12% NuPage Bis-Tris pre-cast gels (Invitrogen). Approx. 2 μl of MagicMark XP (Invitrogen) was loaded for standard molecular mass markers. Following electrophoresis, the proteins were transferred on to a nitrocellulose membrane and stained with Ponceau S to evaluate equal protein loading into the wells. The blots were blocked with 5% non-fat dried skimmed milk/1% BSA in PBS-Tween followed by incubation with primary antibody in 1% BSA in PBS-Tween overnight at 4°C with gentle rocking. The primary antibodies used were rabbit polyclonal anti-EGFR antibody (Ab 15669, Abcam; used at a 1:250 dilution) or mouse monoclonal anti-phosphotyrosine-100 (Cell Signalling Technologies; used at a 1:2000 dilution). Following three rinses with PBS-Tween the following day, the blots were incubated with the corresponding secondary antibodies such as DAR-HRP (horseradish peroxidase)-conjugated (Jackson Laboratories; used at a 1:10000 dilution) or DAM-HRP conjugated (Jackson laboratories; used at a 1:5000 dilution) antibodies for 2 h at room temperature. The blots were washed and the signal developed with Western Lightning chemiluminescence reagent (PerkinElmer) according to the manufacturer's protocol. The bands were visualized using UVP EpiChem3 and processed with Labworks 4.0 digital quantification software (UVP).
RNA isolation
SVZs were microdissected as outlined above and snap frozen in 0.5 ml of TRIzol® (Invitrogen) per SVZ in a slush of dry ice/ethanol and stored at −80°C. Just prior to extraction, the tissues were thawed and homogenized using a hand-held tissue homogenizer. Chloroform (100 μl) was added per sample and mixed to obtain a cloudy suspension that was centrifuged at 800 g for 5 min, following which the aqueous phase was transferred to a new tube. Approx. 250 μl of 70% ethanol was added and the mixture was applied to an RNeasy Mini-spin column (Qiagen) and the manufacturer's protocol was followed to remove contaminants. The purity and amount of total RNA obtained was determined from the absorbance values measured using a UV–visible spectrophotometer (Becton Dickinson). The total RNA concentration was adjusted to 200 ng/μl and stored at −80°C until further use.
Real-time PCR
RNA from six SVZs was used per condition per group. Total RNA (1 μg) was transcribed to cDNA using the Qiagen Omniscript RT kit with random nanomers (Sigma–Aldrich) and RNaseIN (Promega). Primer pairs for the gene of interest were obtained from Taqman gene expression systems (Applied Biosystems) and the 18S primers were obtained from Lux primer design (Invitrogen, catalogue number 115HM-01). The Platinum-UDP supermix kit (Invitrogen) was used for reactions, which were carried out in 96-well plates and run in an ABI Prism 7700 sequence detection system (Applied Biosystems). Fold-changes in gene expression with reference to the housekeeping gene 18S were analysed using the REST (relative expression software tool) program (Pfaffl et al., 2002)
Thymidine incorporation assay
At 72 h, post-H/I and sham-operated control rat pups were decapitated and the SVZs dissected out as described above. The SVZ cells were dissociated and cultures were set up at 3×105 cells/ml in 20 ng/ml EGF in ProN overnight. The following morning the cells were spiked with 8 μCi of [3H]thymidine. Starting every 4 h, the cells were collected on Whatman 25 mm filters and washed once for 5 min in PBS, followed by three washes for 10 min each with 5% TCA (trichloroacetic acid). The filter papers were allowed to dry and collected in scintillation vials and 5 ml of scintillation fluid was added. The scintillation vials were measured in a Beckman LS6500 Multipurpose Scintillation counter for the amounts of [3H]thymidine incorporated by analysing the d.p.m. obtained. The data were normalized to a percentage of the 4 h control value d.p.m. obtained. Three independent experiments were performed and the graph was plotted as the means±S.E.M. of all three experiments.
Pharmacological EGFR inhibition assay
The SVZ was dissected out from rat pups 72 h post-H/I or sham-operated control P9 rat pups as described above. The cells were digested using trypsin and triturated to generate a uniform cell suspension. Then, 5×104 cells/ml were plated in the following conditions in 6-well tissue culture plates: (i) 2 ng/ml EGF±1 ng/ml FGF-2 in ProN; (ii) 2 ng/ml EGF±1 ng/ml FGF-2±the pharmacological EGFR inhibitor PD153035 (Calbiochem) at 300 nM in ProN; and (iii) 2 ng/ml EGF±1 ng/ml FGF-2±PD153035 at 1 μM in ProN. The cells were cultured 7 DIV (days in vitro) with media changes every 2 days. The number of neurospheres formed was quantified. To ascertain that the decrease in neurospheres obtained upon culturing SVZ cells with PD153035 was not due to cell death, the cells cultured for 7 DIV in media containing the inhibitor PD153035 were transferred to fresh medium containing 2 ng/ml EGF±1 ng/ml FGF-2 in ProN and cultured for an additional 7 DIV. After 1 week, the number of neurospheres obtained was quantified.
EGFR gain-of-function
Plasmid pEF2-Fl-Fc-γR1-IRES-EGFP [where IRES is internal ribosome entry site and EGFP is enhanced GFP (green fluorescent protein)] was digested with NheI and SpeI followed by heat inactivation of the restriction enzymes. The digested ends were dephosphorylated with shrimp alkaline phosphatase to avoid vector re-ligation. Plasmid pEf2-FLAF-EGFR was digested with NheI and XbaI to obtain the EGFR fragment. This fragment was ligated to the vector to obtain pEF2-fl-Fc-EGFR-IRES-EGFP. The resulting plasmid was transfected into COS cells and the transfection resulted in increased levels of phosphorylated EGFR as a proof of concept (results not shown).
Primary neurospheres were generated from rat pup SVZs and dissociated with accutase to yield single cells. Approx. 2×106 cells/condition were used for pEGFR_IRES_EGFP and pEGFP plasmid using the rat nucleofector kit (Lonza, catalogue number VPG-1005). Mock transfection controls had cells that were pulsed using the Amaxa system, but with no added DNA. Fluorescence in the transfected cells was readily evident by 24 h. At this time, experiments were performed to assess cell division. Cells were diluted to 5×104 cells/well in neurosphere growth medium, cultured in a six-well tissue culture dish and allowed to form neurospheres that were quantified at 3 days and 7 days post-transfection. Other cells were incubated with 10 μM BrdU (bromodeoxyuridine) and samples were collected 6 and 12 h post-BrdU addition and processed for BrdU incorporation analysis using the BD Pharmingen BrdU Flow Kit.
Statistical analysis
Data were analysed using one-way ANOVA followed by Tukey's post-hoc test or Student's t test to detect significant differences between the means with P<0.05. Analyses were performed using SAS (Cary). For real-time PCR data, the REST program was used to detect significant differences between the means of treatment groups and untreated controls based on P values of 0.05.
RESULTS
Neurosphere number and size increase following H/I in the presence of EGF, but not FGF-2
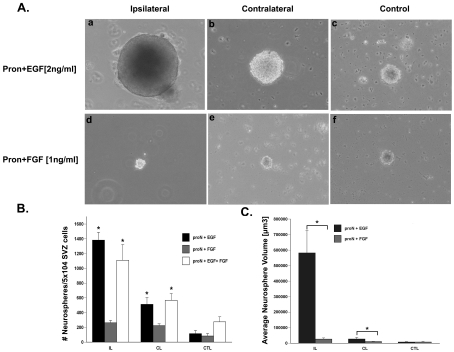
The neurosphere has remained a standard assay for measuring numbers of neural stem cells in the SVZ, therefore we isolated SVZ cells to determine whether there was an increase in the number of sphere-forming cells after neonatal H/I. Neurosphere cultures of rat SVZs were established from ipsilateral, contralateral and sham-operated control animals 72 h post-H/I. Spheres were propagated in a chemically defined serum-free medium (ProN) supplemented with either EGF (2 ng/ml) or FGF-2 (1 ng/ml). After 7 DIV, the number of neurospheres obtained from the ipsilateral SVZ following injury was 5-fold higher in medium supplemented with EGF as compared with FGF-2 [1381.7±105.2 compared with 261.5±32.4 (means±S.E.M.), n = 6 brains] (Figure 1B). There was a significant difference in the number of neurospheres generated from the ipsilateral SVZ compared with the contralateral SVZ in EGF-supplemented media (1381±105.2 compared with 511.5±90.3, n = 6 brains), but no significant difference in the number of spheres obtained from the ipsilateral SVZ compared with the contralateral SVZ when cultured in FGF-2-supplemented media (261.5±32.4 compared with 223.10±24, n = 6 brains). There was no difference in the number of neurospheres obtained from sham-operated control SVZs in medium supplemented with EGF as compared with FGF-2 (114.8±42.2 compared with 82.9±33.3, n = 6 brains). The addition of FGF-2 to the EGF-containing medium had no effect on the outcome of this assay (Figure 1A).
Figure 1. Neurospheres grow larger in the presence of EGF, but not FGF-2.
(A) SVZ cells were dissociated from ipsilateral (a and d), contralateral (b and e) and control (c and f) hemispheres at 3 days recovery from H/I and cultured for 7 DIV in ProN medium supplemented with either 2 ng/ml EGF (a–c) or 1 ng/ml FGF-2 (d–f). Phase-contrast images of representative neurospheres were captured for quantification. (B) Quantification of the average number of neurospheres obtained per 50 000 SVZ cells. (C) Quantification of the average neurosphere size from at least 30 spheres. *P<0.05 using ANOVA and Tukey's post-hoc tests. Values are the mean number/size of neurospheres±S.E.M. of three experiments with six animals per experiment. IL, ipsilateral; CL, contralateral; CTL, control.
Measuring the volume of the neurosphere can provide insights into the proliferative capacity of the NSPs; therefore we measured the diameter of at least ten neurospheres from each culture condition (n = 6 SVZs per condition). The size of the neurospheres obtained from the ipsilateral hemisphere of the injured brain cultured in 2 ng/ml EGF was 17-fold larger (P<0.05) compared with neurospheres from the ipsilateral SVZs cultured in 1 ng/ml FGF-2 (470143.9±79422.2 μm3 compared with 26676.2±7397.6 μm3, n = 6 brains). Contralateral SVZ cells cultured in EGF-supplemented medium were 2.5-fold larger (P<0.05) in size compared with their counterparts cultured in FGF-2-supplemented medium (27065.2±9611.3 μm3 compared with 11055.8±1583.7 μm3, n = 6 brains). There was no significant difference in sham-operated control SVZ neurosphere sizes between EGF- and FGF-2-supplemented media (7579.2±2275.7 μm3 compared with 7833.4±3273.4 μm3). Lastly, there was a 62-fold change in neurosphere size difference between ipsilateral SVZ- and control SVZ-generated spheres in EGF-supplemented media (ipsilateral neurosphere, 470143.7±79422 μm3; control neurosphere, 7579.2±2275.7 μm3) as compared with a 3.4-fold change in FGF-2-supplemented media (ipsilateral neurosphere, 26676.2±7397.6 μm3; control neurosphere, 7833.4±3273.4 μm3) (Figures 1A and 1C).
Pharmacologically inhibiting EGFR abrogates the increase in precursors following neonatal H/I
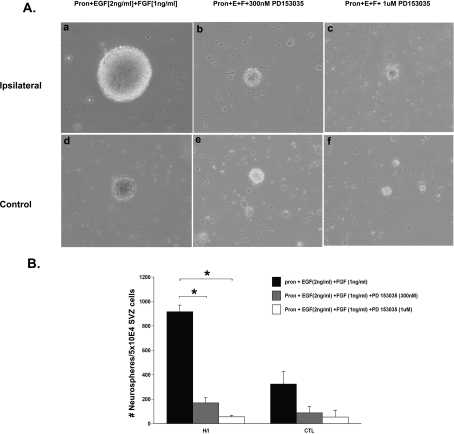
To test the hypothesis that an increase in EGFR signalling was responsible for the expansion of the neural progenitors during recovery from neonatal H/I, we administered a commercially available selective inhibitor of the EGFR, PD153035 (alternatively named AG1517 or Compound 32) (Bos et al., 1997; Lichtner et al., 2001). PD153035 is a competitive inhibitor of ATP binding and, hence, prevents functional activation of the receptor. This inhibitor also locks the receptors into inactive dimers and thus reduces EGFR signalling. At 72 h post-H/I, SVZs from ipsilateral and contralateral hemispheres, along with sham-operated control SVZs, were dissected out and cultured in ProN media supplemented with EGF and FGF-2 alone or with the addition of 300 nM or 1 μM PD153035. The neurosphere numbers obtained were quantified at 7 DIV. PD153035 at both 300 nM and 1 μM abrogated the increase in neurosphere number and size measured using the neurosphere assay. The number of neurospheres obtained 7 DIV from the ipsilateral SVZ at 3 day recovery from H/I cultured in growth-factor-supplemented media containing 300 nM and 1 μM PD153035 were 5.5-fold and 16-fold lower (P<0.05) respectively, compared with their counterparts cultured in the absence of the inhibitor (ipsilateral SVZ cells: growth media, 916.8±53.8; +300 nM PD153035, 169.6±43.2; +1 μM PD153035, 55.9±12.3; n = 3 with six animals per experiment) (Figure 2). Analogously, at 7 DIV, the number of neurospheres obtained from the sham-operated control SVZs at 3 days of recovery cultured in growth-factor-supplemented media containing 300 nM and 1 μM PD153035 were 3.6-fold and 6-fold lower respectively (P<0.05) compared with their counterparts cultured in growth-factor-supplemented media in the absence of the inhibitor (control SVZ cells: growth media, 324.4±104.06; +300 nM PD153035, 88.5±52.6; +1 μM PD153035, 54.1±32.4; n = 3 with six animals per experiment). The number of neurospheres obtained from ipsilateral SVZs in 300 nM PD153035 was not different from that of uninjured control SVZs cultured in growth media containing EGF and FGF-2 (ipsilateral SVZ cells in 300 nM inhibitor, 169.6±43.2; control SVZ cells in growth media, 324.4±104.1; n = 3 with six animals per experiment). Finally, the numbers of neurospheres obtained in the presence of either doses of the inhibitor (300 nM and 1 μM PD153035) were not significantly different between injured ipsilateral SVZ and sham-operated control SVZ at 3 day recovery from H/I (at 300 nM inhibitor: ipsilateral, 169.6±43.1 and control, 88.5±52.6; at 1 μM inhibitor: ipsilateral, 55.9±12.3 and control, 54.1±32.4). Notably, FGF-2 at 1 ng/ml [the Kd of FGF-2/FGFR (FGF receptor) 1 is 50 pM] was unable to recapitulate the EGF effect upon inhibition of the EGFR activity (Figure 2).
Figure 2. Pharmacologically inhibiting EGFR abrogates the in vitro NSP response observed following neonatal H/I.
(A) SVZ cells were dissociated from ipsilateral (a–c) and control (d–f) hemispheres at 3 days recovery from H/I and cultured for 7 DIV in ProN medium supplemented with 2 ng/ml EGF or 1 ng/ml FGF-2 along with the EGFR inhibitor PD153035 at 300 nM (b and e) or 1 μM (c and f) or with no inhibitor (a and d). (B) Quantification of the average number of neurospheres generated from ipsilateral and control SVZ after 72 h recovery from H/I upon culturing in growth-factor-supplemented medium with or without the EGFR-specific inhibitor PD153035. Values are the means±S.E.M. of three independent experiments with n = 6 animals per experiment. *P<0.05, as measured using a Student's t test.
H/I recruits more SVZ cells into the cell cycle and they divide more rapidly in response to EGF
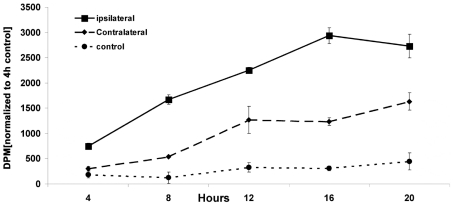
To determine whether the increase in neurosphere size obtained from the ipsilateral SVZ in EGF-supplemented medium was the result of altered cell-cycle kinetics, we performed a cumulative [3H]thymidine incorporation assay. Cells isolated from ipsilateral, contralateral and sham-operated control SVZs at 3 days of recovery from H/I were cultured overnight in EGF-supplemented medium spiked with 8 μCi of [3H]thymidine. The DNA was acid precipitated and the radioactivity was quantified at 4 h intervals. This assay revealed that a significantly greater number of cells in the ipsilateral hemisphere achieved a higher level of [3H]thymidine incorporation compared with cells from the contralateral and control hemispheres. By 16 h, the slope of the rate of [3H]thymidine from the ipsilateral SVZ began to plateau with maximum incorporation of radioactivity reaching approx. 2.5-fold and 10-fold higher levels compared with contralateral and control respectively (at 16 h: ipsilateral, 2935.2 d.p.m.; contralateral, 1233 d.p.m.; control, 307.7 d.p.m.] (Figure 3). Analysing the slopes of the curves, ipsilateral cells showed a 2.5-fold and 18-fold greater slope between 4 and 16 h compared with contralateral and sham-operated control SVZ cells respectively, indicating that the cells in the ipsilateral SVZ cells have reduced cell-cycle time following injury compared with sham-operated controls (slope of ipsilateral, 182.58; slope of contralateral, 77.41; slope of control, 10.09; between 4–16 h) (Figure 3).
Figure 3. NSPs proliferate more rapidly in the presence of EGF following neonatal H/I.
Animals were killed 3 days after H/I and neurospheres were cultured from ipsilateral (▪), contralateral (♦) and control (•) SVZs in 20 ng/ml EGF overnight. The following morning [3H]thymidine was added to the culture medium (8 μCi/ml). At 4 h intervals, cells were collected on Whatman filter discs by vacuum, the DNA was precipitated with TCA and [3H]thymidine incorporation was quantified. This Figure shows one representative experiment from three repetitions, normalized to the 4 h control. Values are means±S.E.M.
Neonatal H/I increases the expression of EGFR mRNA and protein levels, but not EGFR ligands
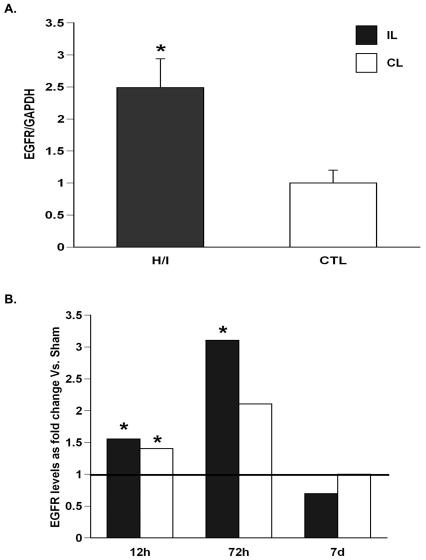
To determine whether the observed increase in neural stem/progenitor numbers and alteration in cell-cycle kinetics subsequent to injury could be attributed to increased EGF responsiveness, we stained brain sections for EGFR at 48 h after H/I and found that cells in the most medial aspect of the SVZ, immediately subjacent to the ependymal cells, had the pronounced increase in EGFR expression. Increased EGFR expression, however, was also seen within most of the cells of the dorsolateral SVZ. We also analysed EGFR mRNA expression between 12 and 72 h of recovery. At 12 h post-H/I there was a 1.5-fold increase (P<0.05) in EGFR mRNA in both the ipsilateral and contralateral SVZs compared with sham-operated controls (Figure 4). At 3 days recovery from H/I the ipsilateral EGFR mRNA was increased by 3-fold (P<0.05) relative to sham-operated controls, whereas the level of EGFR expression in the contralateral hemispheres was not statistically different from sham-operated controls. By 7 days post-H/I injury, the levels of EGFR mRNA in the ipsilateral hemisphere returned to levels comparable with those of sham-operated controls (Figure 4). Surprisingly, there was no change in the levels of the EGF or HB-EGF mRNAs, and the level of the major EGFR brain ligand, TGF-α (transforming growth factor-α), decreased to 50% in the ipsilateral hemisphere compared with controls (Supplementary Figure S1 at http://www.asnneuro.org/an/001/an001e009add.htm).
Figure 4. H/I increases EGFR mRNA levels.
Ipsilateral H/I and control SVZs were dissected and total RNA was extracted and amplified by (A) semi-quantitative PCR using primers specific for EGFR and normalized to the expression of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) after 72 h of recovery; (B) real-time PCR using primers specific for EGFR and normalized to expression of 18S after 2 h, 72 h and 7 day recovery (n = 6). The solid line represents EGFR expansion in sham-operated animals. *P<0.05 as measured using the Student's t test (A) and REST (B).
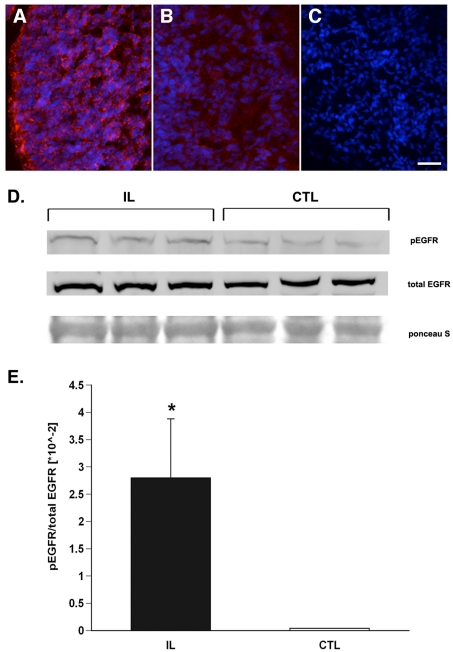
To establish whether the observed increase in EGFR mRNA translated into greater EGFR protein expression, we performed Western blot analysis on subfractionated SVZ homogenates isolated from ipsilateral hemispheres 3 days post-H/I and sham-operated control rat pups. The SVZ tissue was homogenized and fractionated into cytosolic, nuclear and membrane fractions. An analysis of the membrane fractions revealed a 2.8-fold increase (P<0.05) in phosphorylated EGFR in the ipsilateral hemisphere relative to sham-operated control SVZs at 72 h post-injury (Figure 5).
Figure 5. H/I increases the levels of activated EGFR protein.
(A–C) Cryostat sections (12 μm) from animals killed 48 h after H/I by intracardiac perfusion were stained for EGFR and counterstained with DAPI. Panels are representative of the ipsilateral hemisphere (A), contralateral hemisphere (B) and an immunostaining control (C). The scale bar represents 40 μm. To evaluate EGFR activation, H/I and control SVZs were dissected after 72 h recovery and the membranes were subfractionated (see the Materials and methods section) and 30 μg of each membrane fraction was analysed by Western blot for total EGFR and for phosphorylated EGFR (D). The Western blots were quantified and normalized to total receptor levels (E). Results are averaged from three independent experiments (n = 6). *P<0.05 (as measured using a Student's t test).
Injury increases EGFR expression in SVZ stem/progenitor cells
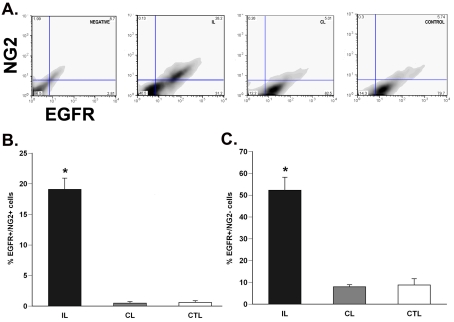
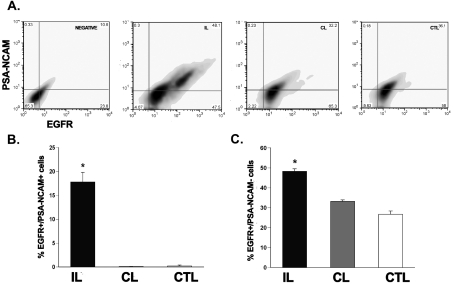
To investigate EGFR expression levels on stem and progenitor cells, we performed flow cytometry for EGFR expression on progenitors (PSA-NCAM and NG2 for rats) and stem cells (LeX for mice). We used anti-NG2 and anti-PSA-NCAM antibodies as they label several types of progenitors within the SVZ. At 3 days recovery from H/I, there was a 20-fold increase (P<0.05) in EGFR+/NG2+ cells and a 17-fold increase (P<0.05) in the EGFR+/PSA-NCAM+ cells in the ipsilateral SVZ as compared with controls (Figures 6A, 6B, 7A and 7B). There was also a 7-fold increase (P<0.05) in EGFR+/NG2− and a 1.5-fold increase (P<0.05) in EGFR+/PSA-NCAM− cells in the damaged SVZ as compared with controls (Figures 6C and 7C). No significant change was found in the double-positive EGFR/NG2 cells or EGFR/PSA-NCAM cells between the 72 h post-H/I contralateral hemisphere and sham-operated control SVZ cells (Figures 6B and 7B). Previously we found that progenitors, expressing PSA-NCAM, are deleted early after H/I injury (Romanko et al., 2004). The increase in the number of PSA-NCAM+/EGFR+ cells probably reflects the effort to replace these progenitors by augmenting their sensitivity to EGFR ligands.
Figure 6. H/I increases the number of EGFR-positive NG2-expressing neural precursors in the rat SVZ.
(A) Dot plot of EGFR+/NG2+ cells from the ipsilateral, contralateral and control SVZs after 72 h recovery from H/I. (B) Quantification of the EGFR+/NG2+ neural precursors in the SVZ. (C) Quantification of EGFR+/NG2− population, encompassing neural stem cells, in the SVZ after injury. Values are the mean number±S.E.M. and are from one representative experiment (n = 6 animals per experiment). *P<0.05, measured using one-way ANOVA and Tukey's post-hoc tests. IL, ipsilateral; CL, contralateral; CTL, control.
Figure 7. H/I increases the number of EGFR+ PSA-NCAM-expressing progenitor neuroblasts in the rat SVZ.
(A) Dot plot of EGFR+/PSA-NCAM+ cells from the ipsilateral, contralateral and control SVZs after 72 h recovery from H/I. (B) Quantification of the EGFR+/ PSA-NCAM+ neural precursor population in the SVZ. (C) Quantification of the EGFR+/PSA-NCAM− population, encompassing neural stem cells, in the SVZ after injury. Values represent the mean number±S.E.M. and are from one representative experiment with n = 6 animals per experiment. *P<0.05, measured using one-way ANOVA and Tukey's post-hoc tests. IL, ipsilateral; CL, contralateral; CTL, control.
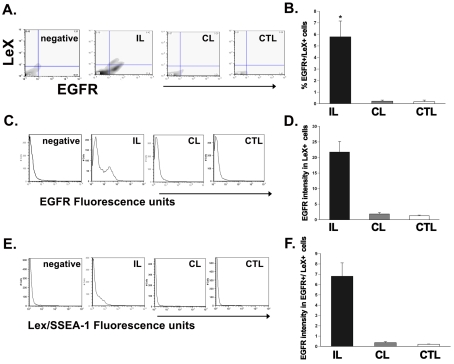
To evaluate whether EGFR is induced on neural stem cells, we used a mouse model of neonatal H/I wherein we could identify putative mouse stem cells using the LeX/SSEA-1 marker. LeX is a fucose-containing trisaccharide that has been reported to mark SVZ cells in the mouse brain that possess stem cell properties (Capela and Temple, 2002). Studies from our laboratory have shown that the murine SVZ is similarly affected by H/I (Brazel et al., 2004), and that there is an increase in murine neural stem/progenitor cells following injury as observed by an increase in the numbers of neurospheres generated from the damaged hemisphere compared with controls (Alagappan, 2008). Therefore cells acutely dissociated from P12 mouse SVZ were stained for EGFR and LeX and analysed by flow cytometry. This analysis revealed a 27-fold increase (P<0.05) in the number of EGFR+/LeX+ cells in ipsilateral SVZs compared with contralateral and sham-operated control SVZs (Figure 8) (5.59±1.4 compared with 0.2±0.04; 5.59±1.4 compared with 0.2±0.1; n = 6 brains). There was no statistical difference in the EGFR+/LeX+ population between the contralateral and sham-operated control SVZ cells (contralateral, 0.2±0.04; control, 0.2±0.1; n = 6 brains).
Figure 8. H/I increases the EGFR sensitivity on neural precursors in the rat SVZ.
(A) Histogram plot of EGFR intensity within the neural-precursor-positive cells from the ipsilateral, contralateral and control SVZs after 72 h recovery from H/I. (B) Quantification of the EGFR intensity (in a.u.) within the neural precursor population in the SVZ after injury. (C) Quantification of the EGFR intensity (in a.u.) within the neural-precursor-negative, putative stem-cell-encompassing population in the SVZ after injury. Values are the mean number±S.E.M. of three independent experiments with n = 6 animals per experiment. *P<0.05, measured using one-way ANOVA and Tukey's post-hoc tests. IL, ipsilateral; CL, contralateral; CTL, control.
Recovery from H/I increases EGFR intensity on SVZ stem/progenitors
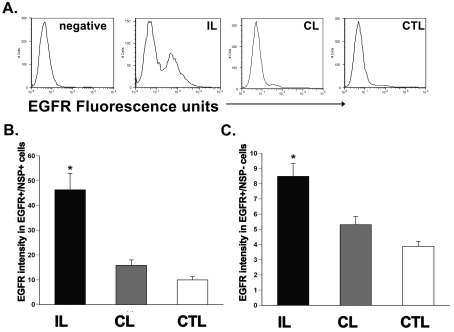
To determine whether H/I also increases the amount of EGFR expressed per cell, we measured the geometric mean fluorescence intensity of EGFR labelling in the progenitor marker-positive as well as the LeX-positive stem cell population in the ipsilateral and contralateral hemisphere SVZs at 72 h post-H/I as compared with sham-operated control SVZs in rats and mice. In rats, EGFR+/progenitor+ ipsilateral SVZ cells expressed 3-fold greater (P<0.05) EGFR intensity compared with contralateral and sham-operated control SVZ [ipsilateral, 46.3±6.6 a.u. (arbitrary units); contralateral, 15.9±2.2 a.u.; control, 9.9±1.4 a.u.; n = 6 brains] (Figures 9A and 9B). In mice, EGFR+/LeX+ ipsilateral SVZ cells expressed 11-fold greater (P<0.05) EGFR intensity compared with contralateral and sham-operated control SVZ (ipsilateral, 21.7±3.4 a.u.; contralateral, 1.9±0.5 a.u.; control, 1.3±0.2 a.u.; n = 6 brains) (Figures 9C and 9D). Not surprisingly, in rats, EGFR+/progenitor− cells in the ipsilateral SVZ showed a 1.5-fold increase (P<0.05) in the EGFR intensity compared with the contralateral hemisphere and sham-operated controls (ipsilateral, 8.5±0.9 a.u.; contralateral, 5.3±0.5 a.u.; control, 3.9±0.3 a.u.; n = 6 brains) (Figure 9C). In addition, LeX intensity was also affected by H/I. EGFR+/LeX+ ipsilateral SVZ cells expressed 17-fold greater (P<0.05) LeX intensity compared with their counterparts in the contralateral and sham-operated control SVZs (ipsilateral, 6.8±1.3 a.u.; contralateral, 0.4±0.1 a.u.; control, 0.2±0.02 a.u.; n = 6 brains) (Figures 8E and 8F). There was no significant difference in LeX intensity or EGFR intensity between contralateral hemisphere and sham-operated control hemispheres at 3 day recovery from H/I (LeX intensity: contralateral, 0.4±0.1 a.u.; control, 0.2±0.02 a.u.; EGFR intensity: contralateral, 1.9±0.5 a.u.; control, 1.3±0.2 a.u.; n = 6 brains) (Figures 8D and 8F).
Figure 9. H/I increases the expression of EGFR-positive LeX-expressing putative stem cells in murine SVZ.
(A) Dot plot of EGFR+/LeX+ cells from the ipsilateral, contralateral and control SVZs after 72 h recovery from H/I. (B) Quantification of the EGFR+/LeX+ neural stem population in the SVZ after 72 h recovery from H/I. (C) Histogram plot of EGFR intensity within the LeX-positive cells from the ipsilateral, contralateral and control SVZs after 72 h recovery from H/I. (D) Quantification of the EGFR intensity (in a.u.) within the LeX-positive stem cell population in the SVZ after injury. (E) Histogram plot of LeX intensity from the ipsilateral, contralateral and control SVZs after 72 h recovery from H/I. (F) Quantification of the LeX intensity (in a.u.) of SVZ cells after injury. Values represent the mean number±S.E.M. of three independent experiments with n = 6 animals per experiment. *P<0.05, measured using one-way ANOVA and Tukey's post-hoc tests. IL, ipsilateral; CL, contralateral; CTL, control.
EGFR overexpression is sufficient to recruit cells into the cell cycle and decrease their cell-cycle time
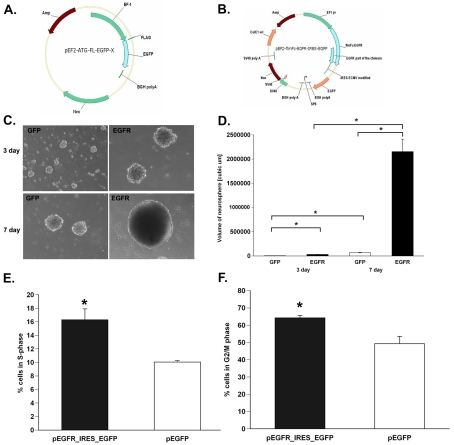
To establish whether overexpressing EGFR is sufficient to recruit cells into the cell cycle and to increase the rate at which they traverse through the cell cycle, we compared BrdU incorporation into SVZ stem/progenitor cells transfected with a plasmid containing a constitutively active EGFR or a control vector. The constitutively active EGFR plasmid increased the number of cells in S-phase by 1.65-fold compared with controls (pEGFR-IRES_EGFP, 16.6±1.6; pEGFP, 10.1±0.4; n = 3 independent experiments) following a 6 h pulse with BrdU (Figure 10E). EGFR overexpression also increased the number of cells in G2/M-phase by 1.3-fold compared with control cells (pEGFR-IRES_EGFP, 64.3±1.4; pEGFP, 49.3±4.3; n = 3 independent experiments) in a 12 h period (Figure 10F).
Figure 10. EGFR overexpression is sufficient to increase cell-cycle kinetics of SVZ NSPs.
(A and B) pEF2-ATG-FL-EGFP and pEF2-fl-Fc-EGFR-IRES-EGFP plasmids. (C) Neurospheres generated at 3 days and 7 days following transfection with pEGFP and pEGFR-IRES-EGFP into SVZ NSPs. (D) Quantification of neurosphere size obtained from (C). (E) Percentage of cells in S-phase after a 6 h BrdU pulse and in G2/M-phase (F). Values represent the mean number±S.E.M. of three independent experiments with n = 6 animals per experiment. *P<0.05, measured using a Student's t test.
A volumetric analysis of neurosphere size was performed by measuring the diameter of at least 30 neurospheres from each transfection condition per experiment. The size of the neurospheres obtained from the constitutively active EGFR-transfected cells were 5-fold and 32-fold larger (P<0.05) compared with EGFR-transfected cells after 3 days and 7 days in culture post-transfection respectively (3 day: 31609.67±2820.4138 compared with 5831.27±492.87 μm3; 7 day: 2154943±256683.7 compared with 66929.55±8118.92 μm3; n = 6 brains) (Figures 10C and 10D).
DISCUSSION
Many factors including TGF-α, FGF-2, insulin-like growth factor 1, platelet-derived growth factor, neurotrophins, ephrins, LIFR–CNTFR–gp130 complex, Notch 1, Sonic Hedgehog and Wnts regulate the formation of the SVZ from the ventricular zone in the developing brain (Carson et al., 1993; Vescovi et al., 1993; Weickert and Blum, 1995; Craig et al., 1996; Johe et al., 1996; Kuhn et al., 1997; Shimazaki et al., 2001; Doetsch et al., 2002; Giuliani et al., 2004; Hsieh et al., 2004; Israsena et al., 2004; Palma et al., 2005; Felling et al., 2006). These factors also regulate the proliferation and self-renewal of SVZ stem/progenitors and control the production of neurons and macroglia. Currently it has not been established which factor(s) among this pool of molecules is/are critically involved in the expansion and proliferation of the endogenous SVZ stem/progenitors for brain repair following injury. In the present study we show that following neonatal H/I: (i) quiescent SVZ cells enter the cell cycle; (ii) the increased proliferation of these precursors occurs in response to EGF, but not FGF-2; (iii) EGFR mRNA and protein expression increases in SVZ; (iv) EGFR expression increases on progenitors and stem cells within the SVZ; (v) EGFR acquisition increases the rate that SVZ cells traverse the cell cycle; and (vi) pharmacologically inhibiting EGFR, even in the presence of FGF-2, reduces the in vitro stem/progenitor expansion observed following injury, confirming that FGF-2 stimulation cannot compensate for the EGF-mediated regenerative response.
Both EGF and FGF-2 are mitogenic for adult neural stem/progenitors. SVZ cells express FGF-2, FGFRs, EGF and EGFRs, and signal transduction through these receptors is important for their proliferation and self-renewal during embryonic and postnatal development (Richards et al., 1992; Gritti et al., 1996; Johe et al., 1996; Kuhn et al., 1997; Doetsch et al., 1999; Gritti et al., 1999; Doetsch et al., 2002; Anton et al., 2004; Fox and Kornblum, 2005). Intraventricular injection of EGF expands the numbers of nestin+ precursors in the SVZ (Craig et al., 1996) and forced EGFR acquisition confers migratory capacity to non-migratory postnatal neural progenitors (Aguirre et al., 2005). In parallel, studies on FGF-2 signalling have shown that FGF-2 is mitogenic for multipotential adult murine forebrain precursors, maintains the number of slowly dividing stem cells within the anterior SVZ and FGF-2 knockouts have reduced numbers of dividing neural stem cells and fewer olfactory bulb neurons (Gritti et al., 1996; Zheng et al., 2004). Similar to the results of the present study, the expression of the EGFR increases within nestin+ cells located adjacent to ependymal cells during recovery from middle cerebral artery occlusion in adult rats, with peak expression occurring at 3 days post-ischaemia. Furthermore, infusing EGF for 7 days into the cerebral ventricles beginning 2 days after inducing ischaemia stimulated striatal neurogenesis 100-fold compared with vehicle-infused animals (Teramoto et al., 2003). Robust induction of FGF-2 and FGFRs has been documented following chronic hypoxia and this has been correlated with increased neurogenesis (Ganat et al., 2002; Fagel et al., 2006). However, we did not observe any increased cellular responses to FGF-2 using the neurosphere assay, nor did FGF-2 infusion stimulate enhanced neurogenesis after ischaemia in adult rats (Teramoto et al., 2003) Thus the precise role and requirement for FGF signalling in SVZ cellular responses to injury remain incomplete.
In the present study we evaluated whether functional activation of the EGFR is necessary and sufficient for the proliferative response initiated by stem/progenitor cells of the SVZ subsequent to brain injury. Studies from our laboratory, as well as others, have documented a regenerative response from the SVZ following neonatal H/I (Plane et al., 2004; Felling et al., 2006). During the first week of recovery from a moderate H/I insult, there is increased proliferation of cells in the most medial aspect of the SVZ, as revealed by increased BrdU uptake, as well as by increased numbers of nestin+/PCNA+ (proliferating-cell nuclear antigen) cells in the most medial aspect of the dorsolateral SVZ. This increase in cells in S-phase within the region of the SVZ that harbours the stem cells correlates with a doubling of the number of tripotential, self-renewing NSPs as measured using the neurosphere assay. Fate-mapping studies have shown that there is a subsequent emigration of neuronal and glial progenitors from the SVZ to repopulate subcortical and neocortical structures (Plane et al., 2004; Ong et al., 2005; Felling et al., 2006; Yang and Levison, 2006; Yang et al., 2007; Yang and Levison, 2007). However, the extent of stem cell expansion and the resulting regeneration following injury is modest. Therefore our most recent studies have focused on understanding the signals that regulate these processes towards achieving a more robust amplification of the stem/progenitor cell pool and more significant repair.
In a key experiment we abolished the in vitro expansion of SVZ NSPs by pharmacologically inhibiting functional EGFR activation in the presence of FGF-2. We used the quinazoline PD153035, a reversible tyrosine kinase inhibitor specific to EGFR. In vitro, PD153035 inhibits EGF-dependent cellular processes completely at doses ranging up to 1 μM whereas PDGF-, FGF-2-, and serum-dependent mitogenesis is inhibited only at concentrations higher than 5 mM (Fry et al., 1994). PD153035 is the parent compound of several 4-aniloquinazolines in the clinic such as Iressa (Astra-Zeneca), Tarceva (Roche/Genentech), GW 2016 (GlaxoSmithKline) and CI-1033 (Pfizer) (Boschelli, 2002). In the present study, we used 300 nM PD153035 to block EGFR activation during stimulation with FGF-2. At this dose, we found that the number of neurospheres obtained in the presence of the inhibitor at 72 h post-H/I from the damaged ipsilateral hemisphere was comparable with sham-operated control SVZs indicating that FGF-2 signalling cannot compensate for the loss of EGFR signalling, and EGFR activation is necessary for the observed reactive expansion of the NSPs subsequent to an ischaemic insult. The above compounds gain access to the CNS, whereas PD153035 is excluded. It would have been informative to use these inhibitors for in vivo experiments; however, the manufacturers of these drugs denied our requests for these reagents.
Complementary studies to the loss-of-function experiment were performed using a novel technology to introduce a constitutively active EGFR into the NSPs. Acquisition of this functionally active EGFR enhanced the number of cells traversing the cell cycle. This was manifested as an increase in neurosphere size obtained from EGFR-overexpressing SVZ NSPs compared with GFP-transfected cells. Thus augmenting EGFR signalling is sufficient to recapitulate in vitro the increased regenerative NSP response observed ex vivo after injury.
A subset of tripotential neural progenitors in the postnatal brain expressing the NG2 proteoglycan can migrate to the subcortical white matter and to the olfactory bulb via the rostral migratory stream. Several studies have shown that acquisition of EGFR by neural progenitors correlates with their ability to proliferate and migrate (Aguirre and Gallo, 2004; Aguirre et al., 2005; Ivkovic et al., 2008). SVZ cells migrate into damaged white and grey matter after H/I as we, and others, have shown. These studies have implicated chemokines such as MCP-1 (monocyte chemoattractant protein-1) and SDF-1α (stromal-cell-derived factor-1α) as stimulators of progenitor cell emigration post-stroke (Miller et al., 2005; Yang et al., 2007). In light of the above studies substantiating a role for the EGFR in the migration of glial progenitors in remyelination, our results suggest a previously unanticipated role for the EGFR in progenitor cell migration post-ischaemic injury.
To establish whether EGFR acquisition following injury is conserved across species, and to determine whether it is induced on neural stem cells, we evaluated the expression of EGFR on LeX+ SVZ cells. LeX/SSEA-1 is a surface carbohydrate moiety expressed by a small population of mouse SVZ cells that retain sustained proliferation and self-renewal capabilities characteristic of SVZ stem cells (Capela and Temple, 2002; Maric et al., 2003; Imura et al., 2006). Upon injury, the numbers of EGFR+/LeX+ cells increased significantly in the regenerating SVZ. We have also confirmed in the rat that the EGFR increases in the progenitor marker-negative population using the strategy developed by Maric et al. (2003) and have observed a similar increase of EGFR+ cells that are Aldefluor™+ in the neonatal SVZ after H/I (Alagappan, 2008). These results are consistent with our previous in situ and neurosphere analyses that indicate that the brain initiates an active expansion of SVZ stem cells, which is not simply an activation of pre-existing quiescent stem cells for repair.
We employed a cumulative thymidine incorporation assay to evaluate cell-cycle kinetics acutely following injury. This analysis affirmed an alteration in stem/progenitor behaviour upon EGF treatment. In this assay, the height at which the curve plateaus is a measure of the number of cells recruited into cell cycle and the slope of the curve provides an index of the cell-cycle time, with a steeper slope indicating a shorter cell-cycle time (Yu et al., 1996; Khanna, 2005; Khanna and Mehra, 2006). We specifically adopted cumulative thymidine labelling compared with pulse labelling because serum starvation, typically used to synchronize cells prior to cell-cycle analysis, is not technically feasible with NSPs. Averaging across experiments, approx. ten times as many cells from the injured brain entered the cell cycle and they divided significantly faster compared with uninjured controls in the presence of EGF. SVZ cells from the contralateral SVZ also divided more rapidly, analogous to previously reported neurosphere assay data (Felling et al., 2006). Taken together, these results suggest that acquisition of EGFR by the SVZ cells following injury not only expands the numbers of stem cells, but that it recruits quiescent cells to enter the cell cycle and stimulates these precursors to transit through the cell cycle faster compared with uninjured controls. These results are reminiscent of published studies in cancer biology that show that activation of EGFR destabilizes cell-cycle inhibitor proteins to enable quiescent cells to enter the cell cycle (Lenferink et al., 2000; Boerner et al., 2005).
In conclusion, the present study demonstrates that the EGFR is a key mediator of the regenerative response elicited from the stem cell and progenitor cell pools within the SVZ subsequent to brain injury. EGFR expression is elevated in SVZ cells, which recruits relatively quiescent cells to cycle more rapidly. The in vitro response of the SVZ stem/progenitors is manifested by an increase in neurosphere size and number, which can be abolished by a pharmacological inhibitor specific to EGFR in the presence of FGF-2 and is mimicked by overexpressing the EGFR. The increase in EGFR sensitivity acquired will promote their expansion and hence can participate in their migration into pallial and subpallial structures to aid repair. We also show that EGFR levels return to control levels within a few days, therefore strategies to augment EGFR signalling may represent important avenues to pursue to initiate a robust response from the resident SVZ NSPs following injury for functional brain recovery.
Online data
FUNDING
This work was supported by the National Institutes of Health [grant numbers MH 059950 (to S.W.L.) and NS 0469903 (to R.J.F.)].
REFERENCES
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Rizvi TA, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J Neurosci. 2005;25:11092–11106. doi: 10.1523/JNEUROSCI.2981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagappan D. Brain injury expands the numbers of neural stem cells and progenitors in the SVZ by enhancing their responsiveness to EGF. Ph.D. Thesis, UMDNJ New Jersey Medical School, Graduate School of Biomedical Sciences. 2008 doi: 10.1042/AN20090002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KCK, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Boerner JL, Biscardi JS, Silva CM, Parsons SJ. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinogen. 2005;44:262–273. doi: 10.1002/mc.20138. [DOI] [PubMed] [Google Scholar]
- Bos M, Mendelsohn J, Kim YM, Albanell J, Fry DW, Baselga J. PD153035, a tyrosine kinase inhibitor, prevents epidermal growth factor receptor activation and inhibits growth of cancer cells in a receptor number-dependent manner. Clin Cancer Res. 1997;3:2099–2106. [PubMed] [Google Scholar]
- Boschelli DH. 4-Anilino-3-quinolinecarbonitriles: an emerging class of kinase inhibitors. Curr Top Med Chem. 2002;2:1051–1063. doi: 10.2174/1568026023393354. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Rosti RT, Boyce S, Rothstein RP, Levison SW. Perinatal hypoxia/ischemia damages and depletes progenitors from the mouse subventricular zone. Dev Neurosci. 2004;26:266–274. doi: 10.1159/000082143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Burrows RC, Lillien L, Levitt P. Mechanisms of progenitor maturation are conserved in the striatum and cortex. Dev Neurosci. 2000;22:7–15. doi: 10.1159/000017422. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/SSEA-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Caric D, Raphael H, Viti J, Feathers A, Wancio D, Lillien L. EGFRs mediate chemotactic migration in the developing telencephalon. Development. 2001;128:4203–4216. doi: 10.1242/dev.128.21.4203. [DOI] [PubMed] [Google Scholar]
- Carson M, Behringer R, Brinster R, McMorris F. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Fagel DM, Ganat Y, Silbereis J, Ebbitt T, Stewart W, Zhang H, Ment LR, Vaccarino FM. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z, Givogri MI, Bongarzone ER, Levison SW. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci. 2006;26:4359–4369. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J Neurosci Res. 2005;79:584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM. Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience. 2002;112:977–991. doi: 10.1016/s0306-4522(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Giuliani A, D'Intino G, Paradisi M, Giardino L, Calzà L. p75NTR-Immunoreactivity in the subventricular zone of adult male rats: expression by cycling cells. J Mol Histol. 2004;35:749–758. doi: 10.1007/s10735-004-9609-2. [DOI] [PubMed] [Google Scholar]
- Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Gritti A, Parati EA, Cova L, Frolichsthal P, Galli R, Wanke E, Faravelli L, Morassutti DJ, Roisen F, Nickel DD, Vescovi AL. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J Neurosci. 1996;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Nakano I, Kornblum HI, Sofroniew MV. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–293. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- Israsena N, Hu M, Fu W, Kan L, Kessler JA. The presence of FGF2 signaling determines whether β-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–231. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Canoll P, Goldman JE. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J Neurosci. 2008;28:914–922. doi: 10.1523/JNEUROSCI.4327-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- Junier M-P. What role(s) for TGFα in the central nervous system? Prog Neurobiol. 2000;62:443–473. doi: 10.1016/s0301-0082(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Justicia C, Planas AM. Transforming growth factor-α acting at the epidermal growth factor receptor reduces infarct volume after permanent middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1999;19:128–132. doi: 10.1097/00004647-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Khanna AK. Reciprocal role of cyclins and cyclin kinase inhibitor p21WAF1/CIP1 on lymphocyte proliferation, allo-immune activation and inflammation. BMC Immunol. 2005;6:22. doi: 10.1186/1471-2172-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna AK, Mehra MR. Targeted in vitro and in vivo gene transfer into T lymphocytes: potential of direct inhibition of allo-immune activation. BMC Immunol. 2006;7:26. doi: 10.1186/1471-2172-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum H, Hussain R, Bronstein JM, Gall C, Lee D, Seroogy K. Prenatal ontogeny of the epidermal growth factor receptor and its ligand, transforming growth factor α, in the rat brain. J Comp Neurol. 1997;380:243–261. doi: 10.1002/(sici)1096-9861(19970407)380:2<243::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenferink AEG, Simpson JF, Shawver LK, Coffey RJ, Forbes JT, Arteaga CL. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu+MMTV/TGF-α bigenic mice. Proc Natl Acad Sci USA. 2000;97:9609–9614. doi: 10.1073/pnas.160564197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner RB, Menrad A, Sommer A, Klar U, Schneider MR. Signaling-inactive epidermal growth factor receptor/ligand complexes in intact carcinoma cells by quinazoline tyrosine kinase inhibitors. Cancer Res. 2001;61:5790–5795. [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci. 2003;23:240–251. doi: 10.1523/JNEUROSCI.23-01-00240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JT, Bartley JH, Wimborne HJ, Walker AL, Hess DC, Hill WD, Carroll JE. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J, Plane JM, Parent JM, Silverstein FS. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediatr Res. 2005;58:600–606. doi: 10.1203/01.PDR.0000179381.86809.02. [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Altaba ARi. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci USA. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanko MJ, Rothstein RP, Levison SW. Neural stem cells in the subventricular zone are resilient to hypoxia/ischemia whereas progenitors are vulnerable. J Cereb Blood Flow Metab. 2004;24:814–825. doi: 10.1097/01.WCB.0000123906.17746.00. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Gall CM, Lee DC, Kornblum HI. Proliferative zones of postnatal rat brain express epidermal growth factor receptor mRNA. Brain Res. 1995;670:157–164. doi: 10.1016/0006-8993(94)01300-7. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Volpe J. Perintal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Blum M. Striatal TGF-α: postnatal developmental expression and evidence for a role in the proliferation of subependymal cells. Dev Brain Res. 1995;86:203–216. doi: 10.1016/0165-3806(95)00026-a. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Colvin SM, Herman MM, Hyde TM, Weinberger DR, Kleinman JE. Localization of epidermal growth factor receptors and putative neuroblasts in human subependymal zone. J Comp Neurol. 2000;423:359–372. doi: 10.1002/1096-9861(20000731)423:3<359::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yang Z, Levison SW. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience. 2006;139:555–564. doi: 10.1016/j.neuroscience.2005.12.059. [DOI] [PubMed] [Google Scholar]
- Yang Z, Levison SW. Perinatal hypoxic/ischemic brain injury induces persistent production of striatal neurons from subventricular zone progenitors. Dev Neurosci. 2007;29:331–340. doi: 10.1159/000105474. [DOI] [PubMed] [Google Scholar]
- Yang Z, Covey MV, Bitel CL, Ni L, Jonakait GM, Levison SW. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;6:199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yu S-M, Tsai S-Y, Guh J-H, Ko F-N, Teng C-M, Ou JT. Mechanism of catecholamine-induced proliferation of vascular smooth muscle cells. Circulation. 1996;94:547–554. doi: 10.1161/01.cir.94.3.547. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.