Abstract
MyD88 KO (knockout) mice are exquisitely sensitive to CNS (central nervous system) infection with Staphylococcus aureus, a common aetiological agent of brain abscess, exhibiting global defects in innate immunity and exacerbated tissue damage. However, since brain abscesses are typified by the involvement of both activated CNS-resident and infiltrating immune cells, in our previous studies it has been impossible to determine the relative contribution of MyD88-dependent signalling in the CNS compared with the peripheral immune cell compartments. In the present study we addressed this by examining the course of S. aureus infection in MyD88 bone marrow chimaera mice. Interestingly, chimaeras where MyD88 was present in the CNS, but not bone marrow-derived cells, mounted pro-inflammatory mediator expression profiles and neutrophil recruitment equivalent to or exceeding that detected in WT (wild-type) mice. These results implicate CNS MyD88 as essential in eliciting the initial wave of inflammation during the acute response to parenchymal infection. Microarray analysis of infected MyD88 KO compared with WT mice revealed a preponderance of differentially regulated genes involved in apoptotic pathways, suggesting that the extensive tissue damage characteristic of brain abscesses from MyD88 KO mice could result from dysregulated apoptosis. Collectively, the findings of the present study highlight a novel mechanism for CNS-resident cells in initiating a protective innate immune response in the infected brain and, in the absence of MyD88 in this compartment, immunity is compromised.
Keywords: bone marrow chimaera mice, brain abscess, central nervous system, MyD88, Staphylococcus aureus, Toll-like receptor
Abbreviations: CFU, colony forming unit; CNS, central nervous system; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; Ier3/IEX, immediate early response 3; IL, interleukin; IL-1R etc., IL-1 receptor; KO, knockout; Lcn2, lipocalin-2; NF-κB, nuclear factor κB; Pacsin3, protein kinase C and casein kinase substrate in neurons 3; Pfc, complement factor properdin; qRT-PCR, quantitative real-time RT (reverse transcriptase)-PCR; ROS, reactive oxygen species; SOCS3, suppressor of cytokine signalling 3; TLR, Toll-like receptor; TNF-α, tumour necrosis factor-α; WT, wild-type
INTRODUCTION
In the CNS (central nervous system), brain abscesses arise from a parenchymal infection by pyogenic bacteria and represent a serious life-threatening condition. Brain abscesses can form by bacterial perforation of the thin bony structures separating the brain from neighbouring sites of chronic infection occurring in the paranasal sinuses, middle ear, or upper molars. Other routes include seeding of the brain with bacterial emboli originating from systemic sites of infection (i.e. endocarditis or septicemia) (Mathisen and Johnson, 1997) where the frontal and temporal lobes are most commonly affected (McClelland et al., 1978; Carpenter et al., 2007), penetrating trauma to the head, or following neurosurgery (Tenney, 1986; Schliamser et al., 1988). Although brain abscesses ensue in response to a diverse array of pathogens, streptococcal species and Staphylococcus aureus represent the most common aetiological agents of infection in humans.
Innate immunity plays an essential role in the host response to bacterial infections. Among the central players in anti-bacterial immunity are members of the TLR (Toll-like receptor) family of pattern recognition receptors (Akira et al., 2006; Trinchieri and Sher, 2007). These receptors recognize conserved motifs from a wide range of pathogens that are inherently resistant to mutation based on their essential nature for pathogen survival (Medzhitov and Janeway, 2002). When considering the array of TLRs that could be triggered during S. aureus infection in the brain, several candidates emerge, since the bacterium presents an arsenal of distinct immunostimulatory motifs. For example, bacterial lipoproteins and PGN (peptidoglycan) can trigger TLR1 and TLR2, whereas bacterial DNA can stimulate TLR9 in endosomal compartments (Akira et al., 2006; Trinchieri and Sher, 2007). Based on this complexity it is expected that numerous receptors are engaged following bacterial infection in the brain. This is supported by our previous studies demonstrating that brain abscess pathogenesis following S. aureus infection was not markedly affected by the loss of TLR2 (Kielian et al., 2005). Therefore a broader role for additional recognition molecules sensing bacterial infection was apparent.
MyD88 is a central adaptor molecule for the majority of TLRs, with the exception of TLR3 (Akira, 2006; O'Neill and Bowie, 2007). This molecule is also responsible for transducing activation signals emanating from the IL-1R [IL (interleukin)-1 receptor] and IL-18R (Wesche et al., 1997; Adachi et al., 1998; Burns et al., 1998; Medzhitov et al., 1998). Since IL-1 and IL-18 have been shown to have important roles in anti-bacterial immunity, coupled with the pivotal role of MyD88-dependent pathways in bacterial recognition and the induction of downstream cytokine signalling networks, MyD88 represents a central converging point in the innate inflammatory pathway. Indeed, recent studies from our laboratory have demonstrated the essential role of MyD88-dependent mechanism(s) in mounting a productive host innate immune response during the acute stage of brain abscess development (Kielian et al., 2007). Studies by other groups have also established the importance of MyD88-dependent pathways in the innate immune response to Gram-positive infections in both the CNS and periphery (Takeuchi et al., 2000; Koedel et al., 2004; Miller et al., 2006; Fremond et al., 2007).
Although our previous report demonstrated an essential and non-redundant role for MyD88 in eliciting an innate immune response during brain abscess development (Kielian et al., 2007), it remained unclear whether MyD88 expression was more important in CNS-resident compared with infiltrating immune cells since the molecule was globally absent in KO (knockout) mice. To address this question, we engineered radiation bone marrow chimaera mice where MyD88 was differentially expressed in the CNS compared with the peripheral immune cell compartments. Unexpectedly, the results demonstrated that MyD88 expression in the CNS was required to mount an innate immune response equivalent to WT (wild-type) during the acute stage of brain abscess development. The requirement for MyD88 in CNS-resident cells was reinforced by the finding that neutrophil influx into the infected brain was only achieved in chimaeric mice where MyD88 was present in the CNS. This is probably due to the fact that numerous neutrophil chemokines were restored to WT levels only in animals where MyD88 was expressed in the CNS compartment.
Curiously, our previous study demonstrated that, despite global defects in innate immunity, bacterial burdens remained relatively consistent between MyD88 KO and WT mice, suggesting that mechanisms other than bacterial burdens themselves were responsible for the enhanced susceptibility of MyD88 KO mice to CNS S. aureus infection (Kielian et al., 2007). In the present study, we performed transcriptional profiling with Illumina microarrays to identify pathways that were differentially regulated by MyD88 during brain abscess development that might provide some insights into why these animals succumb so quickly to infection, despite having relatively equivalent bacterial burdens as WT animals. These studies revealed a preponderance of differentially regulated genes involved in apoptotic pathways, suggesting that the extensive tissue damage characteristic of brain abscesses from MyD88 KO mice could result from dysregulated apoptosis. Collectively, these results reveal an essential role for MyD88 in CNS-resident cells that triggers a protective innate immune response within the first 24 h following intracerebral infection with S. aureus.
MATERIALS AND METHODS
Mice
All animal use protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health guidelines for the use of rodents. MyD88 gene KO mice (originally from Dr Shizuo Akira, Osaka University, Japan) (Adachi et al., 1998) have been previously backcrossed with C57BL/6 mice for over ten generations (Kawai et al., 1999; Fremond et al., 2004). Age- and sex-matched C57BL/6 mice (Harlan Laboratories) were used as WT controls. For the generation of bone marrow chimaeras, B6/SJL mice that are congenic for the CD45 allele (CD45.1) on a C57BL/6 background were purchased from Jackson Laboratories. Mice were used between 10 and 12 weeks of age for all brain abscess studies.
Generation of MyD88 radiation bone marrow chimaera mice
For generating MyD88 bone marrow chimaeras, CD45 congenic B6/SJL mice were used where only one allele, CD45.1, originates from the SJL strain, whereas the remainder of the genome is derived from C57BL/6 mice. These animals represent the WT strain since they express functional MyD88. MyD88 KO mice are on a C57BL/6 background and express the CD45.2 allele, allowing for the discrimination between donor- compared with recipient-derived leucocytes based on staining with antibodies specific for CD45.1 and CD45.2. The following radiation chimaeras were generated in these experiments (donor bone marrow→irradiated recipient): WT→WT, KO→KO, WT→KO and KO→WT. The experimental chimaeras were WT→KO and KO→WT, whereas the other two groups (WT→WT and KO→KO) represented controls to rule out any non-specific effects of irradiation on immune activation. The latter could be verified by confirming that the inflammatory profiles of irradiated control groups obtained in the present study (WT→WT and KO→KO) were similar to those observed in non-irradiated MyD88 WT and KO mice as reported recently (Kielian et al., 2007). CNS parenchymal cells are radiation-resistant and maintain the host phenotype following radiation exposure (Hickey and Kimura, 1988; Ajami et al., 2007). Reconstitution of irradiated MyD88 KO animals with bone marrow from WT recipients ensured that cells derived from the bone marrow expressed this molecule along with CNS perivascular cells (Hickey and Kimura, 1988; Bechmann et al., 2001). In contrast, parenchymal microglia would not express MyD88 as a result of their radioresistance. The procedure for bone marrow chimaera generation was based on previously published studies with minor modifications (Hickey and Kimura, 1988; Byram et al., 2004; Zehntner et al., 2004; Chakravarty and Herkenham, 2005). Briefly, bone marrow donor mice were euthanized with an overdose of inhaled isoflurane and marrow was isolated from the long bones by flushing with sterile 1×PBS. Recipient mice were placed on antibiotic-supplemented water (1 g/l neomycin and 125 mg/l polymyxin) for 4 days prior to bone marrow transfer and subjected to irradiation (1000 rad) using a J.L. Shepherd Model i45 caesium irradiator to destroy the marrow. Within 4 h following irradiation, recipient mice received an i.v. (intravenous) injection of 2×107 bone marrow cells supplemented with 1×107 cells from the spleen to serve as an immediate source of immune cells. Engraftment was allowed to take place over a 6–8 week period and chimaeric animals were maintained on antibiotic-supplemented water for the first 2 weeks to provide protection during transient immunocompromise. At 6–8 weeks post-transplant, chimaeric mice were bled retro-orbitally and cells were stained for flow cytometric analysis using CD45.1 and CD45.2. Only animals that displayed chimaerism of greater than 90% were used in brain abscess studies. Animals were evaluated for their responses to an intracerebral inoculation of S. aureus at approx. 10–12 weeks following bone marrow transfer, a period that we, and others (Hassan-Zahraee et al., 2000; Becher et al., 2001; Zehntner et al., 2004), have established to be sufficient for establishing chimaerism. We found that bone marrow chimaeras generated with B6/SJL and MyD88 KO mice did not exhibit any evidence of graft versus host disease, indicating that this was not a confounding issue.
Generation of experimental brain abscess
Live S. aureus (strain RN6390) was encapsulated in agarose beads prior to implantation in the brain as previously described (Kielian et al., 2001). Previous studies from our laboratory have established that the introduction of sterile agarose beads does not induce overt inflammation or peripheral immune cell infiltrates (Kielian et al., 2001; Baldwin and Kielian, 2004). To induce brain abscesses, mice were anaesthetized with 2.5% avertin i.p. (intraperitoneally) and a 1 cm longitudinal incision was made along the vertex of the skull extending from the ear to the eye. A rodent stereotaxic apparatus equipped with a Cunningham mouse adaptor (Stoelting) was used to implant S. aureus-encapsulated beads into the caudate/putamen region using the following co-ordinates relative to bregma: +1.0 mm rostral, +2.0 mm lateral, and −3.0 mm deep from the surface of the brain. A burr hole was made and a 5 μl Hamilton syringe fitted with a 26-gauge bevelled needle was used to slowly deliver 2 μl of beads [104 CFUs (colony forming units)] into the brain parenchyma. The needle remained in place for 2.5 min following injection to minimize bead efflux and potential leakage into the meninges. The incision was closed using surgical glue.
Preparation of brain abscess homogenates
To prepare brain abscess homogenates for downstream protein and RNA analysis, lesion sites were visualized by the stab wound created during injections and were sectioned within 1–2 mm on all sides. Upon recovery, brain abscesses were homogenized in 500 μl of PBS supplemented with a Complete™ protease inhibitor cocktail tablet (Roche) and 160 units/ml RNAse inhibitor (Promega) using a Polytron homogenizer (Brinkmann Instruments). At this point, a 20 μl aliquot of abscess homogenate was removed for quantitative culture of viable bacteria as described below. Subsequently, homogenates were centrifuged at 21 000 g for 15 min at 4°C to pellet membrane material, and supernatants were removed and stored at −70°C until ELISA and multi-plex cytokine microbead array analysis as described below.
Quantification of viable bacteria from brain abscesses
To quantify the numbers of viable bacteria associated with brain abscesses, serial 10-fold dilutions of abscess homogenates were plated on to blood agar plates. Titres were calculated by enumerating colony growth and were expressed as CFUs per ml of homogenate.
ELISA
Protein levels of CXCL2 were quantified in brain abscess homogenates using an ELISA kit according to the manufacturer's instructions (DuoSet, R&D Systems; level of sensitivity = 15.6 pg/ml). Results were normalized to the amount of total protein extracted from tissues to correct for differences in sampling size as previously described (Baldwin and Kielian, 2004; Kielian et al., 2004a).
Multi-analyte microbead array to detect pro-inflammatory mediator expression
To expand the analysis of inflammatory mediators differentially expressed between the various MyD88 bone marrow chimaera mice, a mouse 20-plex cytokine microbead array system was used according to the manufacturer's instructions (BioSource International). This microbead array allowed for the simultaneous detection of 20 individual inflammatory molecules in a single 50 μl brain abscess homogenate sample including IL-1α, IL-1β, TNF-α (tumour necrosis factor-α), IFN-γ (interferon-γ), IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12 p40/p70, IL-13, IL-17, IP-10 (chemokine CXCL10), MIG (monokine induced by IFN-γ), MCP-1 (monocyte chemotactic protein-1), KC (chemokine CXCL1), GM-CSF (granulocyte/macrophage colony-stimulating factor), VEGF (vascular endothelial growth factor) and bFGF (basic fibroblast growth factor). Results were analysed using a Bio-Plex Workstation (Bio-Rad) and adjusted based on the amount of total protein extracted from abscess tissues for normalization. The level of sensitivity for each microbead cytokine standard curve ranged from 1 to 35 pg/ml.
Immunofluorescence staining and confocal microscopy
Neutrophil and microglia/macrophage accumulation in brain abscesses from MyD88 bone marrow chimaera mice were evaluated by immunofluorescence staining using Gr-1 and Iba-1 respectively. For these studies, mice were infected with 104 CFUs of S. aureus RN6390 engineered to express GFP (green fluorescent protein) under the control of the RNAIII promoter (a gift of Dr Ambrose Cheung, Dartmouth Medical School, Hanover, NH, U.S.A.) to visualize bacterial dissemination in tissue sections. At the indicated time points post-infection (i.e. 18 or 24 h), MyD88 bone marrow chimaera mice were perfused transcardially to eliminate leucocytes from the vasculature, whereupon brains were removed and immediately flash-frozen on dry ice. These early time intervals were required since the survival period of MyD88 KO mice did not extend much beyond this time point (Kielian et al., 2007). Prior to cryostat sectioning, brain tissues were embedded in OCT (optimal cutting temperature) medium, whereupon serial 10 μm sections were made throughout the entire lesioned tissue, mounted on to SuperFrost Plus slides (Fisher Scientific), air-dried, and stored at −80°C until use. For Gr-1 staining, fresh frozen tissues were analysed; however, to detect Iba-1 immunoreactivity, tissue sections were post-fixed in 4% (w/v) paraformaldehyde for 1 h, since strong reactivity with this antibody was not detected in freshly frozen sections. Tissues were incubated with either rat anti-mouse Gr-1 (BD Biosciences) or rabbit anti-mouse Iba-1 (Biocare Medical) antibodies overnight at 4°C in a humidified chamber. Following numerous rinses in PBS, Gr-1 staining was detected with a mouse anti-rat IgG-HRP (horseradish peroxidase)-conjugated antibody (Invitrogen) for 1 h at room temperature (25°C) and visualized using a TSA-Alexa Fluor® 594 kit (Invitrogen). For Iba-1 detection, a rabbit ImmPRESS kit (Vector Laboratories) was used in conjunction with a TSA-Alexa Fluor® 594 kit. Upon completion of the staining protocols, slides were coverslipped using the Prolong anti-fade reagent (Invitrogen) and sealed using nail polish. Slides were imaged using a Zeiss laser-scanning confocal microscope (LSM 510; Carl Zeiss Microimaging). Specific staining of antibodies was confirmed by the absence of fluorescence signal following incubation of brain abscess tissues with secondary antibodies alone (results not shown).
RNA isolation and Illumina oligonucleotide microarray
Total RNA from brain abscesses of MyD88 KO and WT mice was isolated using TRIzol® reagent (Invitrogen) and subjected to DNAse treatment prior to use in microarray studies. RNA concentrations and integrity were determined with an Agilent 2100 bio-analyser using Agilent RNA6000 Nano kits.
Transcriptional profiling of changes in gene expression between S. aureus-infected MyD88 KO and WT mice was determined using Illumina Sentrix MouseRef-8 Expression BeadChips (Illumina). For these experiments, RNA samples from four individual animals/group/time point were analysed to account for biological variability. Total RNA was used to generate biotin-labelled cRNA using the Illumina TotalPrep RNA Amplification Kit (Ambion, catalogue number IL1791). Briefly, 0.5 μg of total RNA was first converted into single-stranded cDNA with reverse transcriptase using an oligo-dT primer containing the T7 RNA polymerase promoter site and then copied to produce double-stranded cDNA molecules. The double-stranded cDNA was cleaned and concentrated with the supplied columns and used in an overnight in vitro transcription reaction where single-stranded RNA (cRNA) was generated and labelled by incorporation of biotin-16-UTP. A total of 0.75 μg of biotin-labelled cRNA was hybridized at 58°C for 16 h to Illumina's Sentrix MouseRef-8 Expression BeadChips. Each BeadChip has 24 000 well-annotated RefSeq transcripts with approx. 30-fold redundancy. The arrays were washed, blocked, and the labelled cRNA was detected by staining with streptavidin-Cy3. The arrays were scanned using an Illumina BeadStation 500X Genetic Analysis Systems scanner and the image data was extracted using the Illumina BeadStudio software, version 3.0.
Microarray data analysis and statistical methods
The expression data were filtered to include only probes with a consistent signal on each chip; the probe original signal filter value was established at a detection P value<0.02. The resulting dataset was next analysed with DIANE 6.0, a spreadsheet-based microarray analysis program. An overview of DIANE can be found online at http://www.grc.nia.nih.gov/branches/rrb/dna/diane_software.pdf. Using DIANE, the results were normalized with a Z-score transformation (Cheadle et al., 2003). Z-normalized data were then analysed with PCA (principal component analysis). To determine the gene expression changes caused by each specific RNA comparison, Z-scores for paired treatment groups were compared using the Z-ratio statistic (Cheadle et al., 2003):
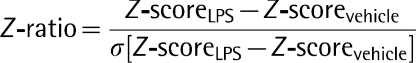 |
Expression changes for individual genes were considered significant if they met four criteria: Z-ratio above 1.5 or below −1.5; FDR (false detection rate) (Tusher et al., 2001) of less than 0.30; a P value statistic for Z-score replicability below 0.05; and mean background-corrected signal intensity greater than zero.
qRT-PCR [quantitative real-time RT (reverse transcriptase)-PCR]
To confirm a subset of differentially expressed genes from microarray studies, qRT-PCR was performed as previously described (Kielian et al., 2004b). ABI Assays on Demand kits were used to examine Lcn2 (lipocalin-2), Pacsin3 (protein kinase C and casein kinase substrate in neurons 3), Ier3/IEX (immediate early response 3), Pfc (complement factor properdin) and SOCS3 (suppressor of cytokine signalling 3) expression, whereas GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers and the TAMRA TaqMan probe were synthesized by ABI based on previously published sequences (Esen et al., 2004; Tanga et al., 2005).
Statistics
Significant differences between the various MyD88 bone marrow chimaera experimental groups were determined using one-way ANOVA followed by the Holm–Sidak method for multiple pair-wise comparisons with Sigma Stat (SPSS Science). For comparisons in gene expression profiles between MyD88 KO and WT mice by qRT-PCR the Student's t test was used.
RESULTS
MyD88 expression in the CNS compartment is essential for achieving maximal inflammatory responses to intracerebral S. aureus infection
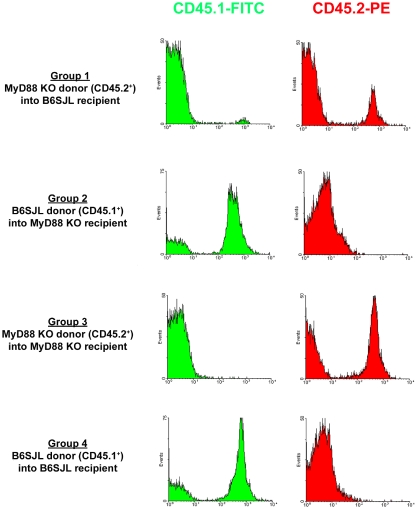
Recent studies from our laboratory have identified an essential role for MyD88-dependent pathways in initiating innate immune responses to S. aureus during the acute stage of brain abscess formation (Kielian et al., 2007). However, the relative importance of MyD88 within the CNS compartment compared with infiltrating peripheral immune cells was uncertain, since prior studies utilized MyD88 KO mice where this adaptor was globally absent. To address this question, we generated chimaeric animals using B6.SJL congenic mice that express the CD45.1 allele, allowing the discrimination between donor and recipient cells based on the fact that MyD88 KO mice are on a C57BL/6 background and express CD45.2. In pilot studies, we carefully titrated the dose of ionizing radiation administered to recipient mice to ablate the bone marrow without inducing toxicity to the gastrointestinal tract (results not shown). Mice were evaluated at 8 weeks following bone marrow transfer to assess the degree of chimaerism by examining the extent of CD45.1 and CD45.2 expression on peripheral blood leucocytes by FACS. A representative FACS screen of bone marrow chimaera mice is provided in Figure 1. For these experiments, a total of four bone marrow treatment groups were compared. The two experimental groups consisted of mice where MyD88 was present in the CNS but not in bone marrow-derived cells (i.e. KO→WT; Group 1) and vice versa (i.e. WT→KO; Group 2), whereas bone marrow transfers into genetically identical mice (i.e. KO→KO and WT→WT; Groups 3 and 4 respectively) were also performed to rule out any non-specific effects of ionizing radiation on the responses obtained. The latter could be verified by confirming that the inflammatory profiles of irradiated control groups obtained in the present study (WT→WT and KO→KO) were similar to those observed in non-irradiated MyD88 WT and KO mice as reported recently (Kielian et al., 2007). Although chimaeric mice were screened at 8 weeks following bone marrow transfer, animals were not infected with S. aureus until 10–12 weeks after transplantation. Any mice that demonstrated incomplete chimaerism (i.e.>10% residual recipient phenotype) were excluded from the study.
Figure 1. Validation of MyD88 bone marrow chimaera mice by FACS analysis.
Peripheral blood leucocytes were recovered from MyD88 bone marrow chimaeric mice at 8 weeks following irradiation and bone marrow reconstitution, whereupon CD45.1 (B6/SJL origin) and CD45.2 (MyD88 KO origin) expression was evaluated by FACS. Results are presented from one bone marrow chimaera study and are representative of five independent experiments.
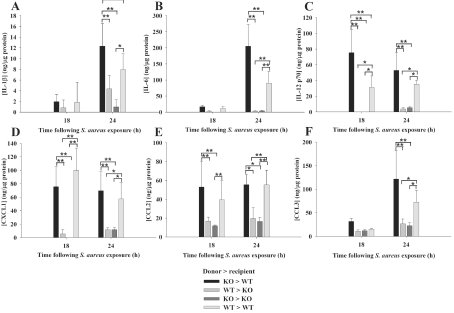
As previously reported, MyD88 KO mice do not survive very long following intracerebral S. aureus infection, with the majority of animals succumbing within 24 h after bacterial exposure with the infectious inoculum used in the earlier and present studies (i.e. 104 CFUs) (Kielian et al., 2007). This fact dictated the time course for analysing the current experiments, since we were restricted by the survival time of mice where MyD88 KO bone marrow was transferred into irradiated MyD88 KO recipients (KO→KO). Therefore we examined the entire cohort of bone marrow chimaera animals at two acute intervals, namely 18 and 24 h following S. aureus infection. As shown in Figure 2, numerous pro-inflammatory cytokines and chemokines were already detected in WT control mice (WT→WT) at these early time points and were significantly attenuated in MyD88 KO control mice (KO→KO) in agreement with our previous report using non-irradiated animals (Kielian et al., 2007), suggesting that the irradiation paradigm itself did not significantly alter the inflammatory phenotype of MyD88 KO and WT mice. Next we assessed which of the experimental chimaeras achieved cytokine expression profiles that were equivalent to WT levels, which would implicate a compartmental-specific contribution for MyD88-dependent signals. As shown in Figure 2, chimaeras where MyD88 was present in the CNS but not in bone marrow-derived cells (KO→WT) were able to mount pro-inflammatory mediator expression profiles equivalent to or exceeding those detected in WT mice at both 18 and 24 h post-infection. This finding implicates CNS MyD88 as essential in eliciting the initial wave of inflammation during the acute response to parenchymal infection. In contrast, the inflammatory phenotype of chimaeric mice where MyD88 was absent in the CNS compartment but present in infiltrating bone marrow-derived cells (WT→KO) was basically identical with MyD88 KO control mice (KO→KO; Figure 2). This finding reinforces the importance of MyD88 in the brain to elicit an effective innate immune response to S. aureus and that MyD88 originating from peripheral immune cells alone is not sufficient to recover inflammatory mediator expression to WT levels.
Figure 2. MyD88 in the CNS compartment is important for regulating pro-inflammatory mediator expression in brain abscesses.
Abscess homogenates from MyD88 bone marrow chimaeras (n = 4–6 mice per group) were prepared at 18 or 24 h following S. aureus infection, whereupon inflammatory mediator expression was analysed at the protein level using multi-plex microbead arrays. Mediator levels were normalized to the amount of total protein to account for differences in tissue sampling size. Significant differences between chimaera groups were determined by one-way ANOVA followed by the Holm–Sidak method for multiple pair-wise comparisons and are denoted with asterisks (*P<0.05; **P<0.001). Results are representative of three independent experiments.
CNS MyD88 dictates the degree of neutrophil influx into S. aureus-induced brain abscesses
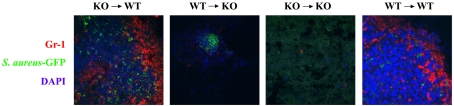
Our previous study with MyD88 KO mice demonstrated that neutrophil influx into brain abscesses was significantly attenuated in these animals (Kielian et al., 2007). In the present study, we examined whether MyD88 in the CNS or peripheral immune cell compartment was important for dictating neutrophil entry into the infected brain. For these studies we utilized immunofluorescence staining and confocal microscopy with an S. aureus strain that constitutively expresses GFP. Importantly, this S. aureus-GFP strain is identical with the isolate used in our previous studies with the exception of the GFP construct. As shown in Figure 3, neutrophil influx was readily apparent in abscesses of WT control mice (WT→WT), whereas very few cells could be visualized in lesions of MyD88 KO control animals (KO→KO), in agreement with our previous report (Kielian et al., 2007). In concordance with the finding that chemokine expression was restored to WT levels in bone marrow chimaera where MyD88 was present in the CNS compartment (KO→WT), significant numbers of neutrophils were associated with brain abscesses of these chimaeras, reaching levels that were equivalent to WT mice (WT→WT; Figure 3). In contrast, minimal neutrophil influx was detected in chimaeras where MyD88 was only present in bone marrow-derived cells and absent in the CNS (WT→KO; Figure 3). We did not perform quantitative measurements of PMN (polymorphonuclear cell) infiltrates by FACS in the various chimaera groups owing to limiting numbers of mice. Collectively, these findings indicate that MyD88 expression in resident CNS cells is critical for dictating subsequent neutrophil efflux into the infected brain. Importantly, these findings mirror chemokine expression profiles (Figure 2), providing additional supportive evidence for the important role of central MyD88 in initiating the inflammatory cascade required for CNS amplification of inflammatory networks to recruit peripheral anti-microbial effector cells.
Figure 3. MyD88 expression in the CNS dictates the degree of neutrophil influx into the infected CNS.
Bone marrow chimaera mice (n = 4–6 per group) received an intracerebral infection with a S. aureus-GFP strain (green) and were euthanized 24 h later, whereupon brain tissues were flash-frozen on dry ice for subsequent cryostat sectioning. Serial 10 μm thick sections were prepared throughout the entire abscess, subjected to immunofluorescence staining for the neutrophil marker Gr-1 (red), and imaged by confocal microscopy (magnification, 40×). Nuclei were visualized by DAPI (4′,6-diamidino-2-phenylindole) staining (blue). Significant numbers of neutrophils can be visualized infiltrating brain abscesses of WT→WT and KO→WT chimaeras. Results are representative of two independent experiments.
Neutrophil and microglia/macrophages occupy distinct anatomical niches during S. aureus infection in the CNS parenchyma
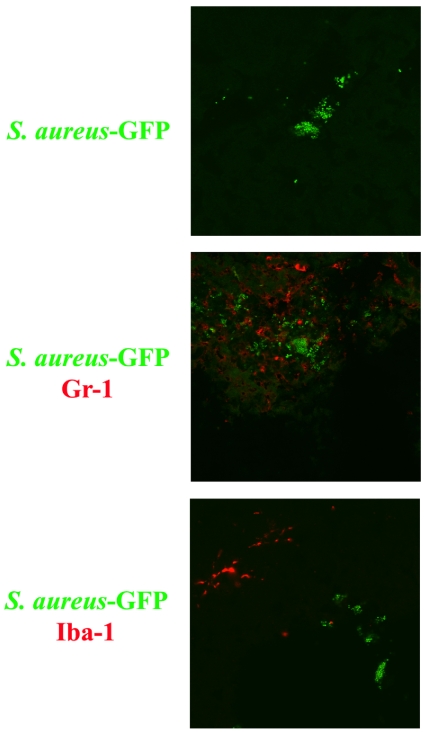
The use of a S. aureus-GFP isolate enabled us to evaluate the cell type(s) intimately associated with bacteria in the CNS parenchyma. A consistent trend surfaced where neutrophils were in direct contact with bacteria (Figure 4), whereas microglia/macrophages were never found associated with S. aureus, but rather were physically removed from the bacteria and localized along the abscess margins. The physical locales of these cell types are probably attributed to their effector functions during brain abscess development. For example, neutrophils are essential for controlling bacterial burdens and the extent of brain abscess dissemination (Kielian et al., 2001), whereas microglia/macrophages are typically localized along the abscess margins (Flaris and Hickey, 1992; Kielian et al., 2008).
Figure 4. Neutrophils and microglia/macrophages occupy distinct anatomical niches during acute S. aureus infection in the brain parenchyma.
WT mice received an intracerebral infection with a S. aureus-GFP strain (green) and were euthanized 24 h later, whereupon brain tissues were flash-frozen on dry ice for subsequent cryostat sectioning. Serial 10 μm thick sections were prepared throughout the entire abscess, subjected to immunofluorescence staining for the neutrophil or microglia/macrophage markers Gr-1 and Iba-1 respectively (red), and imaged by confocal microscopy (magnification, 40×). Neutrophils are found to directly interact with bacteria, whereas microglia/macrophage staining did not overlap with S. aureus. Results are representative of two independent experiments.
Lack of MyD88 expression in the CNS compartment leads to elevated S. aureus burdens
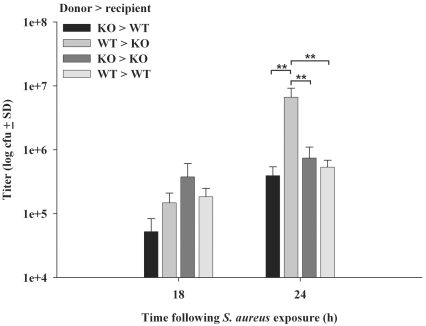
We next examined whether MyD88 expression in CNS parenchymal compared with infiltrating immune cells influences S. aureus survival in the brain. Interestingly, although no significant differences in bacterial burdens were observed at 18 h post-infection, a significant increase in S. aureus titres was detected in chimaeric animals where MyD88 was absent in the CNS but present in bone marrow-derived cells (WT→KO) at 24 h following bacterial exposure (Figure 5). This finding is in agreement with the fact that pro-inflammatory mediator production was depressed in MyD88 WT→KO chimaeras compared with KO→WT and WT→WT animals; however, it is intriguing that elevated bacterial burdens were not also observed in MyD88 KO controls, although the latter is in agreement with our previous findings (Kielian et al., 2007). It is not clear what mechanism(s) are responsible for enhanced bacterial burdens in MyD88 WT→KO chimaeras, but one possibility could relate to the recent observation that some bacterial species possess homologues of the TIR (Toll/IL-1 receptor) domain to subvert host defences (Cirl et al., 2008), and it is conceivable that this mechanism could result in the failure to contain bacterial burdens in the presence of MyD88 WT leucocytes. An alternative explanation is that MyD88-positive leucocytes infiltrating the brain parenchyma do not receive a requisite signal(s) from the brain microenvironment in the absence of MyD88, culminating in ineffective bacterial neutralization. These possibilities remain highly speculative at the present time. Collectively, these results indicate that CNS-derived MyD88 signals influence the efficacy of ensuing bactericidal effector mechanisms.
Figure 5. Lack of MyD88 expression in the CNS compartment leads to elevated S. aureus burdens.
Abscess homogenates from MyD88 bone marrow chimaeras (n = 4–6 mice per group) were prepared at 18 or 24 h following S. aureus infection, whereupon the number of viable bacteria were determined by quantitative plate assays. Significant differences between chimaera groups were determined by one-way ANOVA followed by the Holm–Sidak method for multiple pair-wise comparisons and are denoted with asterisks (**P<0.001). Results are representative of three independent experiments.
Transcriptional profiling of MyD88 KO mice following intracerebral S. aureus infection reveals alterations in apoptosis regulatory pathways
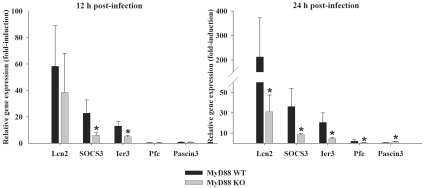
Previous studies with MyD88 KO mice in the experimental brain abscess model revealed that, although these animals were incapable of mounting a significant innate immune response and experienced high mortality rates, paradoxically, these mice did not exhibit significantly elevated bacterial burdens as compared with WT animals (Kielian et al., 2007). This finding demonstrated that bacterial burdens themselves are not the sole determinant in dictating the extent of tissue injury and/or mortality during brain abscess development. To begin to dissect the potential mechanism(s) responsible for the rapid decline of MyD88 KO mice following intracerebral S. aureus infection, transcriptional profiling was performed. Interestingly, only a small subset of genes was found to be differentially expressed by microarray analysis and many of these have been implicated in controlling apoptotic pathways (i.e. Erdr1, Ier3, Pdap1, Calm2, Hspca, Mtch1, and Ifitm1 and 2; Table 1). Dysregulation of apoptotic pathways could conceivably be responsible, in part, for the excessive tissue destruction observed in MyD88 KO mice (Kielian et al., 2007), where a combination of rampant necrotic and apoptotic death leads to the destruction of infected and neighbouring non-infected brain parenchyma respectively. In addition, several genes detected by microarray analysis (i.e. CXCL1, IL-1β) have previously been shown to be down-regulated in brain abscesses of MyD88 KO mice at the protein level (Kielian et al., 2007), confirming the validity of the microarray findings to accurately distinguish changes in gene expression between the groups. However, to further confirm the differential expression patterns detected by microarray analysis, we selected a subset of genes for validation by qRT-PCR. As shown in Figure 6, the expression patterns of the five genes examined (Lcn2, SOCS3, Ier3, Pfc and Pascin3) were in agreement with the microarray findings, indicating that these genes may impact the differential outcome of S. aureus infection and widespread parenchymal destruction in the brains of MyD88 KO mice. It should be noted that the relatively low number of differentially expressed genes detected between MyD88 KO and WT mice is probably the result of our experimental design. Namely, we elected to perform microarrays on brain abscess RNA collected from individual animals at each time point rather than pooling RNA from a group of animals. We felt this was important to account for biological variability between individual mice and, as such, it is highly likely that the breadth of differentially expressed genes was underestimated by this approach. Collectively, these transcriptional profiling studies suggest dysregulation of pro-inflammatory and apoptotic genes in acute brain abscesses of MyD88 KO mice. The functional role for each of these molecules in contributing to the excessive parenchymal destruction observed following S. aureus infection in MyD88 KO mice remains to be determined.
Table 1. Differentially expressed genes between MyD88 KO and WT mice harbouring brain abscesses.
| (a) Ratios of MyD88 KO compared with MyD88 WT at 12 h post-infection | |||
| Gene name | Common name | GenBank® accession number | Z-ratio |
| Lcn2 | Lipocalin 2 | NM_008491.1 | −11.13 |
| Pacsin3 | Protein kinase C and casein kinase substrate in neurons 3 | NM_028733.1 | −3.25 |
| Erdr1 | Erythroid differentiation regulator 1 | NM_133362.1 | −2.62 |
| SOCS3 | Suppressor of cytokine signaling 3 | NM_007707.2 | −2.40 |
| CXCL1 | C-X-C motif ligand 1 | NM_008176.1 | −2.25 |
| Ier3 | Immediate early response 3 | NM_133662.1 | −2.02 |
| Pdap1 | PDGFA-associated protein 1 | XM_132501.2 | −1.93 |
| Calm2 | Calmodulin 2 | NM_007589 | −1.65 |
| Glul | Glutamine synthase | NM_008131.2 | −1.65 |
| Hspca | Heat-shock protein 1α | NM_010480.3 | −1.65 |
| Mtch1 | Mitochondrial carrier homologue 1 | NM_019880.2 | 1.95 |
| Bach | Brain acyl-CoA hydrolase | NM_133348.1 | 1.93 |
| Hba-a1 | Haemoglobin α, adult chain 1 | NM_008218.1 | 1.63 |
| Ndufs2 | NADH dehydrogenase Fe-S protein 2 | NM_153064.3 | 1.55 |
| (b) Ratios of MyD88 KO compared with MyD88 WT at 24 h post-infection | |||
| Gene name | Common name | GenBank® accession number | Z-ratio |
| Ifitm2 | Interferon-induced transmembrane protein 2 | NM_030694 | −3.26 |
| Lcn2 | Lipocalin 2 | NM_008491.1 | −2.39 |
| Pfc | Properdin factor, complement | XM_135820.3 | −2.05 |
| Serpina3n | Serine (cysteine) proteinase inhibitor, clade A, member 3N | NM_009252.1 | −1.87 |
| Socs3 | Suppressor of cytokine signaling 3 | NM_007707.2 | −1.68 |
| IL-1β | Interleukin-1β | NM_008361 | −1.67 |
| Ifitm1 | Interferon-induced transmembrane protein 1 | NM_026820.2 | −1.54 |
| Egr4 | Early growth response 4 | NM_020596.1 | 1.81 |
| Pacsin3 | Protein kinase C and casein kinase substrate in neurons 3 | NM_028733.1 | 1.73 |
Figure 6. qRT-PCR confirms a subset of differentially expressed genes detected by microarray analysis.
MyD88 KO and WT mice (n = 4–5 per group) were infected with S. aureus intracerebrally and euthanized at 12 or 24 h, whereupon total RNA was isolated and evaluated for a subset of genes determined to be differentially expressed by microarray analysis using qRT-PCR. Genes analysed included Lcn2, SOCS3, Ier3, Pfc and Pascin3. Gene expression levels were calculated after normalizing target signals against the housekeeping gene GAPDH and are presented as the change in mRNA expression compared with uninfected animals (mean±S.D.). Significant differences in gene expression levels between MyD88 KO and WT mice are denoted with asterisks (*P<0.05). Results are representative of two independent experiments examining a total of 8–10 individual animals.
DISCUSSION
MyD88-dependent signalling plays a pivotal role in regulating the host innate immune response to bacterial infection (Akira et al., 2006; Trinchieri and Sher, 2007). Not only is this adaptor molecule central in the signalling of the majority of TLRs, but also mediates activation through the IL-1R and IL-18R (Wesche et al., 1997; Adachi et al., 1998; Burns et al., 1998; Medzhitov et al., 1998). The importance of MyD88 in the host response to infectious diseases has been highlighted by several laboratories in diverse infectious disease models (Takeuchi et al., 2000; Koedel et al., 2004; Miller et al., 2006; Fremond et al., 2007).
Our recent study demonstrated that MyD88 KO mice displayed global defects in innate immunity following intracerebral S. aureus infection as typified by the dramatic reduction in pro-inflammatory cytokine and chemokine expression and the inability to recruit significant numbers of neutrophils and macrophages from the periphery (Kielian et al., 2007). One main question that remained to be answered was how important is MyD88 in the CNS compartment? Does CNS MyD88 expression play a role in shaping the subsequent innate immune response following bacterial infection in the brain or are infiltrating leucocytes alone sufficient for establishing immunity? This issue could not be resolved in our previous study since MyD88 was globally absent in MyD88 KO mice and the fact that the brain abscess model is complicated by the involvement of both activated CNS-resident and infiltrating immune cells both of which express this adaptor molecule (Kielian, 2004; Stenzel et al., 2005; Kielian et al., 2007). This conundrum was addressed in the present study by generating radiation bone marrow chimaera mice where MyD88 was specifically expressed in either CNS-resident cells or peripheral bone marrow-derived leucocytes.
The results from MyD88 bone marrow chimaera mice were rather unexpected given the essential nature of MyD88-dependent signalling in peripheral leucocyte activation and the fact that these cells represent the major infiltrate associated with evolving brain abscesses (Kielian, 2004). Indeed, studies by our group and others have shown that neutrophils constitute the main cell type associated with brain abscesses during the early stage of infection (Kielian et al., 2001; Stenzel et al., 2005; Kielian et al., 2007). In essence, our results demonstrated that MyD88 expression in CNS-resident cells, not bone marrow-derived cells, was essential for inducing maximal cytokine/chemokine expression since levels were restored to those observed in WT-infected animals only in MyD88 KO→WT chimaeras. In contrast, animals where MyD88 was present in bone marrow-derived cells, but not the CNS (i.e. WT→KO) did not display WT mediator expression. However, the finding that some inflammatory molecules were more highly expressed in WT→KO chimaeras compared with MyD88 KO controls (i.e. KO→KO) suggests a minor contribution for peripheral MyD88 in shaping the innate immune response during the first 24 h following S. aureus infection. It should be acknowledged that we cannot discount a potential role for MyD88 expression in the periphery during later stages of infection. In fact, this possibility appears likely since resident CNS cells (i.e. microglia and astrocytes), although immune competent, have relatively poor bactericidal activity compared with professional phagocytes (i.e. neutrophils and macrophages). In addition, since many gene products driven by MyD88 impact bacterial survival [i.e. iNOS (inducible NO synthase), ROI (reactive oxygen intermediates) and cytokines] it is likely that, with time, MyD88 signals originating from bone marrow-derived leucocytes may become more important in regulating anti-bacterial immunity in brain abscesses. However, this possibility could not be examined in the current study due to the short survival period of infected MyD88 KO mice.
A similar requirement for MyD88 expression in the CNS compartment was observed when evaluating neutrophil influx into the infected brain. Specifically, WT levels of neutrophil recruitment were only observed in chimaeras with MyD88 expression in the brain parenchyma (i.e. KO→WT). This finding probably stems from the fact that the expression of neutrophil chemokines was also restored to WT levels in these chimaeras. It is important to note that radiation bone marrow chimaera studies are not without potential caveats. For example, irradiation has been shown to induce transient blood–brain barrier compromise and the expression of several pro-inflammatory mediators (Belka et al., 2001; Diserbo et al., 2002; Li et al., 2004; Linard et al., 2004). The likelihood that these factors influenced the results obtained in the present study is minimized by the fact that chimaeras were not infected with S. aureus until 8 weeks post-transplant. Indeed, a recent report has demonstrated that pro-inflammatory molecule expression was similar between radiation chimaera and non-irradiated mice in response to intracerebral LPS (lipopolysaccharide) (Turrin et al., 2007). It has been suggested that irradiation serves to condition the CNS for subsequent colonization by bone marrow-derived cells that eventually transition into microglia-like cells, although some controversy with regard to this latter point still exists (Simard et al., 2006; Ajami et al., 2007; Mildner et al., 2007; Davoust et al., 2008). Therefore it is possible that a minor percentage of parenchymal microglia in the present study originated from bone marrow precursors since we did not shield the head during the irradiation procedure; nonetheless, our results clearly demonstrate an essential role for MyD88 expression by CNS-resident cells in eliciting protective immunity during early stages of brain abscess development.
To further investigate the potential pathway(s) affected by MyD88 loss during early brain abscess development, microarray analysis was performed. Interestingly, many genes that were differentially expressed in MyD88 KO mice were related to apoptosis, which may be due, in part, to the inability to trigger NF-κB (nuclear factor κB) activation, a well known survival signal (Van Antwerp et al., 1996; Li et al., 1999). One such gene was Ier3 (IEX-1), a stress-inducible gene that is rapidly up-regulated in response to a variety of factors including infection, inflammatory cytokines, and transcription factors such as NF-κB (Pietzsch et al., 1997; Domachowske et al., 2000; Arlt et al., 2008). Indeed, a previous study has demonstrated that IEX-1 expression is NF-κB- and TNF-α-dependent (Osawa et al., 2003) and, since both molecules are significantly attenuated in MyD88 KO mice, this is in agreement with the reduction in IEX-1 levels detected by microarray in these animals. Although IEX-1 has been shown to play a pivotal role in promoting cell survival in response to stress (Wu et al., 1998; Garcia et al., 2002; Mittal et al., 2006), its functional impact on cell survival remains controversial and probably depends on cell type, stimulus, and expression levels (Arlt et al., 2001; Schilling et al., 2001; Osawa et al., 2003). Interestingly, a previous study has demonstrated that IEX-1 reduces ROS (reactive oxygen species) production (Shen et al., 2006) and, by extension, attenuated IEX-1 levels in brain abscesses of MyD88 KO mice could conceivably exacerbate ROS accumulation contributing to the massive tissue damage observed in these animals. However, these possibilities remain speculative at the present time.
Another gene that was significantly down-regulated in brain abscesses of MyD88 KO mice is Lcn2 [also referred to as NGAL (neutrophil gelatinase-associated lipocalin)]. Similar to IEX-1, Lcn2 has also been implicated in regulating apoptosis and is produced by numerous cell types including macrophages and neutrophils in response to infection (Kjeldsen et al., 1993; Devireddy et al., 2001; Tong et al., 2005; Lee et al., 2007). Therefore, in the context of our brain abscess model, it is likely that reduced Lcn2 expression in MyD88 KO mice resulted from the fact that neutrophil and macrophage infiltrates are decreased approx. 85% and 15% respectively, compared with WT animals as we previously reported (Kielian et al., 2007). In addition, a previous study has demonstrated that Lcn2 is induced in macrophages in response to heat-killed group B Streptococcus via a TLR2-dependent manner, implying a role for TLRs in Lcn2 induction (Draper et al., 2006). Obviously, TLR2-mediated signalling is ablated in MyD88 KO mice, suggesting that this may represent one mechanism by which Lcn2 expression is reduced during brain abscess formation in these animals. Another gene whose expression was decreased in brain abscesses of MyD88 KO animals was properdin. Properdin activates the alternative pathway of complement by assembling the C3 convertase on target surfaces (Kemper and Hourcade, 2008). Properdin is capable of binding to bacteria to engage the alternative complement pathway and its importance in host defence is illustrated by the enhanced sensitivity of properdin-deficient patients to meningococcal disease (Densen et al., 1987; Emonts et al., 2003). Unlike most complement components, properdin is produced by neutrophils and macrophages (Schwaeble et al., 1994; Wirthmueller et al., 1997) and the fact that the influx of both of these cell types is significantly attenuated in brain abscesses of MyD88 KO mice (Kielian et al., 2007) probably explains the reduction in properdin levels observed in these animals. Another gene that was attenuated in brain abscesses of MyD88 KO mice was SOCS3, which is a negative regulator of cytokine signalling elicited by JAK (Janus kinase)/STAT (signal transducer and activator of transcription), as well as TLR signalling pathways (Dimitriou et al., 2008). It is interesting to note that SOCS3 has been reported to block IL-10 production, as well as induce classical macrophage activation (Dimitriou et al., 2008; Liu et al., 2008). Our previous study demonstrated that macrophages and microglia isolated from brain abscesses of MyD88 KO mice expressed equivalent levels of IL-10 compared with WT cells, and this fact could be explained by the reduction in SOCS3 levels observed here. In addition, TNF-α has been reported to stabilize SOCS3 mRNA expression (Ehlting et al., 2007), and the failure to induce significant TNF-α expression in brain abscesses of MyD88 KO mice could be another factor contributing to reduced SOCS3 levels in these animals.
Importantly, our microarray analysis revealed several genes that we have previously reported to be significantly attenuated in brain abscesses of MyD88 KO mice at the protein level including IL-1β and CXCL1 (KC) (Kielian et al., 2007). This provides further confidence in the accuracy of our microarray analysis. Collectively the propensity of apoptosis-related genes that are differentially expressed in brain abscesses of MyD88 KO mice is highly suggestive that the expansive tissue damage that occurs in these animals is influenced by accelerated/dysregulated apoptotic pathways. This possibility remains to be directly tested in future studies with MyD88 KO mice in the brain abscess model.
One important point to emphasize is that, although we have identified a critical role for MyD88 in the CNS compartment during the early phase of S. aureus infection, several outstanding questions remain. The first is that we cannot determine whether MyD88 mediates its effects through TLRs or the IL-1R or IL-18R since all of these molecules utilize this signalling adaptor. This is important since earlier studies from our laboratory have revealed a pivotal role for IL-1 in innate immunity to CNS S. aureus infection (Kielian et al., 2004a). A second issue relates to the target cells in the brain where MyD88 expression is important. Likely candidates include microglia and astrocytes since both cell types are capable of recognizing S. aureus via a MyD88-dependent mechanism leading to pro-inflammatory mediator release (Esen and Kielian, 2006; T. Kielian, unpublished data). Regardless, the findings of the present study highlight a novel mechanism for CNS-resident cells in initiating a protective innate immune response in the infected brain and, in the absence of MyD88 in this compartment, immunity is compromised.
ACKNOWLEDGEMENTS
We thank Cathy Gurley, Gail Wagoner and Jennifer Johnson for excellent technical assistance. We are also grateful to Dr Monica Carson (Division of Biomedical Sciences, University of California, Riverside, CA, U.S.A.) for sharing her protocol for generating bone marrow chimaera mice.
FUNDING
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [grant number RO1 NS NS055385 (to T.K.)]; the National Institute of Neurological Disorders and Stroke supported Core Facility at the University of Arkansas for Medical Sciences [grant number P30 NS047546]; and the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
REFERENCES
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Arlt A, Vorndamm J, Breitenbroich M, Folsch UR, Kalthoff H, Schmidt WE, Schafer H. Inhibition of NF-κB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene. 2001;20:859–868. doi: 10.1038/sj.onc.1204168. [DOI] [PubMed] [Google Scholar]
- Arlt A, Rosenstiel P, Kruse ML, Grohmann F, Minkenberg J, Perkins ND, Folsch UR, Schreiber S, Schafer H. IEX-1 directly interferes with RelA/p65 dependent transactivation and regulation of apoptosis. Biochim Biophys Acta. 2008;1783:941–952. doi: 10.1016/j.bbamcr.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Baldwin AC, Kielian T. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuroimmunol. 2004;151:24–32. doi: 10.1016/j.jneuroim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J Exp Med. 2001;193:967–974. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity: molecular and cellular mechanisms. Br J Cancer. 2001;85:1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer JL, Di Marco F, French L, Tschopp J. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1–11. doi: 10.1007/s10096-006-0236-6. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using Z score transformation. J Mol Diagn. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, Svanborg C, Miethke T. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008;14:399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–234. doi: 10.1016/j.it.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Densen P, Weiler JM, Griffiss JM, Hoffmann LG. Familial properdin deficiency and fatal meningococcemia. Correction of the bactericidal defect by vaccination. N Engl J Med. 1987;316:922–926. doi: 10.1056/NEJM198704093161506. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- Dimitriou ID, Clemenza L, Scotter AJ, Chen G, Guerra FM, Rottapel R. Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol Rev. 2008;224:265–283. doi: 10.1111/j.1600-065X.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- Diserbo M, Agin A, Lamproglou I, Mauris J, Staali F, Multon E, Amourette C. Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study. Can J Physiol Pharmacol. 2002;80:670–678. doi: 10.1139/y02-070. [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Bonville CA, Mortelliti AJ, Colella CB, Kim U, Rosenberg HF. Respiratory syncytial virus infection induces expression of the anti-apoptosis gene IEX-1L in human respiratory epithelial cells. J Infect Dis. 2000;181:824–830. doi: 10.1086/315319. [DOI] [PubMed] [Google Scholar]
- Draper DW, Bethea HN, He YW. Toll-like receptor 2-dependent and -independent activation of macrophages by group B streptococci. Immunol Lett. 2006;102:202–214. doi: 10.1016/j.imlet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Ehlting C, Lai WS, Schaper F, Brenndorfer ED, Matthes RJ, Heinrich PC, Ludwig S, Blackshear PJ, Gaestel M, Haussinger D, Bode JG. Regulation of suppressor of cytokine signaling 3 (SOCS3) mRNA stability by TNF-α involves activation of the MKK6/p38MAPK/MK2 cascade. J Immunol. 2007;178:2813–2826. doi: 10.4049/jimmunol.178.5.2813. [DOI] [PubMed] [Google Scholar]
- Emonts M, Hazelzet JA, de Groot R, Hermans PW. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect Dis. 2003;3:565–577. doi: 10.1016/s1473-3099(03)00740-0. [DOI] [PubMed] [Google Scholar]
- Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J Immunol. 2006;176:6802–6811. doi: 10.4049/jimmunol.176.11.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- Flaris NA, Hickey WF. Development and characterization of an experimental model of brain abscess in the rat. Am J Pathol. 1992;141:1299–1307. [PMC free article] [PubMed] [Google Scholar]
- Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- Garcia J, Ye Y, Arranz V, Letourneux C, Pezeron G, Porteu F. IEX-1: a new ERK substrate involved in both ERK survival activity and ERK activation. EMBO J. 2002;21:5151–5163. doi: 10.1093/emboj/cdf488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan-Zahraee M, Tran EH, Bourbonniere L, Owens T. Elevated interferon-γ in CNS inflammatory disease: a potential complication for bone marrow reconstitution in MS. J Neuroimmunol. 2000;108:40–44. doi: 10.1016/s0165-5728(99)00263-5. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kemper C, Hourcade DE. Properdin: new roles in pattern recognition and target clearance. Mol Immunol. 2008;45:4048–4056. doi: 10.1016/j.molimm.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-α play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004a;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- Kielian T, McMahon M, Bearden ED, Baldwin AC, Drew PD, Esen N. S. aureus-dependent microglial activation is selectively attenuated by the cyclopentenone prostaglandin 15-deoxy-Delta12,14- prostaglandin J2 (15d-PGJ2). J Neurochem. 2004b;90:1163–1172. doi: 10.1111/j.1471-4159.2004.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Haney A, Mayes PM, Garg S, Esen N. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun. 2005;73:7428–7435. doi: 10.1128/IAI.73.11.7428-7435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, Johnson J. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J Immunol. 2007;178:4528–4537. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Syed MMd, Liu S, Phillips N, Wagoner G, Drew PD, Esen N. The synthetic PPAR-γ agonist ciglitazone attenuates neuroinflammation and accelerates encapsulation in bacterial brain abscesses. J Immunol. 2008;180:5004–5016. doi: 10.4049/jimmunol.180.7.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- Koedel U, Rupprecht T, Angele B, Heesemann J, Wagner H, Pfister HW, Kirschning CJ. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain. 2004;127:1437–1445. doi: 10.1093/brain/awh171. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee J, Kim S, Park JY, Lee WH, Mori K, Kim SH, Kim IK, Suk K. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007;179:3231–3241. doi: 10.4049/jimmunol.179.5.3231. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Chen P, Jain V, Reilly RM, Wong CS. Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat Res. 2004;161:143–152. doi: 10.1667/rr3117. [DOI] [PubMed] [Google Scholar]
- Linard C, Marquette C, Mathieu J, Pennequin A, Clarencon D, Mathe D. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-κB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427–434. doi: 10.1016/j.ijrobp.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Liu Y, Stewart KN, Bishop E, Marek CJ, Kluth DC, Rees AJ, Wilson HM. Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J Immunol. 2008;180:6270–6278. doi: 10.4049/jimmunol.180.9.6270. [DOI] [PubMed] [Google Scholar]
- Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–779. doi: 10.1086/515541. quiz 780–761. [DOI] [PubMed] [Google Scholar]
- McClelland CJ, Craig BF, Crockard HA. Brain abscesses in Northern Ireland: a 30 year community review. J Neurol Neurosurg Psychiatry. 1978;41:1043–1047. doi: 10.1136/jnnp.41.11.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Mittal A, Papa S, Franzoso G, Sen R. NF-kappaB-dependent regulation of the timing of activation-induced cell death of T lymphocytes. J Immunol. 2006;176:2183–2189. doi: 10.4049/jimmunol.176.4.2183. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Osawa Y, Nagaki M, Banno Y, Brenner DA, Nozawa Y, Moriwaki H, Nakashima S. Expression of the NF-κB target gene X-ray-inducible immediate early response factor-1 short enhances TNF-α-induced hepatocyte apoptosis by inhibiting Akt activation. J Immunol. 2003;170:4053–4060. doi: 10.4049/jimmunol.170.8.4053. [DOI] [PubMed] [Google Scholar]
- Pietzsch A, Buchler C, Aslanidis C, Schmitz G. Identification and characterization of a novel monocyte/macrophage differentiation-dependent gene that is responsive to lipopolysaccharide, ceramide, and lysophosphatidylcholine. Biochem Biophys Res Commun. 1997;235:4–9. doi: 10.1006/bbrc.1997.6715. [DOI] [PubMed] [Google Scholar]
- Schilling D, Pittelkow MR, Kumar R. IEX-1, an immediate early gene, increases the rate of apoptosis in keratinocytes. Oncogene. 2001;20:7992–7997. doi: 10.1038/sj.onc.1204965. [DOI] [PubMed] [Google Scholar]
- Schliamser SE, Backman K, Norrby SR. Intracranial abscesses in adults: an analysis of 54 consecutive cases. Scand J Infect Dis. 1988;20:1–9. doi: 10.3109/00365548809117210. [DOI] [PubMed] [Google Scholar]
- Schwaeble W, Huemer HP, Most J, Dierich MP, Strobel M, Claus C, Reid KB, Ziegler-Heitbrock HW. Expression of properdin in human monocytes. Eur J Biochem. 1994;219:759–764. doi: 10.1111/j.1432-1033.1994.tb18555.x. [DOI] [PubMed] [Google Scholar]
- Shen L, Guo J, Santos-Berrios C, Wu MX. Distinct domains for anti- and pro-apoptotic activities of IEX-1. J Biol Chem. 2006;281:15304–15311. doi: 10.1074/jbc.M600054200. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Stenzel W, Soltek S, Miletic H, Hermann MM, Korner H, Sedgwick JD, Schluter D, Deckert M. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J Neuropathol Exp Neurol. 2005;64:27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney JH. Bacterial infections of the central nervous system in neurosurgery. Neurol Clin. 1986;4:91–114. [PubMed] [Google Scholar]
- Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441–448. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Plante MM, Lessard M, Rivest S. Irradiation does not compromise or exacerbate the innate immune response in the brains of mice that were transplanted with bone marrow stem cells. Stem Cells. 2007;25:3165–3172. doi: 10.1634/stemcells.2007-0508. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- Wirthmueller U, Dewald B, Thelen M, Schafer MK, Stover C, Whaley K, North J, Eggleton P, Reid KB, Schwaeble WJ. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol. 1997;158:4444–4451. [PubMed] [Google Scholar]
- Wu MX, Ao Z, Prasad KV, Wu R, Schlossman SF. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- Zehntner SP, Bourbonniere L, Hassan-Zahraee M, Tran E, Owens T. Bone marrow-derived versus parenchymal sources of inducible nitric oxide synthase in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;150:70–79. doi: 10.1016/j.jneuroim.2004.01.020. [DOI] [PubMed] [Google Scholar]