Abstract
Because Parkinson’s disease is a progressive degenerative disorder that is mainly confined to the basal ganglia, gene transfer to deliver therapeutic molecules is an attractive treatment avenue. The present review focuses on direct in vivo gene transfer vectors that have been developed to a degree that they have been successfully used in animal model of Parkinson’s disease. Accordingly, the properties of recombinant adenovirus, recombinant adeno-associated virus, herpes simplex virus, and lentivirus are described and contrasted. In order for viral vectors to be developed into clinical grade reagents, they must be manufactured and tested to precise regulatory standards. Indeed, clinical lots of viral vectors can be produced in compliance with current Good Manufacturing Practices (cGMPs) regulations using industry accepted manufacturing methodologies, manufacturing controls, and quality systems. The viral vector properties themselves combined with physiological product formulations facilitate long-term storage and direct in vivo administration.
Keywords: adenovirus, adeno-associated virus, herpes simplex virus, lentivirus, purification, quality control, quality assurance, cleanroom
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is mainly, but not exclusively, characterized by atrophy and death of nigrostriatal dopamine (DA) neurons (Olanow and Tatton, 1999). Gene therapy is a promising avenue to deliver therapeutic molecules to treat PD. Two facts make gene therapy for PD attractive: 1) the symptoms of the disease are caused by pathology in a highly specific localized brain region and 2) due to the fact that PD patients often live with the disease for many years, long-term to permanent delivery is probably required. Given this idea, development of agents that deliver DNA to the brain is key to the ultimate realization of a gene therapy to treat PD. In addition to direct in vivo gene therapy, ex vivo gene therapy using various cells that are transduced in vitro and then transplanted into the PD brain is a viable strategy (Dass et al., 2006). However, the present review is restricted to viral vectors for in vivo gene therapy. The majority of cells in the CNS are post-mitotic, thus, vectors that are capable of gene delivery to quiescent cells are of the most interest for use in PD.
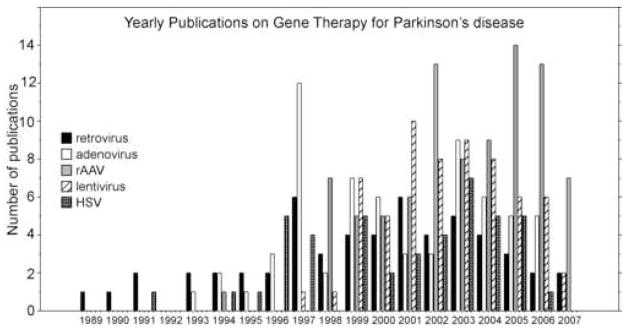
Currently the most efficient method for transferring functional genes directly to the CNS is via viral vectors. Viruses have evolved over millions of years to efficiently transfer genes that allow them to survive and replicate in mammalian hosts (Verma and Weitzman, 2005). Characterization of the life cycle of wild-type viruses has allowed the engineering of recombinant viral vectors that are deficient in replication functions but are capable of infecting the cell to introduce foreign genes. In recent years, investigators have developed techniques for engineering the genome of many different viruses to transfer a gene of interest (transgene). Moreover, production procedures have been devised for these vectors in sufficient quantities and of sufficient purity to enable experimentation in animal models of PD and for clinical trials (Barkats et al., 1998; Burton et al., 2003; Mandel and Burger, 2004; Mandel et al., 2006). To illustrate this trend, publications that report gene transfer to treat or study PD have increased over the years (Fig. 1). Additionally, both commercial entities that sell customized viral vectors (Table 1), and Universities that support vector core facilities to produce vectors for in-house and external investigators (Table 2) exist which enables greater availability of the latest vector reagents.
Figure 1.
Approximate number of viral vector related publications regarding Parkinson’s disease over a period of years. The data is based on pubmed searches for each year. The rAAV publications exclude Ad publications. The data includes review publications. The data may not be perfectly accurate due to the nature of pubmed search terms but is intended to show the trend of increased publications since 1984.
Table 1.
Commercially available viral vectors. These commercial entities were identified via internet search engines. This may not represent a fully comprehensive list of companies that sell viral vectors for research purposes. In addition, this information is not intended to rank the quality of the companies and the quality of the viral vectors for use in the brain is unknown.
| Recombinant Vector | Kit | Custom Produced vector | Specific pre-made vectors | Vector purification | Vector titering | Company |
|---|---|---|---|---|---|---|
| AAV | Yes | No | Yes | No | No | Stratagene, Torrey Pines, CA |
| AAV | no | yes | no | no | no | Vector Biolabs, University of Pittsburgh |
| Ad | yes | yes | Yes | Kit + cell line | Cell Biolabs, San Diego, CA | |
| Ad | No | Yes | No | No | No | Invitrogen, Carlsbad, CA |
| Ad | No | Yes | Yes | No | No | Kelen Biolab, Diego, CA |
| Ad | Yes | yes | Obiogene Adenovirus Technology, Irvine, CA | |||
| Ad | No | Yes | Yes | No | No | BIOgene, Solon, OH |
| Ad | Yes | No | Yes | No | No | Stratagene, Torrey Pines, CA |
| Ad | yes | yes | Vector Biolabs, University of Pittsburgh | |||
| Ad | Yes | Yes | ViraQuest Inc North Liberty, IA | |||
| Lentivirus | yes | yes | kit | Cell Biolabs, San Diego, CA | ||
| Lentivirus | No | Yes | No | No | No | Invitrogen, Carlsbad, CA |
| Lentivirus | No | Yes | No | No | No | Kelen Biolab San Diego, CA |
| MLV retrovirus | yes | Cell Biolabs, San Diego, CA |
Table 2.
This table lists the academic vector cores that could be identified either by search engines or by pubmed searches. We apologize if it is not complete but the goal is to at least give the reader a starting point if they are interested in starting in vivo gene transfer studies.
| Institution | rAAV | Lentivirus | rAd | retrovirus | Non-Viral |
|---|---|---|---|---|---|
| Baylor College of Medicine | ✓ | ✓ | ✓ | ✓ | ✓ |
| Colorado State University, Animal Sciences | ✓ | - | - | - | - |
| University of Cincinnati, Children’s Hospital | ✓ | ✓ | - | ✓ | - |
| University of Florida College of Medicine | ✓ | - | - | - | - |
| Dana Farber Harvard Cancer Center | ✓ | ✓ | ✓ | ✓ | - |
| University of Iowa | - | ✓ | ✓ | - | - |
| University of Michigan | ✓ | ✓ | ✓ | ✓ | plasmids |
| University of North Carolina at Chapel Hill | ✓* | ✓ | - | - | |
| Children’s Memorial Research Center, Northwestern University | ✓ | ✓ | - | - | - |
| University of Pennsylvania | ✓ | ✓ | ✓ | ✓ | - |
| University of Pittsburgh | ✓ | ✓ | ✓ | ✓ | plasmids |
| University of Texas MD Anderson Cancer Center | - | - | ✓ | ✓ | - |
| Tufts-New England Medical Center | - | - | ✓ | - | - |
= AAV1-AAV8
The present review will focus on the most developed viral vectors that could be used to deliver therapeutic genes in PD models. Recombinant adenovirus (rAd), recombinant adeno-associated viruses (rAAV), herpes simplex viruses (HSV) and lentiviruses (Lv) are currently most utilized viral vectors that have been demonstrated to reliably transfer DNA in animal models of PD (Azzouz et al., 2002; Bilang-Bleuel et al., 1997; Choi-Lundberg et al., 1997; Corti et al., 1999; Kordower et al., 2000). Currently, there are at least 3 early stage clinical trials testing the safety of vector-delivery of genes to treat PD (Mandel and Burger, 2004)(http://www.gemcris.od.nih.gov/). Therefore, production of clinical grade vectors becomes an important issue for investigators planning to use these reagents in a clinical trial. Other viruses have been engineered into viral vectors and are in various stages of development but are beyond the scope of this review (Verma and Weitzman, 2005).
Safety
All of the viral vectors that are reviewed here have been engineered to be replication defective. Therefore, when injected into the brain, rAd, rAAV, HSV, and Lv only transduce the cells that they infect, any remaining virus is cleared, and no further spread of the infection can take place. Moreover, these viruses cannot enter their lytic cycle and thus do not kill the cells that they infect. Furthermore, recombination events that might revert the proviral genome to the wild-type virus to induce a productive infection have never been observed.
A significant safety concern, however, is insertional mutagenesis leading to de novo oncogenesis as has been observed in ex vivo gene therapy using retroviruses for the treatment of severe combined immunodeficiency (adenosine deaminase deficiency) and chronic granulomatous disease (Nienhuis, 2006). In general, rAd and HSV do not integrate into the genome and form episomes (Harui et al., 1999; Hillgenberg et al., 2001; Oehmig et al., 2004b). Both rAd and HSV can be engineered to integrate into the genome, thus, addition of a transposon to a helper-dependent (high capacity) rAd, increases the frequency of integration and enhances long-term gene expression (Yant et al., 2002). Likewise, HSV amplicon vectors can be induced to integrate by including sequences from viruses that normally integrate into the genome (Oehmig et al., 2004b).
Wild-type rAAV integrates at a high frequency especially at a specific site in human chromosome 19 (Kotin et al., 1990), see below). Whether rAAV vectors integrate frequently is controversial. Although rAAV has been reported to rarely integrate (Kay et al., 2003), recent reports suggest that when injected in the liver, rAAV integrates at a particular site that encodes for mouse micro-RNAs and can cause alarming rates of insertional mutagenesis, as evidenced by formation of hepatocellular carcinoma (Donsante et al., 2007). Lv efficiently integrates into the genome (Mitchell et al., 2004; Sinn et al., 2005) and therefore, may carry some risk. However, VSV-g pseudotyped Lv and most rAAV serotypes studied to date almost exclusively transduces neurons (Blömer et al., 1997; Burger et al., 2004; Naldini et al., 1996a; Taymans et al., 2007) which preferentially enter an apoptotic pathway rather re-entering the cell cycle which could lead to tumor formation (Klein and Ackerman, 2003). Therefore, insertional mutagenesis as a result of striatal transduction using rAAV or VSV-g pseudotyped lentiviral vectors may not pose a significant problem for PD gene therapy. It should also be noted that although rAAV, rAd, and HSV1 vectors may not integrate, they all can support long-term gene expression (see below). A simplified comparison of the properties of each viral vector with regard to CNS gene delivery is shown in Table 3.
Table 3.
This table presents the characteristics of each reviewed viral vector in the first 4 columns. In the next three columns, generalized ratings are given regarding the vectors’ performance in vivo. These ratings are somewhat subjective and are taken both from the authors’ own experience and from cited review papers. Given that the vector platforms are continuously improving, some of the vector performance ratings may change over time.
| Vector | Deletions | Cell Specificity | Molecular Fate | Transgene Capacity | Immune Response in the CNS | In vivo transgene expression | Safety Concerns |
|---|---|---|---|---|---|---|---|
| rAd | |||||||
| First generation r-Ad | E1a, E1b, E3 | neurons, glia | episomal | ~8kb | High Antigenicity | high protein production variable durability pending promoter and immune response | High Toxicity |
| Gutless “high capacity” | All structural genes | neurons, glia | episomal | <37kb | variable protein production long-term | Toxicity dependent on titer | |
| rAAV | |||||||
| rAAV1–10 | rep, cap | neurons | episomal/integrated | ~4.7kb | Potential antigenicity due to capsid proteins | high protein production long-term | Insertional Mutagenesis?/immune response |
| rHSV | |||||||
| replication-defective | ICP4, ICP22, ICP27, ICP47 | neurons, glia | episomal/latent | ~20kb | Moderate Antigenicity | high protein production short term | Moderate Toxicity |
| Am plicon | |||||||
| All except oris and pac | neurons, glia | episomal/latent | ~20kb | Low Antigenicity | low protein production short-term | Low Toxicity | |
| rLV | |||||||
| 1st generation rLV | vif, vpr, vpu, nef | neurons* | integrated | ~9kb | low | high protein production long term | potential problems from VSV-g, pseudotyped envelope, possible HIV sero-conversion, possible recombination |
| 2st generation rLV | vif, vpr, vpu, nef | neurons* | integrated | ~9kb | low | high protein production long term | potential problems from VSV-g, pseudotyped envelope, possible HIV sero-conversion, possible recombination |
| 3rd generation rLV | vif, vpr, vpu, nef tat, rev | neurons* | integrated | ~9kb | Moderate Antigenicity | high protein production long term | VSV-g, seroconversion, recombination |
| Self-Inactivating | All above and 5′ LTR inactivation | neurons* | integrated | ~9kb | Low Antigenicity | high protein production long term | VSV-g, seroconversion |
Another concern with regard to direct injection of any viral vector is an immune response against the virus. Immune responses to each specific vector are discussed below. However, it should be acknowledged that, although the brain is relatively immunoprivileged, the brain can mount significant immune response to vector injections (Lowenstein et al., 2007). Common to all the vectors discussed below, the level of immune response to the vector is also mediated by vector dose and purity of the vector preparation. Thus, newer methods for production of highly purified vector stocks often results in lower toxicity than has typically been reported previously (Lowenstein et al., 2007).
Direct in vivo injection of viral vectors results in permanent or near permanent transgene expression (Mandel et al., 2006). Therefore, the ability to control the expression of the transgene in the brain after transduction is a significant safety issue. External control of transgene expression is the subject of another report in this special issue (citation pending).
Recombinant adenovirus (rAd)
rAd was one of the earliest viral vectors used with success in the brain (Le Gal La Salle et al., 1993) and in animal models of PD (Barkats et al., 1998; Choi-Lundberg et al., 1997). Wild type Ad (wt-Ad) commonly infects humans and is known to cause respiratory infections, conjunctivitis, and gastroenteritis (Shenk, 1996).
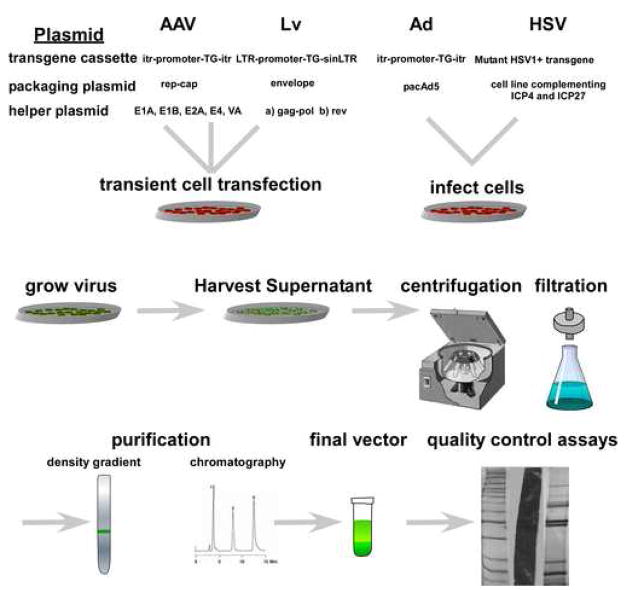
Ad has a large genome consisting of a 36 kb genome of linear double-stranded DNA (Shenk, 1996). Adenoviral infection is a receptor-mediated event and at least some of the receptors have been identified. The Coxsackie and adenovirus receptor (CAR) with alphaV-beta5 or alphaV-beta3 integrin co-receptors allow Ad entry into the cell (Bergelson et al., 1997). The recombinant version of Ad can transduce both mitotic and post-mitotic cells. In the CNS, Ad transduces both neurons and glia (Akli et al., 1993) and rAd supports high levels of transgene expression (Bohn et al., 1999; Gerdes et al., 2000). The wild-type adenoviral genome is composed of two inverted terminal repeats that are required for replication, a packaging signal, early genes and late genes. First generation recombinant Ad vectors have a deletion of the E1A early gene that is responsible for viral growth and induction of late genes. For production of the recombinant form of Ad, the E1 gene product can be provided in trans in another separate plasmid (Danthinne and Imperiale, 2000). This allows for the deletion of the E1A gene which renders the virus replication deficient and allows introduction of therapeutic genes in place of the E1A gene (see Fig. 2). Second generation Ad vectors, where E1A, E3/E4 genes are deleted, successfully delivered GDNF under the control of the cellular GFAP promoter in an animal model of PD (Do Thi et al., 2004).
Figure 2.
A schematic of generalized production procedures of viral vectors for experimental use. There are minor differences that are unique to individual laboratories and this figure is not intended to be used as an exact protocol for recombinant virus production.
However, in these versions of the Ad vector, wild-type viral genes remain in the vector. While expression of these viral genes may be impaired due to the deletion of early genes late genes are not ablated. Thus, in vivo, expression of these wt Ad genes leads to a host inflammatory response which hinders the use of this vector for clinical purposes outside cancer therapy (Byrnes et al., 1995; Byrnes et al., 1996; Dewey et al., 1999; Lowenstein and Castro, 2003; Thomas et al., 2001a; Thomas et al., 2001b; Wood et al., 1996). Moreover, rAd infection induces well characterized innate and adaptive immune responses in the brain (Byrnes et al., 1995; Byrnes et al., 1996; Dewey et al., 1999; Lowenstein and Castro, 2003; Thomas et al., 2001a; Thomas et al., 2001b; Wood et al., 1996).
Newer Ad vectors have been designed that retain only the inverted terminal repeats, i.e., with all viral genes removed; the so-called gutless vectors or high-capacity vectors (Hardy et al., 1997; Parks et al., 1996; Thomas et al., 2001b). The production of this vector requires the use of helper first generation Ad (Hardy et al., 1997; Parks et al., 1996; Thomas et al., 2001b). While the gutless Ad has been demonstrated to transduce cells in the CNS with reduced toxicity, there also seems to be some loss of transduction efficiency (Lowenstein et al., 2002). In addition, at this time, removing all helper Ad from the final gutless Ad preparation is difficult and incomplete (Puntel et al., 2006). The small but significant contamination with helper Ad can contribute to adverse inflammatory reactions in the brain. Finally, there is an innate inflammatory response to Ad infection regardless of its internal genomic structure (Muruve, 2004). Thus, problems dealing with long-term expression and antigenicity of the recombinant vector remain to be resolved prior to its use in gene therapies requiring permanent CNS transduction. To the contrary, Lowenstein and collaborators have performed careful intracerebral studies with gutless rAd that has been highly purified has indicated that vector doses below 108 infectious particles can evade the immune response in the brain (Lowenstein et al., 2007; Thomas et al., 2001a). Moreover, this group routinely achieves long-term brain expression even with second generation E1/E3 deleted rAd (Zermansky et al., 2001).
Applications for brain tumors are however, realistic at this time (Candolfi et al., 2006). Nevertheless, the technical issues that currently face gutless Ad are most likely surmountable. Therefore, because of the large packaging capacity of gutless Ad and its ability to transduce both neurons and glia in the brain, this vector can still be considered for use in PD therapy. Because Ad is produced by viral infection rather than transient transfection (fig. 2), scale-up to clinical levels of vector is not problematic and methods for clinical grade scale-up have been reported(Kamen and Henry, 2004).
Adeno-Associated Virus
Wild-type adeno-associated virus (wt-AAV) is a small virus (≈20 nm) that is a non-pathological member of the parvovirus family (Muzyczka and Berns, 2001). When wt-AAV infects a cell, it normally integrates into the host cell genome and goes into latency at a specific site on chromosome 19 (Muzyczka and Berns, 2001). In order to enter a lytic cycle in which progeny are produced, wt-AAV requires infection of a helper virus and is therefore classified as a dependovirus (Muzyczka and Berns, 2001). Combined with its lack of pathogenicity and its natural proclivity for latency, replication incompetent AAV makes an excellent candidate for a gene therapy vector.
Wt-AAV is a single stranded DNA virus with a genome size of 4.7 kb that contains two genes, rep and cap, both of which are necessary for its life cycle. These two genes are flanked by two inverted terminal DNA repeats (ITR). Recombinant AAV has been stripped of both rep and cap genes and includes the ITRs that are necessary for second strand synthesis and integration and in the cell (see fig. 2). The genes necessary for vector production are provided in trans, by a helper transfection system (Cao et al., 2000; Hauswirth et al., 2000).
Complementary DNAs of up to ~4.7 kb can be inserted between the two ITRs in the viral vector for delivery into the target cell. More than 100 genotypes of AAV have been identified that fall into nine antigenically different genetic clades (Gao et al., 2004). More than 100 genotypes of AAV have been identified that fall into nine different antigenic genetic clades (Gao et al., 2004; Gao et al., 2005) but only AAV1–10 have been engineered into vectors (Davidson et al., 2000; Gao et al., 2005; Gao et al., 2002; Huszthy et al., 2005; Wang et al., 2005). Production of rAAV has been improved recently and extremely high titer pure virus is commonly obtained (Zolotukhin et al., 2002).
rAAV2 has been the most studied serotype in the brain and transduces exclusively neurons in the CNS (Burger et al., 2005). The receptor for AAV2 has been characterized and is composed of the heparan sulfate proteoglycan receptor, and at least one co-receptor, FGF-receptor-1 (FGFr1) (Qing et al., 1999; Summerford et al., 1999; Summerford and Samulski, 1998). The relative abundance of these receptors in the brain probably explains why rAAV2 is more efficient in some brain regions such as globus pallidus and substantia nigra than in most other regions (Burger et al., 2004; Klein et al., 1998). Initially, there was thought to be little immune response to rAAV2 in the brain. However, a large proportion of the human population has circulating antibodies to AAV2 as well as other serotypes (Zaiss and Muruve, 2005). Indeed, neutralizing antibodies are capable of blocking rAAV2 transduction in the brain (Peden et al., 2004; Sanftner et al., 2004). Moreover, there is an innate immune response to rAAV even though no viral genes are expressed (Peden et al., 2004). The innate immune response is likely due to processing and antigen presentation of capsid proteins (Lowenstein, 2004). Other rAAV serotypes that have been investigated in brain appear to be more efficient than rAAV2, especially in the striatum which is the likely target for PD therapies (Burger et al., 2004; Mandel et al., 2006; Sondhi et al., 2007). Thus, rAAV1, rAAV5, rAAV8, and rAAV10 have been shown to transduce more neurons than rAAV2 injected at similar titers and with the same transcriptional cassette (Burger et al., 2004; Reimsnider et al., 2007; Sondhi et al., 2007).
Because rAAV production usually consists of triple transient transfection (fig. 2), scale-up of production to the levels that might be needed for wide distribution of an approved vector for PD treatment has been an issue. Several potential scale-up procedures have been published (Booth et al., 2004; Conway et al., 1997; Kohlbrenner et al., 2005) and all are amenable to cGMP procedures (see below) (Farson et al., 2004; Snyder and Francis, 2005).
Herpes Simplex Virus (HSV)
Wild-type herpes simplex virus is a very large linear double-stranded DNA virus that encodes over 80 genes spanning a genome of about 150 kb (Roizman and Sears, 1987). The wild type virus is the pathogen that is associated with cold sores, corneal blindness, and encephalitis (Carr and Tomanek, 2006). Wt-HSV can infect a wide variety of cells due to the envelope binding to heparan sulfate and herpes virus entry mediators. After endocytosis, the wt-HSV virus is retrogradely transported to the cell body and eventually to the nucleus where it replicates. Wt-HSV can infect cells lytically or establish latency especially in neurons (Millhouse and Wigdahl, 2000). HSV-1 can infect both neurons and glia (Fink and Glorioso, 1997; Fink et al., 1997).
There are two types of HSV-1 vectors: recombinant virus and amplicon vectors (Burton et al., 2005; Tyler et al., 2006). The recombinant HSV virus retains almost the entire wild-type genome, and the gene of interest is inserted in this vector by homologous recombination (Burton et al., 2005). Potentially, up to 150kb could be inserted in this vector if all viral genes could be removed (Fraefel et al., 2000). For example, successful packaging of a HSV amplicon vector containing a human artificial chromosome encoding the entire hypoxanthine phosphoribosyltransferase gene has been reported (Moralli et al., 2006). The amplicon vector system is produced similarly to other recombinant vector systems and contains a viral origin of replication and a packaging signal, while the genes necessary for virus production and replication are provided in trans, by a helper virus/transfection system (Tyler et al., 2006) (fig. 2).
One of the problems of the HSV-1 vectors is their inherent cytotoxicity and their inability to support long-term expression in vivo (Burton et al., 2002; Burton et al., 2005; Burton et al., 2003). Work regarding production methods that allow more viral genes that mediate toxicity to be removed is being continued (Tyler et al., 2006). In addition, the factors controlling long-term in vivo expression are also being elucidated (Burton et al., 2005; Tyler et al., 2006). For example, hybrid HSV amplicon vectors that combine features of other viral vectors that allow long-term transgene expression but retain the large cDNA packaging capacity of HSV are being investigated (Oehmig et al., 2004a; Oehmig et al., 2004b). HSV’s inherent neural tropism, retrograde transport, and large transgene capacity are attractive features to be exploited in the future. Therefore, it is conceivable that HSV vectors can be used to treat PD especially if a gene or combination of genes are required to treat the disease. Finally, scale-up of HSV production for clinical trials has been developed (Burton et al., 2005; Shah et al., 2003).
Lentivirus (Lv)
Lentiviruses belong to the retrovirus family of RNA viruses. In contrast to more commonly studied oncoretroviruses, they can infect both mitotic and post-mitotic cells (Naldini and Verma, 2000; Verma and Weitzman, 2005). Human Immunodeficiency Virus type I (HIV) is classified as a lentivirus. Because HIV is one of the most widelystudied wild-type viruses and production of recombinant retroviruses has the longest history in gene therapy, it was natural to study HIV as a potential viral vector for gene transfer (Naldini et al., 1996a). Similar to all retroviruses, the lentivirus genome consists of the gag, pol, and env coding regions that encode the core proteins, the virion-associated enzymes, and the envelope glycoprotein, flanked by the long terminal repeats (LTR, see fig. 2). The LTRs are important for integration, transcription and polyadenylation. Lentiviruses are more complex than other retroviruses since they contain other regulatory genes such as tat and rev, as well as other accessory genes including vif, vpr, vpu, and nef (Naldini et al., 1996a; Naldini and Verma, 2000; Verma and Weitzman, 2005).
The tropism and stability of the virion of the recombinant version of the HIV virus has been extended by pseudotyping the envelope glycoproteins with the envelope of the vesicular stomatitis virus glycoprotein (VSV-G) (Zhu et al., 1990). A number of non-replicating vectors have been developed that involve three viral transfection constructs: a packaging construct, the vector containing the LTRs, and a plasmid encoding the VSV-g (Kim et al., 1998; Naldini et al., 1996b). Because of the concern about HIV vector biosafety, two new generations of lentivirus vectors have been developed. The first consisted of vector production plasmids with removal all of the accessory HIV genes from the plasmid that are unnecessary for vector propagation (vif, vpr, vpu and nef) and supplying the essential tat gene in a separate plasmid to produce the tat protein in trans (Dull et al., 1998).
A third generation version of lentiviral vector, termed, the self-inactivating lentivirus, was produced by inactivating the 5′ LTR to vastly reduce the probability of a recombination event after the vector genome has integrated into the host (Miyoshi et al., 1998; Zufferey et al., 1998). These self-inactivating viral vectors lack the promoter and enhancer sequences in the LTR to prevent possible replication of the virus during vector production. Lv vectors have been extensively used in the nervous system and show a tropism toward neurons, though it does show expression in a minority of glia as well (Blömer et al., 1997; Naldini et al., 1996a; Naldini et al., 1996b; Palfi et al., 2002; Zufferey et al., 1997).
There is a long history of clinical grade scale-up of retroviral vectors for clinical trials (Verma and Weitzman, 2005) and lentivirus vectors can likewise be scaled-up for clinical grade production (Mitta et al., 2005; Segura et al., 2007).
Conclusion
Currently, viral vectors are powerful vehicles for transferring genes to the brain. This is particularly true for both the substantia nigra and the striatum which are likely targets for a PD gene therapy. Several phase 1 gene therapy trials using rAAV aimed at PD are currently underway. Nevertheless, all the vector systems have room for improved engineering that aids in their safety profiles. In addition, the likely future of gene therapy for PD will likely include most or all of the vector systems reviewed here and each vector will fill a treatment niche that takes best advantage of the properties of the particular vector.
Manufacture of Clinical Grade Viral Vectors
Introduction
Viral vector manufacturing and product testing to support human clinical trials is conducted in compliance with current Good Manufacturing Practices (cGMPs) as outlined in the US Code of Federal Regulations (21CFR) (http://www.gpoaccess.gov/cfr/index.html) and FDA guidelines (http://www.fda.gov/cber/guidelines.htm), European Commission Directive (2003/94/EC) (http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol-1/dir_2003_94/dir_2003_94_en.pdf), International Conference on Harmonization guidelines (ICH Q7A) (http://www.ich.org/), World Health Organization guidelines (http://www.who.int/medicines/en/), or country-specific codes. Given these standards, there are basic principles that have been adopted by industry and research institutions to protect the product during manufacturing. These have been reviewed previously for AAV vectors (Snyder and Francis, 2005), but have been revised and expanded here to include other vector systems. The basic principles include1) adequate cleanroom facilities that provide ample space for materials preparation, manufacturing, testing and storage. 2) process and analytical equipment that is properly installed and validated, routinely calibrated and cleaned, and placed on a preventative maintenance schedule. 3) Personnel who have relevant education or experience, and are trained for specific assigned duties, 4) Raw materials that are traceable, characterized, and meet preset specifications that qualify the materials for use, 5) a process (including production, purification, formulation, filling, storage, and shipping) that is controlled, aseptic, automated, and consistently produces a product that meets preset specifications, and 6) Quality systems that include Quality Control (QC) and Quality Assurance (QA) to ensure regulatory compliance.
Technology Transfer and Process Development
Many protocols have been published for vector production that are suitable for the research laboratory, however, these methods need to be enhanced by the process development group in preparation for clinical manufacturing. Starting with a research-scale process, process development and evaluation is usually required to increase scale, maintain asepsis through the utilization of closed systems, automate specific process steps such as chromatography, and achieve reproducibility of yield and purity. These process development activities involve developing methods for expansion of large numbers of producer cells; identifying proper sampling points that do not compromise process integrity; establishing harvesting (supernatant or cells) and cell lysis parameters; and optimizing purification (centrifugal, filtration, or chromatographic); formulation; and final filtering and filling. Identifying and sourcing raw materials and reagents suitable for human use, but having equivalent performance, is an important aspect of process development. Once the actual methods, automated programs, and raw materials are established, then the documents (batch records, reagent preparation records, test records, standard operating procedures, etc.) and specifications need to be written, and shake-down runs need to be executed at scale to evaluate performance. Working with the clinical team, the formulation of the final product must be established to provide not only the appropriate dose in an acceptable excipient, but also a product that is stable when stored and shipped to clinical sites. In addition, the volume of product filled into vials needs to take into account the actual device used to administer the product in the clinic; this may require an extra amount of product added to the vials to account for the retained volume in the delivery device. In parallel with process development, new and existing assays are developed and qualified to characterize intermediates and final product (see below). Product material generated during process development is utilized by the QC group in the refinement and qualification of the various assays.
It is important to have a perspective that the process and assays will ultimately support product licensure, therefore the origin of cell lines and other materials that form the basis of the manufacturing process should have a documented history. Adaptation to suspension culture and serum-free culture has its advantages, and the manufacturing process can evolve during the clinical phases, but bridging studies to demonstrate product equivalency may be needed.
Facilities and equipment
The manufacturing cleanroom facilities are designed to segregate the progressive steps of the manufacturing process (cell culture, purification, filling), and reduce or eliminate cross contamination by controlling the flow of air, materials, product, waste, and personnel. A suitable layout that is designed in consideration of the process ensures the proper flow of manufacturing activities and an ability to maintain cleanliness. The cleanrooms are each classified (e.g., class 10,000 cleanrooms: ISO class 7) and are pressurized (positive or negative) in a configuration suitable for the specific process and containment. Adjacent to the processing modules are anterooms for gowning and de-gowning, and materials staging. All of the surfaces are cleanable, with hardcore ceilings, recessed and sealed fixtures, and epoxy or stainless steel surfaces that can resist the stringent cleaning agents. All rooms are outfitted with moveable equipment (except for the biosafety cabinets (BSCs) and other hard-piped equipment) and stainless steel furniture to maximize flexibility and ease of cleaning within the facility. Access to the entrances and exits of the facility are restricted with electronic or metal key access. Electronically activated magnetic interlocking doors control personnel movement within the facility and help to maintain room pressure differentials. Air to classified areas is supplied via HEPA filters, and is exhausted and filtered to the exterior through high-velocity exhaust stacks. Open steps during processing are performed exclusively in thimble-ducted ISO 5 BSCs.
A dedicated controlled access QC laboratory that is suitable for analytical and microbiological tests is equipped to perform the in-house reagent, intermediate product, final product, stability, and environmental testing. Additional manufacturing support space houses a clean steam autoclave and depyrogenation oven for materials preparation, cold storage (refrigerators, ,−20°C and −80°C freezers, liquid nitrogen vapor), autoclaves for waste inactivation, and temperature controlled quarantine and release space for raw materials and reagents, and intermediate and final product. Equipment and critical facility systems are calibrated and validated (Installation Qualification and Operational Qualification, and for some equipment, Performance Qualification) for manufacturing and QC operations. A central computerized monitoring system monitors temperature controlled equipment and room pressurization, temperature and humidity. This system also automatically notifies on-call personnel when operating limits are exceeded and they can respond in the event of an equipment failure.
Procedures for cleaning, environmental monitoring, equipment calibration and preventative maintenance, and facility preventative maintenance should be established according to a schedule. Line clearance procedures should be established and are executed to remove materials and reagents used for the manufacture of the previous batch before beginning the manufacture of the next batch. Change over procedures are established and executed between different vector products where extensive cleaning is performed, and equipment reconfigurations and other changes are made.
Organization and Personnel training
The different departments have independent responsibilities but it is imperative that they work together to manufacture, test, and release products. Personnel hired into the various departments need to have appropriate education and/or experience. A reporting structure within each of the manufacturing, facilities, QC, and QA departments is necessary, and each person is assigned specific responsibilities. Manufacturing operators and facilities technicians report to a supervisor who oversees day-to-day operations of the facility. The QC supervisor coordinates technicians who conduct environmental and personnel monitoring and testing of raw materials, in-process and product samples. QA’s independent oversight of all manufacturing and testing activities and especially for test acceptability and product release is key to the quality systems. To eliminate conflict of interest, QA and QC are independent from the manufacturing unit and ensure that production and testing activities are in compliance with cGMPs.
Training
Once personnel are assigned specific responsibilities, training is conducted to ensure that the trainee can properly execute the procedures described in the written documents, and properly record their activities. When individuals can complete the task competently, they have completed training and are allowed to perform the tasks independently. Refresher training for specific procedures is provided on an annual basis to each employee. General training sessions regarding current Good Manufacturing Practices regulations are held for new employees and on an annual basis for all employees. All training and relevant education records are archived by QA in personnel training files.
Quality Control
QC personnel have the responsibility to 1) conduct environmental monitoring of the facility for microbial and particulate contaminants to determine if it is within the specifications for cleanliness, both “at rest” and when personnel are present, 2) monitor the proper gowning and gowning cleanliness of manufacturing personnel during the manufacturing process, 3) sample and test raw materials to ensure adherence to specifications, 4) evaluate the manufacturing process by sampling and testing intermediates, 5) develop and qualify new assays, 6) characterize the product, and 7) perform product safety testing. Data generated by QC is provided to manufacturing and QA for their timely responses to process, facility, and testing results.
Quality Assurance
There are hundreds of documents that are utilized by a manufacturing and testing operation. The majority of these documents describe processes, facility operations, and quality systems that are unique to the operation. In addition, these documents are highly cross referenced since the manufacturing activities, cleaning procedures, testing procedures, and specific equipment and facilities are highly integrated. These include: standard operating procedures, validation protocols and reports, cleaning and use logs, batch records, test records, training records, product release specifications, raw materials specifications; reagent preparation documents, forms, etc. QA together with the manufacturing, facilities, and QC departments develop the protocols and procedures used in manufacturing and testing. QA personnel 1) review, maintain, issue, and archive documents, 2) audit all manufacturing documents and QC data, including those from external vendors, 3) release raw materials for use in manufacturing and final product lots for use in the clinic, 4) issue and control all reagents, product intermediates, and final product labels, 5) document, investigate and correct/prevent process and testing variances, 6) review validation master plans, and all facility, process, and assay validation protocols, including acceptance criteria, specifications and operating limits, and 7) provide change control for changes made to equipment, process, or specifications. These quality systems ensure that a safe, pure, potent, and stable product is manufactured and characterized prior to being administered to patients.
Manufacturing and Testing
There are many similarities in the manufacture and testing of different viral vectors. The basic scheme, as reviewed previously for AAV vectors (Snyder and Francis, 2005), includes sourcing suitable raw materials; utilizing qualified cell and viral banks; producing, purifying, and formulating the vector; vialing the vector; and performing QC tests for vector characterization and safety parameters.
Reagents and Raw Materials
Reagents and raw materials should be chosen that are of high quality, traceable, and readily available. Acceptability criteria and specifications should be established for each item. GMP manufactured, United States Pharmacopoeia (USP), or multi-compendial grade chemicals and water for injection (WFI) should be used whenever possible. Animal derived materials, if needed for production processes, should be accompanied by Certificates of Origin (COO) to ensure the safety of these materials. In some cases, custom reagents and materials need to be made by an outside vendor, this may require detailed product diagrams and specifications, and once in the vendor’s queue, may require several weeks or months to be delivered. Upon receipt from the vendor, these materials are inspected by QC, labeled and placed in quarantine storage, and the Certificate of Analysis (COA) is compared to preset specifications. Once the verification of the specifications is complete, then the materials are over-labeled and moved to the designated released storage area for subsequent use in the manufacturing process. These material management procedures also apply to manufacturing reagents (such as chromatography buffers) that are produced on-site.
Plasmids
Plasmid DNA and E. Coli cell banks are needed for processes that require transient transfection. Plasmid backbones that confer kanamycin resistance (or a non-antibiotic based selection marker (Soubrier et al., 2005) and harbor the elements for replication in E. coli are preferred because β-lactam containing antibiotics may derivatize proteins and generally should not be used in production runs because of the risk of hypersensitization in product recipients (FDA, 1985). Glycerol stabilized Master Cell Banks are produced and plasmid DNA preparations derived from these MCBs are sequenced and analyzed as part of qualification and release prior to vector manufacturing.
Cell Culture reagents
Water for injection, trypsin, and phosphate buffered saline (PBS) of suitable quality are available from commercial sources, and tissue culture media can be pre-formulated, tested and released prior to vector manufacturing.
Cell and Viral Banks
Master Cell Banks (MCBs) and Master Viral Banks (MVBs) are made and tested under cGMPs. These banks should be large enough to meet current and future production needs, and periodically tested for viability and sterility. The components and reagents used during the manufacture of the banks, should be sourced such that appropriate quality standards can be met. Working cell banks (WCBs) and Working Viral Banks (WVBs) should also be made in compliance with cGMP. Cells or virus from the master bank are cultured using qualified reagents and components, and the working bank is tested and characterized to qualify for use.
Process
The size of the batch is determined by the capacity of the equipment and facility, and the duration of the manufacturing campaign is dictated by total amount of vector that is needed for the clinical protocol, product release testing, and stability studies. Process data is recorded recorded in batch records during each step including the lot numbers for raw materials and reagents, execution of process steps including any deviations, and equipment used.
Production and harvest
Vials of the WCB are thawed and seeded into culture vessels, then split until enough cells are generated. Production cells are transfected, infected, or induced to produce vector, and the supernatant (Lentiviral, retroviral, and HSV vectors) or cells (adenoviral, AAV, and HSV vectors) are harvested. At the end of the incubation, cells are pooled and a sample is mixed with spent media for mycoplasma, bioburden, and adventitious agent testing (fig. 2). The remaining cells or supernatant is centrifuged, transferred to a bioprocess container (BPC), and stored until vector purification.
Purification
If vector is cell-associated, then cells are lysed and clarified. Cell lysate or harvested cell supernatant is purified chromatographically, or using centrifugal or filtration methods. All purification steps are carried out using closed systems that can incorporate disposable containers such as flexible bioprocess bags bioprocess bags. During automated chromatography, UV absorbance, pH, system pressure, flow rate, temperature, and conductivity are monitored and recorded by the FPLC system; this data can be attached to the batch record to support process performance. The purified material is designated the “Purified Bulk”. Samples of the Purified Bulk are taken for testing, and the remainder is stored at the proper temperature. Following purification, all process equipment undergoes cleaning and sanitization using appropriate agents such as NaOH or steam according to SOPs.
Formulation and filling
Following testing, Purified Bulk that meets the predetermined specifications is thawed (if necessary), formulated into the correct physiological buffer at the proper concentration, and sterile filtered. Formulation can be achieved by gel filtration chromatography or tangential flow filtration. The Final Filtered Bulk is filled at a clinically useful volume into vials that are labeled and stored at the proper temperature. Samples of the final vialed product are taken for characterization, and safety and stability testing.
Storage, Shipping, and Use
Product vials are stored in an inventoried and locked controlled temperature environment. Vials are packaged on dry ice or wet ice in an insulated container and shipped to the clinical site. The shipping container is outfitted with a temperature monitoring device to ensure appropriate temperatures are maintained during shipping. Upon receipt the product vials are removed immediately from the container and again stored at the proper temperature until required for patient administration. When required for use, vials are removed from storage following pharmacy procedures, thawed, if necessary, and the vector dose is administered to the patient.
Assays
Several assays have been published to evaluate vectors used for research, however, additional assay development may be required to achieve sufficient accuracy, precision, reproducibility, sensitivity, range, and/or limit of detection in order to support clinical product testing. Standards and controls need to be characterized and generated in sufficient quantities to support assay development, assay qualification, and product testing. If available, community recognized reference standards should be used to calibrate in-house standards. When the assay evolves to a mature state, then test records and reagent preparation logs need to be drafted. Once the assay is established, then the assays are qualified to demonstrate that they meet appropriate (robustness, reproducibility, ruggedness, specificity, sensitivity, etc.) for eventual assay validation. In addition, the QC unit should establish a sample receipt and tracking system used for in-house and contract laboratory testing. This system will help organize the testing required for each sample, when samples will be tested, anticipated result dates, and will remove confusion during the testing of multiple samples and similar samples from different batches.
Intermediate and Final Product Testing
Testing and characterization of each lot of the vector is performed under cGMPs prior to product release. Release documentation (a COA, that lists the tests, specifications, and results) for each product lot is submitted to the US Food and Drug Administration (FDA) or other regulatory agency. Once produced and purified, the vector batch is characterized using qualified assays to ensure it meets preset specifications for safety, identity, purity, potency, and stability. These tests can be expensive and consume a significant amount of vector that approaches the amount of vector needed for the clinical doses, thus manufacturing twice as much vector than is actually required in the clinic is a significant additional cost.
Assay for protein purity and identity
Batches are analyzed by silver or Coomassie blue staining of viral vector proteins separated on reduced and non-reduced SDS polyacrylamide gels (Laemmli, 1970). The proper number, molecular weight, and stoichiometry of the viral proteins can be used to positively identify the vector. Protein encoded by the vector transgene can be expressed during vector production and may co-purify with the vectors, possibly leading to immunological response against the vector-encoded protein upon vector administration. Cellular proteins and viral protein fragments are just two other types of impurities that may reside in vector preparations. Identification of protein contaminants or impurities using specific antibodies, mass spectrometry, or protein sequencing can help trace the source (cellular, serum protein, etc.) in order to modify the manufacturing process to reduce or eliminate the contamination or impurity.
Assay for infectious vector
An infectious titer assay or transduction assay using permissive cells is used to determine the infectious titer of the vector (Sastry et al., 2002; Tollefson et al., 2007). Since an accurate titer is desired, it is important that the assay is analyzed prior to a second round of infection that could inflate the titer values.
Assay for replication competent vector (RCV)
Dilutions of the vector preparation are used to infect a non-complementing cell line and only particles in the preparation that have packaged a wild-type or pseudo-wild-type genome are capable of replication. Wild-type virus may contaminate vector preparations, and RCV may be formed during production due to recombination between the vector and the helper sequences found in complementary cell lines or plasmids (Escarpe et al., 2003; Murakami et al., 2002; Nony et al., 2003; Sastry et al., 2005) The presence of wild-type virus or RCV may affect vector expression and mobilization.
Assays for transgene expression and potency
Transgene expression is evaluated by transducing cells in vitro, and harvesting the supernatant or the transduced cells, and testing for the presence of the transgene protein product by ELISA, cell staining, immunoblot, or enzymatic activity assay. Potency assays correlate with titer, but specifically measure the biologic function of the product in vitro or in vivo. Although it might be suggested that testing the product in a small mammal (either mice or rats) might provide an appropriate assay for potency, the human transgene product would need to function in the particular disease model.
DNA hybridization assay, PCR, or optical density for physical particle titer and the particle to infectivity ratio
The DNA hybridization assay is used to determine the titer of virions that contain vector genomes. Plasmid and unpackaged vector DNA is digested with nuclease. Encapsidated vector genomes are liberated by protease digestion, followed by phenol extraction and ethanol precipitation. A dilution series of the vector plasmid DNA that was packaged is prepared. DNA is immobilized by blotting or filtering, and the blots are probed for the transgene or promoter and exposed to film or phospho-imager screen. The vector DNA signal is compared to the signal generated from the plasmid DNA standard curve, and extrapolated to determine a vector genome titer. Alternatively, real-time PCR-based assays can be used to determine the vector genome titer using appropriate primers and fluorescent probes (Clark et al., 1999; Puntel et al., 2006). Lastly, the optical density (A260) can be measured to calculate a particle titer (Mittereder et al., 1996; Sommer et al., 2003). A comparison of the vector genome titer or particle titer to the infectious titer produces the particle:infectious (P:I) ratio.
Appearance, pH, and Osmolarity
Vials of final product are thawed, if necessary, and visually inspected against a white and a black background for discoloration of the liquid and presence of particulates. Vector aggregation can be evaluated by dynamic light scattering. Purified bulk or vials of the final product are tested for pH, conductivity, or osmolality using calibrated testing equipment to ensure proper formulation.
Safety testing
Safety testing is conducted to ensure that process intermediates or final product is free of detectable contaminating agents or process residuals that can pose risks to patients. These assays can be developed for on-site testing, but there are several commercial vendors who offer testing services. These services should be GMP compliant with appropriate controls and validity criteria, and the vendor will indicate the amount and type of sample required for their tests. However, it is still the responsibility of QC and QA to review the vendor test report for validity and accuracy. The primary safety tests include: Adventitious agents (in vitro and in vivo). These assays are designed to detect the presence of infectious viral agents of human or animal origin. A choice of cell lines (e.g. human, murine, bovine, porcine, etc) is available for the in vitro assay where as rodents and embryonated hen’s eggs are commonly used for the in vivo assay. Mycoplasma. The test for the presence of mycoplasma relies on the growth of mycoplasma during the expansion of indicator cells in antibiotic-free conditions and detection of the organism using dye or PCR, as well as growth of mycoplasma in broth or on appropriate agar media. Endotoxin. Endotoxin can be detected using the Limulus Amebocyte Lysate Assay or by using a rabbit pyrogen test. Sterility. This assay determines the absence of bacterial or fungal organisms and must also include the performance of bacteriastasis and fungistasis analysis. General safety. This assay is designed to determine if there are toxins (chemical or biological) present that induce an acute toxicity in animals.
Stability Study
A stability study should be designed to generate data for a purified viral vector at the proper storage temperature, formulation, and fill volume, and in the storage container used for patient doses (i.e. final product). The study is designed to demonstrate genetic and physiochemical stability, and container integrity at specified intervals for at least the duration of the clinical trial. Lastly, studies to ensure that the transportation conditions are maintained are also conducted, and temperature tracking monitors are included in the shipments.
In conclusion, the process controls and quality systems outlined here apply to the manufacture and testing of most viral gene transfer vector products. Referring to the current regulations and regulatory guidance is essential when developing compliant processes, test methods, and procedures, and a perspective that these activities will ultimately support licensure is important. The product specifications, manufacturing methodology, facilities, etc will vary as to the stage of product development, so as the product is evaluated in Phase II, Phase III trials and after licensure (Phase IV), the manufacture and testing of the product requires progressively more stringent controls. Continued review of federal and international regulations, guidances, and other regulatory information sources to ensure that processes and procedures are adapted to meet the current requirements. Incorporation of process and quality controls into product development, manufacture, and testing ensures that patients are protected during clinical trials and commercial distribution.
Acknowledgments
RJM and CB were supported by P01 NS363602. ROS is an inventor on patents related to recombinant AAV technology and owns equity in a gene therapy company that is commercializing AAV for gene therapy applications. To the extent that the work in this manuscript increases the value of these commercial holdings ROS has a conflict of interest. We thank Joyce Francis for helpful comments and discussion. This project was funded, in part, by UF College of Medicine start-up funds and Association Francaise contre les Myopathies (AFM) Award 12263 to R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet LD, Poenaru L, Perricaudet M, Kahn A, Peschanski MR. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3:224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB, Kingsman SM, Kingsman AJ, Mazarakis ND. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson’s disease. J Neurosci. 2002;22:10302–10312. doi: 10.1523/JNEUROSCI.22-23-10302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkats M, Bilang-Bleuel A, Buc-Caron MH, Castel-Barthe MN, Corti O, Finiels F, Horellou P, Revah F, Sabate O, Mallet J. Adenovirus in the brain: recent advances of gene therapy for neurodegenerative diseases. Prog Neurobiol. 1998;55:333–341. doi: 10.1016/s0301-0082(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, Horellou P. Intrastriatal injection of an adenoviral vector expression glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc Natl Acad Sci USA. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blömer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH. Highly efficient and sustained gene transfer in adult neurons and lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MC, Choi-Lundberg DL, Davidson BL, Leranth C, Kozlowski DA, Smith JC, O’Banion MK, Redmond D. Adenovirus-mediated transgene expression in nonhuman primate brain. Hum Gene Ther. 1999;10:1175–1184. doi: 10.1089/10430349950018166. [DOI] [PubMed] [Google Scholar]
- Booth MJ, Mistry A, Li X, Thrasher A, Coffin RS. Transfection-free and scalable recombinant AAV vector production using HSV/AAV hybrids. Gene Ther. 2004;11:829–837. doi: 10.1038/sj.gt.3302226. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk O, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Molecular Therapy. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Burger C, Nash K, Mandel RJ. Recombinant adeno-associated viral vectors in the nervous system. Hum Gene Ther. 2005;16:781–791. doi: 10.1089/hum.2005.16.781. [DOI] [PubMed] [Google Scholar]
- Burton EA, Fink DJ, Glorioso JC. Gene delivery using herpes simplex virus vectors. DNA Cell Biol. 2002;21:915–936. doi: 10.1089/104454902762053864. [DOI] [PubMed] [Google Scholar]
- Burton EA, Fink DJ, Glorioso JC. Replication-defective genomic HSV gene therapy vectors: design, production and CNS applications. Curr Opin Mol Ther. 2005;7:326–336. [PubMed] [Google Scholar]
- Burton EA, Glorioso JC, Fink DJ. Gene therapy progress and prospects: Parkinson’s disease. Gene Ther. 2003;10:1721–1727. doi: 10.1038/sj.gt.3302116. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, Rusby JE, Wood MJA, Charlton HM. Adenovirus gene transfer causes inflammation in the brain. Neurosci. 1995;66:1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, Wood MJ, Charlton HM. Role of T cells in inflammation caused by adenovirus vectors in the brain. Gene Ther. 1996;3:644–651. [PubMed] [Google Scholar]
- Candolfi M, Curtin JF, Xiong WD, Kroeger KM, Liu C, Rentsendorj A, Agadjanian H, Medina-Kauwe L, Palmer D, Ng P, Lowenstein PR, Castro MG. Effective 29 high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol Ther. 2006;14:371–381. doi: 10.1016/j.ymthe.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Liu Y, During MJ, Xiao W. High-titer, wild-type free recombinant adeno-associated virus vector production using intron-containing helper plasmids. J Virol. 2000;74:11456–11463. doi: 10.1128/jvi.74.24.11456-11463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Tomanek L. Herpes simplex virus and the chemokines that mediate the inflammation. Curr Top Microbiol Immunol. 2006;303:47–65. doi: 10.1007/978-3-540-33397-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang LN, Chiang L, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Clark KR, Liu X, McGrath JP, Johnson PR. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- Conway JE, Zolotukhin S, Muzyczka N, Hayward GS, Byrne BJ. Recombinant adeno-associated virus type 2 replication and packaging is entirely supported by a herpes simplex virus type 1 amplicon expressing Rep and Cap. J Virol. 1997;71:8780–8789. doi: 10.1128/jvi.71.11.8780-8789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti O, Sabaté O, Horellou P, Colin P, Dumas S, Buchet D, Buc-Caron MH, Mallet J. A single adenovirus vector mediates doxycycline-controlled expression of tyrosine hydroxylase in brain grafts of human neural progenitors. Nat Biotechnol. 1999;17:349–354. doi: 10.1038/7901. [DOI] [PubMed] [Google Scholar]
- Danthinne X, Imperiale MJ. Production of first generation adenovirus vectors: a review. Gene Ther. 2000;7:1707–1714. doi: 10.1038/sj.gt.3301301. [DOI] [PubMed] [Google Scholar]
- Dass B, Olanow CW, Kordower JH. Gene transfer of trophic factors and stem cell grafting as treatments for Parkinson’s disease. Neurology. 2006;66:S89–103. doi: 10.1212/wnl.66.10_suppl_4.s89. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, Chiorini JA. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ, Southgate TD, Klatzmann D, Lassmann H, Castro MG, Lowenstein PR. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- Do Thi NA, Saillour P, Ferrero L, Dedieu JF, Mallet J, Paunio T. Delivery of GDNF by an E1, E3/E4 deleted adenoviral vector and driven by a GFAP promoter prevents dopaminergic neuron degeneration in a rat model of Parkinson’s disease. Gene Ther. 2004;11:746–756. doi: 10.1038/sj.gt.3302222. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escarpe P, Zayek N, Chin P, Borellini F, Zufferey R, Veres G, Kiermer V. Development of a sensitive assay for detection of replication-competent recombinant lentivirus in large-scale HIV-based vector preparations. Mol Ther. 2003;8:332–341. doi: 10.1016/s1525-0016(03)00167-9. [DOI] [PubMed] [Google Scholar]
- Farson D, Harding TC, Tao L, Liu J, Powell S, Vimal V, Yendluri S, Koprivnikar K, Ho K, Twitty C, Husak P, Lin A, Snyder RO, Donahue BA. Development and characterization of a cell line for large-scale, serum-free production of recombinant adeno-associated viral vectors. J Gene Med. 2004;6:1369–1381. doi: 10.1002/jgm.622. [DOI] [PubMed] [Google Scholar]
- FDA. Office of Biologics Research and Review Center for Drugs and Biologics. 1985. Points to consider in production and testing of new drugs and biologicals produced by recombinant DNA technology. [Google Scholar]
- Fink DJ, Glorioso JC. Engineering herpes simplex virus vectors for gene transfer to neurons. Nat Med. 1997;3:357–359. doi: 10.1038/nm0397-357. [DOI] [PubMed] [Google Scholar]
- Fink DJ, Poliani PL, Oligino T, Krisky DM, Goins WF, Glorioso JC. Development of an HSV-based vector for the treatment of Parkinson’s disease. Exp Neurol. 1997;144:103–121. doi: 10.1006/exnr.1996.6395. [DOI] [PubMed] [Google Scholar]
- Fraefel C, Jacoby DR, Breakefield XO. Herpes simplex virus type 1-based amplicon vector systems. Adv Virus Res. 2000;55:425–451. doi: 10.1016/s0065-3527(00)55011-8. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes CA, Castro MG, Lowenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol Ther. 2000;2:330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harui A, Suzuki S, Kochanek S, Mitani K. Frequency and stability of chromosomal integration of adenovirus vectors. J Virol. 1999;73:6141–6146. doi: 10.1128/jvi.73.7.6141-6146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N. Production and purification of recombinant adeno-associated virus. Methods Enzymol. 2000;316:743–761. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- Hillgenberg M, Tonnies H, Strauss M. Chromosomal integration pattern of a helper-dependent minimal adenovirus vector with a selectable marker inserted into a 27.4-kilobase genomic stuffer. J Virol. 2001;75:9896–9908. doi: 10.1128/JVI.75.20.9896-9908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszthy PC, Svendsen A, Wilson JM, Kotin RM, Lonning PE, Bjerkvig R, Hoover F. Widespread dispersion of adeno-associated virus serotype 1 and adeno-associated virus serotype 6 vectors in the rat central nervous system and in human glioblastoma multiforme xenografts. Hum Gene Ther. 2005;16:381–392. doi: 10.1089/hum.2005.16.381. [DOI] [PubMed] [Google Scholar]
- Kamen A, Henry O. Development and optimization of an adenovirus production process. J Gene Med 6 Suppl. 2004;1:S184–192. doi: 10.1002/jgm.503. [DOI] [PubMed] [Google Scholar]
- Kay MA, Nakai H, Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. Looking into the safety of AAV vectors: AAV serotype 2 vectors preferentially integrate into active genes in mice. Nature: Nat Genet. 2003;424(34):251, 297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]
- Kim VN, Mitrophanous K, Kingsman SM, Kingsman AJ. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J Virol. 1998;72:811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest. 2003;111:785–793. doi: 10.1172/JCI18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Meyer EM, Peel AL, Zolotukhin S, Meyers C, Muzyczka N, King MA. Neuron-specific transduction in the rat septohippocampal or nigrostriatal pathway by recombinant adeno-associated virus vectors. Exp Neurol. 1998;150:183–194. doi: 10.1006/exnr.1997.6736. [DOI] [PubMed] [Google Scholar]
- Kohlbrenner E, Aslanidi G, Nash K, Shklyaev S, Campbell-Thompson M, Byrne BJ, Snyder RO, Muzyczka N, Warrington KH, Jr, Zolotukhin S. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA, McLaughlin S, Muzyczka N, Rocchi M, Berns KI. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Robert JJ, Berrard S, Ridoux V, Stratford-Perricaudet LD, Perricaudet M, Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR. Input virion proteins: cryptic targets of antivector immune responses in preimmunized subjects. Molecular Therapy. 2004;9:771–774. doi: 10.1016/j.ymthe.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR, Castro MG. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: peculiarities, mechanisms, and consequences. Gene Ther. 2003;10:946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR, Mandel RJ, Xiong W-d, Kroeger KM, Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Current Gene Therapy. 2007 doi: 10.2174/156652307782151498. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR, Thomas CE, Umana P, Gerdes CA, Verakis T, Boyer O, Tondeur S, Klatzmann D, Castro MG. High-capacity, helper-dependent, “gutless” adenoviral vectors for gene transfer into brain. Methods Enzymol. 2002;346:292–311. doi: 10.1016/s0076-6879(02)46062-4. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Burger C. Clinical trials in neurological disorders using AAV vectors: promises and challenges. Curr Opin Mol Ther. 2004;6:482–490. [PubMed] [Google Scholar]
- Mandel RJ, Manfredsson FP, Foust KD, Rising A, Reimsnider S, Nash K, Burger C. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol Ther. 2006;13:463–483. doi: 10.1016/j.ymthe.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Millhouse S, Wigdahl B. Molecular circuitry regulating herpes simplex virus type 1 latency in neurons. J Neurovirol. 2000;6:6–24. doi: 10.3109/13550280009006378. [DOI] [PubMed] [Google Scholar]
- Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC, Ecker JR, Bushman FD. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitta B, Rimann M, Fussenegger M. Detailed design and comparative analysis of protocols for optimized production of high-performance HIV-1-derived lentiviral particles. Metab Eng. 2005;7:426–436. doi: 10.1016/j.ymben.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moralli D, Simpson KM, Wade-Martins R, Monaco ZL. A novel human artificial chromosome gene expression system using herpes simplex virus type 1 vectors. EMBO Rep. 2006;7:911–918. doi: 10.1038/sj.embor.7400768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami P, Pungor E, Files J, Do L, van Rijnsoever R, Vogels R, Bout A, McCaman M. A single short stretch of homology between adenoviral vector and packaging cell line can give rise to cytopathic effect-inducing, helper-dependent E1-positive particles. Hum Gene Ther. 2002;13:909–920. doi: 10.1089/10430340252939023. [DOI] [PubMed] [Google Scholar]
- Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Muzyczka N, Berns KI. Parvoiridae; The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott, Williams and Wilkins; New York: 2001. pp. 2327–2360. [Google Scholar]
- Naldini L, Blömer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996a;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996b;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Naldini L, Verma IM. Lentiviral vectors. Adv Virus Res. 2000;55:599–609. doi: 10.1016/s0065-3527(00)55020-9. [DOI] [PubMed] [Google Scholar]
- Nienhuis AW. Assays to evaluate the genotoxicity of retroviral vectors. Mol Ther. 2006;14:459–460. doi: 10.1016/j.ymthe.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Nony P, Chadeuf G, Tessier J, Moullier P, Salvetti A. Evidence for packaging of rep-cap sequences into adeno-associated virus (AAV) type 2 capsids in the absence of inverted terminal repeats: a model for generation of rep-positive AAV particles. J Virol. 2003;77:776–781. doi: 10.1128/JVI.77.1.776-781.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehmig A, Fraefel C, Breakefield XO. Update on herpesvirus amplicon vectors. Mol Ther. 2004a;10:630–643. doi: 10.1016/j.ymthe.2004.06.641. [DOI] [PubMed] [Google Scholar]
- Oehmig A, Fraefel C, Breakefield XO, Ackermann M. Herpes simplex virus type 1 amplicons and their hybrid virus partners, EBV, AAV, and retrovirus. Curr Gene Ther. 2004b;4:385–408. doi: 10.2174/1566523043346129. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Palfi S, Leventhal L, Chu Y, Ma SY, Emborg M, Bakay R, Deglon N, Hantraye P, Aebischer P, Kordower JH. Lentivirally delivered glial cell line-derived neurotrophic factor increases the number of striatal dopaminergic neurons in primate models of nigrostriatal degeneration. J Neurosci. 2002;22:4942–4954. doi: 10.1523/JNEUROSCI.22-12-04942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti-wt-AAV2 Antibodies Inhibit rAAV2-, but not rAAV5-mediated Gene Transfer in the Brain. Journal of Virology. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntel M, Curtin JF, Zirger JM, Muhammad AK, Xiong W, Liu C, Hu J, Kroeger KM, Czer P, Sciascia S, Mondkar S, Lowenstein PR, Castro MG. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum Gene Ther. 2006;17:531–544. doi: 10.1089/hum.2006.17.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Reimsnider S, Manfredsson FP, Muzyczka N, Mandel RJ. Time Course of Transgene Expression After Intrastriatal Pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 Transduction in the Rat. Mol Ther. 2007 doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- Roizman B, Sears AE. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Sanftner LM, Suzuki BM, Doroudchi MM, Feng L, McClelland A, Forsayeth JR, Cunningham J. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol Ther. 2004;9:403–409. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Sastry L, Johnson T, Hobson MJ, Smucker B, Cornetta K. Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther. 2002;9:1155–1162. doi: 10.1038/sj.gt.3301731. [DOI] [PubMed] [Google Scholar]
- Sastry L, Xu Y, Duffy L, Koop S, Jasti A, Roehl H, Jolly D, Cornetta K. Product-enhanced reverse transcriptase assay for replication-competent retrovirus and lentivirus detection. Hum Gene Ther. 2005;16:1227–1236. doi: 10.1089/hum.2005.16.1227. [DOI] [PubMed] [Google Scholar]
- Segura MM, Garnier A, Durocher Y, Coelho H, Kamen A. Production of lentiviral vectors by large-scale transient transfection of suspension cultures and affinity chromatography purification. Biotechnol Bioeng. 2007 doi: 10.1002/bit.21467. [DOI] [PubMed] [Google Scholar]
- Shah AC, Benos D, Gillespie GY, Markert JM. Oncolytic viruses: clinical applications as vectors for the treatment of malignant gliomas. J Neurooncol. 2003;65:203–226. doi: 10.1023/b:neon.0000003651.97832.6c. [DOI] [PubMed] [Google Scholar]
- Shenk T. Adenoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fundamental Virology. Lippincott-Raven Co.; New York: 1996. pp. 979–1016. [Google Scholar]
- Sinn PL, Sauter SL, McCray PB., Jr Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors--design, biosafety, and production. Gene Ther. 2005;12:1089–1098. doi: 10.1038/sj.gt.3302570. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Francis J. Adeno-associated viral vectors for clinical gene transfer studies. Curr Gene Ther. 2005;5:311–321. doi: 10.2174/1566523054065066. [DOI] [PubMed] [Google Scholar]
- Sommer JM, Smith PH, Parthasarathy S, Isaacs J, Vijay S, Kieran J, Powell SK, McClelland A, Wright JF. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol Ther. 2003;7:122–128. doi: 10.1016/s1525-0016(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, Wilson JM, Crystal RG. Enhanced Survival of the LINCL Mouse Following CLN2 Gene Transfer Using the rh.10 Rhesus Macaque-derived Adeno-associated Virus Vector. Mol Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- Soubrier F, Laborderie B, Cameron B. Improvement of pCOR plasmid copy number for pharmaceutical applications. Appl Microbiol Biotechnol. 2005;66:683–688. doi: 10.1007/s00253-004-1729-9. [DOI] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, Wilson JM, Debyser Z, Baekelandt V. Comparative Analysis of Adeno-Associated Viral Vector Serotypes 1, 2, 5, 7, And 8 in Mouse Brain. Hum Gene Ther. 2007 doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001a;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001b;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- Tollefson AE, Kuppuswamy M, Shashkova EV, Doronin K, Wold WS. Preparation and titration of CsCl-banded adenovirus stocks. Methods Mol Med. 2007;130:223–235. doi: 10.1385/1-59745-166-5:223. [DOI] [PubMed] [Google Scholar]
- Tyler CM, Wuertzer CA, Bowers WJ, Federoff HJ. HSV amplicons: neuro applications. Curr Gene Ther. 2006;6:337–350. doi: 10.2174/156652306777592045. [DOI] [PubMed] [Google Scholar]
- Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Wood MJ, Charlton HM, Wood KJ, Kajiwara K, Byrnes AP. Immune responses to adenovirus vectors in the nervous system. Trends Neurosci. 1996;19:497–501. doi: 10.1016/S0166-2236(96)10060-6. [DOI] [PubMed] [Google Scholar]
- Yant SR, Ehrhardt A, Mikkelsen JG, Meuse L, Pham T, Kay MA. Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo. Nat Biotechnol. 2002;20:999–1005. doi: 10.1038/nbt738. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Muruve DA. Immune responses to adeno-associated virus vectors. Curr Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- Zermansky AJ, Bolognani F, Stone D, Cowsill CM, Morrissey G, Castro MG, Lowenstein PR. Towards global and long-term neurological gene therapy: unexpected transgene dependent, high-level, and widespread distribution of HSV-1 thymidine kinase throughout the CNS. Mol Ther. 2001;4:490–498. doi: 10.1006/mthe.2001.0479. [DOI] [PubMed] [Google Scholar]
- Zhu ZH, Chen SS, Huang AS. Phenotypic mixing between human immunodeficiency virus and vesicular stomatitis virus or herpes simplex virus. J Acquir Immune Defic Syndr. 1990;3:215–219. [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJJ, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO. Production and purification of serotype 1, 2, and 5 recombinant adeno- associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient In vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]