Abstract
In many species including humans, antagonists of NMDA-type glutamate receptors such as dextromethorphan, when used at sufficient doses, have been found to be relatively safe and effective antitussives. Similarly, now in five different species (guinea pigs, rabbits, cats, dogs and pigs), neurokinin receptor antagonists have also proven to be safe and effective antitussive agents. Both of these classes of drugs act centrally to prevent cough. A brief review of what is known about the central encoding of cough is presented, as are the advantages of centrally acting antitussives. Also discussed are new insights into cough and NMDA receptor signaling that may lead to the development of more effective antitussive agents with limited side effects and broad application in treating cough associated with a variety of aetiologies.
Keywords: NMDA, dextromethorphan, memantine, D-serine, nitrosylation
1. Introduction
Codeine and dextromethorphan are used extensively and specifically for the treatment of cough and likely exert their effects through central sites of action [1,2]. The neurokinin receptor antagonists, amongst the more promising class of therapeutics evaluated for cough over the past 20 years, also likely act primarily via actions in the central nervous system (CNS) to prevent or reduce coughing in guinea pigs, rabbits, cats, dogs and pigs [3-10]. Despite these past successes at treating cough with centrally-acting drugs, most current drug discovery efforts for cough and the majority of the physiological studies of the cough reflex have focused on the peripheral terminals of vagal afferent nerves [11,12]. We propose that the medullary synapses of the vagal afferents regulating cough are not only the primary site of action of existing antitussive therapies, but that these central terminals are also the most appropriate and desirable site of action of new antitussive therapies. The central synaptic properties of the vagal afferent nerves regulating cough are briefly reviewed and features of these synapses that render them amenable to therapeutic intervention are described.
2. Central terminations of the vagal afferent nerves regulating cough
Coughing is initiated by activation of either C-fibres or subtypes of mechanically-sensitive, acid-sensitive, capsaicin-insensitive vagal afferents innervating the larynx, trachea and large bronchi [13-16]. No direct physiological studies of the central terminations of these afferents have been described. There have been several barriers to completing these experiments. For example, C-fibre dependent cough is prevented by general anaesthesia [13,14,17-20]. Other C-fibre-dependent reflexes can be evoked in anaesthetized animals, but the ability of C-fibre-selective stimuli such as capsaicin and bradykinin to evoke coughing is greatly diminished. Studies of bronchopulmonary C-fibre terminations in the brainstem have thus focused on the C-fibres innervating the peripheral airways [21,22]. But these C-fibers are unlikely to initiate coughing in either awake or anaesthetized animals [13,20,23]. The central synapses of the mechanoreceptors (“cough receptors”) that do initiate coughing upon challenge of the airways of anaesthetized animals have also not been evaluated. It is unlikely that the cough receptor terminations overlap much with those of intrapulmonary rapidly adapting receptors (RARs) or slowly adapting receptors (SARs), which are continually active in eupnoea and do not initiate coughing upon stimulation [13,14,20,24-32].
In cats and rats, the induction of c-fos expression in brainstem structures following repetitive cough challenges has been used in attempts to localize the central terminations of cough promoting afferents [33,34]. The results generated in rats are somewhat dubious inasmuch as it has become increasingly controversial as to whether rats (or mice) are able to cough [35-37]. But in cats, cough has been well defined and several cell groupings in the nucleus tractus solitarius (nTS) were labeled for c-fos following repetitive tussive challenge, including in the interstitial, medial and ventrolateral subnuclei of nTS. Stimulation and nTS lesion studies in guinea pigs also favour a role for rostral nTS subnuclei in cough, including the interstitial and ventrolateral subnuclei [38]. Notably absent in the studies of cough in cats and guinea pigs was strong evidence for the participation of the commissural subnucleus of nTS, a primary site of intrapulmonary C-fibre and RAR termination [21,22,26,28,30-32].
Functional and pharmacological analyses in rabbits and in guinea pigs provide conflicting evidence for the role of the commissural subnucleus of nTS in cough. Mutolo and colleagues report that microinjection of several different drugs (glutamate and neurokinin receptor antagonists, μ-opioid and GABAB receptor agonists) into the commissural subnucleus of nTS prevented coughing evoked by mechanical probing of the carina in rabbits [10,39]. Microinjections of these drugs into adjacent brainstem locations were without effect on subsequently evoked coughing. The authors interpreted the results as evidence that the afferent nerves regulating cough, which they suggest were RARs, terminate in the commissural subnucleus of rabbits.
We have also studied the central termination sites of the afferents regulating cough evoked from the trachea and larynx in anaesthetized guinea pigs [15]. We microinjected a combination of glutamate NMDA and nonNMDA receptor antagonists into various brainstem locations in and around nTS and evaluated their effects on cough and other respiratory reflexes. This microinjection strategy identified a discrete location in nTS rostral and lateral to obex and the commissural subnucleus at which the drugs abolished coughing evoked from the trachea but were without effect on basal respiratory rate (largely dependent upon SAR activity). Reflexes attributed to RAR (histamine-induced tachypnoea) or C-fiber (bradykinin-induced tachypnoea) activation were also unaffected by the microinjections that essentially abolished coughing, suggesting a cough specific location in nTS. Tracing studies confirmed the preferential terminations of the cough receptors in nTS locations rostral and lateral to obex and to the commissural subnucleus of nTS.
3. Why Study Central Processing of Afferent Signaling Regulating Cough?
Stimuli that initiate cough have been reasonably well defined [13-16]. These include mechanical stimuli (e.g. aspirate, accumulated secretions; 3-5, 10, 14, 39), changes in airway surface liquid osmolarity [20,40-45], bradykinin [14,16,46], prostanoids [46-48], inhaled pollutants/irritants (e.g. cigarette smoke, acetone, SO2; 14,49,50), and activators of the TRPV1 [51,52] and TRPA1 [51,53,54] ion channel-receptors (e.g. capsaicin, acid). Acid (from gastric fluid/refluxate aspiration or airway acidification during disease), will also evoke coughing by TRPV1-dependent and independent (perhaps ASIC3-dependent) mechanisms [14,16,20,40,42,44,52,55-58]. These stimuli act on the capsaicin-sensitive C-fibres and/or the capsaicin-insensitive cough receptors. Other mediators such as PGE2, thromboxane, PAR2 agonists (via PGE synthesis), ATP, the cysteinyl-leukotrienes and histamine do not reliably evoke coughing but enhance cough responsiveness (presumably by enhancing bronchopulmonary C-fibre responsiveness; 14,17,29,46,59). Chloride channels [40,45,60-63], potassium channels [40,64,65], sodium channels [17,40, 66], sodium pumps [15] and Na+-K+-2Cl transporters [45,60,62] may also regulate the excitability of the cough receptors and C-fibers. Coincident activation of these afferent nerve subtypes can have synergistic effects on cough, a synergism that may depend on central convergence between these reflex pathways [8,15]. Similar interactions may explain the coughing associated with extrapulmonary disorders such as allergic rhinitis and gastro-oesophageal reflux disease [15,67]. RARs, SARs and C-fibre subtypes may also modulate evoked cough [15,29,67]. In the periphery, then, there are two afferent nerve subtypes (C-fibres, cough receptors) that regulate coughing following activation of multiple receptors and ion channel subtypes (e.g. TRPV1, TRPA1, ASIC3, bradykinin B2 receptors, mechanically-gated ion channels). Moreover, the excitability of the afferents that regulate cough can be modulated by the actions of many autacoids, ion channels and parallel afferent inputs (Fig. 1). This level of complexity and redundancy, and the likelihood that similar transduction mechanisms control other cells and nerves within the airways and lungs makes the development of broadly effective and selective peripherally acting antitussive agents challenging in much the same way that it has proven difficult to target the actions of a single mediator in asthma and provide effective treatment.
Fig. 1.

This schematic illustrates some of the central and peripheral mechanisms regulating the cough reflex. Cough is initiated by activation of capsaicin-sensitive C-fibers and capsaicin-insensitive, acid sensitive mechanoreceptors innervating the larynx, trachea and large bronchi. Stimuli evoking cough include TRPV1 (capsaicin, anandamide, resiniferatoxin, protons) and TRPA1 (ozone, toluene diisocyanate, acrolein, cinnamaldehyde) receptor agonists, acid (through TRPV1-dependent and –independent (perhaps ASIC3-dependent) mechanisms), altered airway surface liquid osmolarity, mechanical stimuli (e.g. aspirate, accumulated mucus), bradykinin, and prostanoids. TRPV1-dependent coughing can be enhanced (dashed lines) by the actions of inflammatory mediators. Coughing can also be modulated by activation of other afferent nerve subtypes and by conscious perception of cough stimulation and by psychosocial influences. Central encoding of cough is regulated by both glutamate (via NMDA and nonNMDA receptor activation) and neurokinins (through actions on NK1, NK2 and/or NK3 receptors).
Abbreviations: nAChR: nicotinic acetylcholine receptors; SP/NKA: substance P/neurokinin A; TDI: toluene diisocyanate; nTS: nucleus tractus solitarius; TxA2: thromboxane A2; LTC4 and LTD4: leukotriene C4 and D4; EP: E series prostanoid receptors; B2: bradykinin2 receptors; ASIC: acid-sensing ion channel; See text for further details.
In contrast to the great complexity and redundancy in cough regulation in the periphery, synaptic transmission at the central synapses of the afferent nerves regulating cough seems far less complex. Only two excitatory transmitter systems (glutamate, neurokinins) have been identified in the cough reflex pathways of animals and humans (Fig. 2). Glutamate receptor antagonists such as dextromethorphan block cough in multiple species and in response to several different tussive stimuli [4,15,39,68-71]. Similarly, neurokinin receptor antagonists are effective antitussives in guinea pigs, cats, rabbits, dogs and pigs [3-10]. The effect of neurokinin receptor antagonism on cough in human subjects is unclear from the limited scope of published studies. But it is interesting that vomiting is treated clinically with neurokinin1 (NK1) receptor antagonists [72]. Neurokinin expression in the periphery is typically confined to capsaicin-sensitive nociceptors, comparable to the capsaicin-sensitive airway and lung nociceptive C-fibres regulating cough [73-75]. Emesis shares many physiologic characteristics with cough (vagally-mediated, peripherally triggered by noxious stimuli, preceded by an urge, threshold regulation, and motor patterns of varying duration and intensity depending upon the stimulus). Based on the effectiveness of neurokinin receptor antagonists in the treatment of emesis, the similarities between emesis and cough, and the efficacy of these drugs against cough in five different species, it would be very surprising if neurokinin receptor antagonists proved ineffective in treating cough in humans.
Fig. 2.

NMDA-type glutamate or neurokinin receptor antagonists prevent experimentally induced coughing in humans and in animals. Coughing was evoked by inhalation of citric acid (humans, pigs, guinea pigs (upper panel)) or capsaicin (rat, guinea pig (lower panel)), or by mechanical probing of the airway mucosa in anesthetized dogs, rabbits and cats. NMDA (dextromethorphan, MK801, APV), NK1 (αNK1; CP99994 or SR140333 (pigs), NK2 (αNK2; SR48968 or NK3 (αNK3; SR142801) receptor antagonists were given systemically (to humans, pigs, dogs, guinea pigs (upper panel)), by icv or central arterial administration (rats, cats, guinea pigs (lower panel)) or by nTS microinjection (rabbits). Comparable effects have been reported with systemic administration of NK3 receptor antagonists in guinea pigs [6,7]. Data are modified from published results [3-5, 10, 39, 69-71].
Evidence for a central site of action of several different classes of antitussive drugs has been reported in studies carried out in cats, rabbits, rats and guinea pigs [1,3,4,10, 39, 76,77]. We have evaluated the ability of putative antitussive agents to prevent coughing in anaesthetized guinea pigs when given by microinjection or given selectively to the trachea via tracheal perfusion. Cough was evoked by topical citric acid (0.001-2M), applied in 100 μL aliquots directly to the tracheal perfusate. Dextromethorphan, the GABAB receptor agonist baclofen, the μ-opioid receptor agonists codeine and DAMGO, the Sigma receptor agonist SKF 10047, and the Na+ channel blocker mexiletine all dose-dependently inhibited cough when microinjected bilaterally into nTS locations identified as the primary sites of cough receptor termination (Fig. 3). Microinjection of these drugs into adjacent brainstem locations was either completely ineffective or less effective at preventing cough. Coughing was prevented by all of these drugs at doses that were without effect on basal respiratory rate. In contrast to their effects upon microinjection, however, only mexiletine displayed any antitussive effects when given selectively to the tracheal perfusate.
Fig. 3.
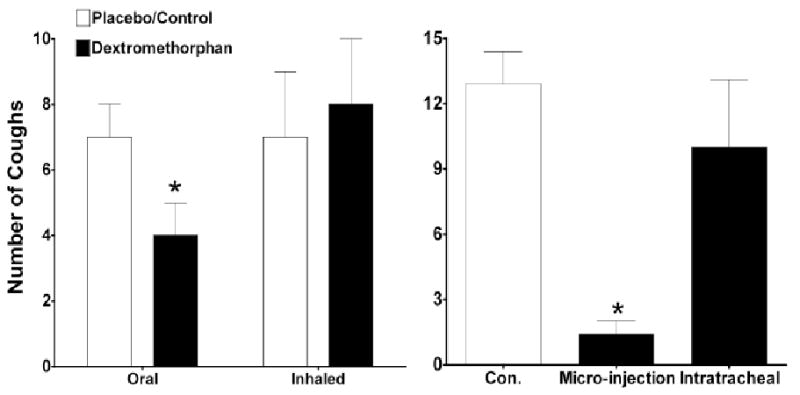
The NMDA receptor antagonist dextromethorphan acts centrally to prevent coughing in humans and in guinea pigs. Dextromethorphan given orally but not by inhalation prevented citric acid evoked coughing in human subjects (left panel). Similarly, dextromethorphan administered by microinjection in nTS but not delivered by perfusion directly to the trachea prevented coughing evoked by citric acid applied topically to the tracheal mucosa (right panel). Comparable results have been obtained in studies with other putative antitussive agents. Data are the author's unpublished results or derived from Grattan et al. [69].
There are several reports of putative antitussive drugs failing to prevent coughing when applied topically to airway mucosa despite evidence that these same drugs can act peripherally and prejunctionally to prevent neurokinin release from airway C-fibres [16, 78,79]. One explanation could be that the afferent nerves responsible for the axon reflex are not the same as those regulating cough. Alternatively, there may be a dissociation of the processes regulating axon potential formation and peripheral neurotransmitter release. Published data are consistent with this latter notion. Removing extracellular Ca++ prevents neurotransmitter release from the peripheral terminals of C-fibres in the airways but greatly increases their responsiveness to mechanical and chemical stimulation [80.81]. Conversely, drugs that prevent action potential generation such as tetrodotoxin are without effect on capsaicin-evoked neuropeptide release from airway C-fibres [82-84]. Autacoids such as adenosine, PGE2 and epinephrine also prevent neuropeptide release but activate or increase airway C-fibre excitability [59,85-89]. Surprisingly, there have been no published studies evaluating the ability of drugs such as codeine, dextromethorphan, baclofen, nociceptin or cannabanoid CB2 receptor agonists to prevent airway afferent nerve discharge despite evidence that they act peripherally to limit coughing [16,58,68,76,77,90,91]. Undem and colleagues have found no evidence for a direct inhibition of vagal afferent discharge (C- or A∂-fibres) in guinea pigs by delta-opioid receptor agonists, nociceptin or CB2 receptor agonists (Undem, personal communication). These sorts of results add further doubt as to whether cough can be inhibited peripherally by G-protein coupled receptor activation.
Regardless of afferent nerve subtype stimulated, glutamate seems to be the primary excitatory transmitter acting centrally during cough, with neurokinins playing a more modulatory role. Provided the many potential side effects of glutamate receptor antagonism can be avoided, the essential role of glutamate in synaptic transmission in the nTS in response to all tussive stimuli and irrespective of underlying illness predicts the potential for broad spectrum cough relief with drugs that act selectively to limit the actions of this excitatory amino acid transmitter. Several unique characteristics of the encoding of cough, including the need for high frequency afferent drive, threshold regulation and NMDA receptor recruitment predict that effective cough therapy can be achieved with minimal side effects.
4. Pharmacology and Central Integration of the Afferent Drive for Cough
Studies of synaptic transmission in nTS evoked by the activation of vagal afferent nerves reveal a primary role for nonNMDA glutamate receptors with minimal if any role for NMDA receptors [92,93]. A primary role for nonNMDA receptors in regulating synaptic transmission initiated by airway and lung afferent nerves has also been published [24,28,94]. It is thus a striking and important observation that NMDA receptor antagonists have proven to be highly effective antitussive agents in several species including humans [4,15,68-71]. Given the Mg++ block at baseline that is characteristic and defining for NMDA receptors, it seems unlikely that the effectiveness of NMDA receptor antagonists in cough reflects the unique involvement of a population of synapses utilizing predominantly or exclusively NMDA receptors for signaling. Rather, we hypothesize that the effectiveness of NMDA receptor antagonism in cough is due to the unique importance of sustained high frequency afferent drive needed to reconfigure a respiratory motor pattern into a cough motor pattern. This high frequency activation likely recruits NMDA receptors for the initiation of cough. The need for high frequency afferent drive might have been predicted from the existence of both an urge-to-cough and cough thresholds [17,95]. In this sense, cough differs from the vagal reflexes controlling respiratory rate, heart rate, airway smooth muscle tone and blood pressure, all of which change on a continual, breath-to-breath basis, and do not display the almost binary regulation that is characteristic of cough and other reflexes such as emesis, ejaculation and sneezing. In these ways, cough is regulated much like the sensation of pain [96].
We studied the role of afferent nerve action potential frequency in the encoding of cough in anaesthetized guinea pigs (Fig. 4). Cough was evoked electrically with an electrode placed on the tracheal mucosa. Ten second stimulus trains of optimal intensity (determined in preliminary studies as 8 V, 1 msec pulse duration) were delivered every minute for 10 minutes at frequencies of 5-64 Hz. We quantified failures at evoking cough at each frequency. Notably, 10 second trains at stimulation frequencies of 5-8 Hz (50-80 action potentials in 10 seconds, targeting all afferent nerves within the current field (∼3 mm diameter)) consistently failed to evoke coughing. Frequencies of ≥10Hz were required to reach cough threshold. Pharmacologically, then, a therapeutic that reduces synaptic transmission only marginally (mimicking a reduction in stimulation frequency from 10 Hz to 5 Hz, or 16 Hz to 8 Hz) may have a disproportionally large effect on coughing (70-100% reduction).
Figure 4.

The cough reflex requires sustained, high frequency vagal afferent nerve activation. Coughing was evoked in anesthetized guinea pigs by electrically stimulating the tracheal mucosa at optimal stimulation intensity (8 V, 1 msec pulse duration) and various frequencies with 10 second trains every minute for 10 minutes. The percentage of stimulations failing to evoke cough are presented. Data are mean ± sem of 6-10 experiments.
When used at sufficient doses, the NMDA receptor antagonist dextromethorphan has proven safe and effective at reducing cough in several species and in human subjects [4,15,68-71]. Dextromethorphan is also a ligand for the peculiar Sigma receptor, and while Sigma receptor binding may contribute to the known antitussive effects (68,71), Sigma receptor binding may also be contribute to the side effect profile of this drug. NMDA receptors may be selectively targeted by several additional approaches in cough. Comprised of two subunits, NMDA receptors are unique amongst cell surface receptors, requiring two agonists for optimal receptor/ion channel function [97,98]. Thus, in addition to a binding site for glutamate, an additional binding site that recognizes glycine must be activated for optimal NMDA receptor dependent signaling. NMDA receptor subtypes have also been described, on observation that might be exploited to further limit side effects of these centrally-acting drugs (98). D-serine, formed from L-serine by serine racemase, is the most likely endogenous agonist for the glycine binding site on the NMDA receptors at many synapses [99]. Drugs that modify D-serine/glycine signaling through the NMDA receptor have shown promise in studies of nociception [100,101]. The NMDA receptor as well as serine racemase may also be nitrosylated, with nitrosylation reducing serine racemase enzymatic activity and reducing NMDA receptor signaling [97,102-104]. Therapeutic agents that coincidentally bind the NMDA receptor and carry a nitrosylating moiety to the receptor are under development [97]. Nitrosylation may occur endogenously, as NMDA receptors are coupled to the activation of neuronal nitric oxide synthase (nNOS; 105,106). Indeed, in preliminary studies, we have found that the non-selective NO synthase inhibitor L-NNA and the nNOS-selective inhibitor L-N-propyl-arginine both prevent citric acid-induced coughing in anaesthetized guinea pigs when microinjected bilaterally into nTS. The activation of NO synthase and resulting nitrosylation may also contribute to the rapid desensitization of the cough reflex seen upon repetitive tussive challenge (unpublished observations).
Because of its function as an ion channel, NMDA receptor-dependent activation of neurones may also be limited by targeting channel opening. Several drugs have been identified as use dependent blockers of the NMDA receptor channel [97,107]. One drug of particular interest is memantine, an orally active use dependent NMDA receptor-channel blocker used clinically in the treatment of Alzheimers disease. What is especially intriguing about drugs like memantine is that they are more effective at preventing NMDA receptor activation by high concentrations of glutamate than they are at preventing suboptimal NMDA receptor activation [97,107]. Given the unique role of NMDA receptors in cough and the need for sustained, high frequency afferent drive, it seems possible that compounds such as memantine may be highly effective at limiting cough but without a substantial side effect profile when given at doses that only slightly reduce afferent signaling in nTS but profoundly inhibit coughing.
Drugs like codeine block cough centrally by presynaptic inhibition of glutamate release or by postsynaptic inhibition of nTS relay neurone excitability. Unless an inhibitory receptor type differentially expressed on the central terminations of the afferent nerves regulating cough can be identified, it is difficult to imagine that the antitussive effects of centrally penetrant drugs such as codeine or baclofen can be improved upon. Consequently, although such drugs may limit coughing, their utility is limited by side effects such as sedation and respiratory depression.
5. Conclusions
New drugs for cough are needed. The most commonly used antitussives are known to act centrally to limit synaptic transmission in the brain stem, which in turn limits coughing. We propose that future research should build upon the successes of drugs such as dextromethorphan and improve upon them by exploiting our increased understanding of the physiology and pathophysiology of cough. Evaluating new therapies that take advantage of the prominent and unique role of NMDA receptor signaling in cough and the need for sustained, high frequency activation of the afferents regulating this reflex may lead to the discovery of broadly effective antitussive agents with limited side effect profiles. The drug memantine is available clinically and has pharmacological properties predictive of few side effects and broad application in treating cough attributed to a variety of underlying disorders. As drugs such as memantine are evaluated for the treatment of pain, the insights gained should help identify other novel therapeutic strategies for cough.
Acknowledgments
Work summarized in this article was supported by grant from the NIH (HL083192)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolser DC. Central mechanisms II: pharmacology of brainstem pathways. Handb Exp Pharmacol. 2009;(187):203–17. doi: 10.1007/978-3-540-79842-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung KF. Clinical cough VI: the need for new therapies for cough: disease-specific and symptom-related antitussives. Handb Exp Pharmacol. 2009;(187):343–68. doi: 10.1007/978-3-540-79842-2_18. [DOI] [PubMed] [Google Scholar]
- 3.Bolser DC, DeGennaro FC, O'Reilly S, McLeod RL, Hey JA. Central antitussive activity of the NK1 and NK2 tachykinin receptor antagonists, CP-99,994 and SR 48968, in the guinea-pig and cat. Br J Pharmacol. 1997;121(2):165–70. doi: 10.1038/sj.bjp.0701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86(3):1017–2. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 5.Chapman RW, House A, Liu F, Celly C, Mei H, Hey JA. Antitussive activity of the tachykinin NK1 receptor antagonist, CP-99994, in dogs. Eur J Pharmacol. 2004;485(13):329–32. doi: 10.1016/j.ejphar.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Daoui S, Cognon C, Naline E, Emonds-Alt X, Advenier C. Involvement of tachykinin NK3 receptors in citric acid-induced cough and bronchial responses in guinea pigs. Am J Respir Crit Care Med. 1998;158(1):42–8. doi: 10.1164/ajrccm.158.1.9705052. [DOI] [PubMed] [Google Scholar]
- 7.Hay DW, Giardina GA, Griswold DE, Underwood DC, Kotzer CJ, et al. Nonpeptide tachykinin receptor antagonists. III. SB 235375, a low central nervous system-penetrant, potent and selective neurokinin-3 receptor antagonist, inhibits citric acid-induced cough and airways hyper-reactivity in guinea pigs. J Pharmacol Exp Ther. 2002;300(1):314–23. doi: 10.1124/jpet.300.1.314. [DOI] [PubMed] [Google Scholar]
- 8.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol. 2005;569(Pt 2):559–73. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreaux B, Nemmar A, Vincke G, Halloy D, Beerens D, Advenier C, Gustin P. Role of substance P and tachykinin receptor antagonists in citric acid-induced cough in pigs. Eur J Pharmacol. 2000;408(3):305–12. doi: 10.1016/s0014-2999(00)00763-9. [DOI] [PubMed] [Google Scholar]
- 10.Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R243–51. doi: 10.1152/ajpregu.00184.2008. [DOI] [PubMed] [Google Scholar]
- 11.McAlexander MA, Carr MJ. Peripheral mechanisms I: plasticity of peripheral pathways. Handb Exp Pharmacol. 2009;(187):129–54. doi: 10.1007/978-3-540-79842-2_7. [DOI] [PubMed] [Google Scholar]
- 12.Spina D, McFadzean I, Bertram FK, Page CP. Peripheral mechanisms II: the pharmacology of peripherally active antitussive drugs. Handb Exp Pharmacol. 2009;(187):155–86. doi: 10.1007/978-3-540-79842-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009;(187):23–47. doi: 10.1007/978-3-540-79842-2_2. [DOI] [PubMed] [Google Scholar]
- 14.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557(Pt 2):543–58. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152(3):223–42. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson JA, Fuller RW. Pharmacological regulation of the cough reflex--from experimental models to antitussive effects in Man. Pulm Pharmacol Ther. 1999;12(4):215–28. doi: 10.1006/pupt.1999.0207. [DOI] [PubMed] [Google Scholar]
- 17.Canning BJ. Encoding of the cough reflex. Pulm Pharmacol Ther. 2007;20(4):396–401. doi: 10.1016/j.pupt.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatar M, Sant'Ambrogio G, Sant'Ambrogio FB. Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol. 1994;76(6):2672–9. doi: 10.1152/jappl.1994.76.6.2672. [DOI] [PubMed] [Google Scholar]
- 19.Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol. 1988;402:411–20. doi: 10.1113/jphysiol.1988.sp017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou YL, Scarupa MD, Mori N, Canning BJ. Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1572–84. doi: 10.1152/ajpregu.90382.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonham AC, Joad JP. Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J Physiol. 1991;441:95–112. doi: 10.1113/jphysiol.1991.sp018740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubin L, Kimura H, Davies RO. The medullary projections of afferent bronchopulmonary C fibres in the cat as shown by antidromic mapping. J Physiol. 1991;435:207–28. doi: 10.1113/jphysiol.1991.sp018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Cough reflex responses during pulmonary C-fibre receptor activation in anesthetized rabbits. Neurosci Lett. 2008;448(2):200–3. doi: 10.1016/j.neulet.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 24.Bonham AC, Coles SK, McCrimmon DR. Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J Physiol. 1993;464:725–45. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol. 1990;427:261–80. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies RO, Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol. 1986;373:63–86. doi: 10.1113/jphysiol.1986.sp016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies RO, Kubin L, Pack AI. Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. J Physiol. 1987;383:571–85. doi: 10.1113/jphysiol.1987.sp016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezure K, Tanaka I, Miyazaki M. Electrophysiological and pharmacological analysis of synaptic inputs to pulmonary rapidly adapting receptor relay neurons in the rat. Exp Brain Res. 1999;128(4):471–80. doi: 10.1007/s002210050870. [DOI] [PubMed] [Google Scholar]
- 29.House A, Celly C, Skeans S, Lamca J, Egan RW, Hey JA, Chapman RW. Cough reflex in allergic dogs. Eur J Pharmacol. 2004;492(23):251–8. doi: 10.1016/j.ejphar.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 30.Kalia M, Richter D. Rapidly adapting pulmonary receptor afferents: I. Arborization in the nucleus of the tractus solitarius. J Comp Neurol. 1988;274(4):560–73. doi: 10.1002/cne.902740406. [DOI] [PubMed] [Google Scholar]
- 31.Kalia M, Richter D. Rapidly adapting pulmonary receptor afferents: II. Fine structure and synaptic organization of central terminal processes in the nucleus of the tractus solitarius. J Comp Neurol. 1988;274(4):574–94. doi: 10.1002/cne.902740407. [DOI] [PubMed] [Google Scholar]
- 32.Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol. 1991;443:55–77. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gestreau C, Bianchi AL, Grélot L. Differential brainstem Fos-like immunoreactivity after laryngeal-induced coughing and its reduction by codeine. J Neurosci. 1997;17(23):9340–52. doi: 10.1523/JNEUROSCI.17-23-09340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakus J, Poliacek I, Halasova E, Murin P, Knocikova J, Tomori Z, Bolser DC. Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir Physiol Neurobiol. 2008;160(3):289–300. doi: 10.1016/j.resp.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belvisi MG, Bolser DC. Summary: animal models for cough. Pulm Pharmacol Ther. 2002;15(3):249–50. doi: 10.1006/pupt.2002.0349. [DOI] [PubMed] [Google Scholar]
- 36.Canning BJ. The cough reflex in animals: relevance to human cough research. Lung. 2008;186 1:S23–8. doi: 10.1007/s00408-007-9054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatar M, Karcolova D, Pecova R, Brozmanova M. The role of partial laryngeal denervation on the cough reflex in awake guinea-pigs, rats and rabbits. Pulm Pharmacol. 1996;9(56):371–2. doi: 10.1006/pulp.1996.0051. [DOI] [PubMed] [Google Scholar]
- 38.Ohi Y, Yamazaki H, Takeda R, Haji A. Functional and morphological organization of the nucleus tractus solitarius in the fictive cough reflex of guinea pigs. Neurosci Res. 2005;53(2):201–9. doi: 10.1016/j.neures.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Mutolo D, Bongianni F, Fontana GA, Pantaleo T. The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull. 2007;74(4):284–93. doi: 10.1016/j.brainresbull.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Canning BJ, Farmer DG, Mori N. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R454–63. doi: 10.1152/ajpregu.00862.2005. [DOI] [PubMed] [Google Scholar]
- 41.Koskela HO, Purokivi MK, Kontra KM, Taivainen AH, Tukiainen HO. Hypertonic saline cough provocation test with salbutamol pre-treatment: evidence for sensorineural dysfunction in asthma. Clin Exp Allergy. 2008;38(7):1100–7. doi: 10.1111/j.1365-2222.2008.02996.x. [DOI] [PubMed] [Google Scholar]
- 42.Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. 1995;79(4):1082–7. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 43.Lavorini F, Pantaleo T, Geri P, Mutolo D, Pistolesi M, Fontana GA. Cough and ventilatory adjustments evoked by aerosolised capsaicin and distilled water (fog) in man. Respir Physiol Neurobiol. 2007;156(3):331–9. doi: 10.1016/j.resp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Lowry RH, Wood AM, Higenbottam TW. Effects of pH and osmolarity on aerosol-induced cough in normal volunteers. Clin Sci (Lond) 1988;74(4):373–6. doi: 10.1042/cs0740373. [DOI] [PubMed] [Google Scholar]
- 45.Ventresca PG, Nichol GM, Barnes PJ, Chung KF. Inhaled furosemide inhibits cough induced by low chloride content solutions but not by capsaicin. Am Rev Respir Dis. 1990;142(1):143–6. doi: 10.1164/ajrccm/142.1.143. [DOI] [PubMed] [Google Scholar]
- 46.Choudry NB, Fuller RW, Pride NB. Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis. 1989;140(1):137–41. doi: 10.1164/ajrccm/140.1.137. [DOI] [PubMed] [Google Scholar]
- 47.Costello JF, Dunlop LS, Gardiner PJ. Characteristics of prostaglandin induced cough in man. Br J Clin Pharmacol. 1985;20(4):355–359. doi: 10.1111/j.1365-2125.1985.tb05077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardiner PJ, Copas JL, Elliott RD, Collier HO. Tracheobronchial irritancy of inhaled prostaglandins in the conscious cat. Prostaglandins. 1978;15(2):303–15. doi: 10.1016/0090-6980(78)90170-3. [DOI] [PubMed] [Google Scholar]
- 49.Lee LY, Burki NK, Gerhardstein DC, Gu Q, Kou YR, Xu J. Airway irritation and cough evoked by inhaled cigarette smoke: role of neuronal nicotinic acetylcholine receptors. Pulm Pharmacol Ther. 2007;20(4):355–64. doi: 10.1016/j.pupt.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Widdicombe JG. Respiratory reflexes from the trachea and bronchi of the cat. J Physiol. 1954;123(1):55–70. doi: 10.1113/jphysiol.1954.sp005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Materazzi S, Nassini R, Gatti R, Trevisani M, Geppetti P. Cough sensors. II. Transient receptor potential membrane receptors on cough sensors. Handb Exp Pharmacol. 2009;(187):49–61. doi: 10.1007/978-3-540-79842-2_3. [DOI] [PubMed] [Google Scholar]
- 52.Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, Morice AH, Geppetti P. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax. 2004;59(9):769–72. doi: 10.1136/thx.2003.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faruqi S, Mann V, Morice A. Cinnamaldehyde: a novel tussive agent in man. Eur Respir Soc. ( http://www.ersnet.org/learning_resources_player/abstract_print_08/main_frameset.htm)
- 54.Taylor-Clark, et al. this issue. [Google Scholar]
- 55.Bolser DC, Aziz SM, Chapman RW. Ruthenium red decreases capsaicin and citric acid-induced cough in guinea pigs. Neurosci Lett. 1991;126(2):131–3. doi: 10.1016/0304-3940(91)90536-3. [DOI] [PubMed] [Google Scholar]
- 56.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L58–65. doi: 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543(Pt 2):591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee MG, Undem BJ, Brown C, Carr MJ. Effect of nociceptin in acid-evoked cough and airway sensory nerve activation in guinea pigs. Am J Respir Crit Care Med. 2006;173(3):271–5. doi: 10.1164/rccm.200507-1043OC. [DOI] [PubMed] [Google Scholar]
- 59.Ho CY, Gu Q, Hong JL, Lee LY. Prostaglandin E(2) enhances chemical and mechanical sensitivities of pulmonary C fibers in the rat. Am J Respir Crit Care Med. 2000;162(2 Pt 1):528–33. doi: 10.1164/ajrccm.162.2.9910059. [DOI] [PubMed] [Google Scholar]
- 60.Fox AJ, Barnes PJ, Dray A. Stimulation of guinea-pig tracheal afferent fibres by non-isosmotic and low-chloride stimuli and the effect of frusemide. J Physiol. 1995;482(Pt 1):179–87. doi: 10.1113/jphysiol.1995.sp020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee MG, Macglashan DW, Jr, Undem BJ. Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol. 2005;566(Pt 1):205–12. doi: 10.1113/jphysiol.2005.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazzone SB, McGovern AE. Na+-K+-2Cl- cotransporters and Cl- channels regulate citric acid cough in guinea pigs. J Appl Physiol. 2006;101(2):635–43. doi: 10.1152/japplphysiol.00106.2006. [DOI] [PubMed] [Google Scholar]
- 63.Oh EJ, Weinreich D. Bradykinin decreases K(+) and increases Cl(-) conductances in vagal afferent neurones of the guinea pig. J Physiol. 2004;558(Pt 2):513–26. doi: 10.1113/jphysiol.2004.066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fox AJ, Barnes PJ, Venkatesan P, Belvisi MG. Activation of large conductance potassium channels inhibits the afferent and efferent function of airway sensory nerves in the guinea pig. J Clin Invest. 1997;99(3):513–9. doi: 10.1172/JCI119187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McAlexander MA, Undem BJ. Potassium channel blockade induces action potential generation in guinea-pig airway vagal afferent neurones. J Auton Nerv Syst. 2000;78(23):158–64. doi: 10.1016/s0165-1838(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 66.Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing J, Meeker S, Undem BJ. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J Physiol. 2008;586(5):1321–36. doi: 10.1113/jphysiol.2007.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanacek J, Tatar M, Widdicombe J. Regulation of cough by secondary sensory inputs. Respir Physiol Neurobiol. 2006;152(3):282–97. doi: 10.1016/j.resp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 68.Brown C, Fezoui M, Selig WM, Schwartz CE, Ellis JL. Antitussive activity of sigma-1 receptor agonists in the guinea-pig. Br J Pharmacol. 2004;141(2):233–40. doi: 10.1038/sj.bjp.0705605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grattan TJ, Marshall AE, Higgins KS, Morice AH. The effect of inhaled and oral dextromethorphan on citric acid induced cough in man. Br J Clin Pharmacol. 1995;39(3):261–3. doi: 10.1111/j.1365-2125.1995.tb04446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamei J, Tanihara H, Igarashi H, Kasuya Y. Effects of N-methyl-D-aspartate antagonists on the cough reflex. Eur J Pharmacol. 1989;168(2):153–8. doi: 10.1016/0014-2999(89)90560-8. [DOI] [PubMed] [Google Scholar]
- 71.Kotzer CJ, Hay DW, Dondio G, Giardina G, Petrillo P, Underwood DC. The antitussive activity of delta-opioid receptor stimulation in guinea pigs. J Pharmacol Exp Ther. 2000;292(2):803–9. [PubMed] [Google Scholar]
- 72.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482–94. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 73.Jancsó G, Hökfelt T, Lundberg JM, Kiraly E, Halász N, et al. Immunohistochemical studies on the effect of capsaicin on spinal and medullary peptide and monoamine neurons using antisera to substance P, gastrin/CCK, somatostatin, VIP, enkephalin, neurotensin and 5-hydroxytryptamine. J Neurocytol. 1981;10(6):963–80. doi: 10.1007/BF01258524. [DOI] [PubMed] [Google Scholar]
- 74.Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992;49(3):715–37. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- 75.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol. 2005;563(Pt 3):831–42. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bolser DC, DeGennaro FC, O'Reilly S, Chapman RW, Kreutner W, Egan RW, Hey JA. Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol. 1994;113(4):1344–8. doi: 10.1111/j.1476-5381.1994.tb17145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLeod RL, Parra LE, Mutter JC, Erickson CH, Carey GJ, et al. Nociceptin inhibits cough in the guinea-pig by activation of ORL(1) receptors. Br J Pharmacol. 2001;132(6):1175–8. doi: 10.1038/sj.bjp.0703954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belvisi MG, Chung KF, Jackson DM, Barnes PJ. Opioid modulation of non-cholinergic neural bronchoconstriction in guinea-pig in vivo. Br J Pharmacol. 1988;95(2):413–8. doi: 10.1111/j.1476-5381.1988.tb11661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belvisi MG, Ichinose M, Barnes PJ. Modulation of non-adrenergic, non-cholinergic neural bronchoconstriction in guinea-pig airways via GABAB-receptors. Br J Pharmacol. 1989;97(4):1225–31. doi: 10.1111/j.1476-5381.1989.tb12582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hua XY, Yaksh TL. Release of calcitonin gene-related peptide and tachykinins from the rat trachea. Peptides. 1992;13(1):113–20. doi: 10.1016/0196-9781(92)90148-v. [DOI] [PubMed] [Google Scholar]
- 81.Undem BJ, Oh EJ, Lancaster E, Weinreich D. Effect of extracellular calcium on excitability of guinea pig airway vagal afferent nerves. J Neurophysiol. 2003;89(3):1196–204. doi: 10.1152/jn.00553.2002. [DOI] [PubMed] [Google Scholar]
- 82.Maggi CA, Patacchini R, Giuliani S, Santicioli P, Meli A. Evidence for two independent modes of activation of the ‘efferent’ function of capsaicin-sensitive nerves. Eur J Pharmacol. 1988;156(3):367–73. doi: 10.1016/0014-2999(88)90282-8. [DOI] [PubMed] [Google Scholar]
- 83.Szolcsányi J. Tetrodotoxin-resistant non-cholinergic neurogenic contraction evoked by capsaicinoids and piperine on the guinea-pig trachea. Neurosci Lett. 1983;42(1):83–8. doi: 10.1016/0304-3940(83)90426-3. [DOI] [PubMed] [Google Scholar]
- 84.Szolcsányi J, Barthó L. Capsaicin-sensitive non-cholinergic excitatory innervation of the guinea-pig tracheobronchial smooth muscle. Neurosci Lett. 1982;34(3):247–51. doi: 10.1016/0304-3940(82)90183-5. [DOI] [PubMed] [Google Scholar]
- 85.Aikawa T, Sekizawa K, Itabashi S, Sasaki H, Takishima T. Inhibitory actions of prostaglandin E1 on non-adrenergic non-cholinergic contraction in guinea-pig bronchi. Br J Pharmacol. 1990;101(1):13–4. doi: 10.1111/j.1476-5381.1990.tb12080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gu Q, Lin YS, Lee LY. Epinephrine enhances the sensitivity of rat vagal chemosensitive neurons: role of beta3-adrenoceptor. J Appl Physiol. 2007;102(4):1545–55. doi: 10.1152/japplphysiol.01010.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lagente V, Barlinski J, Cano E, Frossard N. Adenosine reduces airway excitatory non-cholinergic (e-NC) contraction through both A1 and A2 adenosine receptor activation in the guinea pig. Fundam Clin Pharmacol. 1997;11(6):494–500. doi: 10.1111/j.1472-8206.1997.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 88.Morimoto H, Yamashita M, Imazumi K, Matsuda A, Ochi T, Seki N, Mizuhara H, Fujii T, Senoh H. Effects of adenosine A2 receptor agonists on the excitation of capsaicin-sensitive afferent sensory nerves in airway tissues. Eur J Pharmacol. 1993;240(23):121–6. doi: 10.1016/0014-2999(93)90889-p. [DOI] [PubMed] [Google Scholar]
- 89.Verleden GM, Belvisi MG, Rabe KF, Miura M, Barnes PJ. Beta 2-adrenoceptor agonists inhibit NANC neural bronchoconstrictor responses in vitro. J Appl Physiol. 1993;74(3):1195–9. doi: 10.1152/jappl.1993.74.3.1195. [DOI] [PubMed] [Google Scholar]
- 90.Belvisi MG, Hele DJ. Cough sensors. III. Opioid and cannabinoid receptors on vagal sensory nerves. Handb Exp Pharmacol. 2009;(187):63–76. doi: 10.1007/978-3-540-79842-2_4. [DOI] [PubMed] [Google Scholar]
- 91.Karlsson JA, Lanner AS, Persson CG. Airway opioid receptors mediate inhibition of cough and reflex bronchoconstriction in guinea pigs. J Pharmacol Exp Ther. 1990;252(2):863–8. [PubMed] [Google Scholar]
- 92.Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259(4 Pt 2):H1307–11. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- 93.Gordon FJ, Leone C. Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Res. 1991;568(12):319–22. doi: 10.1016/0006-8993(91)91418-z. [DOI] [PubMed] [Google Scholar]
- 94.Wilson CG, Zhang Z, Bonham AC. Non-NMDA receptors transmit cardiopulmonary C fibre input in nucleus tractus solitarii in rats. J Physiol. 1996;496(Pt 3):773–85. doi: 10.1113/jphysiol.1996.sp021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davenport PW. Clinical cough I: the urge-to-cough: a respiratory sensation. Handb Exp Pharmacol. 2009;(187):263–76. doi: 10.1007/978-3-540-79842-2_13. [DOI] [PubMed] [Google Scholar]
- 96.Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci USA. 1999;96(14):7680–6. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97(6):1611–26. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 98.Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11(1):37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- 99.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97(9):4926–31. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Childers WE, Jr, Baudy RB. N-methyl-D-aspartate antagonists and neuropathic pain: the search for relief. J Med Chem. 2007;50(11):2557–62. doi: 10.1021/jm060728b. [DOI] [PubMed] [Google Scholar]
- 101.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30(6):325–33. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 102.Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS, Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci. 2000;3(1):15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- 103.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364(6438):626–32. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 104.Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc Natl Acad Sci USA. 2007;104(8):2950–5. doi: 10.1073/pnas.0611620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86(22):9030–3. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chianca DA, Jr, Lin LH, Dragon DN, Talman WT. NMDA receptors in nucleus tractus solitarii are linked to soluble guanylate cyclase. Am J Physiol Heart Circ Physiol. 2004;286(4):H1521–7. doi: 10.1152/ajpheart.00236.2003. [DOI] [PubMed] [Google Scholar]
- 107.Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12(11):4427–36. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


