Abstract
Cervical mucosal expression of cytokines involved in mediating cellular immunity is believed to influence the persistence of human papillomavirus (HPV) infection, a necessary prerequisite for the development of cervical intraepithelial neoplasia (CIN). Additionally, regulatory T (Treg) cells are increasingly understood to be important modulators of cellular immunity. Using quantitative RT-PCR, we measured, in cross-sectional design, the cervical mRNA expression of IFN-γ, IL-10, and IL-12, as well as the Treg transcription factor Forkhead box P3 (Foxp3), in a cohort of young women representing CIN 1, 2, and 3 as well as benign histology. Higher levels of IFN-γ and IL-10 were significantly (p≤0.05) associated with decreased odds of having high-grade cervical disease (CIN 2 or 3) in multivariate logistic regression models. In contrast, higher levels of mucosal Foxp3 expression were associated with increased odds of having CIN 2 or 3 (p=0.004). In a multivariate model including cervical infection with HPV16 and/or another high-risk HPV type, Foxp3 remained higher in the CIN 2/3 group, but the difference was notably less significant (p=0.05). These findings support a model in which diminished cellular immunity in the cervical mucosa and mucosal enrichment of Treg cells both contribute to the development of high-grade lesions.
Keywords: cervical intraepithelial neoplasia, papillomavirus infections, cytokines, regulatory T cells, mucosal immunology
INTRODUCTION
Human papillomavirus (HPV) persistence is necessary for the development of cervical intraepithelial neoplasia (CIN) 2 and 3 as well as cervical cancer. Among factors that influence the likelihood of a cervical HPV infection becoming persistent, the host’s cell-mediated immune response and the T helper type 1 (Th1) cytokine axis are likely to be key. Studies have shown diminished reactivity against HPV antigens in peripheral blood of women with high-grade CIN compared with women with HPV infections but without lesions.1,2 Because immune parameters, such as cytokine levels, don’t necessarily correlate between peripheral blood and the cervical mucosa,3 the study of local responses is regarded as important in fully understanding the host response to HPV and cervical disease.4 We’ve previously measured cytokine mRNA expression in exfoliated cervical cells and demonstrated that a Th1 cytokine profile is associated with subsequent HPV clearance.5
In the past few years, a subset of cells that are able to modulate immune responses has come under intensive inquiry. These CD4+CD25hi regulatory T (Treg) cells appear to act principally via a contact-dependent mechanism of suppression.6 Intracellular expression of the Forkhead box P3 (Foxp3) transcription factor is considered to be the most reliable marker to date for Treg cells.7,8 Various lines of evidence suggest that Treg cells are able to dampen anti-tumor immunity in a variety of human cancers,9 and have been reported to be increased in both stroma and intraepithelial tissues in cervical cancer.10 HPV-specific Treg cells have also been isolated from both tumor-draining lymph nodes and tumor tissue from cervical cancer patients, and were able to suppress alloreactive CD4+ responder cells in vitro.11
In the present study, we measured, in cross-sectional design, cervical mRNA expression of 3 key cytokines involved in cell-mediated immunity in a cohort of young women representing normal histology and all grades of CIN. The cytokines examined were interferon (IFN)-γ, the signature cytokine of Th1 responses; interleukin (IL)-12, a key cytokine involved in inducing and maintaining such responses; and IL-10, a potent modulator of cell-mediated immune responses. We also measured Foxp3 mRNA expression in a similar cross-sectional sample set.
MATERIALS AND METHODS
Study Subjects and Visit Procedures
Women aged 14–25 years with abnormal cervical cytology referred for colposcopy at nine Kaiser Permanente Medical Group of Northern California clinics were eligible to participate in a prospective cohort study of the natural history of CIN 2 in adolescents. Women with a history of immunosuppression or who were pregnant or planning to leave the area within three years were excluded. Informed consent was obtained according to the guidelines set forth and approved by the Committee for Human Research, at The University of California, San Francisco and Kaiser Permanente Institutional Review Boards. All consenting women were seen for a screening visit; since women without CIN 2 on biopsy were not followed beyond the screening visit, only data from this screening visit were used in the present analysis.
During the screening study visit, a face-to-face interview was conducted to obtain behavioral history, following which specimens were collected prior to biopsy. These included cervical samples using a cytology brush for RNA isolation followed by another cervical brush for cytology and HPV testing. HPV testing was performed on samples collected into liquid cytology medium (PreservCyt, Cytyc Corporation, Marlborough, Massachusetts) using PCR amplification with the PGMY 09/11 primer system followed by typing via reverse line blot (Roche Molecular Diagnostics, Inc., Alameda, California), as previously described.12
Histologic Diagnosis
All histology samples were sent for review to a centralized laboratory and were classified as benign or CIN 1, 2, or 3. No cancers were diagnosed in this group.
Sample Collection and RNA Isolation
Cervical specimens were collected for cytokine testing and RNA isolated as previously described.12 Briefly, cytobrush specimens were transported in RNAlater (Ambion Inc., Austin TX) RNA stabilization medium and stored at −80 °C. Cells were lysed in TRI Reagent RNA isolation reagent (Molecular Research Center, Inc., Cincinnati, OH) and RNA was isolated following the manufacturer’s protocol, with the modifications previously described.12 RNA was solubilized in 1 mM sodium citrate, pH 6.4 (Ambion Inc.), quantitated by measuring absorbance at 260 nm, and then stored at −80 °C until testing.
Real-time RT-PCR
Samples were reverse transcribed in a 100-µl reaction volume using 0.5 µg of total RNA, 10 ng/µl random hexamers, 0.4 U/µl ribonuclease inhibitor, 1mM of each dNTP, and 2.5 U/µl of Moloney murine leukemia virus reverse transcriptase (all reagents from Promega, Madison, WI). Reactions were carried out at 48 °C for 40 min., followed by enzyme inactivation at 95 °C for 5 min. The complementary (c)DNA was stored at −80 °C until used for PCR.
TaqMan real-time PCR technology was employed for quantitative cytokine gene expression measurements. Gene expression was normalized against the reference gene β-glucuronidase (GUS). For cytokine measurements, the following primers and dual-labeled (5' 6-carboxyfluorescein and 3' black hole quencher-1) probes, synthesized by Integrated DNA Technologies (Coralville, IA), were used (5'–3'): IFN-γ forward ACGAGATGACTTCGAAAAGCTG, IFN-γ reverse TTTAGCTGCTGGCGACAGTTC, IFN-γ probe CGGTAACTGACTTGAATGTCCAACGCAA; IL-10 forward GGGAGAACCTGAAGACCCTCA, IL-10 reverse TGCTCTTGTTTTCACAGGGAAG, IL-10 probe CTGAGGCTACGGCGCTGTCATCG; IL-12 forward GCCCAGCTGCTGAGGAGAGT, IL-12 reverse TGGGTGGGTCAGGTTTGATG, IL-12 probe ACGGCATCCACCATGACCTCAATG; and GUS forward CTCATTTGGAATTTTGCCGATT, GUS reverse CCGAGTGAAGATCCCCTTTTTA, GUS probe TGAACAGTCACCGACGAGAGTGCTGG. For Foxp3 quantitation, primers and probe were purchased commercially (TaqMan Inventoried Gene Expression Assay Hs00203958_m1, Applied Biosystems, Foster City, CA). Triplicate 20-µl reactions were set up in 384-well reaction plates and amplification carried out as previously described,12 using 0.025 U AmpliTaq Gold (Applied Biosystems) and 12.5 ng cDNA per reaction. Thermocycling was performed on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) using an initial incubation at 95 °C for 12 min, followed by 45 cycles of 95 °C for 15 sec and 60 °C for 1 min. Quantitative values—expressed as the fractional cycle number (threshold cycle, CT) at which reporter fluorescence first exceeded a user-set threshold level—were obtained during exponential amplification for all reactions. All gene expression levels were normalized using the formula 2ΔCT, where ΔCT is the mean (of triplicates) CT of GUS minus the mean CT of the cytokine or Foxp3.
Statistical Analysis
To model the associations between cytokine expression and cervical lesions, bivariate and multivariate logistic regression models were used, the latter including variables previously shown to affect cervical cytokine mRNA expression (age, days since last intercourse, oral contraceptive pill use, cigarette smoking within last 24 hours, and last menstrual period),12 as well as infection with a high-risk (oncogenic) HPV type. Comparisons were made between each of the histologic categories (CIN 1, 2, and 3 and normal). Since many consider CIN 2 and 3 to be similar high grade diagnoses, and CIN 1 a benign diagnosis, we also made comparisons between CIN 2/3 and normal histology/CIN 1. For the Foxp3 analysis, normal histology and CIN 1 were combined since the number of CIN 1 cases was small. The multivariate analysis for Foxp3 only adjusted for high-risk HPV infection13 since no other factors have been shown to affect Foxp3 expression. Because cytokine and Foxp3 values were log-transformed to alleviate heteroscedasticity, the interpretation of odds ratios is the odds of having higher grade of cervical disease associated with each 1.72-fold change in analyte mRNA expression, where 1.72 is the natural logarithm base, e, minus 1.
Results
Cytokine Expression and Histologic Diagnosis
Cytokine mRNA expression was measured in samples collected from baseline visits from 351 women with a mean age of 20.0 (±2.0, SD) years. One hundred and fifty-seven (45%) had histologic diagnoses read as benign, 106 (30%) as CIN 1, 51 (15%) as CIN 2, and 37 (11%) as CIN 3. The numbers of women testing positive either for HPV 16 or for other high-risk HPV types (but negative for HPV 16) were 23 (62%) and 12 (32%), respectively, among women with CIN 3, 16 (31%) and 29 (57%) among women with CIN 2, 22 (21%) and 47 (45%) among women with CIN 1 (2 with missing HPV data), and 18 (12%) and 66 (43%) among women with normal histology (4 with missing data).
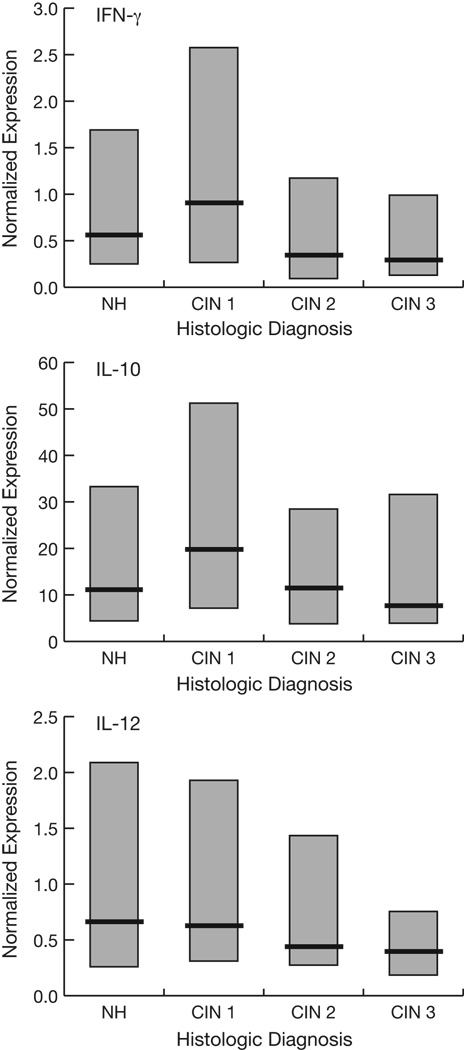
Figure 1 shows the cytokine distributions by histologic diagnosis. To model the effects of cytokine levels on the risk of cervical lesions as well as potential confounding by covariates previously shown to influence cervical cytokine levels,12 bivariate and multivariate logistic regression analyses were performed (Table 1). Because no differences in cytokine expression were associated with altered odds of CIN 3 versus CIN 2 (as shown) and because these high-grade lesions are regarded by many as essentially indistinguishable (see Discussion), they were combined in the models. Higher levels of cervical mRNA expression of IFN-γ were significantly (p≤0.05) associated with decreased odds of having high-grade cervical disease (CIN 2 or 3), with the most significant comparison being high-grade lesions vs. CIN 1. Similar trends were noted in bivariate analysis for IL-10 and IL-12. In the multivariate models, higher levels of both IFN-γ and IL-10 were significantly associated with decreased odds of high-grade lesions.
Fig. 1.
Median (heavy lines) and inter-quartile ranges (boxes) of normalized cytokine expression levels by histologic diagnosis. NH, normal histology.
Table 1.
Relative cytokine mRNA expression as predictor of CIN in bivariate and multivariate logistic regression models.
| IFN-γ1 | IL-101 | IL-121 | ||||
|---|---|---|---|---|---|---|
| Bivariate | Multivariate 2 | Bivariate | Multivariate 2 | Bivariate | Multivariate 2 | |
| CIN 3 vs. CIN 2 | 1.03 (0.78, 1.38) | 1.06 (0.74, 1.51) | 0.99 (0.78, 1.24) | 0.86 (0.64, 1.15) | 1.04 (0.80, 1.35) | 1.02 (0.74, 1.42) |
| CIN 2/3 vs. NH/CIN 1 | 0.72 (0.60, 0.85)*** | 0.73 (0.60, 0.89)** | 0.88 (0.76, 1.00) | 0.79 (0.66, 0.94)** | 0.88 (0.75, 1.04) | 1.00 (0.82, 1.21) |
| CIN 2/3 vs. CIN 1 | 0.65 (0.53, 0.80)*** | 0.68 (0.53, 0.87)** | 0.82 (0.69, 0.97)* | 0.74 (0.60, 0.93)** | 0.88 (0.74, 1.06) | 0.95 (0.77, 1.18) |
| CIN 2/3 vs. NH | 0.76 (0.64, 0.91)** | 0.76 (0.61, 0.96)** | 0.91 (0.78, 1.06) | 0.85 (0.70, 1.03) | 0.90 (0.74, 1.07) | 1.07 (0.85, 1.34) |
| CIN 1 vs. NH | 1.16 (0.99, 1.35) | 1.15 (0.97, 1.36) | 1.13 (0.97, 1.32) | 1.11 (0.93, 1.34) | 1.02 (0.86, 1.22) | 1.07 (0.88, 1.31) |
Odds ratio (95% confidence interval); interpreted as the odds of having the higher grade of disease associated with each 1.72-fold difference in cytokine mRNA expression. All cytokine measures were log-transformed.
The multivariate models included variables previously shown to affect cervical cytokine mRNA expression (age, days since last intercourse, current OCP use, cigarette smoking within last 24 hours, and last menstrual period),12 plus current infection with high-risk HPV type.
p≤0.05
p≤0.01
p≤0.001
NH, normal histology.
Foxp3 Expression and Histologic Diagnosis
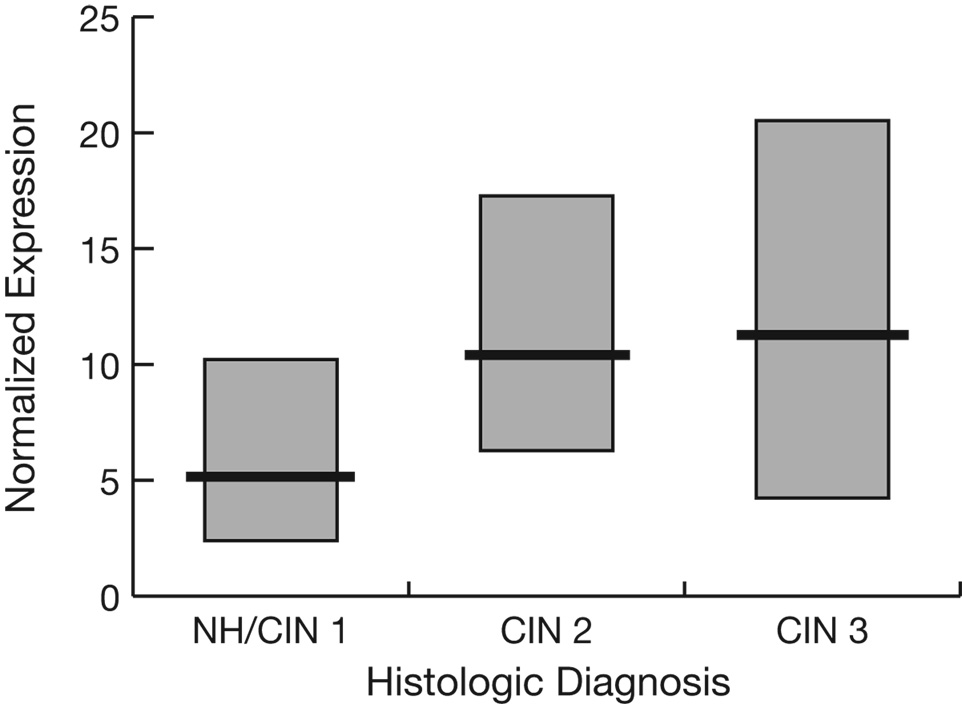
Prompted by our finding of an association between lower IFN-γ expression and elevated odds of having CIN 2/3, as well as recent advances in understanding of the immune modulating role of Treg cells, we next examined the cervical mucosal mRNA expression of Foxp3. Because there wasn’t sufficient RNA remaining from a majority of the original 351 women, Foxp3 was measured in 83 largely non-overlapping samples. Twenty (24%) had histologic diagnoses read as normal, 2 (2%) as CIN 1, 29 (35%) as CIN 2, and 32 (39%) as CIN 3. Figure 2 shows the Foxp3 distributions by histologic diagnosis. In a bivariate regression analysis, higher levels of Foxp3 expression were associated with increased odds of having CIN 2 or 3 vs. CIN 1 or normal histology (p=0.004, Table 2). In contrast, higher Foxp3 expression did not predict CIN 3 vs. CIN 2. In the multivariate logistic regression, Foxp3 was higher in the CIN 2/3 group compared with the CIN 1/normal histology group, although the difference was notably less significant than in the bivariate analysis (p=0.05).
Fig. 2.
Median (heavy lines) and inter-quartile ranges (boxes) of normalized Foxp3 expression levels by histologic diagnosis. Because there were only 2 CIN 1 cases these have been combined with normal histology (NH) cases.
Table 2.
Relative Foxp3 mRNA expression as predictor of CIN in logistic regression models. 1
| Bivariate | Multivariate 2 | |
|---|---|---|
| CIN 2/3 vs. NH/CIN 1 | 2.43 (1.32, 4.48)** | 1.94 (1.00, 3.76)* |
| CIN 3 vs. NH/CIN 1 | 2.44 (1.24, 4.78)** | 1.96 (0.92, 4.17) |
| CIN 3 vs. CIN 2 | 1.33 (0.70, 2.52) | 1.36 (0.69, 2.69) |
Odds ratio (95% confidence interval); interpreted as the odds of having the higher grade of disease associated with each 1.72-fold difference in Foxp3 mRNA expression. Foxp3 measures were log-transformed.
The multivariate model included current infection with HPV16 and/or another high-risk HPV type.
p≤0.05
p≤0.01
NH, normal histology.
Discussion
In the present study, we provide evidence that lower levels of IFN-γ and, to a lesser extent IL-10, are associated with CIN 2 and 3 lesions compared to women with CIN 1 or normal histology. Lower levels of IFN-γ were significantly associated with higher odds of CIN 2 or 3 in both bivariate and multivariate models, and the associations were significant whether the comparison group was CIN 1, normal histology, or both combined. The lack of significant differences between CIN 1 and normal histology is not surprising since CIN 1 is considered to reflect a benign cellular response to acute HPV infection, with over 90% regressing spontaneously.14,15 The trend noted toward higher median IFN-γ levels in CIN 1 compared with normal histology most likely reflects that cellular response. The lack of differences between CIN 2 and CIN 3 is also not surprising since both are reflective of a proliferative phenotype and regarded by many pathologists as largely indistinguishable.15 Patterns for IL-10 appear to roughly parallel those for IFN-γ. Given that IL-10 is produced by a variety of cell types and is part of a critical homeostatic feedback mechanism, it is not necessarily surprising that its expression would change in concert with changes in IFN-γ levels. For IL-10, the association between lower expression levels and CIN 2 or 3 became more evident in the multivariate analysis, suggesting that the influence of one or more covariates masked the association in the bivariate analysis. Furthermore, although trends were noted when the comparison group was normal histology, the association of lower IL-10 with high-grade lesions appears to be mainly driven by differences between CIN 2/3 and CIN 1. Although the validation of biomarkers of high-grade cervical disease was not a goal of the present study, the data presented here suggest that cytokine patterns may help differentiate CIN 2/3 from CIN 1.
We previously showed that a Th1 cytokine profile appears to precede HPV clearance, but is less common in persistent infections.5 While the present study was not designed to examine HPV persistence directly, the association of lower IFN-γ and IL-10 expression with high-grade lesions may be partly reflective of viral persistence and therefore consistent with a hypothesized model of a diminished cell-mediated immune response permitting persistence. As the multivariate analyses adjusted for infection with a high-risk HPV type, however, there appear to also be associations for decreased expression of these cytokines and CIN 2/3 that are independent of the underlying infection. Importantly, as with any cross-sectional study, the patterns reported here do not necessarily imply corresponding longitudinal changes in cytokine expression within a given woman. Certainly, it is plausible that cytokine levels are initially increased in CIN 1 lesions, leading either to eventual regression or, if the initial increase is not sustained, to HPV persistence and associated high-grade lesions. An equally reasonable interpretation is that women who fail to mount a robust cell-mediated response with initial infection are more likely to develop persistence and CIN 2/3. Note that the broad range of IFN-γ and IL-10 levels seen in the CIN 1 group would certainly be consistent with the latter stochastic interpretation.
Other studies have also examined local cytokine expression in cervical tissues from women with HPV-associated lesions. El-Sherif et al., using a competitive RT-PCR assay, reported similar findings to ours in that IFN-γ mRNA expression was significantly lower in CIN biopsies than histologically normal tissue.16 De Gruijl et al. reported that IFN-γ, IL-10, and IL-12 transcripts were all expressed in fewer invasive cervical carcinoma biopsies, as detected by RT-PCR, compared with biopsies representing all 3 grades of CIN,17 suggesting that that the associations we observed for CIN 2/3 become more pronounced in cases that advance to cervical cancer. Like those two studies, the samples and methods employed here allow examination of the immunoregulatory cytokine milieu present in the cervical epithelium, without, however, identifying the cells involved in producing the measured cytokines. It has been previously shown that a wide complement of immune cells can be found in the cervical epithelium, including T cells (major sources of IFN-γ), dendritic and other antigen-presenting cells (major sources of IL-10 and IL-12), and others.18,19 Differences in cytokine mRNA expression suggest but do not prove respective differences in the extent of mononuclear cell infiltration. Nonetheless, given the key immunoregulatory functions associated with these cytokines, snapshots of cytokine milieu contribute to understanding the role of cytokines in the natural history of HPV infection and related disease. Other immunoregulatory cytokines of potential interest include those that are markers of Th2 cells (IL-4, IL-5, and IL-13), which may also be involved in shaping the host response to HPV and associated lesions. Unfortunately, our experience has shown that expression of those cytokines is inordinately low, and even below detection in a substantial percentage of cervical cytology samples (Scott et al.12 and unpublished observations), largely precluding their measurement in these samples.
To investigate a possible role for local Treg cells in high-grade CIN, we examined mRNA expression of the transcription factor Foxp3. Although there is evidence that no single phenotypic marker can identify all T cells with regulatory function, potentially confounding attempts to accurately enumerate frequencies of these heterogeneous cells in tissues,11 Foxp3 is regarded as the best single marker identified to date of the CD4+CD25hi subset of Treg cells.7,8 To the best of our knowledge, this is the first report of increased expression of a Treg cell marker in cervical samples from women with CIN and provides evidence of enrichment of Treg cells in the cervical mucosa. Interestingly, both the magnitude and significance of the associations we found were decreased in the multivariate model that adjusted for cervical infection with a high-risk HPV type. This is not unexpected, as HPV infection is necessary for the development of high-grade cervical disease, and suggests that the elevated Foxp3 expression is associated with the persistence of a high-risk HPV infection. This conclusion is consistent with that of Molling et al.,13 who showed that systemic Treg enrichment is associated with HPV persistence, although our data also suggest that there remains, at the mucosal level, a weak, independent association with CIN 2/3 even after adjusting for infection status.
Another recent study also showed significantly increased Treg cell frequencies among peripheral blood mononuclear cells and CD4+ T cells in women with CIN or cervical cancer.20 The authors further showed that in cervical cancer patients, in vitro depletion of CD25+ T cells enhances HPV 16 E6- and E7-specific helper T cell responses, indicating that intrinsic anti-HPV responses exist but are modulated by Treg cells. These two studies reporting systemic Treg enrichment, plus ours showing increased Foxp3 expression at the cervical mucosal level, support the hypothesis that Treg cells dampen host responses against HPV infection and its sequelae and highlight the interplay between systemic and mucosal immune responses. The recent demonstration of transient, low-level Foxp3 expression in activated T cells,21,22 suggests the possibility that these may also have contributed to the Foxp3 measured here. Notably, however, while this transient Foxp3 expression does not confer suppressor function, development of stable, high-level expression does correlate with acquisition of suppressor function,21,22 supporting the measurement of Foxp3 as a provisional marker of Treg cells. Better mRNA markers of human Treg cells are needed, however, and will allow refinement of this and many other studies that have examined putative Treg roles in cancer and infection. This is likely to be an area of considerable interest, as Treg cells may eventually provide a target for therapeutic intervention in women with HPV persistence or pre-cancerous lesions of the cervix.
Acknowledgments
This work was supported by U.S. Public Health Service grants 2 R01 CA87905, 2 R01 CA51323, and 2 R37 CA051323. The authors acknowledge the contributions of the following staff of the Kaiser Permanente/University of California, San Francisco CIN 2 Study: Amber Dimick-Flores, MA, Cheryl Godwin de Medina, Wanda Griffin, RN, Debra Guisto, RN, Janet Jonte, NP, Katy Kurtzman, MD, Lesley Levine, MD, Anita Levine-Goldberg NP, Carol Lopez, LVN, Ellen McKnight, NP, Karen Milligan-Green, RN, Laura Minikel, MD, Heidi Olander, MD, Mary Phelps, MA, Diane Ragni, RN, Katy Ryan, MD, Debbie Russell, RN, Greg Sacher MD, Mark Seaver, MD, Carolyn Taylor, RN, and Nicole Zidenberg, MD. We also thank Roche Molecular Diagnostics for training and supplies to support the HPV DNA testing.
Grant sponsors: U.S. Public Health Service—National Cancer Institute (grant numbers 2 R01 CA87905, 2 R01 CA51323, and 2 R37 CA051323).
Abbreviations
- cDNA
complementary DNA
- CIN
cervical intraepithelial neoplasia
- CT
threshold cycle
- Foxp3
Forkhead box P3
- GUS
β-glucuronidase
- HPV
human papillomavirus
- IFN
interferon
- IL
interleukin
- Th1
T helper type 1
- Treg
regulatory T cell
Footnotes
Novelty and impact: The study of cervical mucosal responses is important in fully understanding the host response to HPV. Herein we demonstrate that differences in cervical mucosal expression of cytokines, particularly IFN-γ, and the regulatory T cell marker Foxp3 are associated with altered odds of having high-grade cervical disease.
Presented in part at the 23rd International Papillomavirus Conference, September 2006, Prague, Czech Republic.
The authors have no commercial or other associations that might pose a conflict of interest.
References
- 1.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, Kenter G, Offringa R, van der Burg SH. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa M, Stites DP, Farhat S, Sisler JR, Moss B, Kong F, Moscicki AB, Palefsky JM. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J Infect Dis. 1997;175:927–931. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 3.Castle PE, Hildesheim A, Bowman FP, Strickler HD, Walker JL, Pustilnik T, Edwards RP, Crowley-Nowick PA. Cervical concentrations of interleukin-10 and interleukin-12 do not correlate with plasma levels. J Clin Immunol. 2002;22:23–27. doi: 10.1023/a:1014252402630. [DOI] [PubMed] [Google Scholar]
- 4.Castle PE, Giuliano AR. Chapter 4: Genital tract infections, cervical inflammation, and antioxidant nutrients—assessing their roles as human papillomavirus cofactors. J Natl Cancer Inst Monogr. 2003;31:29–34. doi: 10.1093/oxfordjournals.jncimonographs.a003478. [DOI] [PubMed] [Google Scholar]
- 5.Scott M, Stites DP, Moscicki AB. Th1 cytokine patterns in cervical human papillomavirus infection. Clin Diagn Lab Immunol. 1999;6:751–755. doi: 10.1128/cdli.6.5.751-755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006;16:98–105. doi: 10.1016/j.semcancer.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Roncador G, Brown PJ, Maestre L, Hue S, Martínez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 9.Baecher-Allan C, Anderson DE. Immune regulation in tumor-bearing hosts. Curr Opin Immunol. 2006;18:214–219. doi: 10.1016/j.coi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 11.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, Welters MJ, Van Rood JJ, Fleuren GJ, Melief CJ, Kenter GG, Offringa R. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A. 2007;104:12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222–232. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 13.Molling JW, de Gruijl TD, Glim J, Moreno M, Rozendaal L, Meijer CJ, van den Eertwegh AJ, Scheper RJ, von Blomberg ME, Bontkes HJ. CD4+CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int J Cancer. 2007;121:1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- 14.Moscicki AB, Shiboski S, Hills NK, Powell KJ, Jay N, Hanson EN, Miller S, Canjura-Clayton KL, Farhat S, Broering JM, Darragh TM. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678–1683. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 15.Cooper K, Evans M, Mount S. Biology and evolution of cervical squamous intraepithelial lesions: a hypothesis with diagnostic prognostic implications. Adv Anat Pathol. 2003;10:200–203. doi: 10.1097/00125480-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 16.El-Sherif AM, Seth R, Tighe PJ, Jenkins D. Quantitative analysis of IL-10 and IFN-γ mRNA levels in normal cervix and human papillomavirus type 16 associated cervical precancer. J Pathol. 2001;195:179–185. doi: 10.1002/path.929. [DOI] [PubMed] [Google Scholar]
- 17.de Gruijl TD, Bontkes HJ, van den Muysenberg AJ, van Oostveen JW, Stukart MJ, Verheijen RH, van der Vange N, Snijders PJ, Meijer CJ, Walboomers JM, Scheper RJ. Differences in cytokine mRNA profiles between premalignant and malignant lesions of the uterine cervix. Eur J Cancer. 1999;35:490–497. doi: 10.1016/s0959-8049(98)00371-2. [DOI] [PubMed] [Google Scholar]
- 18.Johansson EL, Rudin A, Wassén L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 20.Visser J, Nijman HW, Hoogenboom BN, Jager P, van Baarle D, Schuuring E, Abdulahad W, Miedema F, van der Zee AG, Daemen T. Frequencies and role of regulatory T cells in patients with (pre)malignant cervical neoplasia. Clin Exp Immunol. 2007;150:199–209. doi: 10.1111/j.1365-2249.2007.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hori S. Rethinking the molecular definition of regulatory T cells. Eur J Immunol. 2008;38:928–930. doi: 10.1002/eji.200838147. [DOI] [PubMed] [Google Scholar]
- 22.Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38:925–927. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]