Abstract
This review is the introduction to a special issue concerning, glutathione (GSH), the most abundant low molecular weight thiol compound synthesized in cells. GSH plays critical roles in protecting cells from oxidative damage and the toxicity of xenobiotic electrophiles, and maintaining redox homeostasis. Here, the functions and GSH and the sources of oxidants and electrophiles, the elimination of oxidants by reduction and electrophiles by conjugation with GSH are briefly described. Methods of assessing GSH status in the cells are also described. GSH synthesis and its regulation are addressed along with therapeutic approaches for manipulating GSH content that have been proposed. The purpose here is to provide a brief overview of some of the important aspects of glutathione metabolism as part of this special issue that will provide a more comprehensive review of the state of knowledge regarding this essential molecule.
Keywords: Glutathione, Glutamate cysteine ligase, Hydroperoxide, Xenobiotic, Methods
1. Introduction
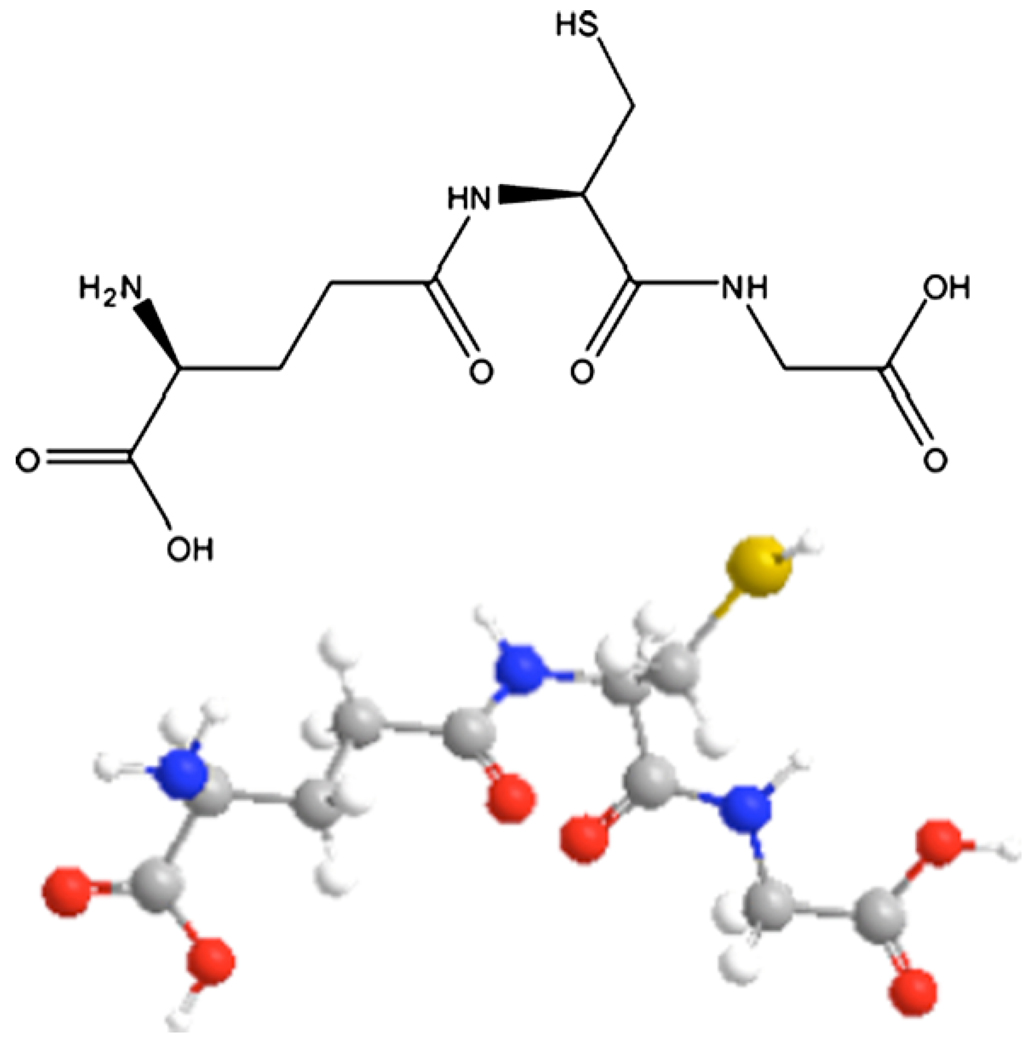
The tripeptide, γ-l-glutamyl-l-cysteinyl-glycine known as glutathione (GSH) (Fig. 1), is the most important low molecular weight antioxidant synthesized in cells. It is synthesized by the sequential addition of cysteine to glutamate followed by the addition of glycine. The sulfhydryl group (−SH) of the cysteine is involved in reduction and conjugation reactions that are usually considered as the most important functions of GSH. These reactions provide the means for removal of peroxides and many xenobiotic compounds; however, GSH is also involved in regulation of the cell cycle (Meister 1992).
Fig. 1.
Glutathione structure. A stereochemical and ball and stick figure showing γ-glutamyl-cysteinyl-glycine are shown.
2. Sources of oxidants
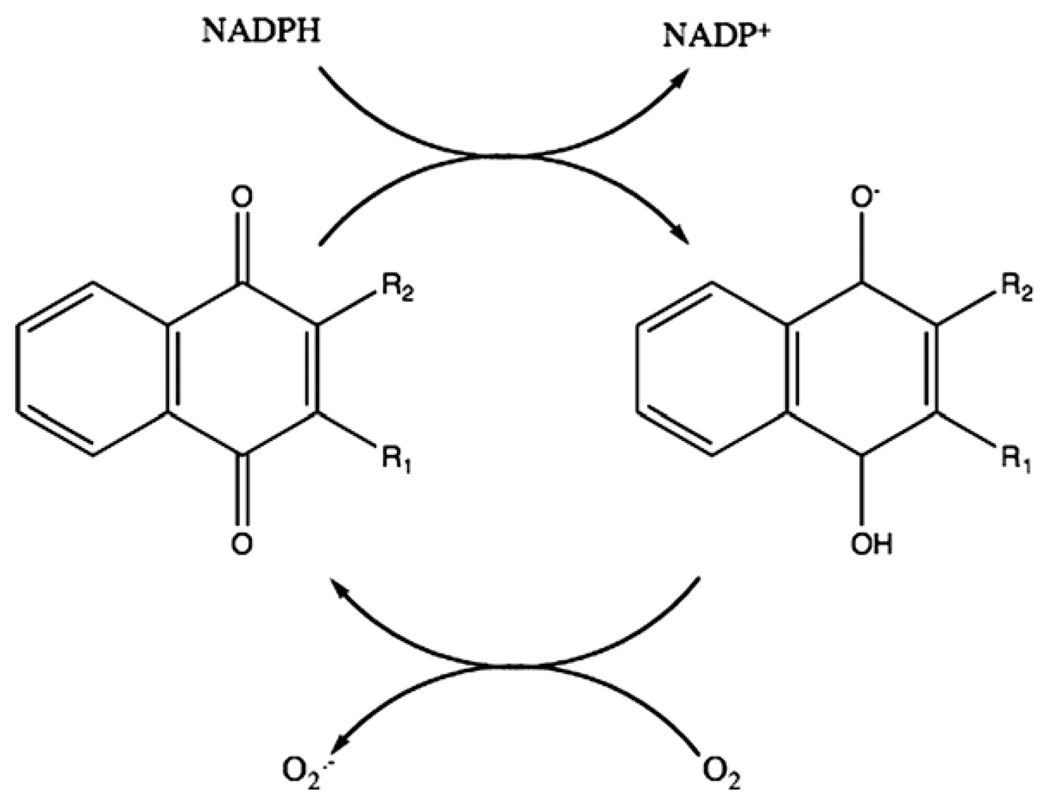
GSH plays a major role in removal of many reactive species. But, before addressing those aspects, it is important to understand from where these reactive species come and their pathological consequences that GSH helps avoid. Quinones are a class of redox cycling molecules that includes some drugs and xenobiotic compounds. Redox cycling in this context refers to the ability to cycle between oxidized and reduced forms and in the process, produce reactive oxygen species, such as superoxide (O2−) and hydrogen peroxide (H2O2). In this reaction (Fig. 2), the quinone is reduced by one electron transport reaction to produce a semiquinone, which is a free radical that can react with oxygen to produce O2−.
Fig. 2.
Redox cycling of 1,4-naphthoquinones. A naphthoquinone with two variable groups (R) can be reduced by NADPH (or NADH, which is not shown) enzymatically to the semiquinone radical and then will react with oxygen to generate superoxide and restore the naphthoquinone.
There are other places in the cells where reactive oxygen species can be generated. In phagocytes, a major part of the mechanism of killing microorganisms involves production of reactive oxygen species (Forman and Thomas, 1986). The first enzyme involved is NADPH oxidase (NOX) that produces O2−. That enzyme is now known to be a member of a class of enzymes found in almost all cells (Vignais 2002). Once O2− is made, it can be dismuted into H2O2 both by a relatively fast non-enzymatic reaction and by very fast reaction catalyzed by one of the superoxide dismutases (SOD). Some phagocytes have the capacity to secrete enzymes called myeleoperoxidases that can catalyze a reaction of H2O2 and halides (chloride or bromide) to produce hypochlorous acid (HOCl) or hypobromous acid (HOBr) (Bakkenist et al., 1980). These hypohalous acids kill bacteria but can also damage normal tissue and thereby contribute to an inflammatory reaction.
The H2O2 formed can also be potentially hazardous if there are reduced metals present in the cells. H2O2 can react with ferrous iron (Fe2+) and produce the hydroxyl radical ( OH). This radical has capability to oxidize Teflon or fluorine and any organic molecule at near diffusion limited rates. In other words, OH can react with any molecule next to where it is produced. O2− can reduce ferric iron (Fe3+) to Fe2+, which suggests that it can play two roles in producing OH; however, reduction of Fe3+can also occur with other reductants such as ascorbic acid (vitamin C).
One of the dangers of producing OH is when it is produced near a membrane. Lipids can be oxidized by OH and start a free radical chain reaction that will damage the membrane. In the initiation of lipid peroxidation by OH, the reaction with a reduced molecule of the lipid produces a lipid radical (L) and water. The L can react with oxygen to produce hydroperoxide radical (LOO ), which then reacts with another lipid molecule, generating a lipid peroxide (LOOH) and another lipid radical L that can continue a chain reaction. One of the dangers from lipid peroxidation besides membrane damage is the production of byproducts such as 4-hydroxy-2-nonenal (HNE). Arachidonic acid is a polyunsaturated fatty acid found in membranes of all cells. When it becomes oxidized, it can break down yielding a large variety of compounds including a, β-unsaturated aldehydes (Poli et al., 1987) These are toxic compounds because they can react with proteins in the cells, particularly at cysteine, lysine or histidine by either Michael addition to the carbon–carbon double bond or by Schiff base formation at the carbon–oxygen double bond (Esterbauer et al., 1991; Eckl, 2003; Schaur, 2003). These reactions can inactivate the function of proteins. For example, reaction with an active site cysteine can destroy the activity of an enzyme.
The final component of oxidative damage considered here is peroxynitrite (ONOO−). This ion is made in a reaction between nitrogen oxide ( NO) and O2−. These two free radicals react at the fastest rate of any reaction known to occur in biology and is the only reaction that is faster than the dismutation reaction of O2− via superoxide dismutases. In its basic form, ONOO− does not react with organic molecule, it breaks down to form nitrite and nitrate But when peroxynitrite is protonated it becomes the highly reactive, peroxynitrous acid (ONOOH) that has the reactivity of nitrogen dioxide ( NO2), a very toxic free radical component in smog and cigarette smoke, and OH.
3. Protective functions of glutathione
3.1. Reduction
GSH is found in the cytosol of cells where it is in the range of 1–10 mM (Meister 1988). In most cells the GSH concentration is about 1–2 mM, while in hepatocytes, which export GSH, the concentration can reach about 10 mM. So why do we need GSH outside of the cells? In plasma GSH is in the micromolar range; however, in some extracellular spaces such as the lining fluid of the lung, a thin layer of fluid covering the air spaces where gas exchange occurs, there is high concentration of GSH that is secreted by epithelial cells (Sutherland et al., 1985; Cantin et al., 1987). In people who smoke or inhale particles or other oxidants, there is potential inflammation that involves invasion of neutrophils from the blood through the endothelial and epithelial cells into the air spaces. As these neutrophils squeeze between the cells, they release HOCl, which can react with GSH secreted from the epithelial cells that normally protects the epithelial cells (Venglarik et al., 2003).
In cystic fibrosis patients, who secrete lower GSH than normal individuals into the lining fluid covering their alveoli, and in smokers, who have exposed their lungs to many oxidants including nitrogen dioxide and H2O2, there is both chronic inflammation and lower than normal GSH (Roum et al., 1993). In that case, HOCl can oxidize proteins in the lining fluid or on the surface of the epithelial cells. It can also react with lipid to produce even more dangerous compounds than are produced by lipid peroxidation itself (Pullar et al., 2000). Fig. 3 shows how GSH reacts with HOCl and removes it (Winterbourn and Brennan, 1997). While many studies of GSH in inflammation have been done of the lungs, these reactions can occur in any organ.
Fig. 3.
Reactions of glutathione with hypochlorous acid. GSH and HOCl can react to produce several different products.
Secretion of GSH to the air space in cystic fibrosis is depressed because of a mutation of a protein called cystic fibrosis transport receptor (CFTR) (Roum et al., 1993). The CFT1 cell line, which is derived from a cystic fibrosis patient, has lower GSH secretion to the apical (air space) side. If the wild type CFTR is transfected into the cells, the rate of GSH secretion is increased to the level seen in normal cells (Gao et al., 1999). The generation of HOCl in the surface fluid covering normal epithelial cells to mimic the action of stimulated neutrophils can decrease in the electrical resistance of that epithelial cell layer; however, the presence of GSH at a concentration similar to normal lining fluid protects against the loss of electrical resistance (Venglarik et al., 2003). Similar events occur during inflammation and are exaggerated in cystic fibrosis patients. There is some evidence that other lung diseases, such as idiopathic pulmonary fibrosis, also have a lower GSH concentration (Cantin et al., 1989). Further studies on the potential contribution of GSH deficiency to these pathologies are needed. Understanding the transport of GSH across the plasma membrane is an important issue that is essential to treatment of diseases involving oxidative stress (see reviews by Ballatori et al., 2008 and by Yuan and Kaplowitz, 2008 in this issue).
Compared to the extracellular environment, what happens inside of the cells is quite different. Glutathione plays major roles in the different cellular compartments. In mitochondria it plays a key role in regulating apoptosis versus necrosis (see review by Yuan and Kaplowitz 2008 in this issue). In the nucleus, GSH is a key regulator of cellular division (see review by Pallardó et al., 2008 in this issue.) While lungs are clearly adversely affected by lowered intracellular and extracellular GSH, the majority of studies on the pathologies involving GSH transport and metabolism have been done in liver. Reviews of the involvement of altered intracellular GSH in lung diseases (Biswas and Rahman, 2008), liver diseases (Yuan and Kaplowitz, 2008) and viral diseases (Fraternale et al., 2008) can be found in this issue.
Most of the GSH in antioxidant defense in cells is utilized by three members of glutathione peroxidase (GPx) family (Brigelius-Flohe, 1999) and by one of the peroxiredoxins (Prdx 6). These enzymes catalyze the reduction of H2O2 by GSH into H2O and GSSG. Prdx 6 also requires GSH S transferase Pi in order to be active (Ralat et al., 2006). Phospholipid hydroperoxide glutathione peroxidase (PHGPx or GPx IV) can reduce lipid peroxides to lipid alcohols (Imai and Nakagawa, 2003). GSSG is potentially toxic to the cells but cells normally contain high glutathione reductase activity, which maintain most of the GSH in the reduced form. Some GSSG is also secreted from cells. During oxidative stress, GSSG could react by disulfide exchange with a protein thiol to produce a protein mixed disulfide (PSSG), which can further exchange with another protein thiol to a protein disulfide (Huang and Huang, 2002). These reactions are actually quite slow unless catalyzed by an enzyme such as protein disulfide isomerase (PDI), an important enzyme that is particularly abundant in the endoplasm reticulum where protein folding occurs. In fact, the cysternae of the endoplasm reticulum is the only part of the cell with a relatively high ratio of GSSG/GSH. In the cytosol formation of PSSG is transient except during oxidative stress.
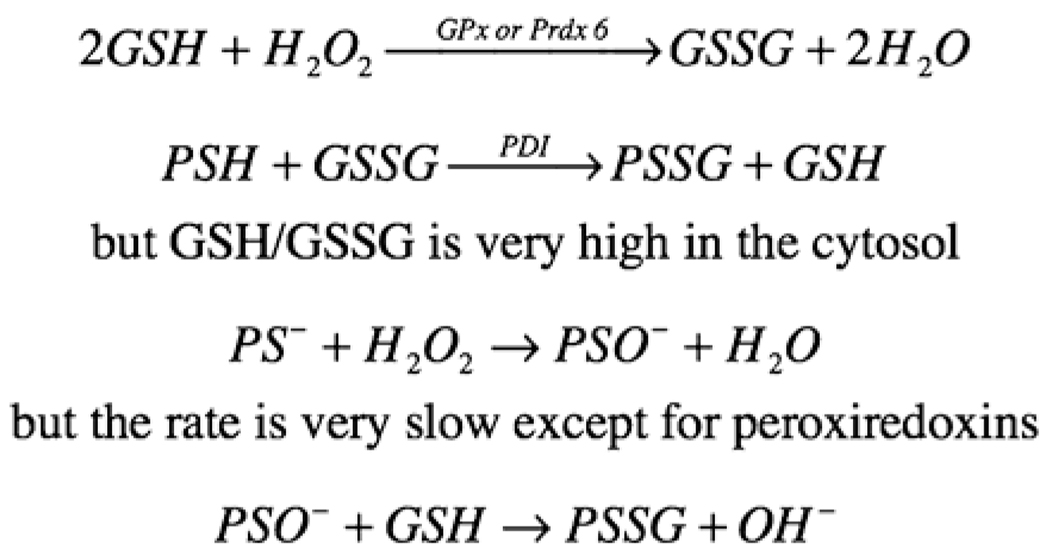
Formation of PSSG with some enzymes may play a role in signal transduction although the exact mechanism of their formation is uncertain. So how might PSSG form during normal metabolism in the cells? While protein disulfide exchange with a thiol can be catalyzed by PDI, some proteins contain a microenvironment in which thiolate (−S−), which is far more reactive than is a thiol in both reaction with H2O2 or disulfide exchange, is formed. This requires that the microenvironment be composed in part by basic amino acids in proximity to the cysteine to allow dissociation of the thiol, which normally has a pKa of around 8.3. GSH peroxidase catalyzes the production of GSSG, which could be potentially exchanged with a thiolate to form mixed disulfide. But in the cytosol, even during oxidative stress, the ratio of GSH/GSSG remains very high, which makes that exchange reaction unfavorable. The enzyme PDI can enhance the rate of that reaction but, like any catalyst, cannot change the equilibrium. Instead, it has been proposed that during physiological signaling when the H2O2 is used as the second messenger, some of protein thiolates could potentially react and form sulfenic acid (PSOH) (Fig. 4); however, for most thiolates including that formed by glutathione, the rate of the non-enzymatic reaction is too slow to account for the inactivation of the enzymes (Forman, 2007). We do know that in the active site of peroxiredoxins, where the reaction of H2O2 with a thiolate can occur up to six orders of magnitude faster than with glutathione in its thiolate form, the reaction can occur. Regardless, once formed, a protein sulfenate would rapidly react with GSH to produce the mixed disulfide, and this could be the mechanism through which PSSG formed for some proteins in the cytosol during oxidative stress when H2O2 is high enough to overcome a slow rate constant.
Fig. 4.
Formation of protein mixed disulfide. Both glutathione peroxidases and peroxiredoxin 6 can catalyze the oxidation of glutathione by hydrogen peroxide to glutathione disulfide and water. GSSG can then undergo an exchange reaction with protein sulfhydryl to form PSSG, which is usually catalyzed by a protein disulfide isomerase. An alternative mechanism is the oxidation of a protein thiolate to a sulfenic acid, which then will react with GSH to form PSSG and water.
3.2. Conjugation
The elimination of many xenobiotic compounds can be accomplished through conjugation with GSH followed by secretion of the adduct from the cell (Boyland and Chasseaud 1969). Although the quinone, menadione, can react with GSH to form an adduct non-enzymatically, an enzymatic catalyzed Michael addition by a glutathione-S transferase (GST) is much faster. The glutathione adduct can then be secreted from cells through a membrane transporter such as the multidrug resistant proteins. The product of the addition of GSH can also rearrange into a quinol that are usually considered to be less toxic than the quinone (see above).
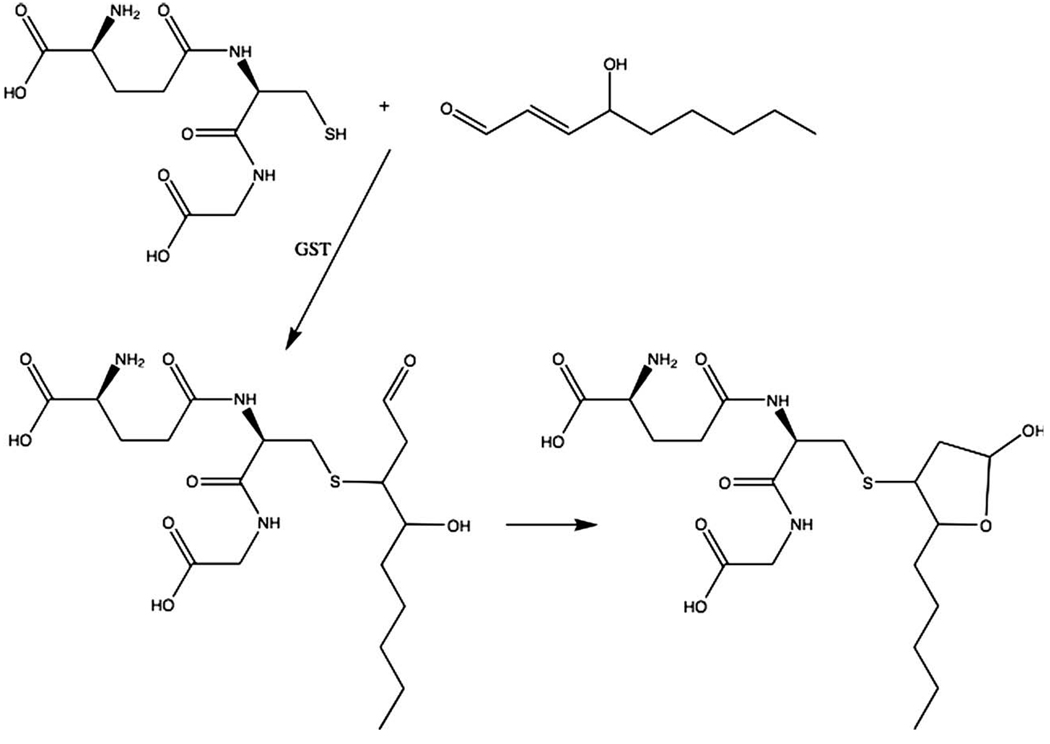
GSH is also used in the elimination of electrophiles such as HNE. Almost all these reactions are catalyzed by GSTs, and there is a specific one in human cells that can cause the conjugation of GSH to HNE at about 100 times faster rate than the non-enzymatic reaction. The conjugate, which is a Michael adduct (because the reaction is a Michael addition), can rearrange to form a cyclic hemiacetal (Fig. 5) (Alary et al., 2003). Both of the compounds however, can be excreted from the cells. This is the major route of elimination of HNE and other electrophiles that conjugate with GSH.
Fig. 5.
Glutathione conjugations with 4-hydroxynonenal. Glutathione S-transferases catalyze the conjugation of GSH with HNE. This is a Michael addition that can slowly occur non-enzymatically.
3.3. Interaction with other non-enzymatic antioxidants
While GSH is the most important small molecular weight antioxidant produced in the cells, there are other small molecular antioxidants obtained from the diet such as vitamins E (α-tocopherol) and C (ascorbic acid). Vitamin E can reduce lipid hydroxyl radicals and lipid peroxides that are produced from polyunsaturated fatty acids. The oxidized vitamin E is then reduced by vitamin C in a non-enzymatic but rapid reaction. The oxidized vitamin C can then be restored to the reduced form by enzymatic reactions, one of which uses GSH as substrate.
4. Measurement of glutathione
One of the important issues in determining the mechanisms of both oxidative stress and redox signaling is the measurement of the different forms of thiols in cells. The predominant forms are the reduced form of GSH and GSSG. Nitrosoglutathione (GSNO) and protein nitrosothiols (PSNO) are also formed in cells and play a role in NO signaling independent of the cyclic GMP pathway. Cysteine is a precursor amino acid of GSH and cystine is the disulfide form of cysteine. Protein thiols exist as cysteine, mixed disulfides between cysteine and GSH or other thiols, and disulfides between two protein cysteines that may be in the same or different protein molecules. It is important to recognize that an increase in the oxidized forms of these thiols in the cytosol will be transient even during oxidative stress. Therefore it can be very difficult to measure thiol oxidation, particularly that occurring in signal transduction.
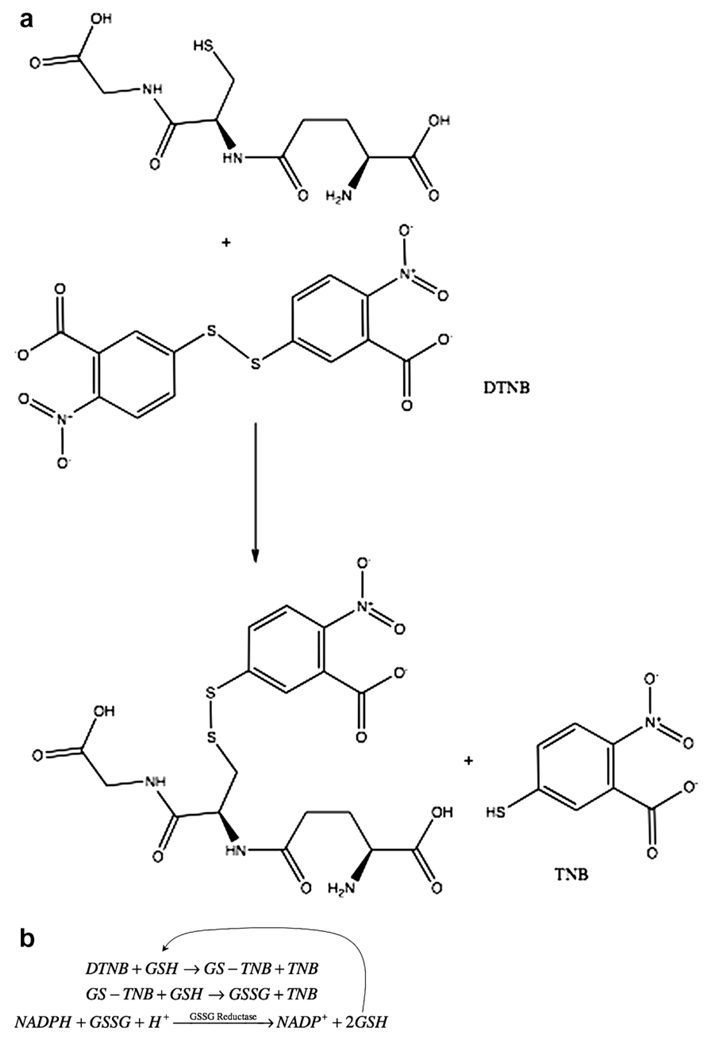
GSH reacts with dithionitrobenzoic acid (DTNB) (Akerboom and Sies, 1981) and by reducing GSSG total GSH (GSH + GSSG) can be measured. DTNB reacts with GSH to produce a conjugate and TNB anion that can be detected by fluorescence or absorbance (Fig. 6a). To measure total GSH, a recycling assay is used in which GSH reacts with the conjugate producing GSSG and another molecule of TNB, which can be increases fluorescence or absorbance (Fig. 6b). The enzyme glutathione reductase then reduces the GSSG releasing the GSH that can react with another molecule of DTNB. Therefore, instead of a single determination of how much DTNB reacts with GSH, the rate of TNB production is measured, as that is proportional to the initial amount of GSH. To measure GSSG however, one must first modify the GSH present at the beginning so it is removed from the recycling assay. Modification of GSH is done with N-ethylmaleimide (NEM) or vinylpyridine. To measure protein mixed disulfides, the GSH can be released from the protein mixed disulfide with sodium borohydride (NaBH4), and the GSH is then measured in the recycling assay.
Fig. 6.
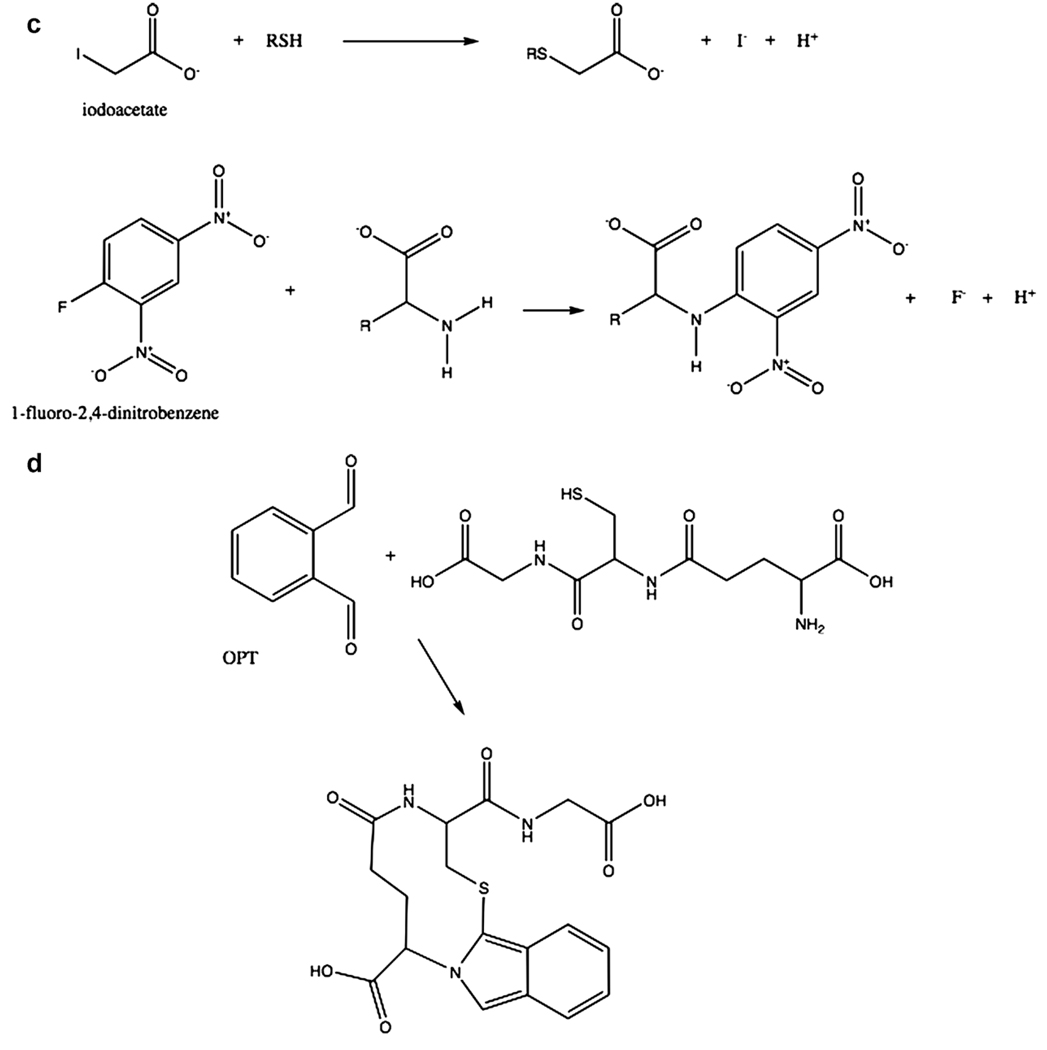
Measurements of thiols. (a) Reaction of GSH with DTNB produces an adduct and TNB, which is measured spectrofluorometrically or spectrophometrically; (b) total glutathione can be determined by recycling of GSSG produced in the reaction in (a) and measuring the rate of TNB; (c) Glutathione and related compounds are first derivatized with iodoacetate followed by a second derivatization with 1-fluoro-2,4-dinitrophenol. The second products are then separated by HPLC and measured spectrofluorometrically; (d) Reaction of glutathione with orthophthaldehyde (OPT) yields a product that can be measured spectrofluorometrically.
A more commonly used procedure for measuring GSH and GSSG now is high performance liquid chromatography (HPLC) (Fariss and Reed, 1987). In this assay, thiol compounds are first modified by the addition of iodoacetate (Fig. 6c). The amino groups on the compound then are modified by 1-fluoro-2, 4-dinitrobenzene. This then allows separation of many compounds that can be identified by their movement on HPLC.
On method that has been developed to measure nitrosoglutathione involves the production of GSH from it followed by reaction with orthophthaldehyde (OPT) to produce a fluorescent compound (Fig. 6d) (Tsikas et al., 1999) while another method uses a biotinylated fluorescent label in a method called the biotin-switch (Gladwin et al., 2006). First however, as with the measurement of GSSG above, it is necessary to first remove any GSH in the original sample with methyl methanethiosulfonate before reducing GSNO to release GSH. Various reagents have been proposed as best for differentially reducing GSNO as well as PSNO especially as the presence of GSSG or protein mixed disulfides can also yield GSH upon reduction (Gladwin et al., 2006). After reaction with OPT the products are separated by HPLC with a fluorescence detector. There are other methods for measuring GSNO such as using 15N labeling (Kluge et al., 1997), but this is not commonly used and requires mass spectrometry.
5. Glutathione synthesis
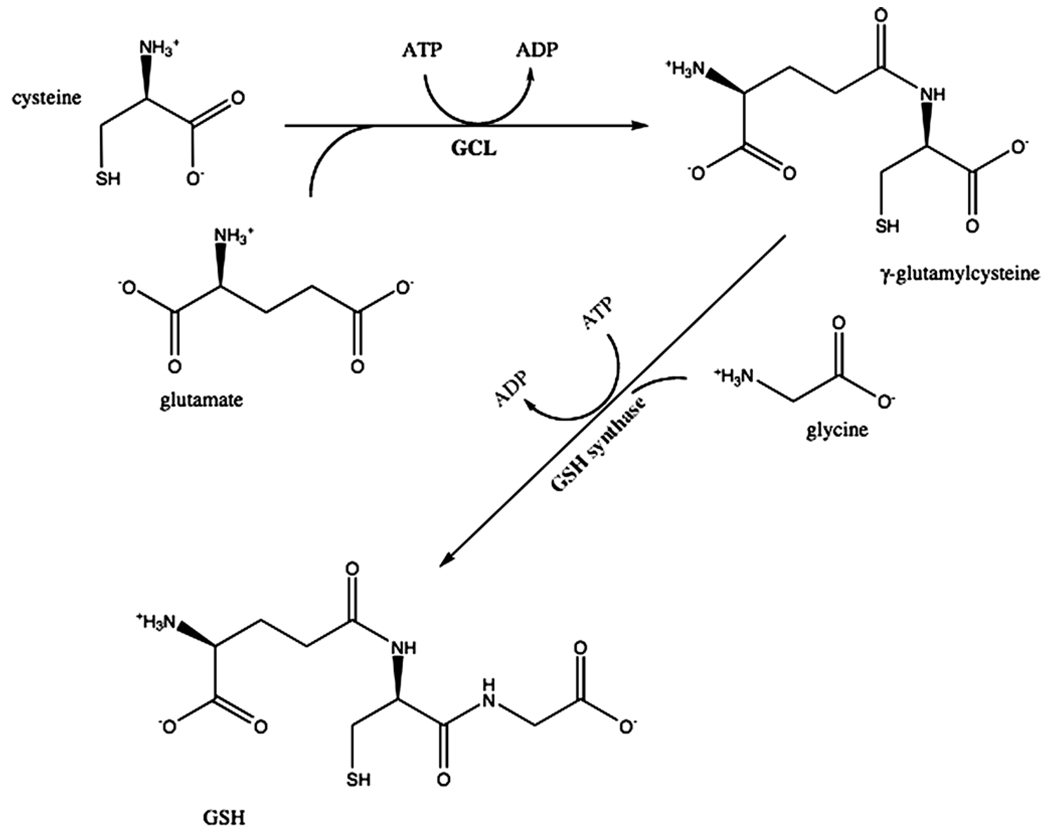
The first step in de novo GSH synthesis involves the combination of cysteine with glutamate to produce γ-glutamylcysteine. This reaction is catalyzed by the enzyme glutamate cysteine ligase (GCL), which is also called γ-glutamylcysteine synthetase (Fig. 7). This enzyme requires coupled ATP hydrolysis to form an amide bond between the γ-carboxyl group of glutamate and the amino group of cysteine (Huang et al., 1993). The next step involves the enzyme glutathione synthetase, responsible for adding glycine to the dipeptide to produce GSH (γ-glutamylcysteinylglycine) and also requires coupled ATP hydrolysis (Meister, 1974).
Fig. 7.
Glutathione synthesis. The sequential ATP dependent formation of amide bonds between cysteine and the γ-carboxyl group of glutamate and then between glycine and cysteine are shown.
GSH can be transported out of cells. This mechanism is physiologically important as hepatocytes supply GSH found in the plasma, which is used as a source of cysteine for GSH synthesis in other cells (Anderson et al., 1980). In fact, GSH in the plasma is maintained at very low concentration because of the metabolism of GSH by many other cells (Sies and Graf, 1985; Hirota et al., 1986). This process requires two enzymes commonly found on the surfaces of cells. The enzyme γ-glutamyl transpeptidase transfers a glutamate to other amino acids releasing cysteinylglycine, which in turn can be broken down by a dipeptidase to produce cysteine and glycine (Kozak and Tate 1982; Hirota et al., 1986). Cysteine and glycine as well as γ-glutamyl amino acids are moved into cells by specific amino acid transporters and used for GSH biosynthesis (Meister, 1991).
5.1. Regulation of glutamate cysteine ligase activity
GCL is regulated at both the level of its enzymatic activity and the expression of its two subunits. One subunit is the relatively heavy (− 73 kDa) subunit, which has competent but low catalytic activity for production of γ-glutamylcysteine. The catalytic subunit, designated as GCLC, can be feedback inhibited by GSH (Huang et al., 1993). The lower molecular weight (− 28 kDa) subunit regulates the activity of the enzyme by reducing the inhibition by GSH (Huang et al., 1993; Choi et al., 2000) and with purified enzyme has been shown to also decrease the KM for glutamate (Huang et al., 1993). This subunit, which is designated as GCLM for its modulatory activity can affect the steady state level of GSH found in cells when GCLM/GCLC expression is altered (Richman and Meister, 1975; Choi et al., 2000; Krzywanski et al., 2004). Thus, increased expression of GCLC will tend to elevate GSH while increasing GCLM/GCLC will further increase GSH. An example of when lowering GCLM/GCLC causes decreased GSH is the expression of the HIV-Tat protein, which suppresses GCLM expression (Choi et al., 2000). Finally, the kinetics of GCL seems to be regulated by phosphorylation of both subunits as well (Sun et al., 1996). The functional roles of the two GCL subunits are reviewed in this issue by Franklin et al. (2008)).
5.2. Regulation of glutamate cysteine ligase expression
The expression of GCL is also regulated at many levels. Oxidant species and electrophiles are able to increase the transcription of both the modulatory and catalytic subunits (Shi et al., 1994; Rahman et al., 1996; Tian et al., 1997) (also see review by Lu, 2008 in this issue). This occurs by the activation of signal transduction pathways involved in the control of transcription of GCLC and GCLM genes but also there is some evidence of mRNA stabilization by oxidants and electrophiles (Liu et al., 1998).
It has been known for almost twenty years that sublethal concentrations of electrophiles could increase GSH production (Ogino et al., 1989; Darley-Usmar et al., 1991); however, it was unclear whether the increase was on the kinetic or the transcriptional level or even whether GSSG reduction was increased. Using redox cycling quinones to increase production of hydrogen peroxide and by measuring transcription by nuclear run-on analysis, it was then shown that a sustained increase the amount of GSH in cells could be achieved by increasing the transcription of GCLC (Shi et al., 1994; Shi et al., 1994). Subsequently many labs showed that a variety of other agents, able to generate an oxidative stress throughH2O2 generation, increasing concentrations of electrophiles or nitric oxide could also induce GCLC or GCLM subunits or both (Rahman et al., 1996; Tian et al., 1997; Galloway and McLellan, 1998; Liu et al., 1998; Moellering et al., 1999; Wild and Mulcahy, 1999).
The GCLC and GCLM promoter sequences were described first from humans and then they were determined in rodents (Gipp et al., 1992; Gipp et al., 1995; Hudson and Kavanagh, 2000; Yang et al., 2001). The human and rodent promoters have some similar cis elements and appear to be regulated somewhat differently than the human genes (Iles and Liu 2005) (see review by Lu, 2008 in this issue). For the human GCL genes, the promoter enhancer regions of the two genes contain several elements able to respond to oxidants and electrophiles (Gipp et al., 1992; Gipp et al., 1995; Yang et al., 2001; Dickinson et al., 2002). One of the important oxidant responsive cis elements (transcription factor binding sites) regulating GCL genes is the AP-1 binding site also called the TRE element. TRE binds members of the Jun and Fos family of transcription factors (Ofir et al., 1990; Binetruy et al., 1991). Another important element in human GCL gene promoters that responds to electrophiles in cells and increases gene expression is the EpRE or electrophile response element (Rushmore et al., 1991; Jaiswal, 1994; Vasiliou et al., 1995). EpRE elements are also present in both human GCLC and GCLM promoters (Gipp et al., 1992; Gipp et al., 1995). Initially EpRE was called the antioxidant response element (ARE) because the first compound, shown to activate ARE was a so-called antioxidant that was subsequently shown to generate H2O2 through redox cycling (Pinkus et al., 1996). The EpRE elements bind proteins members of the Nrf family, Jun family and small Maf family (Venugopal and Jaiswal, 1998;Kong et al., 2001; Moran et al., 2002; Itoh et al., 2004). One of the transcription factors established as able to bind EpRE is Nrf2, which located in the cytosol through the inhibitory interaction with Keap1 in resting cells. Upon stimulation, Nrf2 is translocated into the nucleus after dissociation from Keap1 (Itoh et al., 1999).
While the redox and electrophilic response cis elements have been identified, less has been done to identify the signaling mechanisms that activate the transcription factors that bind to those elements. We will describe here briefly what is understood regarding the signaling by HNE. Darley–Usmar and coworkers have shown that HNE directly modifies Keap1, which allows Nrf2 to avoid degradation and migrate to the nucleus where it can bind to EpRE elements in the promoters of the human GCLC and GCLM genes (Levonen et al., 2004). But, this cannot be the whole story as there are actually multiple EpRE elements in the promoters and not all of them are involved in regulating transcription (Dickinson et al., 2004). While Nrf2 is critical, EpRE binding also involves a partner protein. For the EpRE element that regulates transcription of GCLC in human bronchial epithelial cells that partner has not yet been firmly identified.
More is understood about the TRE element. Interestingly, the TRE element in the human GCLC promoter appears to bind c-Jun dimers preferentially (Rahman et al., 1999). For HNE induction, the activation of the critical AP-1 binding elements in both human GCL genes can is achieved through the Jun N-terminal kinase (JNK) pathway (Dickinson et al., 2002). JNK phosphorylates c-Jun, which translocates into the nucleus, and binds to the TRE element. Inhibition of JNK completely eliminates GCLC and GCLM gene expression in response to HNE in human bronchial epithelial cells while inhibition of the ERK or p38MAPK pathways had no effect. Recently, the activation of the JNK pathway by HNE has been shown to occur upstream at the protein tyrosine phosphatase SHP-1that is inhibited by HNE, which also appears to accelerate the degradation of the enzyme (Rinna and Forman, 2008).
6. Glutathione therapeutics
As an increase in GSH appears to be a ubiquitous response to oxidants and electrophiles and some diseases appear to be exacerbated by decreasing GSH, increasing GSH by using delivery of permeable esters (Levy et al., 1993) or increasing the availability of cysteine using the non-toxic precursor N-acetylcysteine (Thor et al., 1979) have been proposed. Increasing GSH through synthesis would also seem to be useful therapeutically but as oxidants and most electrophiles would not seem appropriate, natural compounds such as curcumin, a principal ingredient of curry powder (Dickinson et al., 2003), and sulforaphane, a potent Phase II gene-inducing compound in broccoli, (Brooks et al., 2001) have been proposed but none of these natural has actually become a major therapeutic agent.
On the other hand, compounds that decrease GSH and increase the susceptibility of tumors to chemotherapy or radiation have been used. GCL can be inhibited by a buthionine sulphoximine quite specifically making it a useful tool in studying GSH metabolism, and useful in cancer chemotherapy (Martensson et al., 1989; Anderson et al., 1997; Gartenhaus et al., 2002). An inhibitor of γ-glutamyl transpeptidase (GGT), acivicin (AT-125) (Griffith and Meister, 1980) was tried in chemotherapy before it was known to inhibit GGT; however, acivicin also inhibits enzymes in purine and pyrimidine biosynthesis, which may be its actual mode of action (Poster et al., 1981; Elliott and Weber, 1985). Thus, there is still much to be done in understanding how GSH synthesis and metabolism may be manipulated to therapeutic advantage. Further information about the use of GSH and related compounds in therapy for a variety of diseases including viral infection, cystic fibrosis and cancer, can be found in the reviews by Biswas and Rahman (2008)) and by Fraternale et al. (2008) in this issue.
Abbreviations
- GSH
glutathione
- −SH
sulfhydryl group
- O2−
superoxide
- H2O2
hydrogen peroxide
- NOX
NADPH oxidase
- SOD
superoxide dismutase
- HOCl
hypochlorous acid
- HOBr
hypobromous acid
- Fe2+
ferrous iron
- OH
hydroxyl radical
- Fe3+
ferric iron
- L
lipid radical
- LOO
hydroperoxide radical
- LOOH
lipid peroxide
- HNE
4-hydroxy-2-nonenal
- ONOO−
peroxynitrite
- NO
nitrogen oxide
nitrite
nitrate
- ONOOH
peroxynitrous acid
- NO2
nitrogen dioxide
- CFTR
cystic fibrosis transport receptor
- GPx
glutathione peroxidase
- PHGPx GPx IV
phospholipid hydroperoxide glutathione peroxidase
- GSSG
glutathione disulfide
- Prdx
peroxiredoxin
- PSSG
protein mixed disulfide
- PDI
protein disulfide isomerase
- −S−
thiolate
- PSOH
sulfenic acid
- GST
glutathione-S transferase
- GSNO
nitrosoglutathione
- DTNB
dithionitrobenzoic acid
- NEM
N-ethylmaleimide
- NaBH4
sodium borohydride
- OPT
orthophthaldehyde
- GCL
glutamate cysteine ligase
- ARE
antioxidant response element
- JNK
Jun N-terminal kinase
- GGT
γ-glutamyl transpeptidase.
References
- Akerboom TPM, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Alary J, Gueraud F, et al. Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways. Mol. Aspects Med. 2003;24(4–5):177–187. doi: 10.1016/s0098-2997(03)00012-8. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Bridges RJ, et al. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves g-glutamyl transpeptidase. Biochem. Biophys. Res. Commun. 1980;96(2):848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Anderson CP, Tsai J, et al. Buthionine sulphoximine alone and in combination with melphalan (L-PAM) is highly cytotoxic for human neuroblastoma cell lines. Eur. J. Cancer. 1997;33(12):2016–2019. doi: 10.1016/s0959-8049(97)00203-7. [DOI] [PubMed] [Google Scholar]
- Bakkenist AR, de Boer JE, et al. The halide complexes of myeloperoxidase and the mechanism of the halogenation reactions. Biochim. Biophys. Acta. 1980;613(2):337–348. doi: 10.1016/0005-2744(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Krance SM, et al. Plasma membrane glutathione transportes and their roles in cell physiology and pathophysiology. Mol. Aspect Med. 2008 doi: 10.1016/j.mam.2008.08.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binetruy B, Smeal T, et al. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Biswas S, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol. Aspects Med. 2008 doi: 10.1016/j.mam.2008.07.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland E, Chasseaud LF. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 1999;27(9–10):951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Brooks JD, Paton VG, et al. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol. Biomarkers Prev. 2001;10(9):949–954. [PubMed] [Google Scholar]
- Cantin AM, North SL, et al. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Cantin AM, Hubbard RC, et al. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Disease. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- Choi J, Liu RM, et al. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J. Biol. Chem. 2000;275(5):3693–3698. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- Darley-Usmar VM, Severn A, et al. Treatment of macrophages with oxidized low-density lipoprotein increases their intracellular glutathione content. Biochem. J. 1991;278:429–434. doi: 10.1042/bj2780429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, et al. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic. Biol. Med. 2002;33(7):974–987. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, et al. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. Faseb. J. 2003;17(3):473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Levonen AL, et al. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 2004;37(8):1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Eckl PM. Genotoxicity of HNE. Mol. Aspects Med. 2003;24(4–5):161–165. doi: 10.1016/s0098-2997(03)00010-4. [DOI] [PubMed] [Google Scholar]
- Elliott WL, Weber G. In vivo inactivation of formylglycinamidine ribonucleotide synthetase in rat hepatoma. Biochem. Pharmacol. 1985;34(2):243–248. doi: 10.1016/0006-2952(85)90131-5. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, et al. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Fariss M, Reed DJ. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- Forman HJ. Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic. Biol. Med. 2007;42(7):926–932. doi: 10.1016/j.freeradbiomed.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Thomas MJ. Oxidant production and bactericidal activity of phagocytes. Annu. Rev. Physiol. 1986;48:669–680. doi: 10.1146/annurev.ph.48.030186.003321. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Backos DS, et al. Functional roles and regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Aspects Med. 2008 doi: 10.1016/j.mam.2008.08.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraternate A, Paoletti AF, et al. GSH and analogs as antiviral drugs. Mol. Aspects Med. 2008 doi: 10.1016/j.mam.2008.09.001. in press. [DOI] [PubMed] [Google Scholar]
- Galloway DC, McLellan LI. Inducible expression of the g-glutamylcysteine synthetase light subunit by t-butylhydroquinone in HepG2 cells is not dependent on an antioxidant-responsive element. Biochem. J. 1998;336(Pt 3):535–539. doi: 10.1042/bj3360535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Kim KJ, et al. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am. J. Physiol. 1999;277:L113–L118. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]
- Gartenhaus RB, Prachand SN, et al. Arsenic trioxide cytotoxicity in steroid and chemotherapy-resistant myeloma cell lines: enhancement of apoptosis by manipulation of cellular redox state. Clin. Cancer Res. 2002;8(2):566–572. [PubMed] [Google Scholar]
- Gipp JJ, Chang C, et al. Cloning and nucleotide sequence of a full-length cDNA for human liver g-glutamylcysteine synthetase. Biochem. Biophys. Res. Commun. 1992;185:29–35. doi: 10.1016/s0006-291x(05)80950-7. [DOI] [PubMed] [Google Scholar]
- Gipp JJ, Bailey HH, et al. Cloning and sequencing of the cDNA for the light subunit of human liver g-glutamylcysteine synthetase and relative mRNA levels for heavy and light subunits in human normal tissues. Biochem. Biophys. Res. Commun. 1995;206:584–589. doi: 10.1006/bbrc.1995.1083. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Wang X, et al. Methodological vexation about thiol oxidation versus S-nitrosation - a commentary on “An ascorbate-dependent artifact that interferes with the interpretation of the biotin-switch assay”. Free Radic. Biol. Med. 2006;41(4):557–561. doi: 10.1016/j.freeradbiomed.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Excretion of cysteine and gamma-glutamylcysteine moieties in human and experimental animal gamma-glutamyl transpeptidase deficiency. Proc. Natl. Acad. Sci. USA. 1980;77(6):3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Nishikawa Y, et al. Characterization of dehydropeptidase I in the rat lung. Eur. J. Biochem. 1986;160(3):521–525. doi: 10.1111/j.1432-1033.1986.tb10070.x. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL. Glutathionylation of proteins by glutathione disulfide S-oxide. Biochem. Pharmacol. 2002;64(5–6):1049–1056. doi: 10.1016/s0006-2952(02)01175-9. [DOI] [PubMed] [Google Scholar]
- Huang C-S, Anderson ME, et al. The function of the light subunit of g-glutamylcysteine synthetase (rat kidney) FASEB J. 1993;7:A1102. [PubMed] [Google Scholar]
- Huang C-S, Chang L-S, et al. Catalytic and regulatory properties of the heavy subunit of rat kidney g-glutamylcysteine synthetase. J. Biol. Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- Hudson FN, Kavanagh TJ. Cloning and characterization of the proximal promoter region of the mouse glutamate-l-cysteine ligase regulatory subunit gene. Biochim. Biophys. Acta. 2000;1492(2–3):447–451. doi: 10.1016/s0167-4781(00)00128-7. [DOI] [PubMed] [Google Scholar]
- Iles KE, Liu RM. Mechanisms of glutamate cysteine ligase (GCL) induction by 4-hydroxynonenal. Free Radic. Biol. Med. 2005;38(5):547–567. doi: 10.1016/j.freeradbiomed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 2003;34(2):145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Tong K-I, et al. Molecular mechanism activating NRF-2-KEAP1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Antioxidant response element. Biochem. Pharmacol. 1994;48(3):439–444. doi: 10.1016/0006-2952(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Kluge I, Gutteck-Amsler U, et al. S-Nitrosoglutathione in rat cerebellum: identification and quantification by liquid chromatography-mass spectrometry. J. Neurochem. 1997;69(6):2599–2607. doi: 10.1046/j.1471-4159.1997.69062599.x. [DOI] [PubMed] [Google Scholar]
- Kong AN, Owuor E, et al. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab. Rev. 2001;33(3–4):255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- Kozak EM, Tate SS. Glutathione-degrading enzymes of microvillus membranes. J. Biol. Chem. 1982;257(11):6322–6327. [PubMed] [Google Scholar]
- Krzywanski DM, Dickinson DA, et al. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem. Biophys. 2004;423(1):116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Levonen AL, Landar A, et al. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378(Pt 2):373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy EJ, Anderson ME, et al. Transport of glutathione diethyl ester into human cells. Proceedings National Academy of Sciences, USA. 1993;90:9171–9175. doi: 10.1073/pnas.90.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RM, Gao L, et al. G-Glutamylcysteine synthetase: mRNA stabilization and independent subunit transcription by 4-hydroxy-2-nonenal. Am. J. Physiol. 1998;275(5 Pt 1):L861–L869. doi: 10.1152/ajplung.1998.275.5.L861. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol. Aspects Med. 2008 doi: 10.1016/j.mam.2008.05.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson J, Jain A, et al. Glutathione metabolism in the lung: Inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc. Natl. Acad. Sci. USA. 1989;86:5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974;15(2):177–190. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharm. Therapeut. 1991;51:155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Meister A. Biosynthesis and function of glutathione, an essential biofactor. J. Nutrit. Sci. Vitaminol. 1992 doi: 10.3177/jnsv.38.special_1. Spec No: 1–6. [DOI] [PubMed] [Google Scholar]
- Moellering D, Mc Andrew J, et al. The induction of GSH synthesis by nanomolar concentrations of NO in endothelial cells: a role for gamma-glutamylcysteine synthetase and gamma-glutamyl transpeptidase. FEBS Lett. 1999;448(2–3):292–296. doi: 10.1016/s0014-5793(99)00371-3. [DOI] [PubMed] [Google Scholar]
- Moran JA, Dahl EL, et al. Differential induction of mafF, mafG and mafK expression by electrophile-response-element activators. Biochem. J. 2002;361(Pt 2):371–377. doi: 10.1042/0264-6021:3610371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir R, Dwarki VJ, et al. Phosphorylation of the C terminus of Fos protein is required for transcriptional transrepression of the c-fos promoter. Nature. 1990;348(6296):80–82. doi: 10.1038/348080a0. [DOI] [PubMed] [Google Scholar]
- Ogino T, Kawabata T, et al. Stimulation of glutathione synthesis in iron-loaded mice. Biochim. Biophys. Acta. 1989;1006:131–135. doi: 10.1016/0005-2760(89)90334-2. [DOI] [PubMed] [Google Scholar]
- Pallardó FV, Markovic J, et al. Role of nuclear glutathione as a key regulator of cell proliferation. Mol. Aspects Med. 2008 doi: 10.1016/j.mam.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Pinkus R, Weiner LM, et al. Role of oxidants and antioxidants in the induction of AP-1, NF-kB, and glutathione S-transferase gene expression. J. Biol. Chem. 1996;271(23):13422–13429. doi: 10.1074/jbc.271.23.13422. [DOI] [PubMed] [Google Scholar]
- Poli G, Albano E, et al. The role of lipid peroxidation in liver damage. Chem. Phys. Lipids. 1987;45(2–4):117–142. doi: 10.1016/0009-3084(87)90063-6. [DOI] [PubMed] [Google Scholar]
- Poster DS, Bruno S, et al. Acivicin. An antitumor antibiotic. Cancer Clin. Trials. 1981;4(3):327–330. [PubMed] [Google Scholar]
- Pullar JM, Vissers MC, et al. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life. 2000;50(4–5):259–266. doi: 10.1080/713803731. [DOI] [PubMed] [Google Scholar]
- Rahman I, Smith CAD, et al. Induction of g-glutamylcysteine synthetase by cigarette smoke is associated with AP-1 in human alveolar epithelial cells. FEBS Lett. 1996;396:21–25. doi: 10.1016/0014-5793(96)01027-7. [DOI] [PubMed] [Google Scholar]
- Rahman I, Antonicelli F, et al. Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor-alpha and dexamethasone in human alveolar epithelial cells. J. Biol. Chem. 1999;274(8):5088–5096. doi: 10.1074/jbc.274.8.5088. [DOI] [PubMed] [Google Scholar]
- Ralat LA, Manevich Y, et al. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry. 2006;45(2):360–372. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- Richman PG, Meister A. Regulation of g-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J. Biol. Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]
- Rinna A, Forman HJ. SHP-1 inhibition by 4-hydroxynonenal activates jun N-terminal kinase and glutamate cysteine ligase. Am. J. Respir. Cell Mol. Biol. 2008 doi: 10.1165/rcmb.2007-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roum JH, Buhl R, et al. Systemic deficiency of glutathione in cystic fibrosis. J. Appl. Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, et al. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Aspects Med. 2003;24(4–5):149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Shi MM, Iwamoto T, et al. G-Glutamylcysteine synthetase and glutathione increase in quinone-induced oxidative stress in bovine pulmonary artery endothelial cells. Am. J. Physiol. 1994;267:L414–L421. doi: 10.1152/ajplung.1994.267.4.L414. [DOI] [PubMed] [Google Scholar]
- Shi MM, Kugelman A, et al. Quinone-induced oxidative stress elevates glutathione and induces g-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J. Biol. Chem. 1994;269:26512–26517. [PubMed] [Google Scholar]
- Sies H, Graf P. Hepatic thiol and glutathione efflux under the influence of vasopressin, phenylephrine and adrenaline. Biochem. J. 1985;226(2):545–549. doi: 10.1042/bj2260545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W-M, Huang Z-Z, et al. Regulation of g-glutamylcysteine synthetase by protein phosphorylation. Biochem. J. 1996;320:321–328. doi: 10.1042/bj3200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MW, Glass M, et al. Oxygen toxicity: loss of lung macrophage function without metabolite depletion. J. Free Radic. Biol. Med. 1985;1:209–214. doi: 10.1016/0748-5514(85)90120-5. [DOI] [PubMed] [Google Scholar]
- Thor H, Moldeus P, et al. Metabolic activation and hepatotoxicity. Effect of cysteine, N-acetylcysteine, and methionine on glutathione biosynthesis and bromobenzene toxicity in isolated rat hepatocytes. Arch. Biochem. Biophys. 1979;192(2):405–413. doi: 10.1016/0003-9861(79)90109-7. [DOI] [PubMed] [Google Scholar]
- Tian L, Shi MM, et al. Increased transcription of the regulatory subunit of g-glutamylcysteine synthetase in rat lung epithelial L2 cells exposed to oxidative stress or glutathione depletion. Arch. Biochem. Biophys. 1997;342(1):126–133. doi: 10.1006/abbi.1997.9997. [DOI] [PubMed] [Google Scholar]
- Tsikas D, Sandmann J, et al. Determination of S-nitrosoglutathione in human and rat plasma by high-performance liquid chromatography with fluorescence and ultraviolet absorbance detection after precolumn derivatization with o-phthalaldehyde. Anal. Biochem. 1999;273(1):32–40. doi: 10.1006/abio.1999.4209. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Shertzer HG, et al. Response of [Ah] battery genes to compounds that protect against menadione toxicity. Biochem. Pharmacol. 1995;50(11):1885–1891. doi: 10.1016/0006-2952(95)02083-7. [DOI] [PubMed] [Google Scholar]
- Venglarik CJ, Giron-Calle J, et al. Hypochlorous acid alters bronchial epithelial cell membrane properties and prevention by extracellular glutathione. J. Appl. Physiol. 2003;95(6):2444–2452. doi: 10.1152/japplphysiol.00002.2003. [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17(24):3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol. Life Sci. 2002;59(9):1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild AC, Mulcahy RT. Pyrrolidine dithiocarbamate up-regulates the expression of the genes encoding the catalytic and regulatory subunits of gamma-glutamylcysteine synthetase and increases intracellular glutathione levels. Biochem. J. 1999;338(Pt 3):659–665. [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC, Brennan SO. Characterization of the oxidation products of the reaction between reduced glutathione and hypochlorous acid. Biochem. J. 1997;326:87–92. doi: 10.1042/bj3260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang J, et al. Cloning and characterization of the 50-flanking region of the rat glutamate-cysteine ligase catalytic subunit. Biochem. J. 2001;357(Pt 2):447–455. doi: 10.1042/0264-6021:3570447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol. Aspects Med. 2008 doi: 10.1016/j.mam.2008.08.003. in press. [DOI] [PubMed] [Google Scholar]