Introduction
Biology and Unanswered Questions Regarding Acute Myeloid Leukemia (AML)
Cancer remains the second leading cause of death in the USA despite recent advances in treatment of patients with anti-neoplastic drugs. Approximately 42,000 people in the USA die each year from leukemias and lymphomas which represent 10% of all cancer deaths. Approximately 11,000 Americans will be diagnosed with AML this year, and about 75% will eventually die from this disease. While improvements in the outcomes have been observed with young patients with AML over the past 40 years, progress in the treatment of older AML patients has not been as significant (Tallmann et al., 2005). Fifty to 75% of adults with AML achieve complete remission with a combination chemotherapy which consists of combination of the deoxycytidine analogue cytarabine and an anthracycline antibiotic (doxorubicin, daunorubicin, idarubicin or the anthracenedione mitoxantrone, which inhibit the enzyme topoisomerase IIa). However, this treatment is not always effective as only approximately 25% of these patients enjoy long term survival (Tallmann et al., 2005). The incidence of AML increase with age, 1.2 cases per 100,000 at age 30 and greater than 20 cases per 100,000 at age 80 (Tallmann et al., 2005). Unfortunately the outcome decreases with age. As the average life span of Americans increases due to improvements in health care and life styles, AML will be an increasing problem in American health care.
While approximately 50% of AML cases have genetic aberrations which can be identified (e.g., deletions such as 5q-, translocations such as t(8;21) AML-ETO, or duplications such as Flt-3 internal tandem duplication [ITD]), the other 50% do not have currently identifiable genetic mutations (Tallmann et al., 2004). Unlike chronic myelogenous leukemia (CML) where the BCR-ABL translocation is present in virtually all patients and the majority of the patients are sensitive to Imatinib, treatment with a targeted “upstream” inhibitor (e.g., Flt-3 inhibitor) would be ineffective in many AML cases. In summary, AML remains a difficult disease to treat due in part to its genetic diversity.
Upregulation of the Ras>Raf>MEK>ERK and PI3K>Akt pathways and phosphorylation of the downstream target Bad are observed frequently in AML specimens and associated with a poorer prognosis than patients lacking these changes (Kornblau et al., 2006; Martelli et al., 2206). Aberrant expression of a single pathway is associated with a poor prognosis and abnormal expression of multiple signaling pathways is associated with an even worse prognosis (Kornblau et al., 2006). Flt-3 ITD mutations have been detected in 20% of AMLs and these patients have a poorer prognosis than patients lacking these mutations (Stone et al., 2004). Dysregulation of the Ras>Raf>MEK>ERK and PI3K>Akt pathways in some AMLs may result from constitutive activation of Flt-3 (Birkenkamp et al., 2004; Stirewalt et al., 2003; Meshinchi et al., 2003). Thus these two signaling pathways provide important clues regarding the mechanisms responsible for autonomous AML growth (Yokota et al., 1998; Hoelzer et al., 2000; Pui et al., 1999; Attwell et al., 2003; McKearn et al., 1985). Targeting these “downstream” pathways may prove effective for AML therapy, especially in those cases where the precise mutation responsible for malignant transformation is unknown.
Drug Resistance and AML
A frequent side effect of treatment of AML patients with chemotherapeutic drugs is the development of drug resistance. After chemotherapeutic drug treatment, drug resistant cells arise which exhibit enhanced efflux of chemotherapeutic drugs (Tallmann et al., 2005,Tallmann et al., 2006;Burnett et al., 2006) Furthermore, the drug resistant cells often exhibit multi-drug resistance as they are resistant to multiple chemotherapeutic drugs which are structurally unrelated. In some cases, this phenomenon has been shown to be due to the increased expression of membrane transporters (Gottesman et al., 2002; Norgaard et al., 2004; van den Heuvel-Eibrink et al., 2000; Ross 2000; Kruh et al., 2003). These transporters belong to a large family of proteins which contains an ATP binding cassette (ABC) domain. Multi-drug resistance protein (Mdr-1 a.k.a., P glycoprotein, Pgp) was one of the first of these molecules to be identified to have a role in drug resistance. Subsequently, additional proteins with this ABC domain were identified and determined to have a role in drug resistance. This family includes: breast cancer resistance protein (BCRP-1), multi drug resistant protein (MRP), MRP1, MRP2, MRP3, MRP4, MRP5, MRP6, MRP7, MRP8 as well as some other proteins. Inhibitors to some of these membrane transporters have been developed and evaluated in clinical trials. Unfortunately, these clinical trials have not yet yielded support for inclusion of these inhibitors in drug resistance therapy (Teodori et al., 2006; Polgar et al., 2005; Ross 2004; Mahadevan et al., 2004). An alternative approach could be to target the growth and survival pathways which become activated in the drug resistant cells. Two pathways frequently implicated in drug resistance are Raf>MEK>ERK and PI3K>Akt (Steelman et al., 2004; Lee et al., 2002; Osaki et al, 2004; Tsuro et al., 2003, Kim et al., 2005). The proposed studies will investigate the roles these pathways play in AML growth, drug resistance and sensitivity to targeted therapy.
The Ras>Raf>MEK>ERK Pathway
The Ras>Raf>MEK>ERK pathway is activated by many cytokines which are important in driving the proliferation and promoting the survival of myeloid cells (Steelman et al., 2004). After receptor ligation, Shc, Src homology (SH)-2, a SH2-domain containing protein, becomes associated with the c-terminus of the cytokine receptor (Matsuguchi et al., 1994; Inhorn et al., 1995; Okuda et al., 1999). Shc recruits the GTP-exchange complex Grb2/Sos resulting in the loading of membrane bound Ras with GTP (Tauchi et al., 1994; Lanfrancone et al., 1995). Ras:GTP then recruits Raf to the membrane where it becomes activated, likely via a Src-family tyrosine kinase (Karin et al., 1994; Lange-Carter et al., 1994; Marais et al., 1995). Raf is responsible for phosphorylation of the mitogen associated/extracellular regulated kinase-1 (MEK1) (Marais et al., 1997; Mason et al., 1999; Xu et al., 1995). MEK1 phosphorylates extracellular regulated kinases 1 and 2 (ERKs 1 and 2) on specific threonine and tyrosine residues (Marais et al., 1997; Mason et al., 1999; Xu et al., 1995). Activated ERK1 and ERK2 serine/threonine kinases phosphorylate and activate a variety of substrates including p90Rsk1 (Cardone et al., 1998; Allan et al., 2003; Davis et al., 1995; Xing et al., 1996; Coutant et al., 2002; Iijima et al., 2002; Blalock et al., 2003). p90Rsk1 can activate the cyclic-AMP response element binding protein (CREB) transcription factor (Xing et al., 1996). Moreover, ERK can translocate to the nucleus and phosphorylate additional transcription factors such as Elk1, CREB and Fos which bind promoters of many genes, including IL-3, a cytokine important in stimulating the growth and survival of early myeloid progenitor cells (Deng et al., 1994; Davis 1995; Robinson et al.,, 1998; Aplin et al., 2001; McCubrey et al., 2000; Tresini et al., 2001; Eblen et al., 2001; Adachi et al., 2002; Wang et al., 1994; Thomas et al., 1997; Ponti et al., 2002; Fry et al., 2002). The Raf>MEK>ERK pathway can also modulate the activity of many proteins involved in apoptosis including: Bcl-2, Bad, Bim, Mcl-1, caspase 9, and Survivin (Deng et al., 2001; Carter et al., 2003; Steelman et al., 2004; Jia et al., 2003; Troppmair et al., 2003; Harada et al., 2004; Marani et al., 2004; Ley et al., 2003; Weston et al., 2003; Domina et al., 2004; Gelinas et al., 2006).
B. 2.1 Roles of the Ras>Raf>MEK>ERK Pathway in Neoplasia
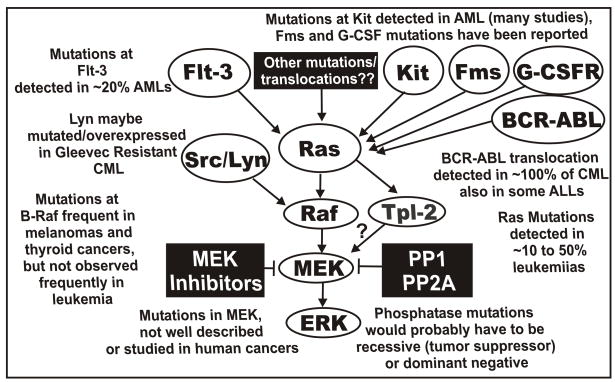
Ras is one of the most frequently mutated oncogenes in human cancer. Ten-50% of individuals diagnozed with myelodysplastic syndrome or AML have Ras mutations (Janssen et al., 1987; Padua et al., 1988; Needleman et al., 1988; Nakagawa et al., 1992; Lubbert et al., 1992; Kubo et al., 1993; Aurer et al., 1994; Vasioukhin et al., 1994; Neubauer et al., 1994; Gougopoulou et al., 1996; Gallagher et al., 1997; Parry 1997; Constantinidou et al., 1997; de Souza Fernandez et al., 1998; Kiyoi et al., 1999; Flotho et al., 1999; Stirewalt et al., 2001; Nakamura et al., 2004; Zebisch et al., 2006; Zebisch et al., 2006; Wellbrock et al., 2004; Garnett et al., 2004). These are often point mutations which alter key residues that affect Ras activity. Mutations which alter Ras activity also perturb the Raf>MEK>ERK kinase cascade. Mutation of B-Raf is frequently observed in melanomas and most thyroid cancers (~70%) but rarely in hematopoietic cancers (<4% in AML & NHL) (Davies et al., 2002; Brose et al., 2002; Lee et al., 2003; Chan et al., 2003; Xu et al., 2003; Mercer et al., 2003; Lilleberg et al., 2004; Kambara et al., 2004; Daniotti et al., 2004; Puxeddu et al., 2004; Wan et al., 2004; Kim et al., 2004; Reifenberger et al., 2004; Fransen et al., 2004; Lee et al., 2004). Activating mutations have been detected at Raf-1 in therapy-induced AML in certain families in Austria (Zebisch et al., 2006). These preexisting Raf-1 mutations are genetically transmitted. MEK and ERK are not thought to be frequently mutated in human cancer; however, the actual published studies which document this are few, although they are listed at the Catalogue of Somatic Mutations in Cancer, COSMIC, http://www.sanger.ac.uk/genetics/CGP/cosmic). Mutations in upstream receptors such as Flt-3 (20 to 30%), Kit (7 to 17% of AMLs), Fms (12% of MDS) and granulocyte colony stimulating factor receptor (G-CSF-R) have been documented in AML and will cause the activation of the Ras>Raf>MEK>ERK pathway (Kiyoi et., 1998; Shimada et al., 2006; Christiansen et al., 2005; Padua et al., 1998, Dong et al., 1997, Dong et al., 1999). Furthermore, over expression of VEGF-R receptors has been observed in AML which could result in activation of this pathway (Hiramatsu et al., 2006). Constitutive activation of the Raf>MEK>ERK pathway has been implicated in invasion (Silberman et al., 1997), metastases (Canman et al., 1995; Keller et al., 2005), angiogenesis (Canman et al., 1995; Simon et al., 1996; Loda et al., 1996; Magi-Galluzzi et al., 1997) and radioresistance (Pirollo et al., 1997). Aberrant activation of the Raf>MEK>ERK cascade has been associated with Bcl-2 and multi-drug resistance gene expression (Kim et al., 1996; Weinstein-Oppenheimer et al., 2001; Arcinas et al., 2001; Wilson et al., 1996; Ji et al., 1996; Nunez et al., 1996; Davis et al., 2003). A diagram of the mutations which can result in activation of the Raf>MEK>ERK cascade is presented in Figure 1.
Fig. 1.
Sites of mutation which can result in activation of the Raf>MEK>ERK pathway. Mutations have been detected in Flt-3, Ras, Kit, Fms, G-CSFR, and at lower frequencies Raf-1 and B-Raf in AML. The BCR-ABL chromosomal translocation is present in virtually all CMLs and some ALLs. These mutations and chromosomal translocations could all result in activation of the Raf>MEK>ERK cascade. A ? is indicated in the connection between Tpl-2 and MEK. This is to indicate that there are other MEK activators besides Raf which can result in MEK activation and may confer sensitivity to MEK inhibitors. Mutations at phosphatase genes could also result in activation of this pathway although they would be predicted to be either tumor suppressor or dominant negative type mutations.
The PI3K>Akt Pathway
Cytokine receptor ligation also leads to rapid activation of phosphatidylinositol 3 kinase (PI3K) (Drexler 1996; Rao et al., 1995; Chang et al., 2003; Steelman et al., 2004). Only Class IA PI3K consists of an 85-kDa regulatory subunit, which contains SH3 Src-homology 2 (SH) and SH3 domains, and a 110-kDa catalytic subunit (Rao et al., 1995; Chang et al., 2003; Steelman et al., 2004). Cytokine stimulation often creates a PI3K binding site on the cytokine receptor. The p85 subunit SH2 domain associates with this site (Rao et al., 1995; Chang et al., 2003; Steelman et al., 2004). The p85 subunit is then phosphorylated, which leads to activation of the p110 catalytic subunit. Activated PI3K phosphorylates the membrane lipid phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] to phosphatidylinositol (3,4,5)-tri-phosphate [PtdIns(3,4,5)P3] which activates PI3K-dependent kinase (PDK1). PDK1 then phosphorylates Akt at threonine 308 (T308) (Steelman et al., 2004). A second kinase phosphorylates Akt on serine 473 (S473) (Cardone et al., 1998; Allan et al., 2003; Troussard et al., 2003; Xu et al., 2003; Persad et al., 2003; Kumar et al., 2004; Songyang et al., 1997).
Akt can transduce an anti-apoptotic signal by phosphorylating downstream target proteins involved in the regulation of cell growth [e.g., glycogen synthase kinase-3β (GSK-3β), Bim, Bad, MDM-2, p21Cip1, X-linked inhibitor of apoptosis (XIAP) and the Foxo3a transcription factor](Songyang et al., 1997; Scheid et al., 1998; del Peso et al., 1997; Nakae et al., 1999; Brunet et al., 1999; Medema et al., 2000; Dijkers et al., 2000; Qi et al., 2006; Mayo et al., 2001; Gottlieb et al., 2002; Zhou et al., 2002, Dan et al., 2004). Phosphorylated Foxo3a loses its ability to induce Fas, p27Kip1, Bim, Noxa, and Puma gene transcription (Nakae et al., 1999; Brunet et al., 1999; Medema et al., 2000; Dijkers et al., 2000; You et al., 2006; Obexer et al., 2006). Akt also phosphorylates I-κK, which subsequently phosphorylates I-κB, resulting in its ubiquitination and subsequent degradation in proteosomes (Ozes et al., 1999; Romashkova et al., 1999; Madrid et al., 20000; Howe et al., 2002, 2004; Hu et al., 2004; Mayo et al., 2000; Shishodia et al., 2004; Du et al., 1998; Arcinas et al., 2001). Disassociation of I-κB from NF-κB enables NF-κB to translocate into the nucleus to promote gene expression that, under certain circumstances, stimulates growth and prevents apoptosis (Du et al., 1998; Arcinas et al., 2001). The PI3K>Akt pathway can also phosphorylate and activate CREB which regulates anti-apoptotic genes including Mcl-1 and Bcl-2 (Du k et al., 1998;Wang et al., 1999).
The PI3K pathway also results in activation of ribosomal protein kinases such as p70S6K (an S6 ribosomal protein kinase) (Mahalingam et al., 1996; Dufner et al., 1999; Romanelli et al., 1999; Harada et al., 2001; Edinger et al., 2004; Panwalkar et al., 2004; Jonassen et al., 2004). p70S6K enhances translation of certain mRNAs, is needed for the early events of cell cycle progression and suppresses apoptosis by phosphorylating Bad (Mahalingam et al., 1996; Dufner et al., 1999; Romanelli et al., 1999; Harada et al., 2001; Edinger et al., 2004). p70S6K is regulated by the mammalian Target of Rapamycin (mTOR) (Ma et al., 2005; Shaw et al., 2006).
The PI3K pathway is negatively regulated by phosphatases. PTEN (phosphatase and tensin homologue deleted on chromosome 10) is considered a tumor suppressor gene (Chang et al., 2003; Steck et al., 1997; Li et al., 1997; Steelman et al., 2004). PTEN is primarily a lipid phosphatase that removes the 3-phosphate from the PI3K lipid product PtdIns (3,4,5)P3 to produce PtdIns (4,5)P2 which prevents Akt activation. PTEN is also reported to be a protein phosphatase, although there is some controversy over the precise protein substrates (Steelman et al., 2004; Mahimainathan et al., 2004; Raftopoulou et al., 2004). Two other phosphatases, SHIP-1 and SHIP-2, remove the 5-phosphate from PtdIns(3,4,5)P3 to produce PtdIns(3,4)P2 (Damen et al., 1996; Kavanaugh et al., 1996; Lioubin et al., 1996; Taylor et al., 2000; Muraille et al., 1999).
Roles of the PI3K>Akt Pathway in Neoplasia
This pathway provides proliferative and anti-apoptotic signals and its dysregulation have often been linked with malignant transformation and drug resistance (Kubota et al., 2004; Cuni et al., 2004). Ras can activate PI3K and some Ras mutations result in deregulated PI3K and downstream Akt activation (Rodriguez-Viciana et al., 1994; Hu et al., 2003; Gire et al., 2000; Sun et al., 2000; Ninomiya et al., 2004). Mutations at the p85 subunit of PI3K have been detected in Hodgkin’s lymphoma cells (Jucker et al., 2002). Recently it was shown that the p110 subunit of PI3K is frequently mutated (~25%) in breast and some other cancers but it has not been reported to be frequently mutated in leukemia (Engelman et al., 2006; Vogt et al., 2006; Bader et al., 2005; Kang et al., 2005; Muller et al., 2006). PTEN negatively regulates Akt activity; hence mutations which result in PTEN loss may lead to persistent elevated Akt levels (Leslie et al., 2000; Dahia et al., 1999; Sakai et al., 1998). Mutations and hemizygous deletions of PTEN have been detected in some primary acute leukemias and non-Hodgkin’s lymphomas (Sakai et al., 1998; Aggerholm et al., 2000; Herranz et al., 2000; Nakahara et al., 1998; Butler et al., 1999). Some hematopoietic cell lines lack or have low PTEN protein expression (Sakai et al., 1998; Aggerholm et al., 2000; Herranz et al., 2000; Nakahara et al., 1998; Butler et al., 1999). Increased Akt expression has also been linked with tumor progression; the Akt-related Akt-2 gene is amplified in some cervical, ovarian, pancreatic cancers and non-Hodgkin’s lymphomas (Graff et al., 2000; Staal 1987; Cheng et al., 1992, 1996). SHIP-1 may also affect Akt activity by controlling the levels of PtdIns(3,4,5)P3 and PtdIns(3,4)P2. SHIP mutations have been detected in certain leukemias including AML (22%). One study reported 22% of AML samples were mutated at SHIP1 (Luo et al., 2003, 2004). Thus the PI3K>Akt pathway is intricately regulated and there are many possible mechanisms which can lead to elevated Akt levels. Hence targeting the PI3K>Akt pathway may prove effective in leukemia therapy.
Interactions Between PI3K>Akt and Raf>MEK>ERK Pathways which Regulate Apoptosis
Akt can phosphorylate Raf-1 on S259 and lead to its inactivation in certain cell types (Rommel et al., 1999; Zimmermann et al., 1999). Akt and serum/glucocorticoid regulated kinase (SGK) can phosphorylate B-Raf which results in its inactivation in certain cell types (Guan et al., 2000; Zhang et al., 2001). Studies in 32D myeloid hematopoietic cells have shown that Akt can activate Raf-1 through a Ras-independent but protein kinase C (PKC)-dependent mechanism which results in the prevention of apoptosis (Majewski et al., 1999). Thus Akt and related proteins phosphorylate Raf family members and either inhibit or enhance their activity and these effects may depend on the cell lineage or environmental cues. Suppression of apoptosis in some cells by Raf and MEK requires PI3K dependent signals (McCubrey et al., 2001; Gelfanov et al., 2001; von Gise et al., 2001; Shelton et al., 2003, 2004).
Both PI3K>Akt and Raf>MEK>ERK pathways contribute to the transcriptional and post-translational regulation of Bcl-2 family members as they can regulate CREB phosphorylation and CREB binds the Mcl-1 and Bcl-2 promoter region (Yang et al., 1995; Pugazhenthi et al., 2000, 1999; Bonni et al., 1999). Moreover, both pathways phosphorylate pro-apoptotic Bcl2 homology (BH)-3 only domain protein Bad which abolishes its apoptotic effects as it is complexed with 14-3-3 proteins and is cytoplasmically localized (Cardone et al., 1998; Allan et al., 2003; Datta et al., 1997; Harada et al., 1999). Another MAPK, Jun N-terminal kinase (JNK) can phosphorylate 14-3-3 proteins which results in their disassociation with phosphorylated Bad proteins and the Bad proteins translocate to the mitochondrion (Sunayama et al., 2005). When Bad associates with Bcl-2 or Bcl-XL, Bad promotes apoptosis by preventing Bcl-2 or Bcl-XL from interacting with Bax (She et al., 2005). Bad is phosphorylated in most AML specimens examined suggesting that inhibition of this molecule is important in AML (Zhao et al., 2004). Interestingly, the anti-apoptotic Mcl-1 protein which is expressed in myeloid cells, is not reported to interact with Bad (Chen et al., 2005).
Both the Raf>MEK>ERK and PI3K>Akt pathways can phosphorylate the BH3 only domain protein Bim (Harada et al., 2004; Qi et al., 2006). When Bim is phosphorylated by ERK and Akt it is targeted for ubiquitination and degradation in the proteosome (Gelinas et al., 2006). ERK also can phosphorylate Mcl-1 which results in its stabilization. Mcl-1 can bind Bim which prevents the activation and mitochondrial translocation of Bak and Bax and it can bind Bim and is able to prevent the activation and mitochondrial translocation of Bak and Bax (Domina et al., 2004). In contrast, JNK can phosphorylate Bim at S65 which enhances its ability to induce Bax activation and hence stimulates apoptosis (Putcha et al., 2003). Mcl-1 can also bind pro-apoptotic Bak (Chen et al., 2005). The Mcl-1:Bax interaction can be disrupted by the binding of the BH3 domain Noxa protein which results in Mcl-1 being ubiquitinated and degraded in the proteosome (Willis et al., 2005). Bak can then form active dimers and induce apoptosis. The stability of Mcl-1 is influenced by both transcriptional (PI3K>Akt) and post-transcriptional (Raf>MEK>ERK) mechanism (Gelinas et al., 2006; Wang et al., 1999).
Cytokines such as IL-3 also induce the Jak/STAT pathway which regulates the transcription of Bcl-XL (Nosaka et al., 1999). Bcl-XL can prevent the formation of Bax:Bax homodimers (Wang et al., 1998). Furthermore JNK can antagonize some of the effects of Raf>MEK>ERK and PI3K>Akt pathways by phosphorylating 14-3-3 proteins which result in released Bad that can translocate to the mitochondrion (Sunayama et al., 2005) or JNK can phosphorylate Bim at different residues than ERK and Akt which results in Bim stabilization. Hence it is clear that the Raf>MEK>ERK, PI3K>Akt, Jak>STAT and JNK pathways regulate many molecules involved in prevention of apoptosis. Dysregulation of these pathways may lead to drug resistance. A diagram of these interactions is presented in Figure 1.
Chemotherapeutic Drugs and Induction of Reactive Oxygen Species (ROS)
Doxorubicin exerts its chemotherapeutic effects through multiple mechanisms. One mechanism is through its interactions with DNA and inhibition of topoisomerase II (Fornari et al., 1994). The other mechanism of action is due to the generation of ROS that occurs via the interaction of doxorubicin with iron (Myers et al., 1986). It is reported that doxorubicin treatment results in the intracellular generation of superoxide anion, hydrogen peroxide, and the hydroxyl radical (Myers et al., 1986; Liu and Tan, 2003). ROS appear to be important for some of the therapeutic effects of doxorubicin as scavenging oxygen radicals using anti-oxidants decreases the ability of doxorubicin to induce apoptosis (Friesen et al., 1999; Gewirtz, 1999; Singal et al., 2000). While ROS are important for some of the activities of doxorubicin they are also are the cause of some of the undesirable side effects of this drug (Hoke et al., 2005).
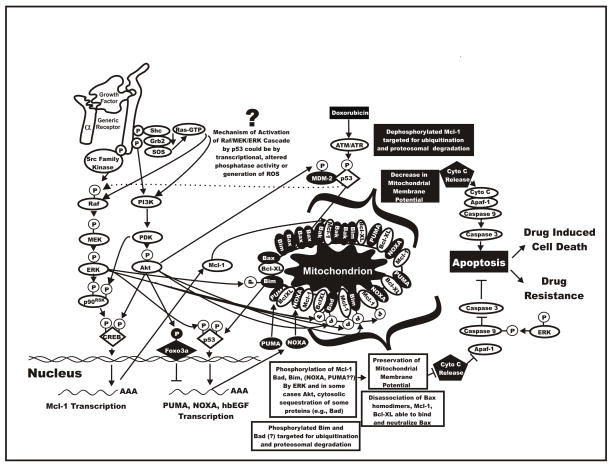
ROS are known to induce the activation of ERK, JNK, p38 and Big MAP Kinase (BMK)/ERK5 signaling pathways. Oxidative stress-induced ERK1/2 activation is reported in a variety of cell types (Jimenez et al., 1997; Tournier et al., 1997; Griffith et al., 1998; Buder-Hoffmann et al., 2001; Kim et al., 2001; Xiao et al., 2002; Blanc et al., 2003; Usatyuk et al., 2003; Conde de la Rosa et al., 2005). In some cases ROS can act directly on receptors, such as the EGFR, and induce the ERK1/2 signaling pathway (Knebel et al., 1996). Triggering of the EGFR is well known to result in the activation of Ras and the subsequent activation of the Raf>MEK>ERK module. ROS can induce the ligand-independent activation of the PDGF receptor and a subsequent increase in Ras and ERK1/2 activity (Knebel et al., 1996). Ligand-independent receptor activation is not the only mechanism by which oxygen radicals activate the ERK1/2 signaling pathway. ROS not only act via growth factor receptors, but also appear to mediate activation of Ras independently of reactive oxygen intermediate-induced receptor activation (Lander et al., 1996). Nor is Ras expression is an absolute requirement for reactive oxygen intermediate activation of the ERK1/2 signaling pathway. ROS will induce the activation of the ERK1/2 signaling pathway in Ras negative cells (Zou et al., 1996). The non-receptor tyrosine kinase, Src, is sensitive to cellular redox and can phosphorylate and activate PLC-γ (Wang et al., 2001). This results in the generation of DAG and increases in intracellular calcium which in turn induce activation of several forms of PKC. Although PKC can lead to Ras activation, it has also been shown to directly activate Raf (Buhl et al., 1995). ROS are also known to inhibit protein phosphatases (Whisler et al., 1995; Rao and Clayton, 2002) and inhibition of phosphatase activity results in activation of the ERK1/2 signaling pathways (Lee and Esselman, 2002). Thus, it would appear that the ERK1/2 kinase signaling cascade can be activated at multiple points by ROS. However, the MEK1 and 2 inhibitors U0126 and PD98059 both block oxidative stress-induced ERK1/2 activation (Lee et al., 2005a;Lee et al., 2005b), indicating that activating actions of oxidative stress on ERK are not direct but instead upstream of ERK. Hydrogen peroxide is able to stimulate ERK5/BMK1 activation in human skin fibroblasts, human vascular smooth muscle cells, and human umbilical vein endothelial cells (Abe et al., 1996). In PC12 cells, hydrogen peroxide-induced ERK5/BMK1 activation requires the activation of a Src kinase (Suzaki et al., 2002). Superoxide anion may play a role in BMK1 activation as superoxide scavengers prevented Angiotensin II- and endothelin-1-induced BMK1 phosphorylation. Since doxorubicin induces ROS, and ROS may induce the ERK signaling pathway. Understanding this pathway may be important in determining how AML cells develop drug resistance. A diagram of the effects of signaling pathways, p53 and ROS and how they may result in drug resistance is presented in Figure 2.
Fig. 2.
Overview of interactions between Raf>MEK>ERK, PI3K>Akt, p53 and apoptotic pathways resulting in drug resistance. The Raf>MEK>ERK and PI3K>Akt pathways can phosphorylate transcription factors which can stimulate gene transcription or apoptotic regulatory molecules which control the induction of apoptosis. Possibly through reactive oxygen species (ROS), doxorubicin can induce Raf>MEK>ERK. Doxorubicin can also activate p53 which can induce the transcription of molecules involved in the regulation of apoptosis. Heparin binding epidermal growth factor (hb-EGF) is a transcriptional target of p53 which could induce activation of the Raf>MEK>ERK cascade. Finally doxorubicin could induce p53 which alters the expression of phosphatases which could lead to prolonged ERK activation. Dysregulation of these cascades can result in the prevention of apoptosis and the induction of drug resistance.
Targeted Therapy in AML
While treatment of some subsets of AML, such as acute promyelocytic leukemia (APL) have shown great success with retinoids and arsenic tri-oxide, a significant problem in the remainder of AML patients is that most chemotherapy does not ultimately work and eventually the patients relapse and succumb to the disease (Tallman et al., 2005). Also another nagging problem in AML therapy is the emergence of drug resistance (Teodori et al., 2006; Polgar et al., 2005; Ross 2004; Mahadevan et al., 2004). Unlike the success stories observed with Gleevec (Imatinib) and Dasatinib in treatment of CML, similar successes have not been observed in AML due in part to the genetic heterogeneity of the disease (Talpaz et al., 2006). Flt-3 inhibitors have been developed, but only approximately 20% of AMLs have mutations at Flt-3 which render them somewhat sensitive to Flt-3 inhibitor monotherapy (Traxier 2003; Markovic et al., 2005; Stone et al., 2005). There have been some combination clinical trials to evaluate the sensitivity of Flt-3 positive AML to chemotherapy and Flt-3 inhibitors.
Materials and methods
Cell line models for identifying signaling pathways involved in hematopoietic drug resistance
The FL5.12 cell line is an IL-3-dependent early hematopoietic progenitor cell line isolated from the fetal liver of mice (McKearn et al., 1985). It is strictly cytokine (interleukin-3[IL-3]) dependent and does not form tumors upon injection into immunocompromised mice. However, FL5.12 cells can be transformed to cytokine-independent and leukemic cells by oncogenes such as v-abl and BCR-ABL (McCubrey et al., 1989). It has wild type (WT) p53 genes.
The effects of the Raf>MEK>ERK and PI3K>Akt pathways on transformation and drug resistance were examined by infecting FL5.12 cells with retroviral vectors encoding activated Akt, activated Raf-1, activated and dominant negative (DN) MEK1, WT and DN p53 as described (Shelton et al., 2003). FL/ΔAkt:ER*(Myr+) + ΔRaf-1:AR are derivatives of FL5.12 cells which grow in response to Akt and Raf activation in the absence of exogenous IL-3 (Shelton et al., 2003). Activated MEK1 (ΔStuMEKLIDEMAN), DN MEK1 encoding retroviral vectors (von Gise et al., 2001) were generously provided by Dr. Jakob Troppmair (Daniel Swarovski Research Laboratory, Innsbruck Medical University, Innsbruck, Austria). The effects of p53 on drug resistance were examined by infecting FL5.12 cells with retroviruses encoding WT and DN p53 (Gottlieb et al., 1994) generously provided by Dr. Moshe Oren, (The Weizmann Institute of Science, Rehovot, Israel). The FL5.12 cells and derivative transformed lines represent models to understand normal and transformed as well as drug resistant early hematopoietic progenitor cells.
Cell lines and growth factors
Cells were maintained in a humidified 5% CO2 incubator with RPMI-1640 [(RPMI) Invitrogen, Carlsbad, CA, USA] supplemented with 5% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA, USA). The IL-3 dependent FL5.12 murine cell line was cultured in this medium supplemented with 10% WEHI-3B(D−) conditioned medium (WCM) as a source of IL-3. Conditionally-transformed FL/ΔAkt:ER*(Myr+) + ΔRaf-1:AR cells were grown in RPMI + 5% FCS + 500 nM 4 hydroxyl tamoxifen (4HT), an estrogen receptor antagonist which activates the ΔAkt:ER*(Myr+) (Sigma, St. Louis, MO, USA), and 100 nM testosterone (Sigma), which activates the ΔRaf-1:AR. ΔAkt:ER*(Myr+) contains a mutated ER domain (ER*) which responds to 4HT 100-fold more efficiently than β-estradiol (Shelton et al., 2003). Thus 4HT as apposed to β-estradiol was used to stimulate Akt activity. ΔRaf-1:AR contains the androgen receptor (AR) hormone binding domain and is activated by testosterone (Shelton et al., 2003).
Limiting dilution analysis in doxorubicin and paclitaxel
FL5.12 and FL/ΔAkt:ER*(Myr+)+ΔRaf-1:AR cells were plated at cell concentrations ranging from 0.1 to 100,000 cells/well in 96 well plates (Corning, Corning NY). Limiting dilution analysis with the parental FL5.12 cells was performed in the presence of IL-3 in doxorubicin (1, 10, 25, 50, 100, 1000 nM) or paclitaxel (0.01, 0.1, 1, 10 and 100 nM). Limiting dilution analysis in the FL/ΔAkt:ER*(Myr+)+ΔRaf-1:AR cells was performed in the presence of IL-3, 4HT, Testosterone or 4HT+Testosterone in doxorubicin (1, 10, 25, 50, 100, 1000 nM) or paclitaxel (0.01, 0.1, 1, 10 and 100 nM). Fresh medium containing the drugs was added every three days and clones isolated from the plates with the least number of colonies after approximately 1 month in culture. After isolation of the clones, they were first expanded in 1 ml cultures in 24 well plates, then subsequently expanded into 5 ml cultures in 25 cm2 tissue culture flasks. The drug resistant cells were grown in medium containing doxorubicin (10 to 100 nM) or paclitaxel (0.1 to 1 nM) with either IL-3 or 4HT+testosterone.
Analysis of Cell Sensitivity to Anticancer Agents
Sensitivity of FL5.12 and FL/ΔAkt:ER* (Myr+)+ ΔRaf-1:AR cells to doxorubicin, paclitaxel, daunorubicin, cisplatin or 5-flurouracil (all purchased from Sigma) were investigated by characterizing effects of these agents on proliferation (Lee et al., 2004). Proliferation assays were performed in order to measure cellular growth under various conditions over a period of 5 days. FL5.12 and FL/ΔAkt:ER*(Myr+)+ΔRaf-1:AR cells were resuspended in phenol red free RPMI containing 5% FBS and either IL-3 (FL5.12 cells) or 4HT, Test, 4HT+Test or no supplement (FL/ΔAkt:ER*(Myr+)+ΔRaf-1:AR cells). Cells were seeded in 96-well cell culture plates (BD Biosciences) at a density of 5,000 cells/well in 100 μL/well of cell culture medium. A 100 μl dose of treatment medium (chemotherapeutic drugs) was added to each well the day after cells were initially seeded. Treatment medium with doxorubicin consisted of 4,000 nM, 2,000 nM, 1,000 nM, 500 nM, 250 nM, 125 nM, 63 nM, 31 nM, 16 nM, 8 nM, 4 nM, or 0 nM in cell culture medium for analysis of proliferation by spectrophotometry. Treatment medium with paclitaxel consisted of 400 nM, 200 nM, 100 nM, 50 nM, 25 nM, 13 nM, 6.3 nM, 3.1 nM, 1.6 nM, 0.8 nM, 0.34 nM, or 0 nM paclitaxel in cell culture medium for analysis of proliferation by spectrophotometry. Treatment medium with daunorubicin consisted of 4,000 nM, 2,000 nM, 1,000 nM, 500 nM, 250 nM, 125 nM, 63 nM, 31 nM, 16 nM, 8 nM, 4 nM, or 0 nM daunorubicin in cell culture medium for analysis of proliferation by spectrophotometry. Treatment medium with cisplatin consisted of 1000 nM, 500 nM, 250 nM, 125 nM, 62.5 nM, 31.3 nM, 15.6 nM, 7.8 nM, 3.9 nM, 2 nM, 1 nM, or 0 nM cisplatin in cell culture medium for analysis of proliferation by spectrophotometry. Treatment medium with 5 flurouracil (5FU) consisted of 200 nM, 100 nM, 50 nM, 25 nM, 12.5 nM, 6.25 nM, 3.1 nM, 1.6 nM, 0.8 nM, 0.4 nM, 0.2 nM, or 0 nM 5FU in cell culture medium for analysis of proliferation by spectrophotometry. Cell culture plates were incubated in a cell culture incubator at 37 °C until extent of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, Sigma) reduction in each well was quantified.
Extent of MTT reduction in each well was measured daily from the day cells were treated until 4 days after treatment. A 40 μl aliquot of MTT medium was added to each well at the end of the treatment period. MTT medium consisted of 3 mg/ml MTT in cell culture medium. MTT medium was sterilized by vacuum filtration before use. After addition of MTT medium, the final volume of medium in each well was 240 μl and the final concentration of MTT was 500 μg/ml. Cell culture plates were incubated in a cell culture incubator for 3 hours at 37 °C to permit MTT reduction by viable cells. MTT reduction produces 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan, which forms crystals that adhere to the bottom of each well because it is insoluble in aqueous solution. Cell culture media was removed after incubation by manually shaking cell culture plates in an inverted position. Crystals of reduced MTT remaining in each well were dissolved in 200 μl of DMSO (Sigma). Cell culture plates were gently shaken for 5 minutes at 37 °C to facilitate dissolution of reduced MTT crystals. Absorbance of each well was measured at 530 nm with a FL600 microplate fluorescence reader (Bio-Tek Instruments, Winooski, VT).
MTT dissolved in DMSO has a yellow color and a visible light absorbance maximum of approximately 410 nm (Plumb et al., 1989). In contrast, MTT reduced by viable cells then dissolved in DMSO has a purple color and a visible light absorbance maximum of approximately 560 nm. It is assumed that absorbance of each well above background at 530 nm is proportional to the number of cells present. Background absorbance at 530 nm was estimated from 56 wells in which no cells were seeded. A 200 μl aliquot of cell culture medium lacking cells was added to each of these wells. These wells were incubated in a cell culture incubator for 1 day at 37 °C before extent of MTT reduction in the absence of cells was determined. Mean absorbance of these 56 wells in which no cells were seeded was subtracted from original absorbance values for all wells containing cells to yield adjusted absorbance. Original absorbance values were adjusted in order to account for background absorbance.
Adjusted absorbance values were normalized by dividing by the mean initial adjusted absorbance. Mean initial adjusted absorbance was measured from wells containing cells incubated for 1 day after seeding. The mean and corresponding standard deviation of normalized adjusted absorbance was calculated from 8 replicate wells for each drug concentration and duration of incubation in order to investigate effects of the drugs and, in some cases, ectopic gene (Raf-1, Akt, MEK1, DN-MEK, p53, DN-p53) expression on cell proliferation rate and sensitivity to the different chemotherapeutic drugs. Relative growth rate was calculated by subtracting mean initial adjusted absorbance from adjusted absorbance after 4 days of treatment then dividing this difference by the increase in mean adjusted absorbance after 4 days of incubation in the absence of the drugs. The mean and standard deviation of relative growth was calculated from 8 replicate wells for each drug concentration to compare effects of the chemotherapeutic drugs on proliferation rates of the different cells.
Inhibitory concentration 50% (IC50) is defined in this context as the concentration of drugs that causes the cells to proliferate at a rate that is half as rapid as cells incubated in the absence of drugs. IC50 values were estimated by linear interpolation of the highest drug concentration yielding a mean relative growth rate greater than 0.5 and the lowest drug concentration yielding a mean relative growth rate less than 0.5.
Annexin V apoptotic assays
AnnexinV/PI binding assays were performed as previously described (Blalock et al., 2000; Bertrand et al., 2006) with a kit purchased from Roche (Indianapolis, IN, USA)
Western blot analysis
Cells were cultured and then protein lysates prepared as described (Blalock et al., 2000). Western blots were performed with antibodies specific for phospho and total MEK, ERK, Akt, JNK, p53, p21Cip-1, p27Kip-1 as we have previously described (Bertrand et al., 2006). The above antibodies were obtained from Cell Signaling (Beverly, MA, USA). Antibodies which recognize total Caspase 3, Bcl-2 and Bcl-XL were obtained from Cell Signaling. An antibody which recognizes Mcl-1 was obtained from Pharmingen (San Diego, CA).
Results and discussion
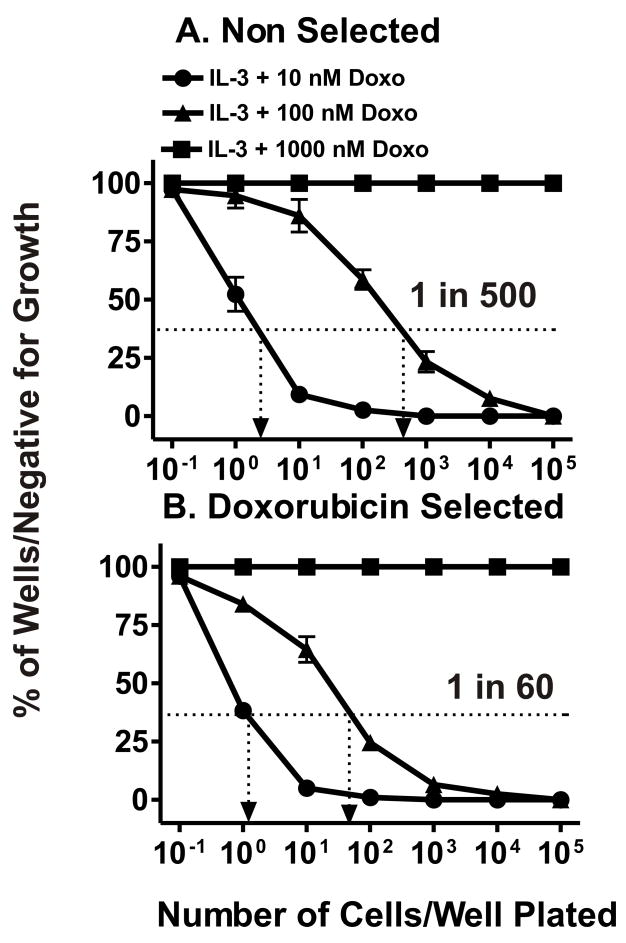
To elucidate the pathways involved in hematopoietic drug resistance, FL5.12 cells were plated in limiting dilution experiments in the presence of different concentrations of doxorubicin in 96 well plates. Doxorubicin resistant cells (FL/Doxo) were isolated in the presence of IL-3 and either 10 or 100 nM doxorubicin but not 1000 nM doxorubicin (Figure 3, Panel A). Approximately 1 in 20 FL5.12 cells would form a colony in the presence of IL-3 + 10 nM doxorubicin while only 1 in 500 (frequency 2 × 10−3) FL5.12 cells would form a colony in the presence of IL-3 + 100 nM doxorubicin. Approximately 25 different clones were isolated, expanded into 200 μl, 1 ml, 5 ml, 10 ml then 25 ml cultures. These individual clones were frozen down. Three different clones were chosen for further study: FL/Doxo-1 FL/Doxo-2, and FL/Doxo-3. These clones have been maintained continuously in 10 to 100 nM doxorubicin for the past two years. The results presented in this manuscript were obtained with FL/Doxo-1, hereafter referred to as FL/Doxo. Similar results were obtained with FL/Doxo-2 and FL/Doxo-3.
Fig. 3.
Isolation of doxorubicin resistant cells from FL5.12 and enhanced subcloning efficiency in doxorubicin. Limiting dilution analysis was performed in the presence of different concentrations of doxorubicin on the FL5.12 (Panel A) and doxorubicin resistant FL/Doxo (Panel B) cells. A dotted line is indicated at 37% of wells negative for growth from which the cloning efficiency can be estimated. These experiments have been performed 4 times and averaged together. Limiting dilution analysis with FL/Doxo-1 is presented in Panel B, similar results were observed with 2 other FL/Doxo clones.
Additional limiting dilution experiments indicated that the doxorubicin resistant cells had an enhanced subcloning efficiency when they were plated in medium containing doxorubicin than the parental cells (Figure 3, Panels A & B). The doxorubicin selected cells that had been maintained in 10 nM doxorubicin had a plating efficiency of 1.6 × 10−2 as 1 out of 60 cells formed a colony when the cells were plated in 100 nM doxorubin. This represents an approximate 8.3-fold increase in cloning efficiency in 100 nM doxorubicin as compared to the unselected FL5.12 cells.
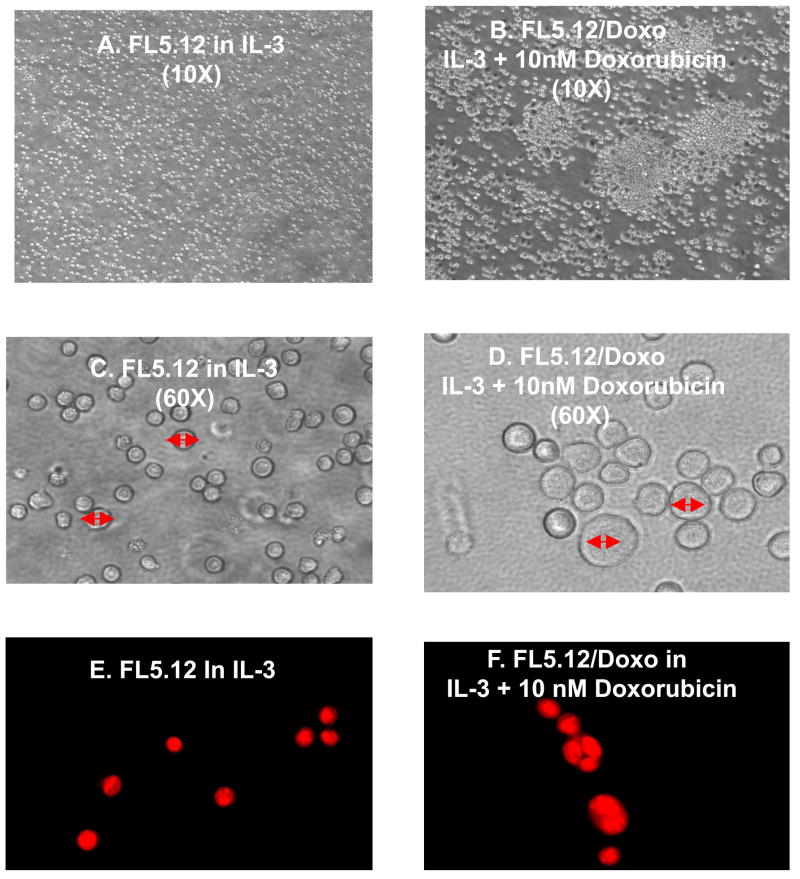
The morphologies of the doxorubicin sensitive (FL5.12) and resistant (FL/Doxo) cells were examined by light microscopy (Figure 4, Panels A & B). The parental cells grew as non adherent individual cells (Panel A). The doxorubicin resistant cells tended to grow in clusters on the bottom of the flask (Panel B). The doxorubicin resistant cells were larger and more blast like (Panel D) than the doxorubicin sensitive cells (Panel C). Furthermore upon staining the cells with acridine orange, which enables visualization of the nucleus, many of the doxorubicin resistant cells had multiple nuclei whereas the parental cells had single nuclei (Figure 4, Panels E & F).
Fig. 4.
Doxorubicin resistant FL5.12 cells are larger, more blast like and some are multinucleate. The morphology of FL/5.12 and FL/Doxo cells was examined by light microscopy (Panels A & B, 10X magnification), (Panels C & D, 60X magnification). The cells were also stained with acridine orange and the nuclear morphology examined (Panels E & F).
The sensitivities of the parental and doxorubicin resistant cells to five common chemotherapeutic drugs were examined. The doxorubicin resistant cells had increased IC50s for doxorubicin, paclitaxel, daunorubicin but not 5-flurouracil (5FU) or cisplatin (Table 1).
Table 1.
Differences in growth IC50s in doxorubicin sensitive and resistant FL5.12 cells1
| Cell Line→ | FL5.12 | FL/Doxo | Fold Difference |
|---|---|---|---|
| Drug↓ | |||
| Doxorubicin | 10 nM | 90 nM | 9X |
| Daunorubicin | 4 nM | 20 nM | 5X |
| Paclitaxel | 1.8 nM | 130 nM | 72X |
| 5-Flurouracil | 800 nM | 1000 nM | 1.3X |
| Cisplatin | 65,000 nM | 85,000 nM | 1.3X |
Determined by plating 2500 cells/well in 96 well plates in phenol red free RPMI+10% FBS+IL-3 and serial 2-fold dilutions (n=12 dilutions) at 8 wells per each drug concentration. MTT analysis was performed after 4 days of incubation and results were normalized to untreated cells.
The effects of these drugs on the induction of apoptosis were determined by the Annexin V/PI binding assay (Table 2). The parental FL5.12 cells were more sensitive to the induction of apoptosis by doxorubicin, paclitaxel and daunorubicin than the doxo resistant FL/Doxo cells. In contrast, the parental and FL/Doxo cells displayed similar sensitivities to 5FU (data not presented). Again, the greatest difference between the sensitive and resistant cells was observed with paclitaxel.
Table 2.
Differences in apoptotic IC50s in doxorubicin sensitive and resistant FL5.12 cells1
| Cell Line→ | FL5.12 | FL/Doxo | Fold Difference |
|---|---|---|---|
| Drug↓ | |||
| Doxorubicin | 10 nM | 100 nM | 10X |
| Daunorubicin | 0.5 nM | 25 nM | 50X |
| Paclitaxel | 0.1 nM | 9 nM | 90X |
| 5-Flurouracil | 1000 nM | 1000 nM | 1X |
Determined by plating 106 cells/well in 6 well plates in RPMI+10% FBS + IL-3 and serial 10-fold dilutions (n=6 dilutions) at 3 wells per each concentration of the different drugs. Annexin V/PI apoptosis analysis was performed after 3 days of incubation and results were normalized to untreated cells.
Evidence for Raf>MEK>ERK Pathway in Drug Resistance
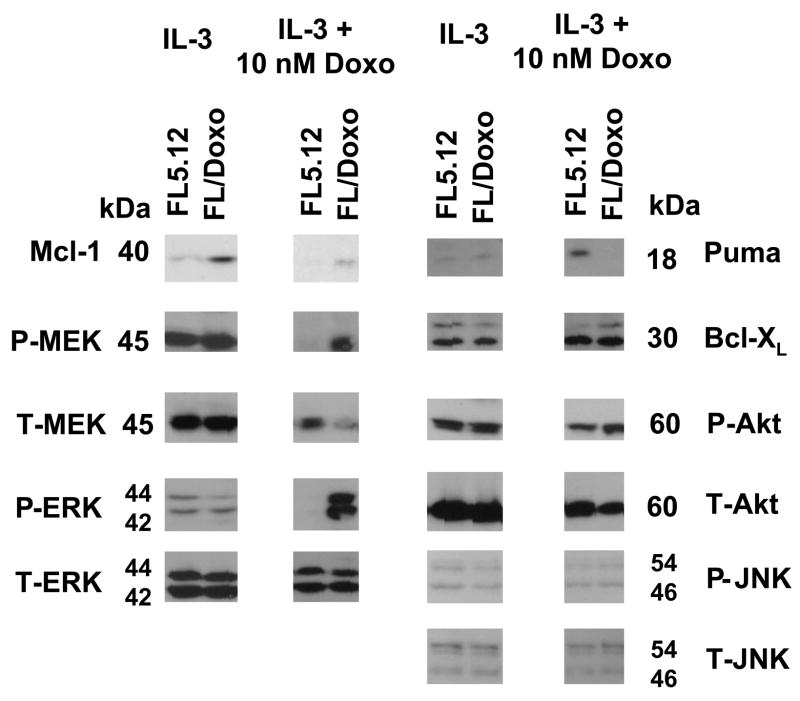
The roles of signal transduction, apoptotic regulatory and p53 pathways were examined in the doxorubicin sensitive and resistant cells. FL5.12 and FL/Doxo cells, which had been growing in IL-3 or IL-3 + 10 nM doxorubicin respectively, were collected, washed twice with PBS and then both cell types were cultured in IL-3 or IL-3 + 10 nM doxorubicin for 24 hrs. When the FL5.12 and FL/Doxo cells were cultured in IL-3 for 24 hrs, similar levels of phospho and total ERK, JNK, Akt and Bcl-XL and Puma proteins were detected. Higher levels of Mcl-1 were detected in the FL/Doxo cells than in FL5.12 cells. In contrast, when the FL5.12 and FL/Doxo cells were culture in IL-3 + 10 nM doxorubicin for 24 hrs, activated MEK and ERK, and total Mcl-1 proteins, were detected at higher levels in the FL/Doxo cells than parental FL5.12 cells (Figure 5). Puma, which was detected at low levels when both cell types were cultured in IL-3, was induced when the FL5.12 cells were cultured in IL-3 + 10 nM doxorubicin, while it was not induced in the doxorubicin resistant cells when they were cultured in IL-3 + 10 nM doxorubicin suggesting that these two cell types may differ in their induction of Puma after doxorubicin treatment. When the doxorubicin sensitive and resistant cell lines were treated with doxorubicin, they both displayed activation of p53, as detected with an antibody which recognized p53 phosphorylated at S15 (data not presented). Thus the doxorubicin resistance of the FL/Doxo cells did not appear to be due to a defective p53 response.
Fig. 5.
Increased activated Mcl-1, pMEK and pERK in doxorubicin resistant FL5.12 cells. FL5.12 and FL/Doxo cells were grown for 24 hours in medium containing IL-3 or IL-3 + 10 nM doxorubicin and then western blot analysis was performed with the indicated antibodies.
Consequences of MEK/ERK and p53 expression on Drug Sensitivity
To further examine the effects of MEK and p53 on the chemosensitivity of the cells, DN MEK and DNp53 constructs were introduced into the cells and the doxorubicin IC50s were determined by MTT analysis (Table 3). Cells were infected with retroviruses encoding DN MEK (MEK-LIDA), DN p53 (p53#661) or as controls an empty retroviral vector (pLXSN) or a WT p53. DN-MEK1 has serine 217 and 221 mutated to alanine which can not be phosphorylated and activated by Raf and is inactive and interferes with endogenous MEK1. DN p53 retrovirus encodes a p53 protein which lacks the DNA binding domain and results in the formation of inactive p53 tetramers.
Table 3.
Effects of DN MEK1 and DNp53 on doxorubicin C501
| Cell Line | ||
|---|---|---|
| Gene Introduced↓ | FL5.12 | FL/Doxo |
| DN-MEK1 | 2 nM | 15 nM |
| Empty Vector | 15 nM | 85 nM |
| DN-p53 | 30 nM | 200 nM |
| WT-p53 | 10 nM | 80 nM |
Determined by MTT analysis as described in the legend to Table 1.
Introduction of DN MEK1 reduced the IC50 for doxorubicin in FL5.12 cells 7.5-fold and in FL/Doxo cells 5.7-fold. Moreover, introduction of the DN MEK1 into the FL/Doxo and FL5.12 cells reduced the cloning efficiency in doxorubicin approximately 3 fold (Data not presented). In contrast, introduction of DN-p53 into FL5.12 or FL/Doxo cells increased the IC50 for doxorubicin approximately two to three fold compared to cells which were transduced with the empty vector or the WT-p53 gene respectively.
The effects of elevated Raf>MEK>ERK expression of the drug resistance of FL5.12 cells was examined by introduction of a constitutive MEK1 gene (ΔStuMEKLIDEMAN (Von Gise et al., 2001), here after referred to MEK-Act (Act = Activated). The FL/Doxo cells with the activated MEK1 gene (FL/Doxo+MEK-Act) had an approximately 5-fold higher doxorubicin IC50 than the FL/Doxo infected with an empty retroviral vector (FL/Doxo+LXSN) cells demonstrating that constitutive MEK activity increased the resistance to doxorubicin.
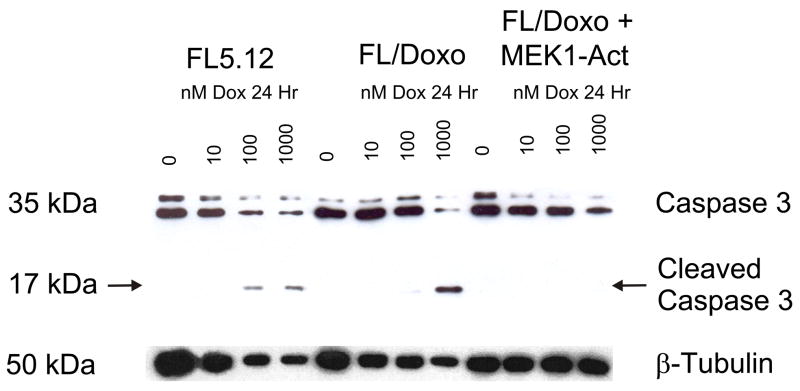
Decreased Caspase 3 Cleavage in Doxorubicin Resistant Cells
Caspase 3 cleavage and activation is one of the last steps in the caspase cascade leading to apoptosis. The extent of caspase 3 cleavage was examined in the doxorubicin sensitive and resistant cells by western blot analysis (Figure 6). Cleavage of caspase 3 was detected in FL5.12 cells in a dose dependent fashion after treatment with 100 or 1000 nM doxorubicin for 24 hrs. In contrast, cleavage of caspase 3 in FL/Doxo cells was only detected after treated with 1000 nM doxorubicin. Furthermore, FL/Doxo+MEK-Act, no cleavage of caspase 3 was detected which correlated with the increased IC50 for doxorubicin in these cells.
Fig. 6.
Decreased caspase 3 cleavage in doxorubicin resistant cells. The extent of cleavage of Caspase 3 was determined in doxorubicin sensitive FL5.12 cells and doxorubicin resistant FL/Doxo and FL/Doxo+MEK1-Act. The cells were incubated in the indicated concentrations of doxorubicin for 24 hours and then protein lysates isolated.
Lack of Elevated Mdr-1/MRP-1 Expression in Doxorubicin resistant FL/Doxo Cells
FL/Doxo cells were shown to be resistant to doxorubicin, paclitaxel, and daunomycin, but not resistant to 5FU or cisplatin. Doxorubicin, paclitaxel and daunomycin can be transported by the membrane transporter proteins Mdr-1 or MRP-1, whereas 5FU and cisplatin are transported by different membrane transporters. A relatively simple means to determine if Mdr-1 or MRP-1 activity is elevated in FL/Doxo cells is to perform a functional Rhodamine 123 dye exclusion assay by FACS analysis. The drug resistant and drug sensitive FL5.12 cells displayed similar levels of drug efflux activity. This assay was performed 4 times. Thus by a functional assay the drug resistant FL/Doxo cells did not appear to have elevated drug efflux when compared to the parental cells.
The expression of these two transporters was examined by RT-PCR and western blot analysis. mRNA levels for MRP-1 were similar in the doxorubicin sensitive and resistant cells. Transcripts encoding Mdr-1 were not detected in ether cell line. Western blot analysis failed to detect the expression of Mdr-1 or MRP-1 proteins in these cells while they were detected in control cell lines. In summary, these results suggest that the drug resistance of FL/Doxo cells is not due to the increased expression of Mdr-1 or MRP-1 but they do not eliminate that possibility that some other transporter is involved in drug resistance.
Interactions between Raf>MEK>ERK and PI3K>Akt Pathways in Induction of Drug Resistance in Hematopoietic Cells
We previously developed a model of hematopoietic cells which proliferate in response to activation of both Raf and Akt (Shelton, et al., 2003). FL5.12 cells were infected with conditional retroviral vectors encoding ΔRaf-1:AR (testosterone–inducible Raf-1) and ΔAkt:ER*(Myr+) (*= tamoxifen, [4HT]-inducible Akt). These cells are named FL/ΔAkt:ER+ΔRaf:AR cells. An advantage of the FL/ΔAkt:ER+ΔRaf:AR cells is the possibility to investigate the effects of Akt and Raf on signal transduction pathways and drug resistance either alone or together.
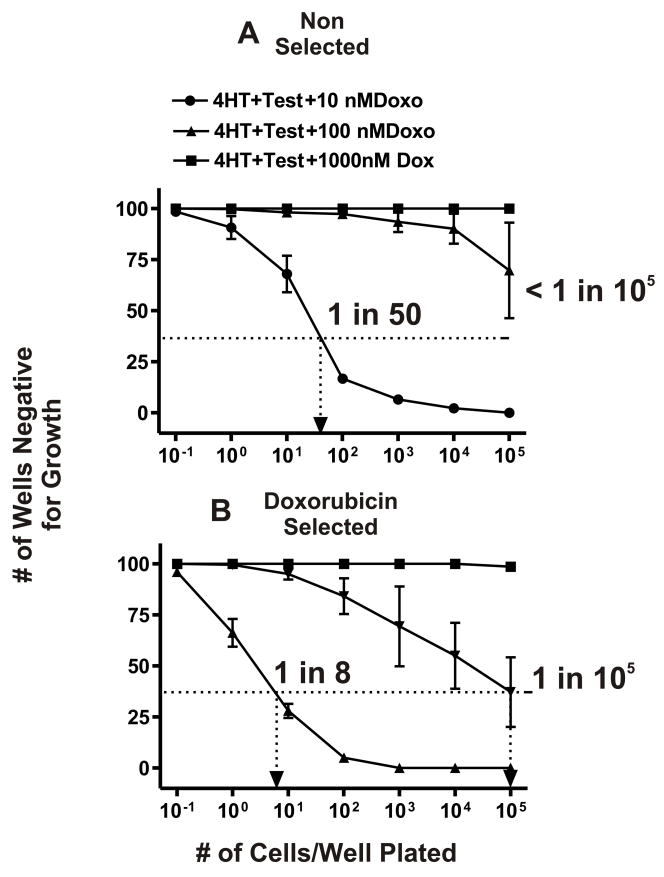
As described earlier with the FL5.12 cells, doxorubicin resistant FL/ΔAkt:ER+ΔRaf-1:AR cells were isolated by culturing the cells in medium containing 10 or 100 nM doxorubicin and 4HT and testosterone. The unselected FL/ΔAkt:ER+ΔRaf-1:AR cells had a subcloning efficiency of approximately 2 × 10−2 (1 in 50 cells would form a colony) in 10 nM doxorubicin. In contrast to the results observed with IL-3 and the parental FL5.12 cells, drug resistant clones were infrequently isolated from unselected FL/ΔAkt:ER+ΔRaf-1:AR cells when they were plated in 100 nM doxorubicin as less than 1 in 105 cells would form a colony. The difference in cloning efficiency in medium containing doxorubicin between in FL5.12 and FL/ΔAkt:ER+ΔRaf-1:AR cells is likely due to the difference in culture conditions, as IL-3 will induce many signaling pathways in addition to Raf>MEK>ERK and PI3K>Akt such as Jak>STAT which can contribute to drug resistance while 4HT and testosterone only induce the Akt and Raf>MEK>ERK pathways.
Additional limiting dilution experiments indicated that the doxorubicin selected FL/ΔAkt:ER+ΔRaf-1:AR cells had an enhanced subcloning efficiency when they were plated in medium containing doxorubicin than the parental FL/ΔAkt:ER+ΔRaf-1:AR cells (Figure 7, Panels A & B). In the doxorubicin selected FL/ΔAkt:ER+ΔRaf-1:AR cells that had been maintained in 10 nM doxorubicin, they had a plating efficiency of 1.25 × 10−1 as 1 in 8 cells would form a colony in 10 nM doxorubicin, an approximate 6.3-fold increase in cloning efficiency. When the doxorubicin selected FL/ΔAkt:ER+ΔRaf-1:AR cells were plated in 100 nM doxorubicin a cloning efficiency of 1 × 10−5 as approximately 1 in 105 cells formed a colony.
Fig. 7.
Isolation of doxorubicin resistant FL/ΔAkt:ER*+ΔRaf-1:AR cells. Limiting dilution analysis was performed in the presence of different concentrations of doxorubicin on FL/ΔAkt:ER+ΔRaf-1:AR cells. These results were obtained when the cells were cultured in medium containing 4HT + testosterone. Additional limiting dilution analyses indicated that neither 4HT nor testosterone were sufficient by themselves to result in the isolation of drug resistant cells which could be expanded into larger cultures. A dotted line is indicated at 37% of wells negative for growth from which the cloning efficiency can be estimated. These experiments have been performed 5 times and averaged together. Limiting dilution analysis with FL/ΔAkt:ER+ΔRaf-1:AR clone 1 is presented in Panel B, similar results were observed with 2 other FL/ΔAkt:ER+ΔRaf-1:AR clones.
The drug sensitivities of the doxorubicin sensitive and resistant FL/ΔAkt:ER+ΔRaf-1:AR cell lines were compared (Table 4).
Table 4.
Differences in IC50 in doxorubicin sensitive and resistant FL/ΔAkt:ER*(Myr+)+ΔRaf-1:AR cells1
| Cell Line→ | Doxo-Sensitive | Doxo-Resistant | Fold Difference |
|---|---|---|---|
| Drug↓ | |||
| Doxorubicin | 25 nM | 75 nM | 3X |
| Daunorubicin | 12 nM | 30 nM | 2.5X |
| Paclitaxel | 3 nM | 18 nM | 6X |
Determined by plating 2500 cells/well in 96 well plates in phenol red free RPMI+10% FBS + 500 nM 4HT +100 nM Test and serial 2-fold dilutions (n=12 dilutions) at 8 wells per each drug concentration. MTT analysis was performed after 4 days of incubation and results were normalized to untreated cells. These experiments differ from those presented in Tables 1, 2 & 3 as in those cases the cells were plated in IL-3.
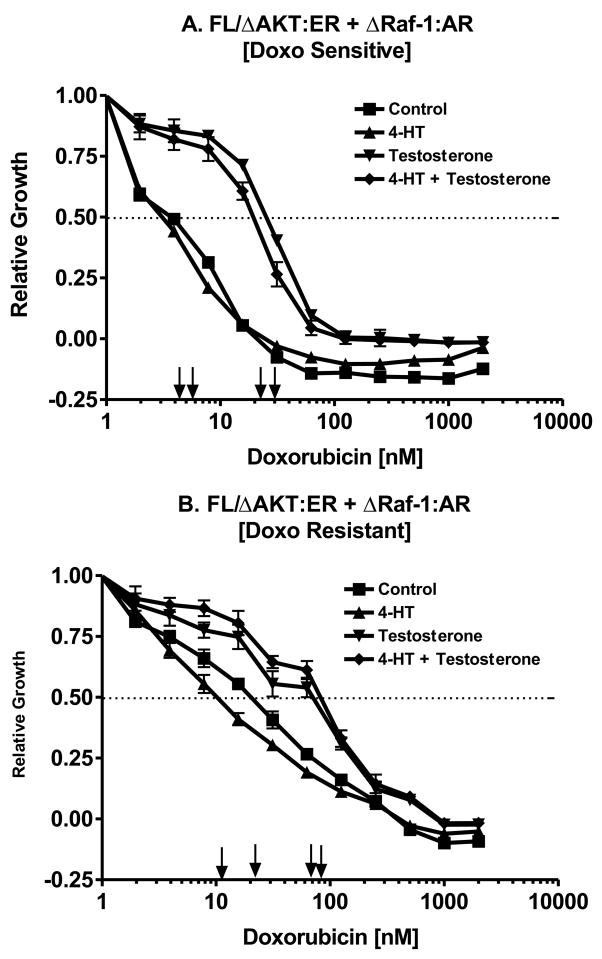
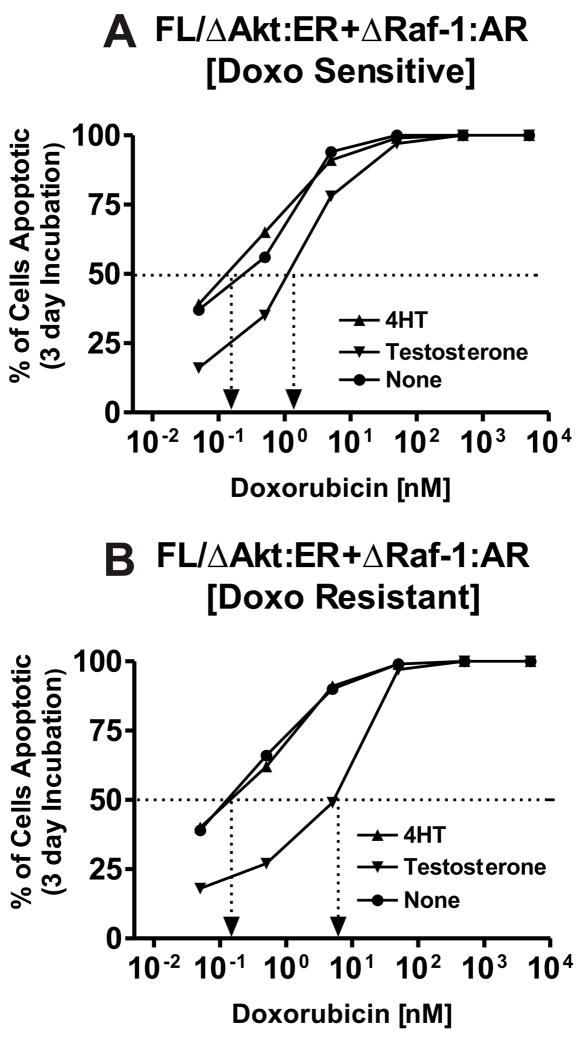
Effects of Raf Activation on the Doxorubicin IC5
The effects Raf and Akt individually on the doxorubicin IC50 were determined by performing the MTT analysis in medium supplement with: no supplement, 4HT, testosterone or the combination of 4HT + testosterone (Figure 8). Activation of Raf increased the IC50 approximately 10-fold, from approximately 3 nM with no supplement or 4HT to 30 nM with testosterone treatment (Panel A).
Fig. 8.
Dominant role of Raf in driving drug resistance. The effects of Raf activation by testosterone and Akt activation by 4HT on the doxorubicin IC50 of non selected and doxorubicin selected FL/ΔAkt:ER+ΔRaf-1:AR cells were examined by MTT analysis in 96 well plates. Activation of Raf increased the IC50 in both the non selected and doxorubicin selected cells.
Likewise in the drug resistant FL/ΔAkt:ER+ΔRaf-1:AR cells, activation of Raf increased the IC50 for doxorubicin from approximately 3-fold from 15 to 25 nM with 4HT or no supplement to approximately 70 nM when Raf was activated. This figure also demonstrates that the drug resistant cells have retained their requirement for Raf for proliferation.
Requirement for Raf for the Prevention of Apoptosis
The effects of Raf and Akt activation on the prevention of apoptosis in response to doxorubicin treatment of doxorubicin sensitive and resistant FL/ΔAkt:ER+ΔRaf-1:AR cells were examined by annexin V/PI assays (Figure 9). The effects Raf and Akt individually on the doxorubicin IC50 were determined by culturing the cells in medium supplement with: no supplement, 4HT, testosterone (Figure 9). Activation of Raf increased the apoptosis IC50 approximately 10-fold in the unselected doxorubicin sensitive FL/ΔAkt:ER+ΔRaf-1:AR, from approximately 0.2 nM with no supplement or 4HT to 2 nM with testosterone treatment (Panel A). Likewise in the drug resistant FL/ΔAkt:ER+ΔRaf-1:AR cells, activation of Raf increased the IC50 for doxorubicin from approximately 80-fold from 0.2 nM with 4HT or no supplement to approximately 8 nM when Raf was activated. This figure also demonstrates that the drug resistant cells have retained their requirement for Raf for prevention of apoptosis.
Fig. 9.
Dominant role of Raf in preventing apoptosis. The effects of Raf activation by testosterone and Akt activation by 4HT on the prevention of apoptosis induced by doxorubicin was determined in non selected and doxorubicin selected FL/ΔAkt:ER+ΔRaf-1:AR cells by the annexin V/PI technique. The extent of apoptosis was determined after incubation of the cells for 3 days in the different concentrations of doxorubicin. Cells were cultured with medium supplemented with 4HT, testosterone (test) on no supplement. Activation of Raf was dominant in the suppression of apoptosis.
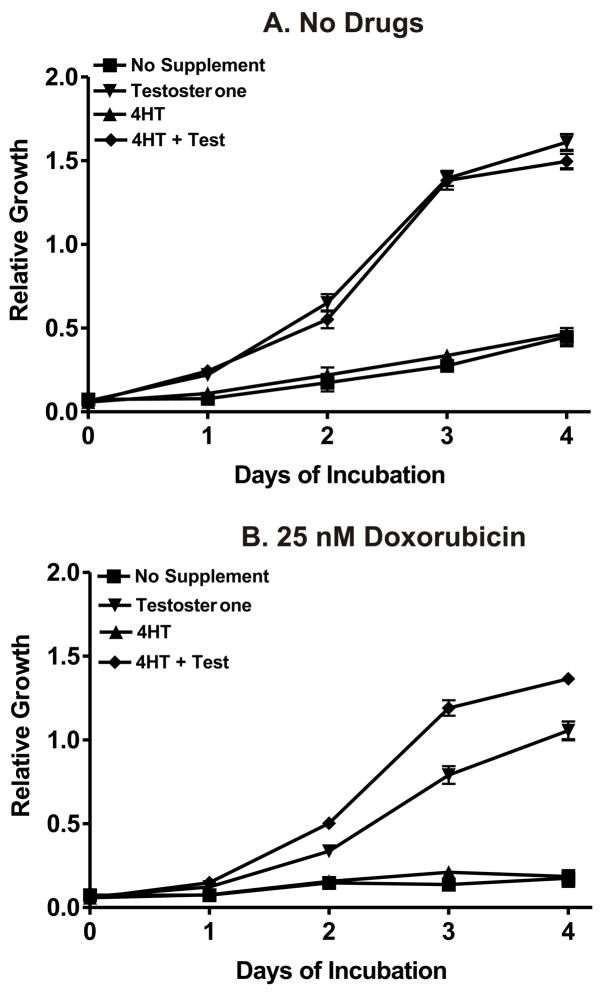
Requirement for Raf and Akt Activation for Optimal Growth in the Presence of Chemotherapeutic Drugs
The requirement of Raf and Akt activation in the growth of the cells in the presence and absence of chemotherapeutic drugs was determined by culturing the cells in 4HT, Test, 4HT + Test or no supplement and then performing MTT analysis (Figure 10). When these cells were cultured in the absence of doxorubicin (Panel A), they proliferated equally well in response to either Raf activation or Raf and Akt activation in 100 μl cultures in 96 well plates as measured by MTT analysis. In contrast, in the presence of just 4HT, which activated Akt, or no supplement, the cells did not proliferate well. Thus, in the absence of drugs, Raf-1 activation was able to induce proliferation as estimated by an MTT assay. In contrast, when the cells were plated in the presence of 25 nM doxorubicin (Panel B), the cells proliferated better when both Raf and Akt were activated as opposed to just activation of Raf-1 by itself. Similar results were observed with daunorubicin and paclitaxel.
Fig. 10.
Requirement of Raf and Akt in drug resistant growth. The effects of Akt activation by 4HT, Raf activation by testosterone and their co-activation in doxorubicin selected FL/ΔAkt:ER+ΔRaf-1:AR cells was determined by MTT analysis in the presence and absence of 25 nM doxorubicin. Activation of Akt was not necessary for growth in 100 μL cultures over a 4 day period in the absence of drugs. In contrast, activation of Akt enhanced the proliferation of the cells when they were cultured in the presence of testosterone and 25 nM doxorubicin. Similar results were observed with paclitaxel and daunorubicin.
Potential Mechanisms for Induction of Drug Resistance
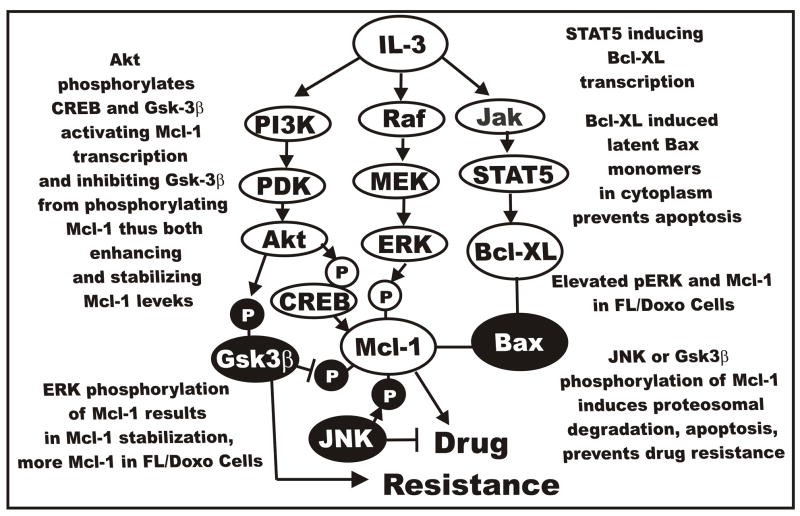
In the following sections, we will briefly summarize potential mechanisms by which interactions between the Raf>MEK>ERK and PI3K>Akt pathways could result in drug resistance. Cytokines such as IL-3 induce multiple signal transduction pathways which can contribute to the prevention of apoptosis (Harada et al., 2004; Qi et al., 2006; Opferman et al., 2003). If their expression becomes deranged, drug resistance may occur. An overview of IL-3 and the different pathways which it induces is presented in Figure 11. Note that all these signaling pathways have roles in the regulation of apoptotic pathways.
Fig. 11.
Cytokine mediated signal transduction pathways and drug resistance. Cytokines such as IL-3 can induce multiple signal transduction pathways which can effect the expression of apoptotic molecules by transcriptional and post-transcriptional mechanisms. Elevated ERK in FL/Doxo cells may phosphorylate Mcl-1 which results in its stabilization. This may result in prolonged binding to Bax, prevent activation of Bax, contribute to the prevention of apoptosis and lead to drug resistance.
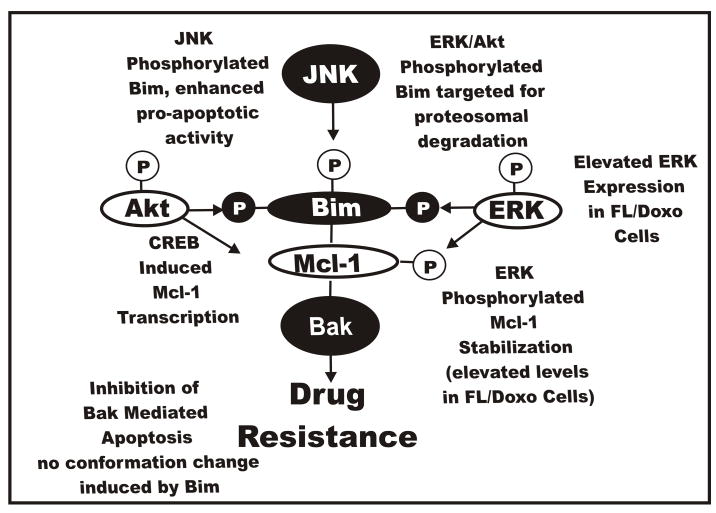
Raf>MEK>ERK Expression Results in Altered Bim Localization
The pro-apoptotic Bim molecule can be phosphorylated by both the Raf>MEK>ERK and PI3K>Akt pathways on multiple residues (Harada et al., 2004; Qi et al., 2006; Opferman et al., 2003). Akt can phosphorylate Bim on S87 in IL-3 dependent cells. ERK induces the phosphorylation of Bim at S55, S65 and S100. Once Bim is phosphorylated it loses its association with Bcl-2 like antiapoptotic proteins associates with 14-3-3 proteins and is ubiquitinated and targeted for degradation in the proteosome. Upon IL-3 withdrawal, non-phosphorylated Bim associates with pro-apoptotic Bax proteins and stimulates apoptosis. JNK also phosphorylates Bim, but this results in enhanced pro-apoptotic activity (Tsuruta et al., 2004; Gao et al., 2005). JNK can also phosphorylate 14-3-3 proteins which may then release cytosolic Bim. The presence of phosphorylated Bim may be elevated in the doxorubicin resistant cells, alternatively, the subcellular localization of Bim may be different. Activation of both Raf>MEK>ERK and PI3K>Akt pathways and hyperphosphorylation of Bim may be necessary for the growth of the drug resistant cells in chemotherapeutic drugs. A diagram depicting these potential interactions is presented in Figure 12.
Fig. 12.
Effects of Raf>MEK>ERK and PI3K>Akt and JNK pathways on Bim phosphorylation and the induction of drug resistance. All three of these pathways can phosphorylate Bim on different residues which affect its activity and interactions with Mcl-1 and Bax and Bak. Phosphorylation events mediated by Raf>MEK>ERK and PI3K>Akt result in the prevention of Bax and Bak activation and lead to Bim being targeted to the proteosome ubiquitination and degradation. In contrast phosphorylation of Bim by JNK results in its dissociation of Bim:Mcl-1 heterodimers, Mcl-1 is targeted to the proteosome, ubiquitination, and degradation and Bim mediated activation of Bax and Bak.
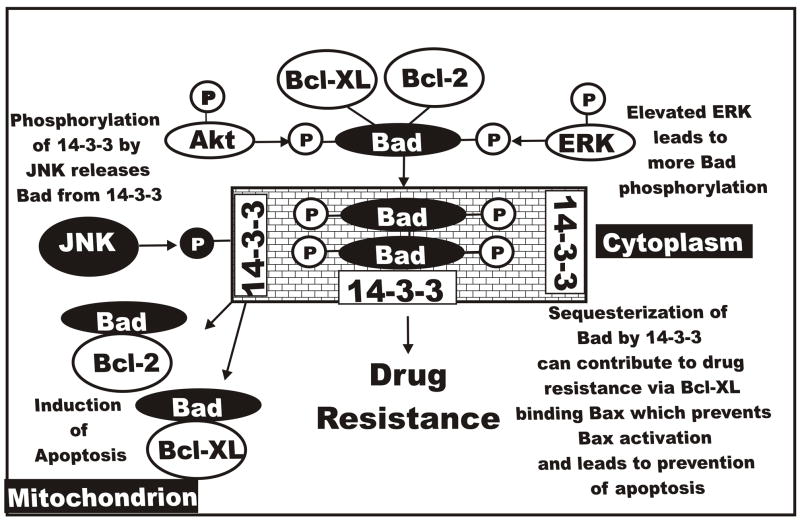
Raf>MEK>ERK Elevates Bad Phosphorylation in Doxorubicin-Resistant Cells
Increased phosphorylation of Bad could be one component of the drug resistance of FL/Doxo cells. Both the Raf>MEK>ERK and PI3K>Akt pathways phosphorylate Bad which results in Bad’s translocation from the mitochondrion and association with 14-3-3 proteins in the cytoplasm. Bcl-2 and Bcl-XL are able to bind Bax and prevent its activation. JNK will phosphorylate 14-3-3 proteins which then release Bad and Bad translocates to the mitochondrion. Bad is then able to bind Bcl-2 and Bcl-XL and Bax is activated and apoptosis is induced. Increased ERK activity in FL/Doxo cells may result in higher levels of Bad phosphorylation. However, this component would be predicted not to involve Mcl-1 as Mcl-1 is not thought to bind Bad whereas, Bcl-1 and Bcl-XL bind Bad. In some scenarios, inhibition of Mcl-1 is not thought to be totally sufficient to induce apoptosis in some cells, as there is thought to be compensatory effect by Bcl-XL. An overview of the interactions of Raf>MEK>ERK, PI3K>Akt, Bcl-XL, Bcl-2 and Bad is presented in Figure 13.
Fig. 13.
Effects of Raf>MEK>ERK and PI3K>Akt and JNK pathways on bad phosphorylation and the induction of drug resistance. All three of these pathways can phosphorylate Bad on different residues which affect its activity and interactions with Bcl-2 and Bcl-XL. Phosphorylation events mediated by Raf>MEK>ERK and PI3K>Akt result in Bad being associated with 14-3-3 proteins and translocation from the mitochondrion to the cytoplasm. Bcl-2 and Bcl-XL remain associated with Bax and Bak which prevent their activation and lead to suppression of apoptosis. In contrast phosphorylation of Bad by JNK results in its dissociation with 14-3-3 proteins and Bad localizes to the mitochondrion and binds Bcl-2 and Bcl-XL. Bax and Bak are then able to induce apoptosis.
However, we do not think that Bad will be the target responsible for drug resistance for two reasons, Bad has been reported to be present at either very low levels or not at all in FL5.12 cells (Yamaguchi et al., 2001), and we did not see a difference in the levels of Bcl-XL in the doxorubicin responsive and resistant cells.
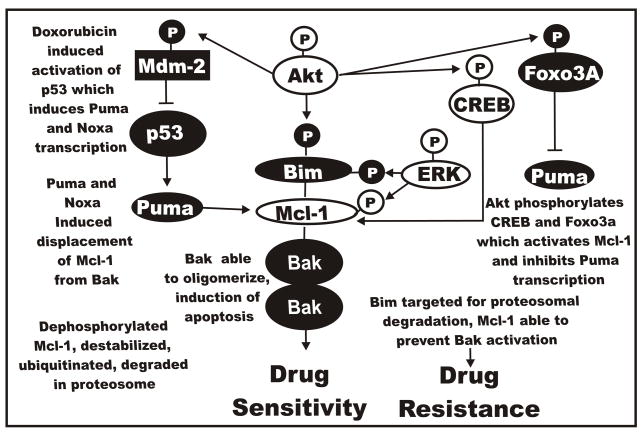
Raf>MEK>ERK Expression Results in Altered Puma/Noxa Localization
Two proteins induced by p53 are the BH3 domain only pro-apoptotic proteins Puma and Noxa (Yu et al., 2005). These proteins are involved in the induction of the caspase cascade by their interactions with Mcl-1 and Bcl-XL. Two Puma proteins are generated from the Puma gene, Puma–α and Puma-β, both are induced by p53 and bind Bcl-XL and Mcl-1. Puma can induce the displacement of Mcl-1 from Bak and Bax Puma then induces conformational changes in Bax which results in Bax’s translocation to the mitochondria, cytochrome C release and apoptosis. An overview of the interactions of Puma, Mcl-1, p53, Bak and Bax is presented in Figure 14.
Fig. 14.
Effects of Raf>MEK>ERK, PI3K>Akt and p53 pathways on noxa and puma and the induction of drug resistance. p53 can induce the BH3 only containing Noxa and Puma proteins which interact with Mcl-1 and other anti-apoptotic proteins. When Mcl-1 is associated with Noxa and Puma that prevents their ability to interact with Bax and Bak. Increased expression of ERK in FL/Doxo cells may result in increased Mcl-1 levels which prevent Noxa and Puma abilities to activate Bax and Bak.
The expression of Puma is under the control of the PI3K/Akt pathway as it has recently been shown that FOXO-3a regulates the expression of Puma (You et al., 2006). Noxa is another BH3-domain protein which can be induced by p53. Noxa has recently been shown to interact specifically with Mcl-1 and A1 (Chen et al.,2005) but not with Bcl-2, Bcl-XL or Bcl-2. The pro-apoptotic Bak molecule associates with Mcl-1 and Bcl-XL but not Bcl-2, Bcl-w or A1 (Willis et al., 2005). Upon induction of Noxa by activation of p53, Noxa binds Mcl-1 and displaces Bak. This leads to Mcl-1 degradation and Bak is free to induce apoptosis.
If the Raf>MEK>ERK pathway increases Mcl-1 protein levels and stability, that may lead to an increase in Mcl-1 associated with Noxa and Puma and a decrease in free Bak levels. Alternatively, PI3K>Akt may phosphorylate FOXO-3a which results in decreased Puma expression. Both of these effects on Noxa and Puma may be required for drug resistance.
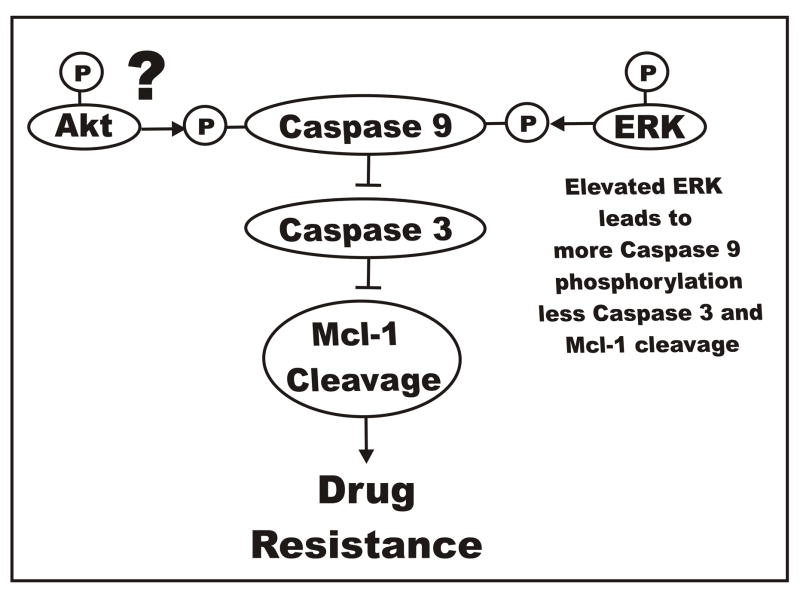
Raf>MEK>ERK Elevates Caspase 9 Phosphorylation in Doxorubicin-Resistant Cells
Human Caspase 9 was originally thought to be phosphorylated by Akt (Cardone et al., 1998), but the murine caspase 9 lacks the Akt consensus phosphorylation site (Allan et al., 2003). Caspase 9 is phosphorylated by the Raf>MEK>ERK pathway at T125 which inhibits activation of the caspase cascade. Elevated phosphorylation of caspase 9 may be responsible for the decreased Caspase 3 detected in the doxorubicin resistant cells.
One of the targets of caspase 3 is Mcl-1 (Weng et al., 2005). Decreased caspase 3 activation could lead to a decrease in Mcl-1 cleavage. The extent of cleavage of Mcl-1 in the doxorubicin sensitive and resistant cells could be different, resulting in the prevention of apoptosis in the doxorubicin resistant cells. An overview of the effects of the effects of Raf>MEK>ERK and PI3K>Akt pathways on the regulation of caspase activity and drug resistance is presented in Figure 15.
Fig. 15.
Effects of Raf>MEK>ERK and PI3K>Akt pathways on caspase 9 phosphorylation and the induction of drug resistance. The Raf>MEK>ERK pathway phosphorylates caspase 9 which prevents activation of caspase 3. The ability of Akt to phosphorylate caspase 9 is controversial as the Akt consensus phosphorylation site is present in mouse but not human caspase 9. Increased phosphorylation of caspase 9 by ERK in FL/Doxo cells could result in less caspase 9 activation, less caspase 3 activation and less Mcl-1 cleavage which could result in the prevention of apoptosis and contribute to drug resistance.
Raf>MEK>ERK Elevates the Phosphorylation of Other Targets Responsible for Drug Resistance
Obviously, there are other downstream targets which elevate Raf>MEK>ERK. These include: p90Rsk, p70S6K, p21Cip1, p27Kip1, Bcl-2 and others. However, in order to keep this discussion focused we have discussed the most direct targets of Raf>MEK>ERK which could lead to drug resistance.
Raf>MEK>ERK Activates the Expression of Membrane Transporters other than Mdr-1/MRP-1 Which Lead to Drug Resistance
A membrane transporter protein other than MDR-1 or MRP-1 may be involved in the drug resistance of the cells (BCRP-1, MRP2, MRP3, MRP4, MRP5, MRP6, MRP7, MRP8).
Summary
We have presented data which documents the importance of the Raf>MEK>ERK and PI3K>Akt pathways in the development of drug resistance in hematopoietic cells. Further understanding of how these pathways interact and induce drug resistance could result in the identification of novel approaches to treat drug resistance in leukemia. Furthermore, p53 played a role in drug resistance in these cells as introduction of a DN-p53 construct increased the resistance of the cells to chemotherapeutic drugs. The drug sensitive and drug resistant FL/ΔAkt:ER+ΔRaf-1:AR cells will allow us the ability to determine not only which downstream components are induced by either Raf>MEK>ERK or PI3K>Akt that are necessary for proliferation and prevention of apoptosis, but also which components are important in drug resistance and how these two pathways can interact to influence drug resistance.
Acknowledgments
JAM, LSS, RAF, SLA, WHC, and EWTW have been supported in part by a grant from the NIH (R01098195). AMM and CE were supported in part from a grant from Associazione Italiana Ricerca sul Cancro (AIRC Regional grants).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–90. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- Adachi T, Kar S, Wang M, Carr BI. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. J Cell Physiol. 2002;192:151–59. doi: 10.1002/jcp.10124. [DOI] [PubMed] [Google Scholar]
- Aggerholm A, Gronbaek K, Guldberg P, Hokland P. Mutational analysis of the tumour suppressor gene MMAC1/PTEN in malignant myeloid disorders. Eur J Haematol. 2000;65:109–13. doi: 10.1034/j.1600-0609.2000.90181.x. [DOI] [PubMed] [Google Scholar]
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 by phosphorylation at Thr125 by ERK MAP kinase. Nature Cell Biol. 2003;5:647–54. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Stewart SA, Assoian RK, Juliano RL. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol. 2001;153:273–82. doi: 10.1083/jcb.153.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcinas M, Heckman CA, Mehew JW, Boxer LM. Molecular mechanisms of transcriptional control of bcl-2 and c-myc in follicular and transformed lymphoma. Cancer Res. 2001;61:5202–6. [PubMed] [Google Scholar]
- Attwell S, Mills J, Troussard A, Wu C, Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell. 2003;12:4813–25. doi: 10.1091/mbc.E03-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurer I, Labar B, Nemet D, Ajdukovic R, Bogdanic V, Gale RP. High incidence of conservative RAS mutations in acute myeloid leukemia. Acta Haematol. 1994;92:123–5. doi: 10.1159/000204200. [DOI] [PubMed] [Google Scholar]
- Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- Bertrand FE, Steelman LS, Chappell WH, Abrams SL, Shelton JG, White ER, Ludwig DL, McCubrey JA. Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-IR-mediated growth in hematopoietic cells. Leukemia. 2006;20:1254–60. doi: 10.1038/sj.leu.2404217. [DOI] [PubMed] [Google Scholar]
- Birkenkamp KU, Geugien M, Schepers H, Westra J, Lemmink HH, Vellenga E. Constitutive NF-kappaB DNA-binding activity in AML is frequently mediated by a Ras/PI3-K/PKB-dependent pathway. Leukemia. 2004;18:103–12. doi: 10.1038/sj.leu.2403145. [DOI] [PubMed] [Google Scholar]
- Blalock WL, Navolanic PM, Steelman LS, Shelton JG, Moye PW, Lee JT, Franklin RA, Mirza A, McMahon M, White MK, McCubrey JA. Requirement for the PI3K>Akt pathway in MEK1-mediated growth and prevention of apoptosis: identification of an Achilles heel in leukemia. Leukemia. 2003;17:1058–67. doi: 10.1038/sj.leu.2402925. [DOI] [PubMed] [Google Scholar]
- Blalock WL, Pearce M, Steelman LS, Franklin RA, McCarthy SA, Cherwinski H, McMahon M, McCubrey JA. A conditionally-active form of MEK1 results in autocrine transformation of human and mouse hematopoietic cells. Oncogene. 2000;19:526–36. doi: 10.1038/sj.onc.1203337. [DOI] [PubMed] [Google Scholar]
- Blanc A, Pandey NR, Srivastava AK. Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by H2O2 in vascular smooth muscle cells: potential involvement in vascular disease (Review) Int J Mol Med. 2003;11:229–34. [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and independent mechanisms. Science. 1999;286:1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, Roth JA, Albelda SM, Davies H, Cox C, Brignell G, Stephens P, Futreal PA, Wooster R, Stratton MR, Weber BL. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2001;24:405–13. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- Buhl AM, Osawa S, Johnson GL. Mitogen-activated protein kinase activation requires two signal inputs from the human anaphylatoxin C5a receptor. J Biol Chem. 1995;270:19828–32. doi: 10.1074/jbc.270.34.19828. [DOI] [PubMed] [Google Scholar]
- Butler MP, Wang SI, Chaganti RS, Parsons R, Dalla-Favera R. Analysis of PTEN mutations and deletions in B-cell non-Hodgkin’s lymphomas. Genes Chromosomes Cancer. 1999;24:322–7. [PubMed] [Google Scholar]
- Canman CE, Gilmer TM, Coutts SB, Kastan MB. Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes & Devel. 1995;9:600–11. doi: 10.1101/gad.9.5.600. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Carter BZ, Milella M, Tsao T, McQueen T, Schober WD, Hu W, Dean NM, Steelman L, McCubrey JA, Andreeff M. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia. 2003;17:2081–89. doi: 10.1038/sj.leu.2403113. [DOI] [PubMed] [Google Scholar]
- Chan TL, Zhao W, Leung SY, Yuen ST. Cancer Genome Project. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003;63:4878–81. [PubMed] [Google Scholar]
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K>Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Molecular Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA. 1992;89:9267–71. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by anti-sense RNA. Proc Natl Acad Sci USA. 1996;93:3636–41. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen DH, Andersen MK, Desta F, Pedersen-Bjergaard J. Mutations of genes in the receptor tyrosine kinase (RTK)RAS-BRAF signal transduction pathway in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2005;19:2232–40. doi: 10.1038/sj.leu.2404009. [DOI] [PubMed] [Google Scholar]
- Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2005;12:12. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Constantinidou M, Chalevelakis G, Economopoulos T, Koffa M, Liloglou T, Anastassiou C, Yalouris A, Spandidos DA, Raptis S. Codon 12 ras mutations in patients with myelodysplastic syndrome: incidence and prognostic value. Ann Hematol. 1997;74:11–14. doi: 10.1007/s002770050248. [DOI] [PubMed] [Google Scholar]
- Coutant A, Rescan C, Gilot D, Loyer P, Guguen-Guillouzo C, Baffet G. PI3K-FRAP/mTOR pathway is critical for hepatocyte proliferation whereas MEK/ERK supports both proliferation and survival. Hepatology. 2002;36:1079–88. doi: 10.1053/jhep.2002.36160. [DOI] [PubMed] [Google Scholar]
- Cuni S, Perez-Aciego P, Perez-Chacon G, Vargas JA, Sanchez A, Martin-Saavedra FM, Ballester S, Garcia-Marco J, Jorda J, Durantez A. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18:1391–1400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- Dahia PL, Aquiar RC, Alberta J, Kum JB, Caron S, Sill H, Marsh DJ, Ritz J, Freedman A, Stiles C, Eng C. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanism in haematological malignancies. Human Mol Gen. 1999;8:185–93. doi: 10.1093/hmg/8.2.185. [DOI] [PubMed] [Google Scholar]
- Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triposphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–93. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–12. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- Daniotti M, Oggionni M, Ranzani T, Vallacchi V, Campi V, Di Stasi D, Torre GD, Perrone F, Luoni C, Suardi S, Frattini M, Pilotti S, Anichini A, Tragni G, Parmiani G, Pierotti MA, Rodolfo M. BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene. 2004;23:5968–77. doi: 10.1038/sj.onc.1207780. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg MA. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Davis JM, Navolanic PM, Weinstein-Oppenheimer CR, Steelman LS, Hu W, Konopleva M, Blagosklonny MV, McCubrey JA. Raf-1 and Bcl-2 induce distinct and common pathways that contribute to breast cancer drug resistance. Clin Cancer Res. 2003;9:1161–70. [PubMed] [Google Scholar]
- Davis RJ, Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–85. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;4:459–67. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- de Souza Fernandez T, Menezes de Souza J, Macedo Silva ML, Tabak D, Abdelhay E. Correlation of N-ras point mutations with specific chromosomal abnormalities in primary myelodysplastic syndrome. Leuk Res. 1998;22:125–34. doi: 10.1016/s0145-2126(97)00112-4. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–89. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994;371:171–75. doi: 10.1038/371171a0. [DOI] [PubMed] [Google Scholar]
- Deng X, Kornblau SM, Ruvolo PP, May WS., Jr Regulation of Bcl2 phosphorylation and potential significance for leukemic cell chemoresistance. J Natl Cancer Inst Monogr. 2001;28:30–7. doi: 10.1093/oxfordjournals.jncimonographs.a024254. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas SH, Lam EWF, Burgering BMT, Raaijmakers JAM, Llammers JWJ, Koenderman L, Coffer PJ. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27Kip1. Mol Cell Biol. 2000;20:9138–48. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domina AM, Vrana J, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERL activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–15. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- Dong F, Brynes RK, Tidow N, Welte K, Lowenberg B, Touw IP. Mutations in the gene for granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N Engl J Med. 1995;333:487–93. doi: 10.1056/NEJM199508243330804. [DOI] [PubMed] [Google Scholar]
- Dong F, Dale DC, Bonilla MA, Freedman M, Fasth A, Neijens HJ, Palmblad J, Briars GL, Carlsson G, Veerman AJ, Welte K, Lowenberg B, Touw IP. Mutations in the granulocyte colony-stimulating factor receptor gene in patients with severe congenital neutropenia. Leukemia. 1997;11:120–5. doi: 10.1038/sj.leu.2400537. [DOI] [PubMed] [Google Scholar]
- Drexler HG. Expression of the FLT-3 receptor and response to FLT3 ligand by leukemic cells. Leukemia. 1996;10:588–99. [PubMed] [Google Scholar]
- Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–9. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- Dufner A, Anjelkovic M, Burgering BMT, Hemmings B, Thomas G. Protein kinase B localization and activation and eukaryotic translational initiation factor 4E-binding protein phosphorylation. Mol Cell Biol. 1999;19:4525–34. doi: 10.1128/mcb.19.6.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblen ST, Catling AD, Assanah MC, Weber MJ. Biochemical and biological functions of the N-terminal, noncatalytic domain of extracellular signal-regulated kinase 2. Mol Cell Biol. 2001;21:249–59. doi: 10.1128/MCB.21.1.249-259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. An activated mTOR mutant supports growth factor-independent, nutrient- dependent cell survival. Oncogene. 2004;23:5654–63. doi: 10.1038/sj.onc.1207738. [DOI] [PubMed] [Google Scholar]
- Engleman JA, Luo J, Canley LC. The evolution phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Flotho C, Valcamonica S, Mach-Pascual S, Schmahl G, Corral L, Ritterbach J, Hasle H, Arico M, Biondi A, Niemeyer CM. RAS mutations and clonality analysis in children with juvenile myelomonocytic leukemia (JMML) Leukemia. 1999;13:32–7. doi: 10.1038/sj.leu.2401240. [DOI] [PubMed] [Google Scholar]
- Friesen C, Fulda S, Debatin KM. Induction of CD95 ligand and apoptosis by doxorubicin is modulated by the redox state in chemosensitive- and drug-resistant tumor cells. Cell Death Differ. 1999;6:471–80. doi: 10.1038/sj.cdd.4400512. [DOI] [PubMed] [Google Scholar]
- Fornari FA, Randolph JK, Yalowich JC, Ritke MK, Gewirtz DA. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 1994;45:649–56. [PubMed] [Google Scholar]
- Fransen K, Klintenas M, Osterstrom A, Dimberg J, Monstein HJ, Soderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–33. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- Gallagher A, Darley R, Padua RA. RAS and the myelodysplastic syndromes. Pathol Biol (Paris) 1997;45:561–8. [PubMed] [Google Scholar]
- Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Review Cancer Cell. 2004;6:313–9. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, Luo Y, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- Gelfanov VM, Burgess GS, Litz-Jackson S, King AJ, Marshall MS, Nakshatri H, Boswell HS. Transformation of interleukin-3-dependent cells without participation of Stat5/bcl-xL:cooperation of akt with raf/erk leads to p65 nuclear factor κB-mediated antiapoptosis involving c-IAP2. Blood. 2001;15:2508–17. doi: 10.1182/blood.v98.8.2508. [DOI] [PubMed] [Google Scholar]
- Gëlinas C, White E. BH3-only proteins in control: specificity regulates MCL-1 and BAK-mediated apoptosis. Genes & Development. 2006;19:1263–8. doi: 10.1101/gad.1326205. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–41. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Gire V, Marshall C, Wynford-Thomas D. PI-3-kinase is an essential anti-apoptotic effector in the proliferative response of primary human epithelial cells to mutant RAS. Oncogene. 2000;19:2269–76. doi: 10.1038/sj.onc.1203544. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. (Review) Nature Reviews Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- Gougopoulou DM, Kiaris H, Ergazaki M, Anagnostopoulos NI, Grigoraki V, Spandidos DA. Mutations and expression of the ras family genes in leukemias. Stem Cells. 1996;14:725–9. doi: 10.1002/stem.140725. [DOI] [PubMed] [Google Scholar]
- Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, Vessella RL, Neubauer BL. Increased Akt activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip-1 expression. J Biol Chem. 2000;275:24500–5. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- Griffith CE, Zhang W, Wange RL. ZAP-70-dependent and -independent activation of Erk in Jurkat T cells. Differences in signaling induced by H2o2 and Cd3 cross-linking. J Biol Chem. 1998;273:10771–6. doi: 10.1074/jbc.273.17.10771. [DOI] [PubMed] [Google Scholar]
- Guan K, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem. 2000;275:27354–9. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. P70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule Bad. Proc Natl Acad Sci USA. 2001;98:9666–70. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Becknell B, Wilm M, Mann M, Huang LJS, Taylor SS, Scott JD, Korsmeyer SJ. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3:413–22. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci U S A. 2004;101:15313–7. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz M, Urioste M, Santos J, Martinez-Delgado JB, Rivas C, Benitez J, Fernandez-Piqueras J. Allelic losses and genetic instabilities of PTEN and p73 in non-Hodgkin lymphomas. Leukemia. 2000;14:1325–7. doi: 10.1038/sj.leu.2401813. [DOI] [PubMed] [Google Scholar]
- Hiramatsu A, Miwa H, Shikami M, Ikai T, Tajima E, Yamamoto H, Imai N, Hattori A, Kyo T, Watarai M, Miura K, Satoh A, Itoh M, Imamura A, Mihara H, Katoh Y, Nitta M. Disease-specific expression of VEGF and its receptors in AML cells; possible autocrine pathway VEGF/type1 receptor of VEGF in t(15;17) AML and VEGF/type2 receptor of VEGF in t(8;21)AML. Leukemia Lymphoma. 2006;47:89–95. doi: 10.1080/10428190500270386. [DOI] [PubMed] [Google Scholar]
- Hoelzer D, Gokbuget N. Recent approaches in acute lymphoblastic leukemia in adults. Crit Rev Oncol Hematol. 2000;36:49–58. doi: 10.1016/s1040-8428(00)00097-4. [DOI] [PubMed] [Google Scholar]
- Hoke EM, Maylock CA, Shacter E. Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic Biol Med. 2005;39:403–11. doi: 10.1016/j.freeradbiomed.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Howe CJ, LaHair MM, Maxwell JA, Lee JT, Robinson PJ, Rodriguez-Mora O, McCubrey JA, Franklin RA. Participation of the calcium/calmodulin-dependent kinases in hydrogen peroxide-induced Ikappa B phosphorylation in human T lymphocytes. J Biol Chem. 2002;277:30469–76. doi: 10.1074/jbc.M205036200. [DOI] [PubMed] [Google Scholar]
- Howe CJ, LaHair MM, McCubrey JA, Franklin RA. Redox regulation of the CaM-kinases. J Biol Chem. 2004;279:44573–81. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- Hu L, Shi Y, Hsu JH, Gera J, Van Ness B, Lichtenstein A. Downstream effectors of oncogenic ras in multiple myeloma cells. Blood. 2003;101:3126–35. doi: 10.1182/blood-2002-08-2640. [DOI] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L, McDermott PJ, Kuppuswamy D. c-Raf>MEK>ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem. 2002;277:23065–75. doi: 10.1074/jbc.M200328200. [DOI] [PubMed] [Google Scholar]
- Inhorn RC, Carlesso N, Durstin M, Frank DA, Griffin JD, Inhorn RC, Carlesso N, Durstin M, Frank DA, Griffin JD. Identification of a viability domain in the granulocyte/macrophage colony-stimulating factor receptor beta-chain involving tyrosine-750. Proc Natl Acad Sci U S A. 1995;92:8665–9. doi: 10.1073/pnas.92.19.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen JW, Lyons J, Steenvoorden AC, Seliger H, Bartram CR. Concurrent mutations in two different ras genes in acute myelocytic leukemias. Nucleic Acids Res. 1987;15:5669–80. doi: 10.1093/nar/15.14.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Mochon E, Arcinas M, Boxer LM. CREB proteins function as positive regulators of the translocated Bcl-2 allele in t(14;18) lymphomas. J Biol Chem. 1996;271:22687–91. doi: 10.1074/jbc.271.37.22687. [DOI] [PubMed] [Google Scholar]
- Jia W, Yu C, Rahmani M, Krystal G, Sausville EA, Dent P, Grant S. Synergistic antileukemic interactions between 17-AAG and UCN-01 involve interruption of RAF/MEK- and AKT-related pathways. Blood. 2003;102:1824–32. doi: 10.1182/blood-2002-12-3785. [DOI] [PubMed] [Google Scholar]
- Jimenez LA, Zanella C, Fung H, Janssen YM, Vacek P, Charland C, Goldberg J, Mossman BT. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. Am J Physiol. 1997;273:L1029–35. doi: 10.1152/ajplung.1997.273.5.L1029. [DOI] [PubMed] [Google Scholar]
- Jonassen AK, Mjos OD, Sack MN. p70S6 kinase is a functional target of insulin activated Akt cell-survival. Biochem Biophys Res Commun. 2004;315:160–5. doi: 10.1016/j.bbrc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Jucker M, Sudel K, Horn S, Sickel M, Wegner W, Fiedler W, Feldman RA. Expression of a mutated form of the p85alpha regulatory subunit of phosphatidylinositol 3-kinase in a Hodgkin’s lymphoma-derived cell line (CO) Leukemia. 2002;16:894–901. doi: 10.1038/sj.leu.2402484. [DOI] [PubMed] [Google Scholar]
- Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, Tanaka N, Higuchi T, Young J, Jass JR, Leggett BA. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;8:1137–44. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bader AG, Zhao L, Vogt PK. Mutated PI 3-kinases: cancer targets on a silver platter. Cell Cycle. 2005;4:578–81. doi: 10.4161/cc.4.4.1586. [DOI] [PubMed] [Google Scholar]
- Karin M, Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–23. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- Kavanaugh WM, Pot DA, Chin SM, Deuter-Reinhard M, Jefferson AB, Norris FA, Masiarz FR, Cousens LS, Majerus PW, Williams LT. Multiple forms of an inositol polyphosphate 5-phosphaatase from signaling complexes with Shc and Grb2. Current Biology. 1996;6:438–45. doi: 10.1016/s0960-9822(02)00511-0. [DOI] [PubMed] [Google Scholar]
- Keller ET, Fu Z, Brennan M. The biology of a prostate cancer metastasis suppressor protein: Raf kinase inhibitor protein. J Cell Biochem. 2005;94:273–8. doi: 10.1002/jcb.20169. [DOI] [PubMed] [Google Scholar]
- Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, Ning J, He L, Yang H, Sun M, Niciosuia SV, Cheng JQ. AKT/PKB signaling mechanisms in cancer and chemoresistance. Frontiers in Bioscience. 2005;10:975–87. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- Kim BY, Han MJ, Chung AS. Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells. Free Radic Biol Med. 2001;30:686–98. doi: 10.1016/s0891-5849(00)00514-1. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee C, Foxworth A, Waldman T. B-Raf is dispensable for K-Ras-mediated oncogenesis in human cancer cells. Cancer Res. 2004;64:1932–7. doi: 10.1158/0008-5472.can-03-3862. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee SH, Kwak NH, Kang CD, Chung BS. Effects of the activated Raf protein kinase on the human multi drug resistance 1 (MDR1) gene promoter. Cancer Letters. 1996;98:199–205. [PubMed] [Google Scholar]
- Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, Asou N, Kuriyama K, Jinnai I, Shimazaki C, Akiyama H, Saito K, Oh H, Motoji T, Omoto E, Saito H, Ohno R, Ueda R. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–80. [PubMed] [Google Scholar]
- Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, Naoe T. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–7. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- Knebel A, Rahmsdorf HJ, Ullrich A, Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. Embo J. 1996;15:5314–25. [PMC free article] [PubMed] [Google Scholar]
- Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, Estey EH, Andreeff M. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–65. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–52. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- Kubo K, Naoe T, Kiyoi H, Fukutani H, Kato Y, Oguri T, Yamamori S, Akatsuka Y, Kodera Y, Ohno R. Clonal analysis of multiple point mutations in the N-ras gene in patients with acute myeloid leukemia. Jpn J Cancer Res. 1993;84:379–87. doi: 10.1111/j.1349-7006.1993.tb00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Ohnishi H, Kitanaka A, Ishida T, Tanaka T. Constitutive activation of PI3K is involved in the spontaneous proliferation of primary acute myeloid leukemia cells: direct evidence of PI3K activation. Leukemia. 2004;18:1438–40. doi: 10.1038/sj.leu.2403402. [DOI] [PubMed] [Google Scholar]
- Kumar AS, Naruszewicz I, Wang P, Leung-Hagesteijn C, Hannigan GE. ILKAP regulates ILK signaling and inhibits anchorage-independent growth. Oncogene. 2004;23:3454–61. doi: 10.1038/sj.onc.1207473. [DOI] [PubMed] [Google Scholar]
- Lander HM, Milbank AJ, Tauras JM, Hajjar DP, Hempstead BL, Schwartz GD, Kraemer RT, Mirza UA, Chait BT, Burk SC, Quilliam LA. Redox regulation of cell signalling. Nature. 1996;381:380–1. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- Lanfrancone L, Pelicci G, Brizzi MF, Aronica MG, Casciari C, Giuli S, Pegoraro L, Pawson T, Pelicci PG, Arouica MG. Overexpression of Shc proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SoS and Grb2/p140 complexes to the beta receptor subunit. Oncogene. 1995;10:907–17. [PubMed] [Google Scholar]
- Lange-Carter CA, Johnson GL. Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science. 1994;265:1458–61. doi: 10.1126/science.8073291. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kim SY, Kwon CH, Kim YK. EGFR-dependent ERK activation triggers hydrogen peroxide- induced apoptosis in OK renal epithelial cells. Arch Toxicol. 2006;80:337–46. doi: 10.1007/s00204-005-0052-2. [DOI] [PubMed] [Google Scholar]
- Lee JT, Jr, McCubrey JA. Targeting the Raf kinase cascade in cancer therapy—novel molecular targets and therapeutic strategies. Review Expert Opinion on Therapeutic Targets. 2002;6:659–78. doi: 10.1517/14728222.6.6.659. [DOI] [PubMed] [Google Scholar]
- Lee JT, Jr, Steelman LS, McCubrey JA. Phosohatidylinositol 3′-kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64:8397–404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- Lee JW, Soung YH, Park WS, Kim SY, Nam SW, Min WS, Lee JY, Yoo NJ, Lee SH. BRAF mutations in acute leukemias. Leukemia. 2004;18:170–2. doi: 10.1038/sj.leu.2403201. [DOI] [PubMed] [Google Scholar]
- Lee JW, Yoo NJ, Soung YH, Kim HS, Park WS, Kim SY, Lee JH, Park JY, Cho YG, Kim CJ, Ko YH, Kim SH, Nam SW, Lee JY, Lee SH. BRAF mutations in non-Hodgkin’s lymphoma. Br J Cancer. 2003;89:1958–60. doi: 10.1038/sj.bjc.6601371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Esselman WJ. Inhibition of PTPs by H(2)O(2) regulates the activation of distinct MAPK pathways. Free Radic Biol Med. 2002;33:1121–32. doi: 10.1016/s0891-5849(02)01000-6. [DOI] [PubMed] [Google Scholar]
- Lee WC, Choi CH, Cha SH, Oh HL, Kim YK. Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem Res. 2005b;30:263–70. doi: 10.1007/s11064-005-2449-y. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Gray A, Pass I, Orchiston EA, Downes Analysis of the cellular functions of PTEN using catalytic domain and C-terminal mutations: differential effects of C-terminal deletion on signaling pathways downstream of phosphoinositide 3-kinase. Biochem J. 2000;346:827–33. [PMC free article] [PubMed] [Google Scholar]
- Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. Journal of Biol Chem. 2003;278:18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–9. [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Lilleberg SL, Durocher J, Sanders C, Walters K, Culver K. High sensitivity scanning of colorectal tumors and matched plasma DNA for mutations in APC, TP53, K-RAS, and BRAF genes with a novel DHPLC fluorescence detection platform. Ann N Y Acad Sci. 2004;1022:250–6. doi: 10.1196/annals.1318.039. [DOI] [PubMed] [Google Scholar]
- Lioubin MN, Algate PA, Tsai S, Carlberg K, Aebersold A, Rohrschneider LR. P150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes & Devel. 1996;10:1084–95. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- Liu QY, Tan BK. Relationship between anti-oxidant activities and doxorubicin-induced lipid peroxidation in P388 tumour cells and heart and liver in mice. Clin Exp Pharmacol Physiol. 2003;30:185–8. doi: 10.1046/j.1440-1681.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- Loda M, Capodieci P, Mishra R, Yao H, Corless C, Grigioni W, Wang Y, Magi-Galluzzi C, Stork PJ. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Amer J Pathol. 1996;149:1553–64. [PMC free article] [PubMed] [Google Scholar]
- Lubbert M, Mirro J, Jr, Kitchingman G, McCormick F, Mertelsmann R, Herrmann F, Koeffler HP. Prevalence of N-ras mutations in children with myelodysplastic syndromes and acute myeloid leukemia. Oncogene. 1992;7:263–8. [PubMed] [Google Scholar]
- Luo JM, Liu ZL, Hao HL, Wang FX, Dong ZR, Ohno R. Mutation analysis of SHIP gene in acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004;12:420–6. [PubMed] [Google Scholar]
- Luo JM, Yoshida H, Komura S, Ohishi N, Pan L, Shigeno K, Hanamura I, Miura K, Iida S, Ueda R, Naoe T, Akao Y, Ohno R, Ohnishi K. Possible dominant-negative mutation of the SHIP gene in acute myeloid leukemia. Leukemia. 2003;17:1–8. doi: 10.1038/sj.leu.2402725. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the Rel A/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–38. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magi-Galluzzi C, Mishra R, Fiorentino M, Montironi R, Yao H, Capodieci P, Wishnow K, Kaplan I, Stork PJ, Loda M. Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab Invest. 1997;76:37–51. [PubMed] [Google Scholar]
- Mahadevan D, List AF. Targeting the multidrug resistance-1 transporter in AML: molecular regulation and therapeutic strategies. Review Blood. 2004;104:1940–51. doi: 10.1182/blood-2003-07-2490. [DOI] [PubMed] [Google Scholar]
- Mahalingam M, Templeton DJ. Constitutive activation of S6 kinase by deletion of amino-terminal autoinhibitory and rapamycin sensitivity domains. Mol Cell Biol. 1996;16:405–13. doi: 10.1128/mcb.16.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahimainathan L, Choudhury GG. Inactivation of platelet-derived growth factor receptor by the tumor suppressor PTEN provides a novel mechanism of action of the phosphatase. J Biol Chem. 2004;279:15258–68. doi: 10.1074/jbc.M314328200. [DOI] [PubMed] [Google Scholar]
- Majewski M, Nieborowska-Skorska M, Salomoni P, Slupianek A, Reiss K, Trotta R, Calabretta B, Skorski T. Activion of mitochondrial Raf-1 is involved in the anti-apoptotic effects of Akt. Cancer Res. 1999;59:2815–9. [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Marshall CJ. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–45. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–83. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- Marani M, Hancock D, Lopes R, Tenev T, Downward J, Lemoine NR. Role of Bim in the survival pathway induced by Raf in epithelial cells. Oncogene. 2004;23:2431–41. doi: 10.1038/sj.onc.1207364. [DOI] [PubMed] [Google Scholar]
- Markovic A, MacKenzie KL, Lock RB. FLT-3: a new focus in the understanding of acute leukemia. Int J Biochem Cell Biol. 2005;37:1168–72. doi: 10.1016/j.biocel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, Cocco L. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–28. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–48. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi T, Salgia R, Hallek M, Eder M, Druker B, Ernst TJ, Griffin JD. Shc phosphorylation in myeloid cells is regulated by granulocyte macrophage colony-stimulating factor, interleukin-3, and steel factor and is constitutively increased by p210BCR/ABL. J Biol Chem. 1994;269:5016–21. [PubMed] [Google Scholar]
- Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 2001;98:10983–5. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo MW, Baldwin AS. The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochem Biophys Acta. 2000;1470:M55–62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Holland G, McKearn J, Risser R. Abrogation of factor-dependence in two IL-3- dependent cell lines can occur by two distinct mechanisms. Oncogene Res. 1989;4:97–109. [PubMed] [Google Scholar]
- McCubrey JA, May WS, Duronio V, Mufson A. Serine/threonine phosphorylation in cytokine signal transduction. Leukemia. 2000;14:9–21. doi: 10.1038/sj.leu.2401657. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lee JT, Steelman LS, Blalock WL, Moye PW, Chang F, Pearce M, Shelton JG, White MK, Franklin RA, Pohnert SC. Interactions between the PI3K and Raf signaling pathways can result in the transformation of hematopoietic cells. Cancer Detection & Prevention. 2001;25:375–93. [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Blalock WL, Lee JT, Moye PW, Chang F, Pearce M, Shelton JG, White MK, Franklin RA, Pohnert SC. Synergistic effects of PI3K>Akt on abrogation of cytokine-dependency induced by oncogenic Raf. Adv Enzyme Regl. 2001;41:289–323. doi: 10.1016/s0065-2571(00)00021-2. [DOI] [PubMed] [Google Scholar]
- McKearn JP, McCubrey JA, Fagg B. Enrichment of hematopoietic precursor cells and cloning of multipotential B lymphocyte precursors. Proc Natl Acad Sci USA. 1985;85:7414–8. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–7. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochem Biophys Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- Meshinchi S, Stirewalt DL, Alonzo TA, Zhang Q, Sweetser DA, Woods WG, Bernstein ID, Arceci RJ, Radich JP. Activating mutations of RTK/ras signal transduction pathway in pediatric acute myeloid leukemia. Blood. 2003;102:1474–9. doi: 10.1182/blood-2003-01-0137. [DOI] [PubMed] [Google Scholar]
- Müller CI, Miller CW, Hofman WK, Gross ME, Walsh CS, Kawamata N, Luong QT, Koeffler HP. Rare mutations of the PIK3CA gene in malignancies of the hematopoietic system as well as endometrium, ovary, prostate and osteosarcomas, and discovery of a PIK3CA pseudogene. Leukemia Research. 2006 doi: 10.1016/j.leukres.2006.04.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Muraille E, Pesesse X, Kuntz C, Erneux C. Distribution of the src-homology-2-domain-containing inositol 5-phosphatase SHIP-2 in both non-haemopoietic and haemopoietic cells and possible involvement in SHIP-2 in negative signaling of B-cells. Biochem J. 1999;342:697–705. [PMC free article] [PubMed] [Google Scholar]
- Myers C, Gianni L, Zweier J, Muindi J, Sinha BK, Eliot H. Role of iron in adriamycin biochemistry. Fed Proc. 1986;45:2792–7. [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–5. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Saitoh S, Imoto S, Itoh M, Tsutsumi M, Hikiji K, Nakamura H, Matozaki S, Ogawa R. Nakao Y Multiple point mutation of N-ras and K-ras oncogenes in myelodysplastic syndrome and acute myelogenous leukemia. Oncology. 1992;49:114–22. doi: 10.1159/000227023. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Nagai H, Kinoshita T, Uchida T, Hatano S, Murate T, Saito H. Mutational analysis of the PTEN/MMAC1 gene in non-Hodgkin’s lymphoma. Leukemia. 1998;12:1277–80. doi: 10.1038/sj.leu.2401099. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Inokuchi K, Yamaguchi H, Dan K. Abnormalities of p51, p53, FLT3 and N-ras Genes and Their Prognostic Value in Relapsed Acute Myeloid Leukemia. J Nippon Med Sch. 2004;71:270–8. doi: 10.1272/jnms.71.270. [DOI] [PubMed] [Google Scholar]
- Needleman SW, Devine SE, Kraus MH. 12th codon mutation resulting in c-N-ras activation in acute myelogenous leukemia. Leukemia. 1988;2:91–3. [PubMed] [Google Scholar]
- Neubauer A, Greenberg P, Negrin R, Ginzton N, Liu E. Mutations in the Ras protogenes in patients with myelodysplastic syndromes. Leukemia. 1994;8:638–41. [PubMed] [Google Scholar]
- Ninomiya Y, Kato K, Takahashi A, Ueoka Y, Kamikihara T, Arima T, Matsuda T, Kato H, Nishida J, Wake N. K-Ras and H-Ras activation promote distinct consequences on endometrial cell survival. Cancer Res. 2004;64:2759–65. doi: 10.1158/0008-5472.can-3487-2. [DOI] [PubMed] [Google Scholar]
- Norgaard JM, Olesen LH, Hokland P. Changing picture of cellular drug resistance in human leukemia. Review Critical Reviews in Oncology-Hematology. 2004;50:39–49. doi: 10.1016/S1040-8428(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui AL, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–65. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez G, Merino R, Simonian PL, Grillot DA. Regulation of lymphoid apoptosis by Bcl-2 and Bcl- XL. Advances Exp Med Biol. 1996;406:75–82. doi: 10.1007/978-1-4899-0274-0_8. [DOI] [PubMed] [Google Scholar]
- Obexer P, Geiger K, Ambros PF, Meister B, Ausseriechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2006;4 doi: 10.1038/sj.cdd.4402017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Okuda K, Foster R, Griffin JD. Signaling domains of the beta c chain of the GM-CSF/IL-3/IL-5 receptor. Ann N Y Acad Sci. 1999;872:305–13. doi: 10.1111/j.1749-6632.1999.tb08474.x. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–6. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Review Apoptosis. 2004;9:667–76. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LLM, Donner DB. NF-kappaB activation by tumor necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Padua RA, Carter G, Hughes D, Gow J, Farr C, Oscier D, McCormick F, Jacobs A. RAS mutations in myelodysplasia detected by amplification, oligonucleotide hybridization, and transformation. Leukemia. 1988;2:503–10. [PubMed] [Google Scholar]
- Padua RA, Guinn BA, Al-Sabah AI, Smith M, Taylor C, Pettersson T, Ridge S, Carter G, White D, Oscier D, Chevret S, West R. RAS, FMS and p53 mutations and poor clinical outcome in myelodysplasias: a 10-year follow-up. Leukemia. 1998;12:887–92. doi: 10.1038/sj.leu.2401044. [DOI] [PubMed] [Google Scholar]
- Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004;100:657–66. doi: 10.1002/cncr.20026. [DOI] [PubMed] [Google Scholar]
- Parry TE. The non-random distribution of point mutations in leukaemia and myelodysplasia--a possible pointer to their aetiology. Leuk Res. 1997;21:559–74. doi: 10.1016/s0145-2126(97)83221-3. [DOI] [PubMed] [Google Scholar]
- Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 2003;22:375–84. doi: 10.1023/a:1023777013659. [DOI] [PubMed] [Google Scholar]
- Pirollo KF, Hao Z, Rait A, Ho CW, Chang EH. Evidence supporting a signal transduction pathway leading to the radiation-resistant phenotype in human tumor cells. Biochem Biophy Res Com. 1997;230:196–201. doi: 10.1006/bbrc.1996.5922. [DOI] [PubMed] [Google Scholar]
- Plum JA, Milroy R, Kaye SB. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989;49:4435–40. [PubMed] [Google Scholar]
- Polgar O, Bates SE. ABC transporters in the balance: is there a role in multidrug resistance? Biochem Soc Trans. 2005;33(Pt 1):241–5. doi: 10.1042/BST0330241. [DOI] [PubMed] [Google Scholar]
- Ponti C, Gibellini D, Boin F, Melloni E, Manzoli FA, Cocco L, Zauli G, Vitale M. Role of CREB transcription factor in c-fos activation in natural killer cells. Eur J Immunol. 2002;32:3358–65. doi: 10.1002/1521-4141(200212)32:12<3358::AID-IMMU3358>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Miller E, Sable C, Young P, Heidenreich KA, Boxer LM, Reusch JE. Insulin-like growth factor-I induces bcl-2 promoter through the transcription factor c-AMP-response element-binding protein. J Biol Chem. 1999;274:27529–35. doi: 10.1074/jbc.274.39.27529. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasly LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–6. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- Pui CH, Evans WE. Genetic abnormalities and drug resistance in acute lymphoblastic leukemia. Adv Exp Med Biol. 1999;457:383–9. doi: 10.1007/978-1-4615-4811-9_40. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson EM., Jr JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Puxeddu E, Moretti S, Elisei R, Romei C, Pascucci R, Martinelli M, Marino C, Avenia N, Rossi ED, Fadda G, Cavaliere A, Ribacchi R, Falorni A, Pontecorvi A, Pacini F, Pinchera A, Santeusanio F. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–20. doi: 10.1210/jc.2003-031425. [DOI] [PubMed] [Google Scholar]
- Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–23. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science. 2004;303:179–81. doi: 10.1126/science.1092089. [DOI] [PubMed] [Google Scholar]
- Rao P, Mufson RA. A membrane proximal domain of the human interleukin-3 receptor beta c subunit that signals DNA synthesis in NIH 3T3 cells specifically binds a complex of Src and Janus family tyrosine kinases and phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:6886–93. doi: 10.1074/jbc.270.12.6886. [DOI] [PubMed] [Google Scholar]
- Rao RK, Clayton LW. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun. 2002;293:610–16. doi: 10.1016/S0006-291X(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Reifenberger J, Knobbe CB, Sterzinger AA, Blaschke B, Schulte KW, Ruzicka T, Reifenberger G. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. Int J Cancer. 2004;109:377–84. doi: 10.1002/ijc.11722. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Stippec SA, Goldsmith E, White MA, Cobb MH. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol. 1998;21:1141–50. doi: 10.1016/s0960-9822(07)00485-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–32. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Romanelli A, Martin KA, Toker A, Bleinis J. p70 S6 Kinase is regulated by protein kinase Cζ and participates in a phosphoinositide 3-kinase-regulated signaling complex. Mol Cell Biol. 1999;19:2921–2928. doi: 10.1128/mcb.19.4.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of Akt in anti-apoptotic PDGF signaling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–41. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Ross DD. Modulation of drug resistance transporters as a strategy for treating myelodysplastic syndrome. Review Bailliere’s BestPractice in Clinical Haematology. 2004;17:641–51. doi: 10.1016/j.beha.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Ross DD. Novel mechanisms of drug resistance in leukemia. Review Leukemia. 2000;14:467–73. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]
- Sakai A, Thieblemont C, Wellmann A, Jaffe ES, Raffeld M. PTEN gene alterations in lymphoid neoplasms. Blood. 1998;92:3410–5. [PubMed] [Google Scholar]
- Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–44. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signaling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- She QB, Solit DB, Ye Q, O’Reillly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JG, Steelman LS, Lee JT, Knapp SL, Blalock WL, Moye PW, Franklin RA, Pohnert SC, Mirza AM, McMahon M, McCubrey JA. Effects of the RAF>MEK>ERK and PI3K>AKT signal transduction pathways on the abrogation of cytokine-dependence and prevention of apoptosis in hematopoietic cells. Oncogene. 2003;22:2478–92. doi: 10.1038/sj.onc.1206321. [DOI] [PubMed] [Google Scholar]
- Shelton JG, Blalock WL, White ER, Steelman LS, McCubrey JA. Ability of the activated PI3K>Akt oncoproteins to synergize with MEK1 and induce cell cycle progression and abrogate the cytokine-dependence of hematopoietic cells. Cell Cycle. 2004;3:503–12. [PubMed] [Google Scholar]
- Shimada A, Taki T, Tabuchi K, Tawa A, Horibe K, Tsuchida M, Hanada R, Tsukimoto I, Hayashi Y. KIT mutations, and not FLT3 internal tandem duplication, are strongly associated with a poor prognosis in pediatric acute myeloid leukemia with t(8;21): a study of the Japanese Childhood AML Cooperative Study Group. Blood. 2006;107:1806–9. doi: 10.1182/blood-2005-08-3408. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res. 2004;119:139–73. doi: 10.1007/1-4020-7847-1_8. [DOI] [PubMed] [Google Scholar]
- Silberman S, Janulis M, Schultz RM. Characterization of downstream Ras signals that induce alternative protease-dependent invasive phenotypes. J Biol Chem. 1997;272:5927–35. doi: 10.1074/jbc.272.9.5927. [DOI] [PubMed] [Google Scholar]
- Simon C, Juarez J, Nicolson GL, Boyd D. Effect of PD 098059, a specific inhibitor of mitogen-activated protein kinase kinase, on urokinase expression and in vitro invasion. Cancer Res. 1996;56:5369–74. [PubMed] [Google Scholar]
- Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000;207:77–86. doi: 10.1023/a:1007094214460. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–50. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–37. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–2. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN: mutation, marker and potential target for therapeutic intervention. Expert Opinion Therapeutic Targets. 2004:8537–50. doi: 10.1517/14728222.8.6.537. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf>MEK>ERK, PI3K>Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, Radich JP. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–95. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–65. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, Grandin W, Lebwohl D, Wang Y, Cohen P, Fox EA, Neuberg D, Clark J, Gilliland DG, Griffin JD. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- Stone RM, O’Donnell MR, Sekeres MA. Acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2004;1:98–117. doi: 10.1182/asheducation-2004.1.98. [DOI] [PubMed] [Google Scholar]
- Sun H, King AJ, Diaz HB, Marshall MS. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr Biol. 2000;10:281–4. doi: 10.1016/s0960-9822(00)00359-6. [DOI] [PubMed] [Google Scholar]
- Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki Y, Yoshizumi M, Kagami S, Koyama AH, Taketani Y, Houchi H, Tsuchiya K, Takeda E, Tamaki T. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: potential role in cell survival following oxidative insults. J Biol Chem. 2002;277:9614–21. doi: 10.1074/jbc.M111790200. [DOI] [PubMed] [Google Scholar]
- Tallman MS, Gillliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–63. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- Tallman MS. New agents for the treatment of acute myeloid leukemia. (Review) Bailliere’s Best Practice in Clinical Haematology. 2006;19:311–20. doi: 10.1016/j.beha.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Tallmann MS. Relevance of pathologic classifications and diagnosis of acute myeloid leukemia to clinical trials and clinical practice. Cancer Treat Res. 2004;21:45–67. doi: 10.1007/1-4020-7920-6_3. [DOI] [PubMed] [Google Scholar]
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes, O’Brien S, Nicaise C, leickardt E, Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP, Sawyers CL. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- Tauchi T, Boswell HS, Leibowitz D, Broxmeyer HE. Coupling between p210bcr-abl and Shc and Grb2 adaptor proteins in hematopoietic cells permits growth factor receptor-independent link to ras activation pathway. J Exp Med. 1994;179:167–75. doi: 10.1084/jem.179.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V, Wong M, Brandts C, Reilly L, Dean NM, Cowsert LM, Moodie S, Stokoe D. 5′phospholipid phosphatase SHIP-2 causes protein kinase B inactivation and cell cycle arrest in glioblastoma cells. Mol Cell Biol. 2000;20:6860–71. doi: 10.1128/mcb.20.18.6860-6871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodori E, Dei S, Martelli C, Scapeechi S, Gualtieri F. The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR) Curr Drug Targets. 2006;7:893–909. doi: 10.2174/138945006777709520. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Tymms MJ, McKinlay LH, Shannon MF, Seth A, Kola I. ETS1, NFkappaB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene. 1997;23:2845–55. doi: 10.1038/sj.onc.1201125. [DOI] [PubMed] [Google Scholar]
- Tournier C, Thomas G, Pierre J, Jacquemin C, Pierre M, Saunier B. Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinase) Eur J Biochem. 1997;244:587–95. doi: 10.1111/j.1432-1033.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- Traxler P. Tyrosine kinases as targets in cancer therapy-successes and failures. Expert Opin Ther Targets. 2003;7:215–34. doi: 10.1517/14728222.7.2.215. [DOI] [PubMed] [Google Scholar]
- Tresini M, Lorenzini A, Frisoni L, Allen RG, Cristofalo VJ. Lack of Elk-1 phosphorylation and dysregulation of the extracellular regulated kinase signaling pathway in senescent human fibroblast. Exp Cell Res. 2001;269:287–300. doi: 10.1006/excr.2001.5334. [DOI] [PubMed] [Google Scholar]
- Troppmair J, Rapp UR. Raf and the road to cell survival: a tale of bad spells, ring bearers and detours. Biochem Pharmacol. 2003;66:1341–5. doi: 10.1016/s0006-2952(03)00483-0. [DOI] [PubMed] [Google Scholar]
- Troussard AA, Mawji NM, Ong C, Mui A, St -Arnaud R, Dedhar S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem. 2003;278:22374–8. doi: 10.1074/jbc.M303083200. [DOI] [PubMed] [Google Scholar]
- Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, Haga N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Review Cancer Science. 2003;94:15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–99. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal. 2003;5:723–30. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- van den Heuvel-Eibrink MM, Sonneveld P, Pieters R. The prognostic significance of membrane transport-associated multidrug resistance (MDR) protein in leukemia. Review International Journal of Clinical Pharmacology & Therapeutics. 2000;38:94–110. doi: 10.5414/cpp38094. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Anker P, Maurice P, Lyautey J, Lederrey C, Stroun M. Point mutations of the N-ras gene in the blood plasma DNA of patients with myelodysplastic syndrome or acute myelogenous leukaemia. Br J Haematol. 1994;86:774–9. doi: 10.1111/j.1365-2141.1994.tb04828.x. [DOI] [PubMed] [Google Scholar]
- Vogt PK, Bader AG, Kang S. Phosphoinositide 3-kinase: from viral oncoprotein to drug target. Virology. 2006;344:131–8. doi: 10.1016/j.virol.2005.09.027. [DOI] [PubMed] [Google Scholar]
- von Gise A, Lorenz P, Wellbrock C, Hemmings B, Berberich-Siebelt F, Rapp UR, Troppmair J. Apoptosis suppression by Raf-1 and MEK1 requires MEK and phosphatidylinositol 3-kinase dependent signals. Mol Cell Biol. 2001;21:2324–36. doi: 10.1128/MCB.21.7.2324-2336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R. Cancer Genome Project. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- Wang CY, Bassuk AG, Boise LH, Thompson CB, Bravo R, Leiden JM. Activation of the granulocyte-macrophage colony-stimulating factor promoter in T cells requires cooperative binding of Elf-1 and AP-1 transcription factors. Mol Cell Biol. 1994;14:1153–9. doi: 10.1128/mcb.14.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–9. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XT, McCullough KD, Wang XJ, Carpenter G, Holbrook NJ. Oxidative stress-induced phospholipase C-gamma 1 activation enhances cell survival. J Biol Chem. 2001;276:28364–71. doi: 10.1074/jbc.M102693200. [DOI] [PubMed] [Google Scholar]
- Weinstein-Oppenheimer CR, Henríquez-Roldán CF, Davis J, Navolanic PM, Steelman LS, Franklin RA, Robinson PJ, Saleh OA, McMahon M, McCubrey JA. Role of the Raf Signal Transduction Cascade in the In Vitro Resistance to the Anticancer Drug Doxorubicin. Clinical Cancer Research. 2001;7:2892–2907. [PubMed] [Google Scholar]
- Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Review Nature Reviews Molecular Cell Biology. 2004;5:875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280:10491–500. doi: 10.1074/jbc.M412819200. [DOI] [PubMed] [Google Scholar]
- Weston CR, Balmanno K, Chalmers C, Hadfield K, Molton SA, Ley R, Wagner EF, Cook SJ. Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene. 2003;22:1281–93. doi: 10.1038/sj.onc.1206261. [DOI] [PubMed] [Google Scholar]
- Whisler RL, Goyette MA, Grants IS, Newhouse YG. Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatases in Jurkat T cells. Arch Biochem Biophys. 1995;319:23–35. doi: 10.1006/abbi.1995.1263. [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes & Development. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BE, Mochon E, Boxer LM. Induction of Bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol. 1996;16:5546–56. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002;282:C926–34. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the Ras-MAPK pathway to gene activation by Rsk2, a growth factor regulated CREB kinase. Science. 1996;273:959–63. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Xu S, Robbins D, Frost J, Dang A, Lange-Carter C, Cobb MH. MEKK1 phosphorylates MEK1 and MEK2 but does not cause activation of mitogen-activated protein kinase. Proc Nat Acad Sci USA. 1995;92:6808–12. doi: 10.1073/pnas.92.15.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. 2003;63:4561–7. [PubMed] [Google Scholar]
- Xu Z, Ma DZ, Wang LY, Su JM, Zha XL. Transforming growth factor-beta1 stimulated protein kinase B serine-473 and focal adhesion kinase tyrosine phosphorylation dependent on cell adhesion in human hepatocellular carcinoma SMMC-7721 cells. Biochem Biophys Res Commun. 2003;312:388–96. doi: 10.1016/j.bbrc.2003.10.130. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Wang HG. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene. 2001;20:7779–86. doi: 10.1038/sj.onc.1204984. [DOI] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a hetro-dimeric partner for Bcl-xL and Bcl-2 displaces Bax and promotes cell death. Cell. 1995;80:285–91. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yokota S, Nakao M, Horiike S, Seriu T, Iwai T, Kaneko H, Azuma H, Oka T, Takeda T, Watanabe A, Kikuta A, Asami K, Sekine I, Matsushita T, Tsuhciya T, Mimaya J, Koizumi S, Miyake M, Nishikawa K, Takaue Y, Kawano Y, Iwai A, Ishida Y, Matsumoto K, Fujimoto T. Mutational analysis of the N-ras gene in acute lymphoblastic leukemia: a study of 125 Japanese pediatric cases. Int J Hematol. 1998;67:379–87. doi: 10.1016/s0925-5710(98)00015-2. [DOI] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Eriacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–63. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biopyhys Res Commun. 2005;331:851–8. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Zebisch A, Staber PB, Delavar A, Bodner C, Hiden K, Fischereder K, Janakiraman M, Linkesch W, Auner HW, Emberger W, Windpassinger C, Schimek MG, Hoefler G, Troppmair J, Sill H. Two transforming C-RAF germ-line mutations identified in patients with therapy-related acute myeloid leukemia. Cancer Research. 2006;66:3401–8. doi: 10.1158/0008-5472.CAN-05-0115. [DOI] [PubMed] [Google Scholar]
- Zebisch A, Troppmair J. Back to the roots: the remarkable RAF oncogene story. Review Cellular & Molecular Life Sciences. 2006;63:1314–30. doi: 10.1007/s00018-006-6005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BH, Tang E, Zhu T, Greenberg M, Vojtek A, Guan KL. Serum and glucocorticoid-inducible kinase SGK phosphorylates and negatively regulates B-Raf. J Biol Chem. 2001;276:31620–6. doi: 10.1074/jbc.M102808200. [DOI] [PubMed] [Google Scholar]
- Zhao S, Konopleva M, Cabreira-Hansen M, Xie Z, Hu W, Milella M, Estrov Z, Mills GB, Andreeff M. Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD and promotes apoptosis in myeloid leukemias. Leukemia. 2004;18:267–75. doi: 10.1038/sj.leu.2403220. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Hung MC. Novel targets of Akt, p21(Cip1.WAF1), and MDM2. Semin Oncol. 2002;29(3 Suppl 11):62–70. doi: 10.1053/sonc.2002.34057. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (Protein Kinase B) Science. 1999;286:1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- Zou Y, Komuro I, Yamazaki T, Aikawa R, Kudoh S, Shiojima I, Hiroi Y, Mizuno T, Yazaki Y. Protein kinase C, but not tyrosine kinases or Ras, plays a critical role in angiotensin II-induced activation of Raf-1 kinase and extracellular signal-regulated protein kinases in cardiac myocytes. J Biol Chem. 1996;271:33592–7. doi: 10.1074/jbc.271.52.33592. [DOI] [PubMed] [Google Scholar]