Abstract
The paradigm of effector T helper cell differentiation into either Th1 or Th2 lineages has been profoundly shaken by the discovery of T cells that secrete IL-17 and other inflammatory cytokines. This subset, referred to as Th17, is centrally involved in autoimmune disease and is important in host defense at mucosal surfaces. In mouse, a series of cytokines, including IL-6, IL-21, IL-23, and TGF-β, function sequentially or synergistically to induce the Th17 lineage. Other cytokines, including IL-2, IL-4, IFNγ, and IL-27, inhibit differentiation of this lineage. Here we review how the nuclear orphan receptor RORγt functions to coordinate the diverse cytokine-induced signals and thus control Th17 cell differentiation.
Keywords: Th17, IL-17, RORγt, mucosal immunity, regulatory T cells
1. Introduction
T helper (Th) cells with diverse effector functions differentiate from naïve CD4+ T cells upon stimulation by antigen in the presence of different cytokines produced by cells of the innate immune system. Until recently, two major cell subsets, Th1 and Th2, were known to provide effector responses to intracellular and extracellular pathogens, respectively, through the production of specific cytokines. Th1 cells produce interferon-γ (IFNγ) and lymphotoxin-α (LTα) while Th2 cells produce interleukin-4 (IL-4), IL-5, IL-13, and other cytokines [1]. Th1 cells, which require IL-12 for their differentiation, had been thought to mediate a series of autoimmune conditions. However, recent studies have clearly shown that T helper lymphocytes that require the IL-12 family member IL-23 to differentiate and secrete pro-inflammatory cytokines, rather than Th1 cells, are major mediators of inflammatory responses in most of these “Th1” autoimmune diseases [2–6]. These cells produce IL-17, IL-17F, IL-21, and IL-22, and are now recognized as belonging to a distinct effector cell subset, the Th17 cells [7]. Our understanding of the normal functions of Th17 cells remains sketchy, although it is thought that they have key roles in providing immunity to various bacteria and fungi, particularly at mucosal surfaces.
In this review, we discuss our current understanding of the regulation of Th17 cell differentiation, with a focus on the cytokine and transcription factor requirements both in vivo and in vitro. We also describe the distribution and potential functions of Th17 cells at sites within the body that are in contact with commensal microorganisms, and focus on the relationship between Foxp3+ regulatory T cells and Th17 cells in such tissues.
2. Th17-inducing cytokine environment
Although the cytokine IL-17 had been known for some time to have potent proinflammatory activity [8], appreciation of its role in inflammatory T cell-mediated autoimmune diseases has come only recently. The key finding in this field was the observation that mice deficient for the p19 subunit of IL-23, a cytokine that shares its other subunit (p40) with IL-12, are resistant to induction of experimental autoimmune encephalomyelitis (EAE) due to lack of IL-17-producing T cells [2,3]. Other studies have shown that IL-23 and, by extension, Th17 cells, are required for induction of a variety of autoimmune diseases in mice, and also for the ability of mice to clear bacterial infections of the intestine and the airways [4–6].
Search for cytokines that stimulate de novo Th17 cell differentiation has been a major focus in the field. Although in vivo studies indicated a key role for IL-23 in Th17 cell induction, IL-23 was shown to be dispensable for in vitro Th17 cell differentiation. Instead, IL-6 and TGF-β were found sufficient for the induction of IL-17 and IL-17F expression in TCR-activated naïve CD4+ T cells [9–11]. Previous studies had shown that IL-23 contributes to Th17 cell differentiation in bulk cultures that contained activated dendritic cells (DCs) that, presumably, served as a source of the other cytokines. IL-23 was therefore proposed to function in the expansion or survival of committed Th17 cells.
Studies with diverse strains of mutant mice have confirmed that IL-6, TGF-β, and IL-23 have essential roles in the differentiation of Th17 cells. In IL-6-deficient mice, Th17 cells were substantially reduced in the small intestinal lamina propria (LP), a tissue in which they are normally particularly abundant among T cells [12] (see below). In mice that express a dominant negative form of the TGF-β receptor, induction of EAE was abrogated, consistent with a defect in the differentiation of Th17 cells [13]. Finally, in IL-23 p19-deficient mice, EAE induction was abrogated and the mice became susceptible to intestinal infection with Citrobacter rodentium [11]; this was observed despite early induction of Th17 cells after infection, as may be expected if IL-23 is not essential in the early phase of Th17 cell differentiation.
3. RORγt: a master regulator of the Th17 cell lineage?
Two independent approaches led to the identification of the retinoic acid-related orphan receptor (ROR)γt as the key transcription factor in the differentiation program of Th17 cells. Genome-wide expression profiling of antigen-stimulated splenocytes revealed up-regulation of RORγt mRNA in response to IL-23 and, in a mouse strain engineered such that RORγt-expressing cells also expressed green fluorescent protein (GFP), most GFP+ T cells, and only a few of the GFP− T cells, expressed intracellular IL-17. These experiments thus first established a correlation between the Th17 cell phenotype and expression of RORγt. Subsequent experiments, described below, demonstrated that RORγt is necessary for differentiation of the Th17 lineage and also sufficient to direct the expression of the hallmark cytokines of this lineage.
RORγt belongs to the nuclear hormone receptor superfamily, the largest family of transcription factors in metazoans [14]. Murine RORγt is encoded by the Rorc gene that is located on chromosome 3. The Rorc gene encodes two isoforms, RORγ and RORγt, with distinct transcriptional start sites. RORγ-specific exons 1 and 2 and RORγt-specific exon 1 are spliced to the common exons 3 to 11, resulting in two proteins that differ only at their N-terminal ends [15–18]. Unlike RORγ, which is expressed in many tissues such as brain, heart, kidney, liver, lung, and muscle, RORγt has been detected exclusively in cells of the lymphoid lineage [19]. RORγt is a molecule that was originally discovered to regulate gene expression during the development of T cells in the thymus, of lymphoid tissue inducer cells (LTi) that are required for the formation of secondary lymphoid organs, such as lymph nodes (LNs) and Peyer’s patches (PPs), and of LTi-like cells in crytopatches (CPs) and isolated lymphoid follicles (ILFs) within the adult intestinal LP [20–22]. Absence of RORγt resulted in early apoptosis of CD4+CD8+ thymocytes and their premature entry into S-phase of the cell cycle. Lymph nodes, PPs, ILFs and CPs also failed to develop in RORγt-deficient mice [20,21].
The more recently appreciated role of RORγt in the differentiation of Th cells became clear only after IL-17 was identified as a lineage-specific T cell cytokine. In mice heterozygous for a knock-in of GFP in place of the RORγt open reading frame, the reporter protein was expressed constitutively in about 10% of the CD4+ T cells in the small intestinal LP [12]. Most of these cells co-expressed the Th17 cytokines, IL-17, IL-17F, and IL-22. In contrast, IL-17 was detected in only a very small fraction of the GFP-cells from the LP. In mice deficient for RORγt, the number of Th17 cells in the LP was markedly reduced. Similarly, under Th17 polarizing conditions in vitro, IL-17 expression was greatly decreased if the CD4+ T cells lacked RORγt. Forced expression of RORγt in naïve CD4+ T cells was sufficient to induce IL-17, IL-17F and IL-22. It is notable that in the absence of RORγt there were residual IL-17+ cells both in the LP and after in vitro polarization. However, the numbers were reduced at least 10-fold in vivo and 50 fold in vitro compared to wild-type cells [12]. Expression of IL-17F, however, was decreased only about 2-fold in vitro in RORγyt-deficient cells, suggesting that IL-17F expression is less dependent on RORγt than IL-17 expression (LZ and DRL, unpublished). These data collectively demonstrate that RORγt is necessary for differentiation of the Th17 lineage and also sufficient for specifying at least part of the lineage program. There is only a partial overlap between genes regulated upon forced expression of RORγt and those regulated following cytokine-induced polarization to the Th17 lineage (LZ, III, and DRL, unpublished). Thus, RORγt is unlikely to serve as a “master regulator” that fully directs the functional differentiation of the Th17 lineage. Other transcription factors that are required for differentiation of the Th17 lineage, as described below, may also contribute to expression of genes that are not regulated by RORγt alone.
The genes that are directly regulated by RORγt have not yet been identified, although conserved ROR responsive elements (ROREs), with the consensus core motif AGGTCA preceded by an A/T-rich sequence [23], have been identified at the IL-17 promoter [12]. Chromatin immunoprecipitation studies will be required to determine whether Il17 and other genes expressed or repressed in the Th17 lineage are direct targets of RORγt.
4. Sequential cytokine function in Th17 cell differentiation: Roles of IL-21 and IL-23
Recent studies have begun to shed some light on why IL-23 is required in vivo, but not in vitro, for Th17 cell differentiation. The function of IL-23 appears to be dependent, at least in part, on another cytokine, IL-21, that is mainly produced by activated CD4+ T cells and activates, through a receptor that contains the common γ chain (γc), the STAT1/STAT3 pathway [24,25]. The mRNA for IL-21 was identified as one of the most highly induced transcripts in expression profile analyses of TCR-stimulated naïve CD4+ T cells stimulated with IL-6 or transduced with a retrovirus expressing RORγt [26,27]. IL-21 further induced its own expression in an autocrine manner. The combination of IL-21 and TGF-β directed the differentiation of TCR-stimulated naïve CD4+ T cells into the Th17 lineage independently of IL-6 [26–28].
The IL-23 receptor (IL-23R), which forms dimers with the IL-12Rβ1 subunit shared by IL-12 and IL-23, is expressed in LP T cells only if IL-6 is present [12]. Like IL-21, IL-23R mRNA is induced after treatment of TCR-activated T cells with IL-6 or IL-21 and is also upregulated by forced expression of RORγt [26,29]. IL-23R expression was abrogated in IL-21R-deficient cells, suggesting that IL-21 is required as an intermediate in IL-6-mediated induction of IL-23R. Accordingly, IL-23R expression was also much lower in RORγt-deficient cells. We demonstrated that, once IL-23R is expressed in T cells, IL-23 can bind to its receptor and thus bypass the requirement for IL-6 to induce IL-17 in the presence of TGF-β [26]. Thus, IL-6 initiates the program that conditions the activated naïve T cell to become receptive to additional cytokine signals required for differentiation towards the Th17 cell lineage (Figure 1). It induces IL-21, which acts in a positive feedback loop to induce more IL-21 expression. This process appears to be independent of RORγt, but requires activation of STAT3 by both the IL-6 and IL-21 receptors. IL-21, in turn, activates expression of IL-23R, a process that is dependent on both STAT3 and RORγt. IL-23, like IL-21 and IL-6, can then synergize with TGF-β-initiated signals to induce transcription of IL-17 and the other Th17 lineage cytokines.
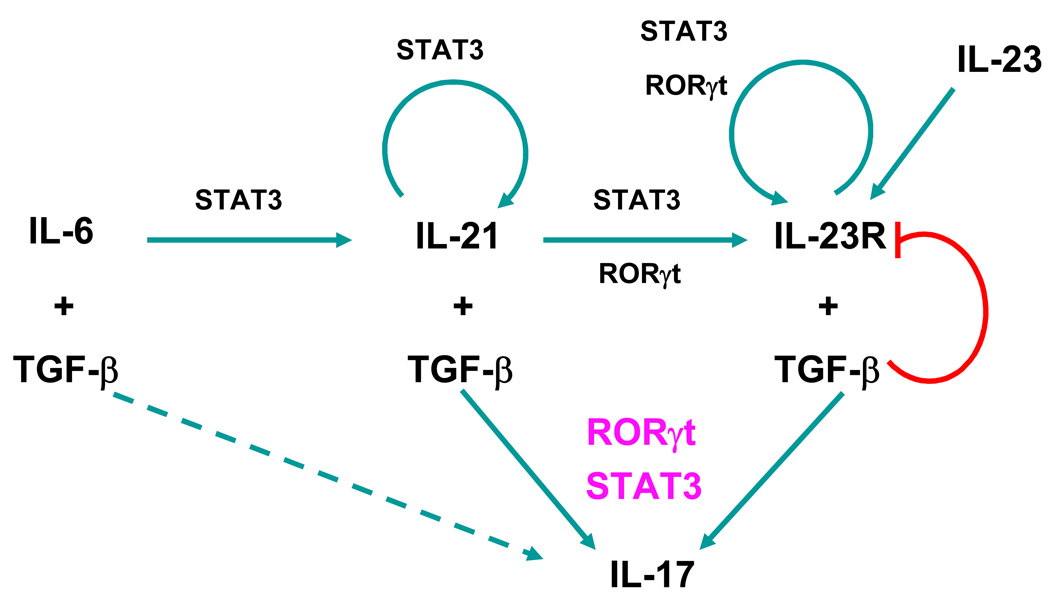
Figure 1. Sequential roles of cytokines in the differentiation of the Th17 cell lineage.
IL-6 induces IL-21, which in turn induces its own transcription in a STAT3-dependent manner. IL-23R expression induced by IL-21 (and IL-23) is dependent on both RORγt and STAT3. IL-21R and IL-23R signaling, in concert with TGF-β, program the naïve T cells into the Th17 lineage independently of IL-6. Full differentiation of Th17 cells requires the cooperative action of RORγt and STAT3. The induction of IL-17 by IL-6 in the absence of IL-21/IL-21R signaling is presented as a dashed arrow.
Th17 cell differentiation, similar to that of Th1 and Th2 cells, is therefore facilitated by several positive feedback loops (Figure 1): (1) The IL-21 loop. IL-21 induces its own gene transcription in a STAT3-dependent manner [26]. IL-21 signaling also up-regulates RORγt expression, which in turn induces more IL-21 and thus contributes to the positive autocrine loop. It is noteworthy, however, that although RORγt is required for IL-23R transcription, it is not necessary for IL-6/IL-21-induced IL-21 expression. (2) The IL-23R loop. Similar to the IL-21 loop, IL-23/IL-23R signaling induces RORγt, which further up-regulates IL-23R expression.
We have additionally observed that TGF-β has a profound influence on IL-23R expression in a concentration-dependent manner (LZ et al. submitted). Low concentrations of TGF-β (pico-gram range) synergize with IL-6 and IL-21 to enhance expression of IL-23R mRNA and IL-17 expression, but high concentrations inhibit IL-23R expression through a mechanism that appears to be mediated by the transcription factor Foxp3 (see below). High levels of active TGF-β thus appear to favor differentiation of Treg cells rather than Th17 cells, but it is not yet known whether such rules apply in vivo. When T cells encounter antigen in an environment with concentrations of IL-6 and TGF-β that favor Th17 cell differentiation, IL-23 produced by DCs activated by distinct microorganisms, e.g. β-glucan-containing fungi [30], would then be able to further enhance and, potentially, stabilize the Th17 cell lineage program.
The studies described above explain why IL-23 was initially found to have little effect on in vitro Th17 cell differentiation [9]. At the concentrations of TGF-β typically used in the in vitro culture systems, expression of IL-23R was inhibited, resulting in loss of responsiveness to IL-23. In vivo, inflammatory cytokines (IL-6/IL-21) are sufficient to induce Th17 cell differentiation, but IL-23/IL-23R signaling appears to be required to maintain or expand these cells and thus control infections or mediate autoimmune disease. IL-23 may provide proliferative or survival signals to cells that have already been directed towards the Th17 lineage, but there are no experimental data to firmly support this hypothesis. An alternative, but not mutually exclusive, explanation is that IL-23R signaling leads to permanent epigenetic marks at the IL-17/IL-17F or IL-22 loci, allowing for stable or heritable transcription of Th17 cytokine genes. Therefore, in the absence of IL-23, early transcription of Th17 cytokine genes would become extinguished and the cells would have the ability to differentiate into other effector lineages.
All of the cytokine pathways involved in Th17 cell differentiation result in up-regulation of RORγt expression. The IL-6/IL-21/IL-23 signaling pathways additionally activate STAT3, which binds directly to the IL-17 and IL-21 promoters [31,32]. When cells were transduced with both RORγt and an active form of STAT3 (STAT3C), there were more IL-17+ cells and a higher level of IL-17 expression per cell, consistent with cooperation of RORγt and STAT3 at transcriptional target sites [26]. A better understanding of the transcriptional regulation in the Th17 cell program awaits chromatin immunoprecipitation studies with these transcription factors.
Chromatin regulation represents an important mechanism of T helper cell differentiation. Active transcription requires an “open” chromatin conformation, allowing subsequent binding of transcriptional activators and recruitment of general transcriptional machinery to critical DNA elements. Until now, the Th1 (IFNγ) and Th2 (IL-4/IL-5/IL-13/Rad50) cytokine gene loci have been the model systems for studying chromatin regulation during T cell differentiation upon external stimulation [33–35]. Both gene promoters and conserved noncoding sequences (CNS) undergo extensive chromatin remodeling and epigenetic changes mediated by covalent histone modification when naive T cells differentiate into Th1 or Th2 cells. These epigenetic changes provide a mechanism whereby cytokine gene transcription is stabilized in a polarized cell lineage. There may be additional trans-chromosomal interactions that mediate activation or repression of cytokine gene loci, perhaps by recruitment of these loci to “transcription factories” [35]. Compared to the substantial knowledge regarding epigenetic modifications and chromosomal interactions at the Th1 and Th2 loci, there is little known about the role of chromatin regulation in Th17 cell differentiation. The genes encoding the signature cytokines of the Th17 lineage (IL-17 and IL-17F) are clustered together on murine chromosome 1, suggesting that they may be co-regulated. In contrast, IL-22, another Th17 cytokine, is encoded on murine chromosome 10. Under Th17-polarizing conditions, histone H3 at the promoter regions of both IL-17 and IL-17F genes becomes hyperacetylated and tri-methylated at Lysine 4. Furthermore, several computationally identified CNS elements in the IL-17-IL-17F locus in polarized Th17 cells become hyperacetylated in histone H3 [36]. These data suggest that chromatin regulation may play an important role in Th17 lineage differentiation. A better understanding of chromatin contribution will require a detailed analysis of the cytokine loci, such as using DNase I hypersensitivity and restriction enzyme accessibility assays.
5. Cytokine-mediated inhibition of the Th17 cell differentiation program
Th17 cells can develop independently of the transcription factors STAT1, T-bet, STAT4 and STAT6, indicating that they represent a distinct T helper cell lineage [37,38]. The Th1 cytokine IFNγ and Th2 cytokines (e.g. IL-4) were the first shown to inhibit the differentiation of the Th17 lineage [39]. Neutralization of IFNγ and IL-4 increased IL-17 expression in cell culture systems. Mice lacking T-bet, a T-box transcription factor required for Th1 cell differentiation and IFNγ production, showed enhanced development of Th17 cells in vitro, suggesting that T-bet may actively suppress IL-17 expression [38,40–43]. Indeed, we observed that forced expression of T-bet in naïve CD4+ T cells prevented IL-17 expression in Th17 polarizing conditions (LZ and DRL, unpublished). T cells isolated from mice overexpressing c-Maf, a Th2 transcription factor important for IL-4 transcription, produce much less IL-17 [38]. Recently, IL-25 (IL-17E), a member of the IL-17 cytokine family, was shown to inhibit IL-17 expression through promoting Th2 differentiation [44,45]. Once again, these reports suggest a cross-regulation in Th1, Th2 and Th17 cell development. However, precise mechanisms by which Th1 and Th2 transcription factors inhibit IL-17 transcription remain to be determined. Moreover, in vivo, many effector cells produce cytokines associated with different lineages. Thus, many of the T helper cells in the central nervous system of mice with experimental autoimmune encephalomyelitis express both IFNγ and IL-17, as do many effector/memory cells in human peripheral blood and human T cells treated in vitro with IL-1β plus IL-6 (see below). These observations suggest that there must be mechanisms that overcome T-bet-mediated inhibition of IL-17 gene expression.
IL-27, a heterodimeric cytokine that belongs to the IL-12 family, was recently shown to have anti-inflammatory activity through its restraint of Th17 cell differentiation [46,47]. IL-27 activates STAT1 and induces T-bet [48,49]. Its suppressive activity was not dependent on either T-bet or IFNγ, but did require STAT1. These results suggest that IL-27 does not suppress Th17 cell development simply by diverting naïve T cells to the Th1 pathway. IL-27 prevented expression of RORγt in response to TGF-β plus IL-6, and IL-27 inhibition of the Th17 lineage was relieved by forced expression of RORγt (LZ and DRL, unpublished). Thus, one mechanism by which IL-27 inhibits Th17 differentiation is by inhibiting induction of RORγt.
Recently, IL-2, a growth factor for most T cells, was shown to inhibit Th17 cell differentiation through a STAT5-mediated pathway [50]. STAT5, which is activated by γc-activating cytokines, including IL-2, suppresses IL-17 expression by direct binding to the IL-17 promoter. The addition of IL-2 resulted in a marked reduction in the expression of RORγt and enhanced TGF-β-induced Foxp3 expression, suggesting that IL-2 might inhibit Th17 differentiation by influencing the RORγt-Foxp3 balance (see below). The positive and negative roles of the diverse cytokines in the differentiation of Th17 cells are summarized in Figure 2.
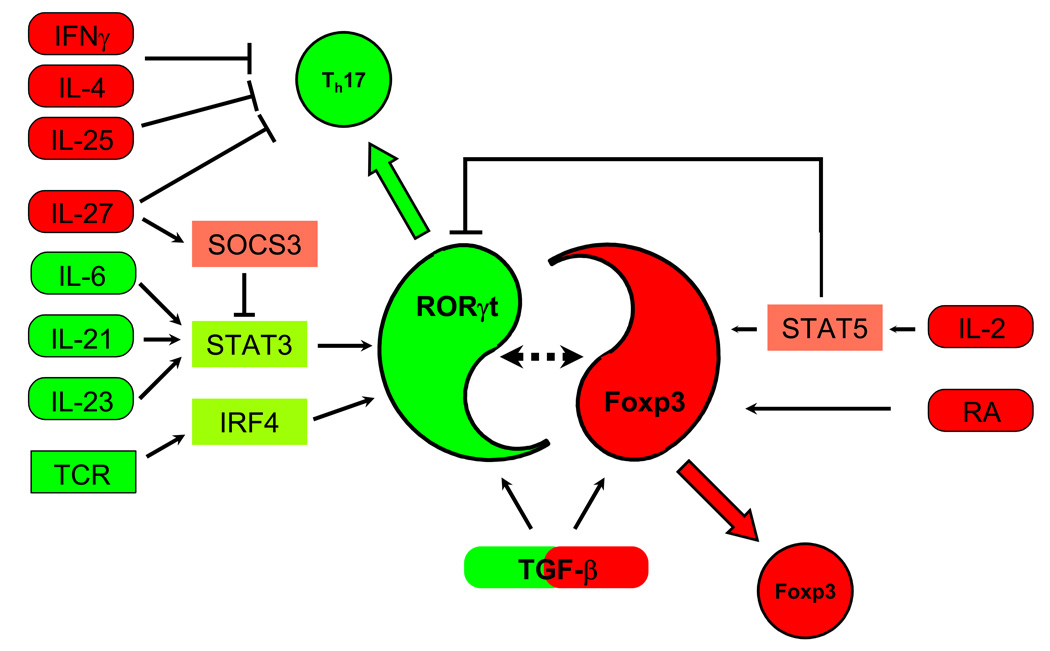
Figure 2. Cytokine pathways and transcription factors in murine Th17 and Treg cell differentiation.
RORγt and Foxp3 are central factors in Th17 and Treg cell differentiation, respectively. Th17 inducing cytokines (in green) induce RORγt through STAT3 (IL-6, IL-21, and IL-23) and IRF4 (induced by TCR activation). However, none of the RORγt-inducing cytokines can induce Th17 differentiation in the absence of TGF-β signaling. Th17 suppressing cytokines (in red) act in several ways. IL-2 acts through STAT5, and IL-27 possibly through SOCS3 and STAT3, inhibiting RORγt-upregulation. Retinoic acid (RA), which promotes Treg development, and TGF-β by itself, which induces Foxp3 expression, decrease Th17 differentiation. In addition, Th1 and Th2 inducing cytokines, such as IL-4, IFNγ, and IL-25 also inhibit Th17 differentiation by unknown mechanisms. TGF-β thus plays a dual role by being absolutely required for Th17 cell differentiation and by also promoting Treg cell development.
6. Regulation of Th17 cell differentiation by a balance of RORγt and Foxp3
TGF-β alone induces the expression of both Foxp3 and RORγt in TCR-stimulated naïve CD4+ T cells [26]. Despite its induction of RORγt, TGF-β is unable to initiate Th17 differentiation in vitro unless pro-inflammatory cytokines, such as IL-6 or IL-21, are also present. This appears to be due to a Foxp3-mediated inhibition of the activity of RORγt, resulting in abrogation of IL-17 and IL-23R expression in the absence of the proinflammatory cytokines (LZ et al. submitted). In humans, two alternatively-spliced isoforms of Foxp3 are expressed [51]. Only the full-length isoform, but not that lacking the sequence encoded by exon 2, was found to co-precipitate with RORγt and to inhibit its function, suggesting that Foxp3 inhibits RORγt-directed IL-17 expression through binding to RORγt via the sequence encoded by exon 2. Furthermore, mutations that abolished Foxp3 DNA binding also blunted its ability to inhibit IL-17 expression, suggesting that Foxp3 inhibits expression of RORγt targets by directly binding to the transcription factor and by also acting upon the target gene (LZ et al. submitted).
In the presence of proinflammatory cytokines, TGF-β-induced Foxp3 expression is greatly reduced and the RORγt level is further up-regulated, thereby favoring Th17 cell differentiation [10,26] (Figure 3). Intriguingly, when Foxp3 was forcibly expressed in T cells along with RORγt, treatment with IL-6 or IL-21 abrogated its inhibition of RORγt-directed IL-17 expression (LZ et al. submitted). We hypothesize that IL-6R/IL-21R signaling results either in post-translational modification of RORγt or Foxp3 and/or alters the association of these proteins with other molecules in a transcriptional complex. Therefore, both the blunting of the Foxp3 inhibitory function and the down-regulation of Foxp3 expression contribute to the emergence of Th17 cells in the appropriate cytokine milieu (Figure 2 and Figure 3).
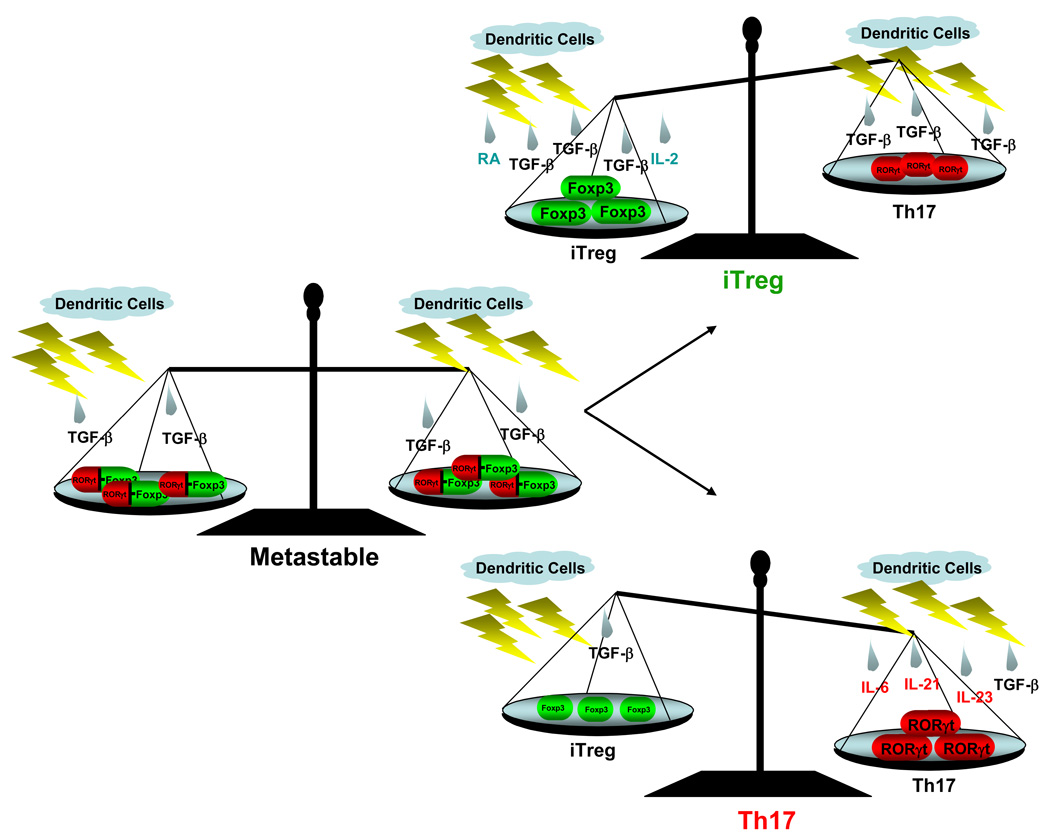
Figure 3. The cytokine milieu influences the balance of differentiating inducible Treg and Th17 cells.
Inductive effects of TGF-β alone (left), with IL-2 and RA (top), and with IL-6, IL-21 and IL-23 (bottom). The left panel represents a hypothetical metastable state, induced by TGF-β, which would allow the cell to differentiate into either the Th17 or Treg lineage depending on the associated cytokines. At the metastable state, TGF-β is able to induce both RORγt and Foxp3, however, RORγt activity is inhibited by Foxp3 through an interaction of the two proteins, which ensures no IL-17 expression. Upon continued stimulation with TGF-β in the absence of proinflammatory cytokines and in the presence of IL-2 and RA, cells will differentiate into the iTreg lineage. On the other hand, if proinflammatory cytokines are present, Foxp3 expression/activity will diminish and RORγt expression will increase, tipping the “balance” towards the Th17 lineage.
7. IRF4: another transcription factor required for Th17 cell differentiation
Most recently, IRF4, a transcription factor previously shown to be important for Th2 cell differentiation, was discovered to also be essential for Th17 cell differentiation [52]. IRF4-deficient mice were protected from EAE and T cells from these animals failed to differentiate into Th17 cells. Furthermore, IRF4-deficient T cells had less RORγt expression and more Foxp3 expression, again highlighting the importance of the RORyt-Foxp3 balance in Th17 cell development. RORγt induction was impaired in IRF4-deficient T cells, but forced expression of RORγt could partially restore induction of IL-17. These data appear to place IRF4 upstream of RORγt. However, it is likely that a complex transcriptional network, rather than a linear process, governs the Th17 differentiation program. Thus, combinatorial interactions of multiple transcription factors, including RORγt, activated STAT3, IRF4, and other factors yet to be described, regulate the genes that define the Th17 lineage (Figure 2). Decoding this transcriptional network will help in the better understanding of Th17 cell differentiation and function and may provide new strategies for treating autoimmune diseases.
8. In vivo development and function of Th17 cells
In wildtype unimmunized mice kept under SPF conditions, CD4+ IL-17-producing Th17 cells are present almost exclusively in the small intestinal LP and other mucosal tissues ([12] and III and DRL unpublished data). This observation suggests that Th17 cells are specialized to handle the unique challenges presented to the immune system in the intestine. The mucosal immune system must maintain tolerance towards the enormous number of resident microbial species and the plethora of food antigens, but must also recognize and react appropriately against the pathogenic organisms that inevitably gain access to the mucosa. It is currently thought that immune responsiveness or unresponsiveness in the intestine involves specific signals transmitted from the intestinal flora in the lumen to T cells and other cells in the LP by way of epithelial cells and the extensive DC network. DC populations in this locale have been shown to produce all of the major cytokines involved in Th17 cell differentiation, including IL-23, TGF-β, and IL-6. Since this specific cytokine environment is very likely generated in response to specific innate immune stimulation from the lumen, Th17 cells are probably induced in the steady state in response to intestinal flora and participate in control of mucosal immunity. It is currently unclear what are the inducing ligands or microorganisms and if these involve both commensal flora and pathogenic bacteria. The LP Th17 cells may be involved in providing immunity against certain classes of pathogenic bacteria and/or in keeping the commensals under control.
The presence of Th17 cells in the LP may also be closely related to the commonality in their cytokine dependence with Tregs. Foxp3+ Tregs represent 20% and 40% of the CD4+ T cells in the LP in the small and large intestine, respectively (III and DRL, unpublished). This makes the LP the largest tissue “reservoir” of Tregs, which is likely explained by the need for immune suppression and regulation in the gut. As mentioned before, Tregs and Th17 cells share TGF-β as a major cytokine controlling their development. Thus, in the TGF-β-rich environment of the gut, T cells may be particularly inclined to differentiate towards the Treg and Th17 programs. The presence of additional cytokines, such as IL-6, IL-21, and IL-23 may then tip the balance towards Th17 [10,26], but, as discussed above, this would also be governed by the local concentration of TGF-β, which is regulated by its binding as a precursor to the extracellular matrix and by interactions with available cell surface integrins, including αvβ6 and αvβ8 [53,54]. The concentration of the cytokines may control the relative levels and functions of RORγt and Foxp3, which, in turn, would determine the balance between Treg and Th17 cells depending on the nature of the required immune response (Figure 3 and Figure 4).
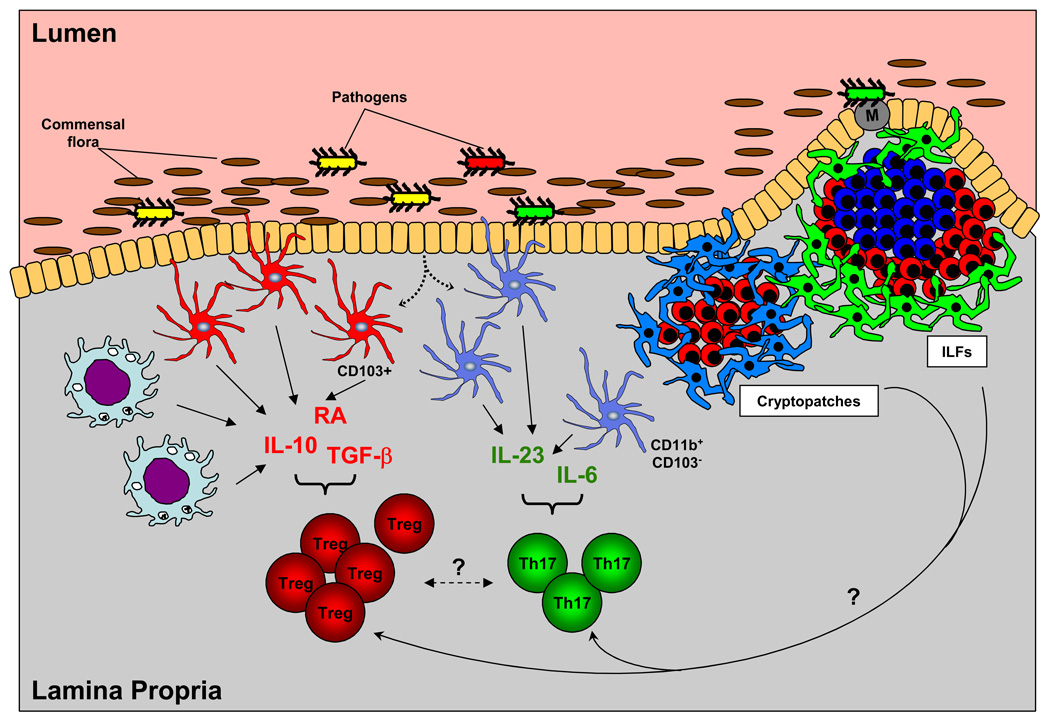
Figure 4. Th17 and Treg cell differentiation in the intestinal lamina propria.
The local cytokine milieu governs the balance between Th17 and Treg cells in the intestine. Th17 lineage-inducing cytokines (e.g., IL-6, L-23) and iTreg cell-inducing cytokines (e.g., TGF-β, IL-10, retinoic acid (RA)) are produced by different subpopulations of antigen presenting cells in response to signals from the lumen of the intestine or from invading microorganisms. Lamina propria macrophages and CD103+ DCs produce Treg-inducing cytokines and CD11b+CD103− DCs produce Th17-inducing cytokines. The Th17:Treg balance may also be influenced by other subpopulations of DCs in the lamina propria or DCs in organized lymphoid structures, such as cryptopatches, where they are closely associated with RORγt-producing lymphoid tissue inducer-like (LTi-like) cells, and isolated lymphoid follicles (ILFs), which contain RORγt+ LTi-like cells, surrounding a single B cell follicle. LP DCs produce cytokines in response to signals from the commensal intestinal flora and pathogenic organisms in the lumen in several ways. They can sample the lumen by extending dendrites through the epithelial layer. Antigens transported through M cells in Peyer’s patches and ILFs also activate the resident DC populations. In addition, epithelial cells have also been shown to participate in DC activation.
The balance between inflammatory and regulatory T cells may be regulated by signals received from different populations of DCs in the LP. Several recent studies reported the ability of mesenteric lymph node and LP DCs to specifically induce Treg cell differentiation by the production of retinoic acid (RA) [55–57]. In turn, RA produced by DCs was shown to inhibit Th17 production, albeit not completely [57]. Most recently, another abundant APC population in the intestine, the LP macrophages, were shown to induce Treg cell differentiation in the presence of TGF-β, through the production of IL-10 and RA [58]. This study reported that CD11b+, but not CD11b−, DCs in the LP preferentially induce Th17 development [58]. CD11b− DCs, in addition, contain most of the CD103+ DC population in the gut, which has also been shown to support Treg cell development [56]. Another recent study reported that CD11b plays a role in oral tolerance by suppressing Th17 responses [59]. Although it has not been examined how CD11b deficiency affects the numbers of different APC subpopulations in the intestine, it does indeed affect the function of these cells [60]. It is therefore becoming clear that specific APC populations in the intestinal LP direct the balance between Treg and Th17 cells (figure 4).
The presence of Th17 cells in the LP in the steady state, as well as the role of LP APCs in this process, support the hypothesis that Th17 differentiation in vivo is induced after DC stimulation by specific microorganisms. IL-17, as well as Th17 cells, have been shown to be important in protective immunity against pathogenic bacteria, such as Klebsiella pneumoniae and Citrobacter rodentium, in the airway and intestine, respectively [11,61]. Th17 responses are induced in specific infections, but the signals involved in recognizing the microbes and inducing appropriate cytokine production by DCs that stimulate Th17 cell differentiation remain unknown. Although TLR ligands were initially shown to promote Th17 differentiation in vitro [9], there are to date no studies on TLR involvement in vivo. In addition, other pattern recognition receptor pathways may be involved, especially in the case of fungal infections. A breakthrough study recently reported that the alternative pattern-recognition pathway through the C-type lectin receptor dectin-1 induced DC maturation and the production of IL-23 and consequent induction of Th17 cells [30]. The authors proposed that Th17 cells play a major protective role in fungal infection with Candida and possibly Aspergillus sp. However, a recent study reported that although Th17 cells were induced in both types of fungal infections, neutralization of the IL-23/IL-17 axis actually led to decreased pathology [62]. Thus, much remains to be learned about the protective role of Th17 cells in immunity and the innate immune signals that lead to protective Th17 responses.
9. Th17 cells in human versus mouse
The Th17 cell differentiation pathway is now recognized as having a key role in a variety of human autoimmune diseases. However, our understanding of human Th17 cell differentiation has lagged behind that of its mouse counterpart. Differences in the pathway between mouse and human T cells have been described, but it has been difficult to interpret these, because the human T cells analyzed are generally antigen-experienced cells, whereas most murine studies have relied on naïve T cells. With this caveat in mind, the main difference described has been in the role of TGF-β in Th17 cell differentiation. Thus, in contrast to mouse T cells, TGF-β and IL-6 failed to induce Th17 cell differentiation in TCR-stimulated CD45RA+ human T cells. Instead, IL-1β was found to induce IL-17 production, and this was enhanced when IL-6 was also included [63]. IL-1β also enhances murine Th17 cell differentiation in vitro [9], and, more importantly, was shown to participate in IL-17-mediated disease in mice [64]. However, it cannot induce the differentiation of naïve mouse T cells into Th17 cells in the absence of TGF-β. TGF-β was found to inhibit human Th17 cell differentiation [63]. However, as discussed earlier, the effects of TGF-β even in the mouse depend to a large extent on the context of the cytokine environment in which Th cell differentiation takes place. It is therefore too early to conclude that mouse and human Th17 cell differentiation involve fundamentally different cytokine signaling pathways.
In humans, many T cells produce both IL-17 and IFNγ. These Th17/Th1 cells are induced in almost all instances during Th17 cell differentiation of human T cells in vitro and exist in human peripheral blood and gut LP [63,65]. This may reflect the relative dearth of true naïve T cells in the periphery in humans [66]. Indeed, such “double producers” are not observed in mouse in vitro cultures of naïve T cells, but are abundant after T cell activation in vivo through immunization or during EAE [12]. In addition, stimulation of mouse Th17 cells from the LP in vitro yields large numbers of double producers (III and DRL, unpublished), and thus the abundance of Th17/Th1 cells is similar between human and mouse.
Despite potential differences in the cytokine requirements for their differentiation, Th17 cells in the human also specifically express RORγt and up-regulate IL-23R [63,65]. In addition, T-bet over-expression as well as IL-12 and high levels of IL-2 inhibit both RORγt-expression and IL-17 production in human Th17 cells, as has been described in mice [63]. Thus, the transcriptional program activated during Th17 cell differentiation is likely to be very similar in the two species. All human Th17 cells express the chemokine receptor CCR6, and many also express CCR4 and elevated levels of CCR5 [65,67]. This specific chemokine profile may reflect the differential migratory and homing phenotype of Th17 cells [67]. Whether murine Th17 cells possess a similar surface phenotype remains to be investigated.
10. Conclusions
Th17 cells are now thought to have key roles in a variety of human autoimmune diseases, including psoriasis, rheumatoid arthritis, and Crohn’s disease. Polymorphisms in the gene encoding IL-23R have been found in strong association with susceptibility or resistance to Crohn’s disease [68], thus providing genetic validation for the Th17 cell differentiation pathway in this disease. Substantial progress has been made in elucidating the cytokine signals and transcription factors that specify the Th17 cell program, but, aside from a few genes encoding effector cytokines, little is known of the targets of these transcriptional regulators. The characterization of the transcriptional program induced by RORγt, the Th17 lineage-specific transcription factor, in collaboration with more widely distributed factors such as STAT3 and IRF4, should provide a better understanding of the mechanisms involved in Th17 cell differentiation as well as new concepts for clinical intervention. In addition, the mechanism by which RORγt is regulated by a yet unknown ligand remains to be elucidated. Such information may facilitate development of small molecule compounds to manipulate the differentiation of Th17 cells and, hence, the ratio of inflammatory and regulatory T cells.
Acknowledgements
This work was supported by fellowships from the Crohns and Colitis Foundation and the Irvington Institute to III and LZ, respectively, by NIH grant RO1 AI033856-14 (DRL), and by the Howard Hughes Medical Institute (DRL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007 doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 14.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 15.He YW, Deftos ML, Ojala EW, Bevan MJ. RORgamma t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9:797–806. doi: 10.1016/s1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medvedev A, Chistokhina A, Hirose T, Jetten AM. Genomic structure and chromosomal mapping of the nuclear orphan receptor ROR gamma (RORC) gene. Genomics. 1997;46:93–102. doi: 10.1006/geno.1997.4980. [DOI] [PubMed] [Google Scholar]
- 17.Medvedev A, Yan ZH, Hirose T, Giguere V, Jetten AM. Cloning of a cDNA encoding the murine orphan receptor RZR/ROR gamma and characterization of its response element. Gene. 1996;181:199–206. doi: 10.1016/s0378-1119(96)00504-5. [DOI] [PubMed] [Google Scholar]
- 18.Hirose T, Smith RJ, Jetten AM. ROR gamma: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem Biophys Res Commun. 1994;205:1976–1983. doi: 10.1006/bbrc.1994.2902. [DOI] [PubMed] [Google Scholar]
- 19.Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 21.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 22.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 23.Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- 24.Mehta DS, Wurster AL, Grusby MJ. Biology of IL-21 and the IL-21 receptor. Immunol Rev. 2004;202:84–95. doi: 10.1111/j.0105-2896.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 25.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 26.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 27.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 28.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 30.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by TH17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007 doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang S, Aune TM. Dynamic changes in histone-methylation 'marks' across the locus encoding interferon-gamma during the differentiation of T helper type 2 cells. Nat Immunol. 2007;8:723–731. doi: 10.1038/ni1473. [DOI] [PubMed] [Google Scholar]
- 34.Schoenborn JR, Dorschner MO, Sekimata M, Santer DM, Shnyreva M, Fitzpatrick DR, et al. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat Immunol. 2007;8:732–742. doi: 10.1038/ni1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- 37.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 38.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, et al. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 43.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 44.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 47.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 48.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 49.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 50.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 53.Sheppard D. Roles of alphav integrins in vascular biology and pulmonary pathology. Curr Opin Cell Biol. 2004;16:552–557. doi: 10.1016/j.ceb.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 58.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 59.Ehirchiou D, Xiong Y, Xu G, Chen W, Shi Y, Zhang L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J Exp Med. 2007;204:1519–1524. doi: 10.1084/jem.20062292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veldhoen M. Oral tolerance: Passing CD11b on the way to tolerance. Immunol Cell Biol. 2007;85:397–398. doi: 10.1038/sj.icb.7100106. [DOI] [PubMed] [Google Scholar]
- 61.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005 doi: 10.1084/jem.20050193. jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 63.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 64.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laurence A, O'Shea JJ. T(H)-17 differentiation: of mice and men. Nat Immunol. 2007;8:903–905. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 67.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 68.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]