Abstract
Schizophrenia, bipolar disorder, and attention deficit/hyperactivity disorder (ADHD) present abnormalities in emotion processing. A previous study showed that the spontaneously hypertensive rats (SHR), a putative animal model of ADHD, present reduced contextual fear conditioning (CFC). The aim of the present study was to characterize the deficit in CFC presented by SHR. Adult male normotensive Wistar rats and SHR were submitted to the CFC task. Sensitivity of the animals to the shock and the CFC performance after repeated exposure to the task were investigated. Pharmacological characterization consisted in the evaluation of the effects of the following drugs administered previously to the acquisition of the CFC: pentylenetetrazole (anxiogenic) and chlordiazepoxide (anxiolytic); methylphenidate and amphetamine (used for ADHD); lamotrigine, carbamazepine, and valproic acid (mood stabilizers); haloperidol, ziprasidone, risperidone, amisulpride, and clozapine (neuroleptic drugs); metoclopramide and SCH 23390 (dopamine antagonists without antipsychotic properties); and ketamine (a psychotomimmetic). The effects of paradoxical sleep deprivation (that worsens psychotic symptoms) and the performance in a latent inhibition protocol (an animal model of schizophrenia) were also verified. No differences in the sensitivity to the shock were observed. The repeated exposure to the CFC task did not modify the deficit in CFC presented by SHR. Considering pharmacological treatments, only the neuroleptic drugs reversed this deficit. This deficit was potentiated by proschizophrenia manipulations. Finally, a deficit in latent inhibition was also presented by SHR. These findings suggest that the deficit in CFC presented by SHR could be a useful animal model to study abnormalities in emotional context processing related to schizophrenia.
Keywords: rats, psychiatric disorder, emotional memory, antipsychotics, amphetamine, mood stabilizers
Introduction
The ability to identify emotionally salient information in the environment and form an appropriate and rapid behavioral response is critical to survival.1 Different psychiatric populations, including schizophrenic, bipolar disorder, and attention deficit/hyperactivity disorder (ADHD) patients, present abnormalities in emotion processing.2,3 In this respect, impaired recognition of facial emotion has been demonstrated in schizophrenia, bipolar disorder, and ADHD patients.2–4
Fear conditioning is one of the most common paradigms used to study the biological basis of emotion.5,6 In this paradigm, an emotionally neutral conditioned stimulus (eg, contextual stimulus) is paired with an aversive unconditioned stimulus, eg, a foot shock, during the acquisition phase. As a result, the contextual stimulus comes to elicit conditioned fear response during expression phase, without foot shock presentation, because it acquires aversive reinforcing properties.5,6 While fear is an adaptative component of response to aversive stimuli, inappropriate fear responses may be present in many common psychiatric problems. For instance, diminished or exaggerated emotional response to aversive stimuli may be observed in schizophrenia, ADHD, and bipolar disorder2 and may trigger nonadaptative response like suicide.7 In this context, a deficit in contextual fear conditioning (CFC) presented by rats submitted to a excitotoxic lesion of the hippocampus is reversed by neuroleptics and has been associated with cognitive deficits presented by schizophrenia.8–11
The spontaneously hypertensive rats (SHR) strain has been suggested as an animal model of ADHD.12,13 This strain presents all the behavioral characteristics of the ADHD: it has sustained attention problems, shows hyperactivity in a variety of behavioral paradigms, and is impulsive.13,14 In addition, a previous study reported that the emotional memory of SHR, assessed by the duration of freezing response in the presence of a conditioned aversive stimulus, is reduced in relation to normotensive rats.15
The first aim of the present study was to replicate the deficit in the CFC test presented by the SHR in comparison to normotensive Wistar rats (NWR). In order to verify if SHR would be less sensitive to a shock stimulus, we also evaluated the vocalization after the shock presentation and the freezing response in animals submitted or not to the shock. The subsequent experiment was designed in order to evaluate whether retraining to CFC would diminish a possible novelty-induced anxiety and distraction effect and, consequently, facilitate the conditioning between the context and the aversive stimulus improving the SHR performance.
In sequence, a pharmacological screening of the effects of different drugs on the deficit in the CFC presented by SHR was performed. To this purpose, the doses used were chosen on the basis of previous studies showing their effectiveness in animal models of anxiety,16–18 ADHD,19,20 bipolar disorder,21,22 and schizophrenia.23–26 An anxiogenic (pentylenetetrazole) and an anxiolytic (chlordiazepoxide) drug were tested to determine the effects of alterations in anxiety levels on the deficit in the CFC presented by SHR. We also evaluated the effects of amphetamine and methylphenidate (used for the treatment of ADHD), mood stabilizers (lamotrigine, carbamazepine, and valproic acid—used for the treatment of bipolar disorder), and typical (haloperidol) and atypical (ziprasidone, risperidone, amisulpride, and clozapine) neuroleptic drugs (used for the treatment of schizophrenia) on the deficit in CFC presented by SHR. Finally, because neuroleptic drugs specifically improved the CFC presented by SHR, we also investigated the effects of dopamine antagonists without antipsychotic properties (metoclopramide, dopamine D2 antagonist, and SCH 23390, dopamine D1 antagonist), ketamine (that induces schizophrenia-related behaviors in animal models 27), and paradoxical sleep deprivation (reported to worsen psychotic symptoms28) on the CFC response of SHR and NWR. In addition, we also compared the response of NWR and SHR to a latent inhibition protocol (a paradigm extensively used to evaluate the impaired ability to ignore irrelevant stimuli associated with schizophrenia 29,30).
Methods and Materials
Subjects
Male NWR and SHR, 5 month old, of our own colony were housed under conditions of controlled temperature (22°C–23°C) and lighting (12/12 h light/dark cycle, lights on at 7:00 AM). Groups of 5–6 animals were kept in Plexiglas cages (41 × 34 × 16.5 cm), with free access to food and water. The animals were maintained in accordance with the guidelines of the Committee on Care and Use of Laboratory Animal Resources, National Research Council, United States.
Drugs
Chlordiazepoxide, pentylenetetrazole, amphetamine, SCH 23390, valproic acid, ketamine (Sigma—St Louis, MO), risperidone, lamotrigine (Torrent—São Paulo, Brazil), metoclopramide (Le Petit—São Paulo, Brazil), and methylphenidate (Novartis—São Paulo, Brazil) were diluted in 0.9% saline. Haloperidol (Sigma) and amisulpride (Sanofi-Synthélab—São Paulo, Brazil) were dissolved in lactic acid and then diluted in distilled water. Ziprasidone (Pfizer—São Paulo, Brazil) and carbamazepine (Sigma) were dissolved in Tween 80 and then diluted in distilled water. Clozapine (Novartis) was dissolved in acetic acid and then diluted in distilled water. Saline, distillated water plus acid lactic or Tween were used as control solution depending on the drugs used in each experiment. All the solutions were injected intraperitoneally in a volume of 1 ml/kg of body weight. In all the experiments, the solutions were administered before the training session of the CFC task.
CFC Task
On the first day (training session), the rats were individually placed in a dark chamber with a grid floor (22 × 22 × 22 cm). After 150 seconds, 0.4-mA foot shocks lasting 5 seconds were applied every 30 seconds for the subsequent 150 seconds. Thirty seconds after the last foot shock, the animal was removed from the apparatus. The contextual conditioning test (test session) was performed 24 hours after the training. Each animal was placed in the same dark chamber, without receiving foot shocks. The freezing duration (defined as complete immobility of the animal, with the absence of vibrissae movements and sniffing) was quantified during 5 minutes. Each animal was used in only one experiment.
Evaluation of Vocalization
Vocalization during the presentation of foot shocks was quantified. Scores ranging from 0 to 4 were attributed to the animal's vocalization every time the shock was delivered. The grading system was as following: 0—no vocalization, 1—until 2 seconds of vocalization, 2—more than 2 seconds of vocalization, and 3—vocalization and jump. In each group, total sum of vocalization scores for each animal was used for statistical analysis.
Paradoxical Sleep Deprivation
Each rat was placed inside a water chamber (22 × 22 × 35 cm) onto a platform of 7 cm in diameter immersed in water until 1 cm of its surface. When the rat enters the paradoxical phase of sleep, it looses the tonus of muscles, touches the water, and reawakens. Control rats were placed inside the water chamber, but, instead of water, the chamber was filled with sawdust bedding.31 Paradoxical sleep–deprived and control rats were kept inside the chambers for 4 days and had free access to food and water.
Experimental Design
Experiment 1—Vocalization Response to the Shock and Freezing Response to the CFC.
NWR and SHR were subjected to the training session of the CFC with the presentation or not of the shock (n = 8–9). During the shock presentation in this session, the vocalization was quantified. The freezing duration was quantified in the test session performed as described above.
Experiment 2—Effects of Reexposure to the CFC
NWR and SHR (n = 7) were subjected to the training and test sessions (trial 1) of the CFC. Seven days later, they were reexposed to a second training and test sessions (trial 2) of the CFC task.
Experiment 3—Effects of an Anxiogenic (Pentylenetetrazole) and an Anxiolytic (Chlordiazepoxide) Drug on the CFC
NWR and SHR (n = 7–8) were treated with saline, 5 mg/kg chlordiazepoxide, or 10 mg/kg pentylenetetrazole. Thirty minutes after chlordiazepoxide or 5 minutes after pentylenetetrazole injections, the rats were submitted to the CFC training session.
Experiments 4 and 5—Effects of Amphetamine or Methylphenidate on the CFC
NWR and SHR (n = 8–9) were treated with saline or 2.5 mg/kg amphetamine (experiment 4) or with saline or 2.5 mg/kg methylphenidate (experiment 5). After fifteen minutes, the rats were submitted to the CFC training session.
Experiment 6—Effects of Mood Stabilizers on the CFC
NWR and SHR (n = 7–8) were treated with saline, 30 mg/kg carbamazepine, 200 mg/kg valproic acid, or 20 mg/kg lamotrigine. Thirty minutes after lamotrigine or carbamazepine and fifteen minutes after valproic acid injections, the rats were submitted to the CFC training session.
Experiment 7—Effects of a Typical (Haloperidol) and Atypical (Ziprasidone, Risperidone, Amisulpride) Antipsychotic Drugs on the CFC
NWR and SHR (n = 10) were treated with saline, 0.5 mg/kg haloperidol, 2 mg/kg ziprasidone, 0.5 mg/kg risperidone, or 50 mg/kg amisulpride. Thirty minutes later, the rats were submitted to the CFC training session.
Experiment 8—Effects of a Dopamine D2 Antagonist (Metoclopramide) and a Dopamine D1 Antagonist (SCH 23390) on the CFC
NWR and SHR (n = 7–8) were treated with saline, 10 mg/kg metoclopramide, or 0.5 mg/kg SCH 23390. Thirty minutes after metoclopramide or fifteen minutes after SCH 23390 injections, the rats were submitted to the CFC training session. The dose and schedules of treatment used for metoclopramide and SCH 23390 have proven to be effective in animal models of dopamine receptor blockade, such as apomorphine-induced stereotyped behavior.32,33
Experiments 9 and 10—A Dose-Response Curve of Amphetamine and Methylphenidate on the CFC
In order to verify if the effect of these psychostimulants would not be related to the dose chosen, dose-response curves were performed.
NWR and SHR (n = 8–9) were treated with saline or 0.5 mg/kg, 1 mg/kg, 2.5 mg/kg, or 5 mg/kg amphetamine (experiment 9) or with saline, 0.5 mg/kg, 2.5 mg/kg, or 5 mg/kg methylphenidate (experiment 10). After fifteen minutes, the rats were submitted to the CFC training session.
Experiments 11 and 12—A Dose-Response Curve of Haloperidol and Clozapine on the CFC
In order to verify if the effect of neuroleptic drugs would not be related to the dose chosen, dose-response curves were performed.
NWR and SHR (n = 8) were treated with saline or 0.25 mg/kg, 0.5 mg/kg, 1 mg/kg haloperidol (experiment 11), or with saline or 2.5 mg/kg, 5 mg/kg, or 10 mg/kg clozapine (experiment 12). After thirty minutes, the rats were submitted to the CFC training session.
Experiment 13—Effects of Ketamine on the CFC
NWR and SHR (n = 10) were treated with saline or 10 mg/kg ketamine. After fifteen minutes, the rats were submitted to the CFC training session. The dose and schedules of treatment used for ketamine have proven to be effective in animal models of schizophrenia.27
Experiment 14—Effects of Paradoxical Sleep Deprivation on the CFC
NWR and SHR (n = 5–6) were sleep deprived or not during 96 hours. Immediately after sleep deprivation, the rats were submitted to the CFC training session. The schedules of the experimental procedure used for paradoxical sleep deprivation has proven to be effective in enhancing dopamine-related behaviors such as apomorphine-induced aggressive behavior and stereotypy.34–37
Experiment 15—Comparison of Latent Inhibition, A Putative Animal Model of Schizophrenia, in NWR and SHR
NWR and SHR (n = 8–9) were previously exposed or nonexposed to the apparatus for 5 minutes, without shock presentation, during 3 consecutive days. Twenty-four hours after the third exposure, the animals were submitted to the CFC training session.
Experiment 16—Effects of Ketamine, Haloperidol, and Clozapine on Freezing Behavior Observed in a Different Context
This experiment was designed in order to verify whether some drugs used would change the freezing scores when the animals were tested in a context different from the box where they received the foot shocks.
NWR and SHR (n = 6) were treated with saline, 10 mg/kg ketamine, 0.5 mg/kg haloperidol, and 2.5 mg/kg clozapine. Thirty minutes after haloperidol or clozapine and fifteen minutes after ketamine injections, the rats were submitted to the CFC training session. Twenty-four hours later, each animal was placed in the open-field apparatus (a circular wooden box with 97 cm in diameter and 32.5 cm high and an open top) and was observed for freezing quantification.
Statistical Analysis
Data were analyzed by multivariate analysis of variance (MANOVA) (time × strain) or 2-way analysis of variance (ANOVA) (strain × treatment) followed by appropriate post hoc analyses: Duncan test or Student t test or Student paired t test. The P < .05 was used as a criterion for statistical significance.
Results
Experiment 1
No differences between NWR and SHR were found in the sum of scores of vocalization (figure 1A). Concerning freezing behavior in the test session, 2-way ANOVA revealed significant shock effect (F1,30 = 283.6; P < .05), strain effect (F1,30 = 143.7; P < .05), and shock × strain interaction (F1,30 = 130.3; P < .05). Post hoc analysis revealed that NWR and SHR that had received shock in the training session presented enhanced freezing response when compared with the respective groups that had not received the stimulus. However, the freezing response observed in SHR previously exposed to the shock was significantly lower than that observed in NWR submitted to the same procedure (figure 1B).
Fig. 1.
(A) Shock-Induced Vocalization of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) During the Training Session of Contextual Conditioning. (B) Freezing response (s) of NWR and SHR that received (shock) or not (nonshock) the shock in the training session. *P < .05 compared with nonshock group. #P < .05 compared with NWR group. (A) Student t test and (B) Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
Experiment 2
MANOVA revealed significant time effect (F1,12 = 11.17; P < .05) and time × strain interaction (F1,12 = 6.75; P < .05) on freezing response. In trial 1, SHR presented less freezing response when compared with NWR (t = 4.63; P < .05). In trial 2, no differences between strains were observed. The comparisons between trials revealed a decrease in freezing response in trial 2 only for NWR (t = 3.93, P < .05—figure 2).
Fig. 2.
Freezing response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) During the First (Trial 1) and Second (Trial 2) Test Session. *P < .05 compared with trial 1. #P < .05 compared with NWR group. Multivariate analysis of variance followed by Student t test between strains and paired t test between trials. Data are reported as mean ± SE.
Experiment 3
Two-way ANOVA showed significant treatment effect (F2,41 = 123.3; P < .05), strain effect (F1,47 = 387.0; P < .05), and treatment × strain interaction (F2,47 = 164.4; P < .05). Post hoc analysis revealed that the freezing response was significantly reduced in SHR group when compared with NWR group treated with saline, as previously observed. Moreover, chlordiazepoxide decreased and pentylenetetrazole increased the freezing response in NWR when compared with the saline-treated group. However, no differences were seen among SHR groups treated with these drugs (table 1).
Table 1.
Freezing Response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) Treated With Saline (SAL), 5 mg/kg Chlordiazepoxide (CDZ), or 10 mg/kg Pentylenetetrazole (PTZ)—Experiment 3; SAL, 30 mg/kg Carbamazepine (CBZ), 200 mg/kg Valproic Acid (VPA), or 20 mg/kg Lamotrigine (LAM)—Experiment 6
| NWR | SHR | ||
| Experiment | Treatment | Mean ± SE | Mean ± SE |
| 3 | SAL | 93.32 ± 7.68 | 40.05 ± 1.89# |
| CDZ | 48.57 ± 5.09* | 37.32 ± 2.35 | |
| PTZ | 232.86 ± 9.34*,+ | 26.25 ± 5.23# | |
| 6 | SAL | 157.63 ± 15.32 | 33.25 ± 5.95# |
| CBZ | 28.12 ± 10.60*,° | 23.87 ± 5.81 | |
| VPA | 19 ± 7.06*,° | 25 ± 11.79 | |
| LAM | 81 ± 25.25* | 13.5 ± 4.19# |
Note: Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
*P < .05 compared with SAL group; +P < .05 compared with CDZ group; °P < .05 compared with LAM group; #P < .05 compared with NWR group.
Experiment 4
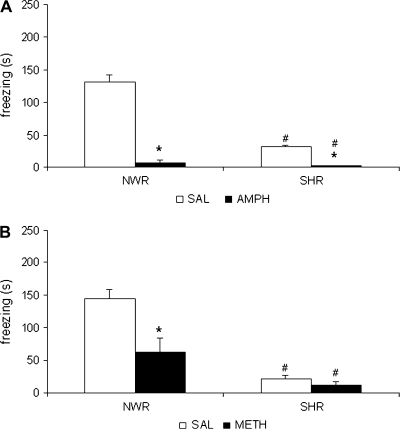
Two-way ANOVA showed significant treatment effect (F1,31 = 219.8; P < .05), strain effect (F1,31 = 101.8; P < .05), and treatment × strain interaction (F1,31 = 84.5; P < .05). Post hoc analysis showed that the freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. The amphetamine decreased the freezing response in both strains when compared with the respective control groups (figure 3A).
Fig. 3.
(A) Freezing response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) Treated With Saline (SAL) or 2.5 mg/kg Amphetamine (AMPH) or (B) Saline (SAL) or 2.5 mg/kg Methylphenidate (METH) Before the Training Session. *P < .05 compared with SAL group. #P < .05 compared with NWR group. Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
Experiment 5
Two-way ANOVA showed significant treatment effect (F1,32 = 11.0; P < .05), strain effect (F1,32 = 38.9; P < .05), and an interaction between these factors (F1,32 = 6.8; P < .05). Post hoc analysis revealed that freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. The methylphenidate significantly decreased the freezing response in NWR groups. No differences were seen among SHR groups treated with saline or methylphenidate. In addition, the SHR group treated with 2.5 mg/kg methylphenidate presented diminished freezing response when compared with the respective NWR group (figure 3B).
Experiment 6
Two-way ANOVA showed significant treatment effect (F3,54 = 15.9; P < .05), strain effect (F3,54 = 30.9; P < .05), and an interaction between these 2 factors (F3,54 = 12.8; P < .05). Post hoc analysis showed that freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. Although all mood stabilizers decreased freezing response in NWR, they did not modify freezing response in SHR. The freezing response in NWR treated with lamotrigine was higher than that presented by carbamazepine- and valproic acid–treated NWR and SHR treated with lamotrigine (table 1).
Experiment 7
Two-way ANOVA showed significant treatment effect (F4,90 = 24.0; P < .05), strain effect (F1,90 = 151.7; P < .05), and an interaction between these 2 factors (F4,90 = 26.8; P < .05). Post hoc analysis showed that freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. The haloperidol treatment significantly reduced the freezing response of NWR while enhanced this response of SHR, when compared with the respective control groups. The treatment with risperidone, ziprasidone, and amisulpride was able to increase the freezing response in both strains when compared with the respective saline groups. In addition, NWR treated with these drugs presented higher freezing responses when compared with SHR treated with these atypical neuroleptics (figure 4).
Fig. 4.
Freezing response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) Treated With Saline (SAL), 0.5 mg/kg Haloperidol (HAL), 2.0 mg/kg Ziprasidone (ZIPRA), 0.5 mg/kg Risperidone (RISP), or 50 mg/kg Amisulpride (AMI) Before the Training Session. *P < .05 compared with SAL group. #P < .05 compared with NWR group. +P < .05 compared with HAL. °P < .05 compared with RISP group. aP < .05 compared with ZIPRA. Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
Experiment 8
Two-way ANOVA showed significant treatment effect (F2,39 = 205.8; P < .05), strain effect (F1,39 = 398.7; P < .05), and an interaction between these 2 factors (F2,39 = 100.3; P < .05). Post hoc analysis showed that freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. Metoclopramide did not modify the freezing response of both strains when compared with the respective saline groups. However, the SCH 23390 decreased the freezing response of NWR and SHR groups when compared with the rats treated with saline and metoclopramide (figure 5).
Fig. 5.
Freezing Response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) Treated With Saline (SAL), 10 mg/kg Metoclopramide (METO), or 0.5 mg/kg SCH 23390 before the training session. *P < .05 compared with SAL group. #P < .05 compared with NWR group. +P < .05 compared with METO. Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
Experiment 9
Figure 6A shows the dose-response curve of amphetamine on the CFC. Two-way ANOVA showed significant treatment effect (F4,77 = 142.24; P < .05), strain effect (F1,77 = 96.69; P < .05), and an interaction between these factors (F4,77 = 58.41; P < .05). Post hoc analysis showed that the freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. All the amphetamine doses decreased the freezing response in both strains when compared with the respective control groups. In addition, the 0.5 mg/kg amphetamine-treated NWR presented an increased freezing response when compared with NWR treated with all the other doses and when compared with SHR treated with 0.5 mg/kg amphetamine.
Fig. 6.
(A) Freezing Response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) Treated With Saline (SAL), 0.5 (AMPH 0.5), 1 (AMPH 1), 2.5 (AMPH 2.5), or 5 (AMPH 5) mg/kg Amphetamine or (B) Saline (SAL), 0.5 (METH 0.5), 2.5 (METH 2.5), or 5.0 (METH 5) mg/kg Methylphenidate Before the Training Session. *P < .05 compared with SAL group. #P < .05 compared with NWR group. +P < .05 compared with AMPH 0.5 or METH 0.5 group. Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
Experiment 10
Figure 6B shows the dose-response curve of methylphenidate on the CFC. Two-way ANOVA showed significant treatment effect (F3,64 = 8.11; P < .05), strain effect (F1,64 = 53.57; P < .05), and an interaction between these factors (F3,64 = 6.95; P < .05). Post hoc analysis revealed that freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. All the doses significantly decreased the freezing response in NWR when compared with the saline-treated group. The freezing response presented by 5 mg/kg methylphenidate-treated NWR was significantly lower than that presented by 0.5 mg/kg methylphenidate-treated NWR. No differences were seen among SHR groups treated with saline or methylphenidate. In addition, the SHR groups treated with saline or 0.5 or 2.5 mg/kg methylphenidate presented diminished freezing response when compared with the respective NWR groups.
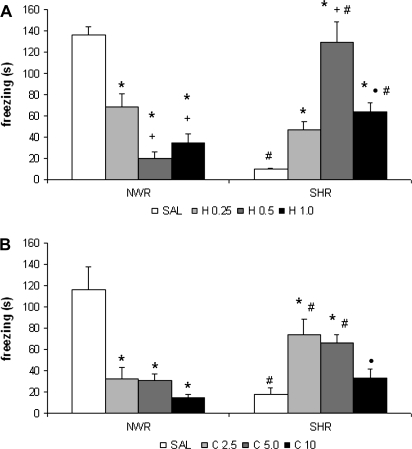
Experiment 11
Figure 7A shows the dose-response curve of haloperidol on the CFC. Two-way ANOVA showed significant treatment effect (F1,56 = 3.01; P < .05) and an interaction between strain and treatment factors (F1,56 = 50.82; P < .05). Post hoc analysis revealed that freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. Post hoc analysis revealed that all the doses of haloperidol decreased freezing response in NWR when compared with the saline-treated group. On the other hand, all the doses of haloperidol increased the freezing response of SHR when compared with the saline-treated group. The SHR groups treated with 0.5 or 1.0 mg/kg haloperidol presented higher freezing response when compared with NWR treated with these same doses.
Fig. 7.
(A) Freezing Response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) Treated With Saline (SAL), 0.25 (H 0.25), 0.5 (H 0.5), or 1 (H 1) mg/kg Haloperidol or (B) saline (SAL), 2.5 (C 2.5), 5 (C 5), or 10 (C 10) mg/kg Clozapine Before the Training Session. *P < .05 compared with SAL group. #P < .05 compared with NWR group. +P < .05 compared with H 0.25 group. •P < .05 compared with H 0.5 or C 2.5 and C 5. Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
Experiment 12
Figure 7B shows the dose-response curve of clozapine on the CFC. Two-way ANOVA showed significant treatment effect (F1,56 = 5.29; P < .05) and an interaction between strain and treatment factors (F1,56 = 17.37; P < .05). Post hoc analysis revealed that freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. All the clozapine doses decreased freezing response presented by NWR. On the other hand, 2.5 and 5.0 mg/kg clozapine enhanced the freezing response presented by SHR when compared with the saline-treated group and with NWR with the same treatments.
Experiment 13
Two-way ANOVA revealed significant treatment effect (F1,36 = 173.3; P < .05), strain effect (F1,36 = 75.3; P < .05), and an interaction between these 2 factors (F1,36 = 40.0; P < .05). Post hoc analysis showed that freezing response was significantly reduced in SHR group when compared with NWR group treated with saline. The ketamine treatment reduced the freezing response in NWR and SHR groups when compared with respective saline group. No difference was seen between NWR and SHR treated with ketamine (figure 8A).
Fig. 8.
Freezing Response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) Treated With Saline (SAL) or 10 mg/kg Ketamine (KET) (A) or Submitted to 96 h of Sleep Deprivation (SD) or Not (NSD) (B) Before the Training Session. *P < .05 compared with SAL or NSD groups. #P < .05 compared with NWR group. Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
Experiment 14
Two-way ANOVA revealed significant sleep deprivation effect (F1,17 = 141.6; P < .05), strain effect (F1,17 = 54.5; P < .05), and an interaction between these 2 factors (F1,17 = 24.9; P < .05). Post hoc analysis showed that freezing response was significantly reduced in non–sleep-deprived SHR when compared with NWR. Sleep deprivation significantly decreased the freezing response in both strains. No differences were seen between sleep-deprived SHR and NWR (figure 8B).
Experiment 15
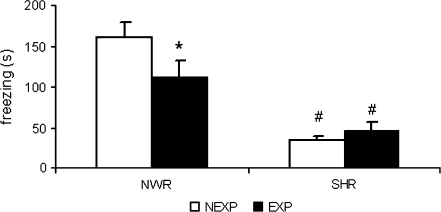
Two-way ANOVA revealed significant strain effect (F1,30 = 40.1; P < .05) and exposure × strain interaction (F1,30 = 4.2; P < .05) on freezing response. Post hoc analysis revealed that the NWR, but not the SHR, previously exposed to the apparatus presented lower freezing response when compared with the respective nonexposed group. Both SHR groups presented lower freezing response when compared with the respective NWR groups (figure 9).
Fig. 9.
Freezing Response (s) of Normotensive Wistar Rats (NWR) and Spontaneously Hypertensive Rats (SHR) Preexposed (EXP) or Not (NEXP) to the Apparatus Before the Training Session. *P < .05 compared with trial 1 or NEXP group. #P < .05 compared with NWR group. Two-way analysis of variance followed by Duncan test. Data are reported as mean ± SE.
Experiment 16
Two-way ANOVA showed no significant effects (table 2). No differences in the freezing response were seen among the groups.
Table 2.
Freezing Response (s) Observed in a Different Context of Normotensive Wilstar Rats (NWR) and Spontaneously Hypotensive Rats (SHR) Treated With Saline (SAL), 10 mg/kg Ketamine (KET), 0.5 mg/kg Haloperidol (HAL), or 2.5 mg/kg Clozapine (CLO)
| NWR | SHR | ||
| Experiment | Treatment | Mean ± SE | Mean ± SE |
| 16 | SAL | 5.66 ± 1.56 | 3.83 ± 1.42 |
| KET | 3.33 ± 52.58 | 2.16 ± 0.7 | |
| HAL | 4.33 ± 2.43 | 2.0 ± 0.68 | |
| CLO | 4.66 ± 1.89 | 2.33 ± 1.05 |
Note: Two-way analysis of variance. Data are reported as mean ± SE.
Discussion
The decrease in the performance of SHR in the CFC task observed in this study corroborates a previous work.15 This reduced fear response could be related to a diminished sensitivity to the shock. In this respect, a number of studies indicate that SHR have abnormal sensitivity to pain, suggesting both a decrease and an increase in the reactivity to painful stimuli, indicating that the pain phenotype of the SHR varies with nociceptive tests.38–40 The strain difference in CFC in this study did not appear to be related to pain sensitivity because the vocalization during the shock presentation was the same for both strains. In addition, although SHR presented a decrease in freezing response, it is important to note that they are able to exhibit this behavior (increase in freezing response induced by previous exposure to the shock compared with animals that were not exposed to this aversive stimulus). In parallel, attention deficits have been described as a prominent feature of SHR. In this respect, procedures that could facilitate the learning of the task could ameliorate the deficit in CFC presented by SHR. Contrary to this idea, repeated conditioning training did not prevent the deficit in CFC presented by SHR.
Fear conditioning has been used to study fear learning in certain anxiety disorders.5,6 Indeed, reduced levels of anxiety can decrease fear-related responses.41,42 In accordance, the acquisition of CFC in NWR was improved by the anxiogenic drug pentylenetetrazole and impaired by the anxiolytic drug chlordiazepoxide. It is well known that SHR show low indices of basal anxiety–like behavior.17,43–46 Hence, a low basal anxiety could account for the deficit of CFC presented by SHR. This does not seem to be the case considering that neither pentylenetetrazole nor chlordiazepoxide modified this deficit.
Abnormalities in emotion processing have been related to schizophrenia, bipolar disorder, and ADHD.2,3 In this context, the next series of experiments addressed the effects of different drugs classically used to treat these pathologies.
Amphetamine and methylphenidate are recommended medications for the ADHD treatment. These drugs were not able to ameliorate the deficit presented by the SHR at any dose tested (Figures 3A and 3B and 6A and 6B). Indeed, these drugs even decreased the acquisition of CFC in both NWR and SHR. Thus, CFC deficit presented by SHR did not seem to be related to the emotional processing impairment presented by ADHD. In accordance, although SHR strain has been proposed as an animal model to study this pathology, 13,14 other groups have also reported the absence of beneficial effects of methylphenidate on ADHD-like behaviors in SHR.47,48
To address if the deficit in the acquisition of CFC presented by SHR would be related to bipolar disorder, we tested 3 mood stabilizers: valproic acid, lamotrigine, and carbamazepine. Contrary to this possibility, these drugs did not alter the deficit in the acquisition of CFC presented by this strain. In addition, they impaired the acquisition of CFC in NWR.
Typical and atypical neuroleptic drugs are the conventional treatment for schizophrenia. In NWR, all the doses of the typical neuroleptic haloperidol and the atypical neuroleptic clozapine impaired the acquisition of the CFC and the atypical neuroleptic drugs risperidone, ziprasidone, and amisulpride improved it. In accordance, an impairment in the acquisition of the CFC produced by haloperidol and clozapine was previously reported.49 On the other hand, the deficit in CFC presented by SHR was reverted by all the neuroleptic drugs used here. Noteworthy, all the doses of haloperidol and clozapine induced different effects on the acquisition of CFC in NWR (impairment) and in SHR (improvement). These opposite effects indicate that the deficit in CFC presented by SHR could be related to a deficit in emotional memory processing characteristic of schizophrenia. In this respect, the atypical neuroleptic quetiapine was able to revert the deficit in CFC presented by animals submitted to an excitotoxin-induced hippocampal lesion,8 an animal model of schizophrenia.9
Nevertheless, these results could merely reflect a modulatory effect of dopaminergic agents on the acquisition of the CFC.6 In order to verify this hypothesis, we evaluated the effects of a D1 (SCH 23390) and a D2 (metoclopramide) antagonist without antipsychotic action on the deficit of CFC presented by this strain. Corroborating the association of this deficit with schizophrenia-related emotional processing impairments, the acquisition of the CFC in SHR was not improved by these dopamine antagonists. In fact, as described previously for normotensive rats,50,51 SCH 23390 impaired the acquisition of the CFC in both strains.
Strengthening the hypothesis that the deficit in CFC presented by SHR is related to schizophrenia impairments, opposite to the effects produced by neuroleptic drugs, the administration of ketamine as well as sleep deprivation potentiated this deficit. In addition, these manipulations also impaired the acquisition of the CFC in NWR. In this way, ketamine induces schizophrenia-related behaviors in animal models27 and reinstates psychosis in remitted schizophrenic patients,52 evidences that support the glutamatergic hypothesis of schizophrenia.53 Additionally, paradoxical sleep deprivation has been reported to worsen psychotic symptoms,28 improve depressive symptoms,54 and impair attention.55 In accordance with this line of reasoning, amphetamine also potentiated the deficit in CFC presented by SHR. In this respect, amphetamine precipitates or aggravates psychosis.56 Within this context, while the release of striatal dopamine induced by amphetamine is increased in schizophrenic patients,57 the release of dopamine from striatal slices induced by this drug is increased in SHR when compared with normotensive rats.58
Based on the above, we also compared the latent inhibition expression in NWR and SHR. Latent inhibition is defined by a decrement in the conditioning between a conditioned stimulus (eg, the context) and an unconditioned stimulus (eg, the shock) when the conditioned stimulus is previously presented without being paired to the unconditioned stimulus.59 This attentional learning has been suggested to correspond to our ability to selectively attend to important information in our environment and ignore the irrelevant stimuli.60 In this context, an absence of latent inhibition process has been described for schizophrenic61 and is one of the most used paradigms to study attentional deficits in animal models of schizophrenia.30 Supporting this rationale, latent inhibition is disrupted by amphetamine, an effect that is reversed by neuroleptic drugs (see Moser et al29and Weiner30). Our data show that latent inhibition process was expressed by NWR but not by SHR (experiment 15). In this respect, the impairment in CFC presented only by NWR submitted to 2 trials of the CFC task (experiment 2—training/test followed by training/test) could be related to processes underlying interferences in associative learning as the latent inhibition phenomenon or the impairment to induce a conditioned response after a preexposure to the unconditioned stimulus,62 a phenomenon similar to latent inhibition that is also disrupted by amphetamine.63 The interpretation of these data in the context of the results obtained from the dose-response curves of psychostimulants and neuroleptic drugs reinforces the schizophrenia component of the CFC deficit presented by SHR.
Corroborating the schizophrenic-like profile of at least some behaviors of SHR strain, deficits in prepulse inhibition of startle64–67—a paradigm to study sensorimotor gating deficits in schizophrenia68—have been described in this strain. In addition, while the prevalence of tardive dyskinesia, a late side effect of long-term treatment with neuroleptic drugs,69 is decreased in schizophrenia when compared with affective disorders,70 we have recently described that the SHR did not develop oral dyskinesia in animal models of tardive dyskinesia.71,72
In summary, the deficit in CFC presented by the SHR strain is specifically reverted by neuroleptic drugs and potentiated by proschizophrenia manipulations. These effects are contextual specific because the administration of neuroleptic drugs or ketamine did not change the freezing scores observed in a context different from that where the foot shock was conditioned (experiment 16). In addition, although the acquisition of the CFC in NWR is diversely altered, all the proschizophrenia interventions impaired it. Taken as a whole, this body of evidence strongly suggests that the deficit in CFC presented by SHR is related to emotional processing abnormalities in schizophrenia. In this context, an impaired recognition of fear in schizophrenia related to an abnormal functional integration in amygdala networks has been suggested.73–75
The mechanisms linking the core symptoms of schizophrenia and their biological bases are not well established. As advocated by Hemsley,76,77 a comprehensive cognitive model proposes that a weakening of contextual influences is central to this pathology. This model proposes “that it is a weakening of the influence of stored memories of regularities of previous input on current perception which is basic to the schizophrenic condition.”76 In this respect, the appropriate stored regularities would not be correctly activated by spatial and temporal context.78,79 These abnormalities in the information processing modulated by context have been linked to the core symptoms of schizophrenia as delusions, hallucinations, disorganization, loss of sense of personal identity, negative symptoms as well as schizophrenics’ disturbances in latent inhibition, and prepulse inhibition paradigms (reviewed in Hemsley76,77). In parallel, a disjunction in emotional processing has been linked to the core symptoms of schizophrenia. Specifically, the relationships between the expression and the perception of emotions and/or emotional experiences are altered, and these disturbances seem to be linked to the negative as well as the positive symptoms of schizophrenia (reviewed by Aleman and Kahn 80). In this sense, the rationale of impairment in emotional contextual processing could explain the deficit in CFC presented by the SHR strain.
Animal models can undoubtedly contribute to the understanding of the complexity of schizophrenia. The functioning of context perception has been addressed in behavioral paradigms such as latent inhibition and prepulse inhibition of startle. In this way, the present study suggests that the deficit in CFC presented by the SHR strain could be useful to shed light on the disturbances in the processing of emotional context associated with schizophrenia.
Funding
Fundação de Amparo a Pesquisa de São Paulo (CEPID); Conselho Nacional de Desenvolvimento Científico e Tecnológico (303691/2005-0); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Associação Fundo de Incentivo à Psicofarmacologia; Fundo de Auxílio aos Docentes e Alunos da Universidade Federal de São Paulo.
Acknowledgments
The authors would like to thank Mrs Teotila R. R. Amaral and Mr Cleomar S. Ferreira for capable assistance. All the authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Darwin C. Chicago, Ill: University of Chicago Press; 1872. The Expression of the Emotions in Man and Animals. [Google Scholar]
- 2.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 3.Marsh PJ, Williams LM. ADHD and schizophrenia phenomenology: visual scanpaths to emotional faces as a potential psychophysiological marker? Neurosci Biobehav Rev. 2006;30:651–665. doi: 10.1016/j.neubiorev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res. 1998;32:171–181. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 5.LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 6.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Progress Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Hawton K, Sutton L, Haw C, Sinclair J, Deeks JJ. Schizophrenia and suicide: systematic review of risk factors. Br J Psychiatry. 2005;187:9–20. doi: 10.1192/bjp.187.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Martin MV, Dong H, Bertchume A, Csernansky JG. Low dose quetiapine reverses deficits in contextual and cued fear conditioning in rats with excitotoxin-induced hippocampal neuropathy. Pharmacol Biochem Behav. 2005;82:263–269. doi: 10.1016/j.pbb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Bardgett ME, Jacobs JL, Taylor GT, Csernansky JG. Kainic acid decreases hippocampal neuronal number and increases dopamine receptor binding in the nucleus accumbens: an animal model of schizophrenia. Behav Brain Res. 1995;70:153–164. doi: 10.1016/0166-4328(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 10.Stubley-Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 1996;716:29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- 11.Yin H, Bardgett ME, Csernansky JG. Kainic acid lesions disrupt fear-mediated memory processing. Neurobiol Learn Mem. 2002;77:389–401. doi: 10.1006/nlme.2001.4037. [DOI] [PubMed] [Google Scholar]
- 12.Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder—from brain dysfunctions to behaviour. Behav Brain Res. 1998;94:1–10. [PubMed] [Google Scholar]
- 13.Russell VA. Neurobiology of animal models of attention-deficit hyperactivity disorder. J Neurosci Methods. 2007;161:185–198. doi: 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Sagvolden T, Metzger MA, Schiørbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- 15.LeDoux JE, Sakaguchi A, Reis DJ. Strain differences in fear between spontaneously hypertensive and normotensive rats. Brain Res. 1983;277:137–143. doi: 10.1016/0006-8993(83)90915-0. [DOI] [PubMed] [Google Scholar]
- 16.Calzavara MB, Patti CL, Lopez GB, Abilio VC, Silva RH, Frussa-Filho R. Role of learning of open arm avoidance in the phenomenon of one-trial tolerance to the anxiolytic effect of chlordiazepoxide in mice. Life Sci. 2004;76:2235–2246. doi: 10.1016/j.lfs.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Calzavara MB, Lopez GB, Abilio VC, Silva RH, Frussa-Filho R. Role of anxiety levels in memory performance of spontaneously hypertensive rats. Behav Pharmacol. 2005;15:545–553. doi: 10.1097/00008877-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Anseloni VZ, Brandão ML. Ethopharmacological analysis of behaviour of rats using variations of the elevated plus-maze. Behav Pharmacol. 1997;8:533–540. doi: 10.1097/00008877-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Myers MM, Musty RE, Hendley ED. Attenuation of hyperactivity in the spontaneously hypertensive rat by amphetamine. Behav Neural Biol. 1982;34(1):42–54. doi: 10.1016/s0163-1047(82)91397-8. [DOI] [PubMed] [Google Scholar]
- 20.Yang PB, Swann AC, Dafny N. Acute and chronic methylphenidate dose-response assessment on three adolescent male rat strains. Brain Res Bull. 2006;71(1–3):301–310. doi: 10.1016/j.brainresbull.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arban R, Maraia G, Brackenborough K, et al. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav Brain Res. 2005;158:123–132. doi: 10.1016/j.bbr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Lamberty Y, Margineanu DG, Klitgaard H. Effect of the new antiepileptic drug levetiracetam in an animal model of mania. Epilepsy Behav. 2001;2:454–459. doi: 10.1006/ebeh.2001.0254. [DOI] [PubMed] [Google Scholar]
- 23.Feldon J, Weiner I. The latent inhibition model of schizophrenia attention disorder. Haloperidol and sulpiride enhance ratś ability to ignore irrelevant stimuli. Biol Psychiatry. 1991;29:635–646. doi: 10.1016/0006-3223(91)90133-7. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Monim Z, Reynolds GP, Neill JC. The effects of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res. 2006;169:263–273. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Weiner I, Schiller D, Gaisler-Salomon I. Disruption and potentiation of latent inhibition by risperidone: the latent inhibition model of atypical antipsychotic action. Neuropsychopharmacology. 2003;28:499–509. doi: 10.1038/sj.npp.1300069. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt U, Abou El-Ela A, Guo LJ, et al. Cyclosporine A (CsA) affects the pharmacodynamics and pharmacokinetics of the atypical antipsychotic amisulpride probably via inhibition of P-glycoprotein (P-gp) J Neural Transm. 2006;113:787–801. doi: 10.1007/s00702-005-0367-4. [DOI] [PubMed] [Google Scholar]
- 27.Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 28.Herz MI, Melville C. Relapse in schizophrenia. Am J Psychiatry. 1980;137:801–805. doi: 10.1176/ajp.137.7.801. [DOI] [PubMed] [Google Scholar]
- 29.Moser PC, Hitchcock JM, Lister S, Moran PM. The pharmacology of latent inhibition as an animal model of schizophrenia. Brain Res Rev. 2000;33:275–307. doi: 10.1016/s0165-0173(00)00026-6. [DOI] [PubMed] [Google Scholar]
- 30.Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology. 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- 31.Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Frussa-Filho R, Palermo-Neto J. Effects of single and long-term metoclopramide administration on open field and stereotyped behavior of rats. Eur J Pharmacol. 1988;10:323–329. doi: 10.1016/0014-2999(88)90663-2. [DOI] [PubMed] [Google Scholar]
- 33.Hess EJ, Norman AB, Creese I. Chronic treatment with dopamine receptor antagonists: behavioral and pharmacologic effects on D1 and D2 dopamine receptors. J Neurosci. 1988;8:2361–2370. doi: 10.1523/JNEUROSCI.08-07-02361.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hipólide DC, Tufik S. Paradoxical sleep deprivation in female rats alters drug-induced behaviors. Physiol Behav. 1995;57:1139–1143. doi: 10.1016/0031-9384(94)00377-h. [DOI] [PubMed] [Google Scholar]
- 35.Troncone LR, Ferreira TM, Braz S, Silveira Filho NG, Tufik S. Reversal of the increase in apomorphine-induced stereotypy and aggression in REM sleep deprived rats by dopamine agonist pretreatments. Psychopharmacology. 1988;94:79–83. doi: 10.1007/BF00735885. [DOI] [PubMed] [Google Scholar]
- 36.Tufik S. Changes of response to dopaminergic drugs in rats submitted to REM-sleep deprivation. Psychopharmacology. 1981;72:257–260. doi: 10.1007/BF00431826. [DOI] [PubMed] [Google Scholar]
- 37.Andersen ML, Tufik S. The effects of dopaminergic agonists on genital reflexes in paradoxical sleep-deprived male rats. Physiol Behav. 2005;84:205–210. doi: 10.1016/j.physbeh.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Taylor BK, Peterson MA, Basbaum AI. Exaggerated cardiovascular and behavioral nociceptive responses to subcutaneous formalin injection in the spontaneously hypertensive rat. Neurosci Lett. 1995;201:9–12. doi: 10.1016/0304-3940(95)12157-y. [DOI] [PubMed] [Google Scholar]
- 39.Taylor BK, Roderick RE, Basbaum AI. Brainstem noradrenergic control of nociception is abnormal in the spontaneously hypertensive rat. Neurosci Lett. 2000;291:139–142. doi: 10.1016/s0304-3940(00)01389-6. [DOI] [PubMed] [Google Scholar]
- 40.Taylor BK, Roderick RE, Lezin EST, Basbaum AI. Hypoalgesia and hyperalgesia with inherited hypertension in the rat. Am J Physiol Regulatory Integrative Comp Physiol. 2001;280:R345–R354. doi: 10.1152/ajpregu.2001.280.2.R345. [DOI] [PubMed] [Google Scholar]
- 41.Helmstetter FJ. Stress-induced hypoalgesia and defensive freezing are attenuated by application of diazepam to the amygdale. Pharmacol Biochem Behav. 1993;44:433–438. doi: 10.1016/0091-3057(93)90487-e. [DOI] [PubMed] [Google Scholar]
- 42.Graeff FG, Netto FC, Zangrossi H. The elevated T-maze as an experimental model of anxiety. Neurosci Biobehav Rev. 1998;23:237–246. doi: 10.1016/s0149-7634(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 43.Gentsch C, Lichtsteiner M, Feer H. Open field and elevated plus-maze: a behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effects of chlordiazepoxide. Behav Brain Res. 1987;25:101–107. doi: 10.1016/0166-4328(87)90003-9. [DOI] [PubMed] [Google Scholar]
- 44.Goto SH, Conceição IM, Ribeiro R de A, Frussa-Filho R. Comparison of anxiety measured in the elevated plus-maze, open-field and social interaction tests between spontaneously hypertensive rats and Wistar EPM-1 rats. Braz J Med Biol Res. 1993;26:965–969. [PubMed] [Google Scholar]
- 45.Conceição IM, Goto SH, Frussa-Filho R. Evaluation of memory in an elevated T maze: a comparison between spontaneously hypertensive, Wistar-Kyoto and Wistar EPM-1 rats. Braz J Med Biol Res. 1994;27:731–735. [PubMed] [Google Scholar]
- 46.Ramos A, Kangerski AL, Basso PF, et al. Evaluation of Lewis and SHR rat strain as a genetic model for the study of anxiety and pain. Behav Brain Res. 2002;129:113–123. doi: 10.1016/s0166-4328(01)00337-0. [DOI] [PubMed] [Google Scholar]
- 47.Van den Bergh FS, Bloemarts E, Chan JSW, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Bizot JC, Chenault N, Houze B, et al. Methylphenidate reduces impulsive behaviour in juvenile Wistar rats, but not in adult Wistar, SHR and WKY rats. Psychopharmacology. 2007;193(2):215–223. doi: 10.1007/s00213-007-0781-4. [DOI] [PubMed] [Google Scholar]
- 49.Inoue T, Tsuchiya K, Koyama T. Effects of typical and atypical antipsychotic drugs on freezing behavior induced by conditioned fear. Pharmacol Biochem Behav. 1996;55:195–201. doi: 10.1016/s0091-3057(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 50.Inoue T, Izumi T, Maki Y, Muraki I, Koyama T. Effect of the dopamine D1/5 antagonist SCH 23390 on the acquisition of conditioned fear. Pharmacol Biochem Behav. 2000;66:573–578. doi: 10.1016/s0091-3057(00)00254-9. [DOI] [PubMed] [Google Scholar]
- 51.de Oliveira AR, Reimer AE, Brandão ML. Dopamine D2 receptor mechanisms in the expression of conditioned fear. Pharmacol Biochem Behav. 2006;84:102–111. doi: 10.1016/j.pbb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 53.Bressan RA, Pilowsky BELS. Glutamatergic Hypothesis Of Schizophrenia. Rev Bras Psiquiatr. 2003;25:177–183. doi: 10.1590/s1516-44462003000300011. [DOI] [PubMed] [Google Scholar]
- 54.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361–377. [PubMed] [Google Scholar]
- 55.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 56.Janowsky DS, Risch C. Amphetamine psychosis and psychotic symptoms. Psychopharmacology. 1979;65:73–77. doi: 10.1007/BF00491982. [DOI] [PubMed] [Google Scholar]
- 57.Laruelle M, Abi-Dargham A, Van Dyck CH, et al. Neurobiology Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russel V, De Villiers A, Sagvolden T, Lamn M, Taljaard J. Differences between eletrically-, ritalin and D-amphetamine-stimulated release of [3H]dopamine from brain slices suggest impaired vesicular storage of dopamine in animal model of Attention-Deficit Hyperactivity Disorder. Behav Brain Res. 1998;94:163–171. doi: 10.1016/s0166-4328(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 59.Lubow RE. Construct validity of the animal latent inhibition model of selective attention deficits in schizophrenia. Schizophr Res. 2005;31:139–153. doi: 10.1093/schbul/sbi005. [DOI] [PubMed] [Google Scholar]
- 60.Lubow RE. Latent Inhibition and Conditioned Attention Theory. New York, NY: Cambridge University Press; 1989. [Google Scholar]
- 61.Gray NS, Hemsley DR, Gray JA. Abolition of latent inhibition in acute, but not chronic, schizophrenics. Neurol Psychiatry Brain Res. 1992;1:83–89. [Google Scholar]
- 62.Randich A, LoLordo VM. Associative and nonassociative theories of the UCS preexposure phenomenon: implications for Pavlovian conditioning. Psychol Bull. 1979;86:523–548. [PubMed] [Google Scholar]
- 63.Chang T, Meyer U, Feldon J, Yee BK. Disruption of the US pre-exposure effect and latent inhibition in two-way active avoidance by systemic amphetamine in C57BL/6 mice. Psychopharmacology. 2007;191:211–221. doi: 10.1007/s00213-006-0649-z. [DOI] [PubMed] [Google Scholar]
- 64.Palmer AA, Dulawa SC, Mottiwala AA, Conti LH, Geyer MA, Printz MP. Prepulse startle deficit in the Brown Norway rat: a potential genetic model. Behav Neurosci. 2000;114:374–388. doi: 10.1037//0735-7044.114.2.374. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson SA, Cada AM. Spatial learning/memory and social and nonsocial behaviors in the spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rat strains. Pharmacol Biochem Behav. 2004;77:583–594. doi: 10.1016/j.pbb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 66.Kinkead B, Selz KA, Owens MJ, Mandell AJ. Algorithmically designed peptides ameliorate behavioral defects in animal model of ADHD by an allosteric mechanism. J Neurosci Methods. 2006;151:68–81. doi: 10.1016/j.jneumeth.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 67.Vendruscolo LF, Terenina-Rigaldie E, Raba F, Ramos A, Takahashi RN, Mormede P. A QTL on rat chromosome 7 modulates prepulse inhibition, a neuro-behavioral trait of ADHD, in a Lewis x SHR intercross. Behav Brain Funct. 2006;2:21. doi: 10.1186/1744-9081-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 69.Casey DE. Tardive dyskinesia. In: Meltzer HY, editor. Psychopharmacology: The Third Generation of Progress. New York, NY: Raven Press; 1987. pp. 1411–1419. [Google Scholar]
- 70.Gardos G, Cole JO. Neuroleptic-treatment and tardive dyskinesia. In: Yassa R, Nair PV, Jeste DV, editors. Neuroleptic-Induced Movement Disorders. New York, NY: Cambridge University Press; 1997. pp. 104–116. [Google Scholar]
- 71.Queiroz CM, Piovezan RD, Frussa-Filho R. Reserpine does not induce orofacial dyskinesia in spontaneously hypertensive rats. Eur J Pharmacol. 1998;356:105–108. doi: 10.1016/s0014-2999(98)00546-9. [DOI] [PubMed] [Google Scholar]
- 72.Abilio VC, Silva RH, Carvalho RC, et al. Important role of striatal catalase in aging- and reserpine-induced oral dyskinesia. Neuropharmacology. 2004;47:263–272. doi: 10.1016/j.neuropharm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 73.Williams LM, Das P, Liddell BJ, et al. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007;155:29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 74.Das P, Kemp AH, Flynn G, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 75.Williams LM, Das P, Harris AW, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 76.Hemsley DR. The development of a cognitive model of schizophrenia: placing it in context. Neurosci Biobehav Rev. 2005;29:977–988. doi: 10.1016/j.neubiorev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Hemsley DR. The schizophrenic experience: taken out of context? Schizophr Bull. 2005;31:43–53. doi: 10.1093/schbul/sbi003. [DOI] [PubMed] [Google Scholar]
- 78.Broadbent DE. Perceptional Communication. London: Pergamon; 1958. [Google Scholar]
- 79.Broadbent DE. Decision and Stress. London: Academic Press; 1971. [Google Scholar]
- 80.Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77(5):283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]