Abstract
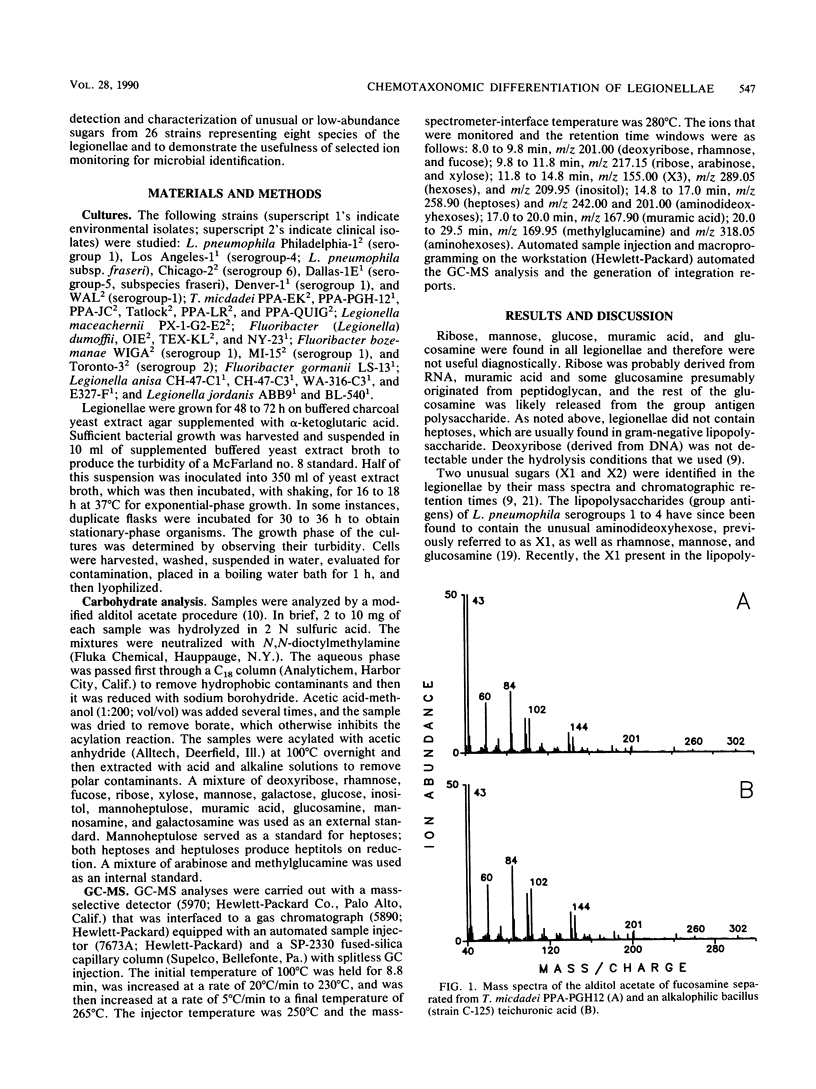
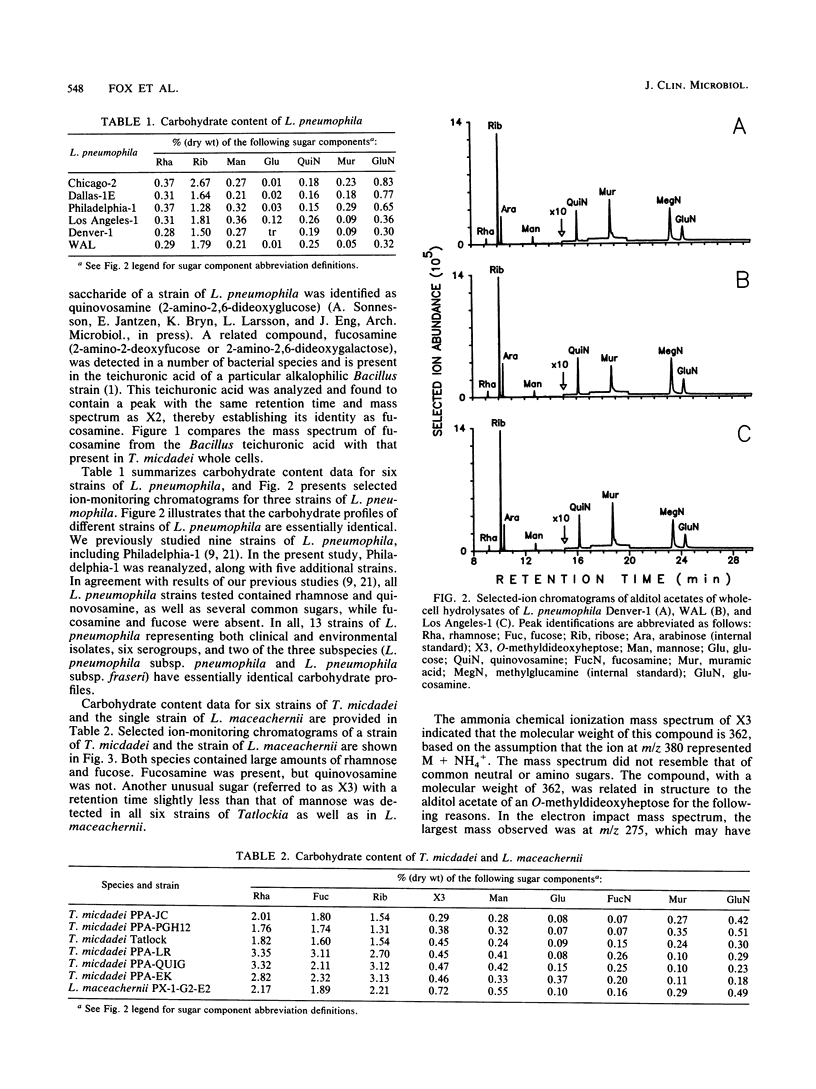
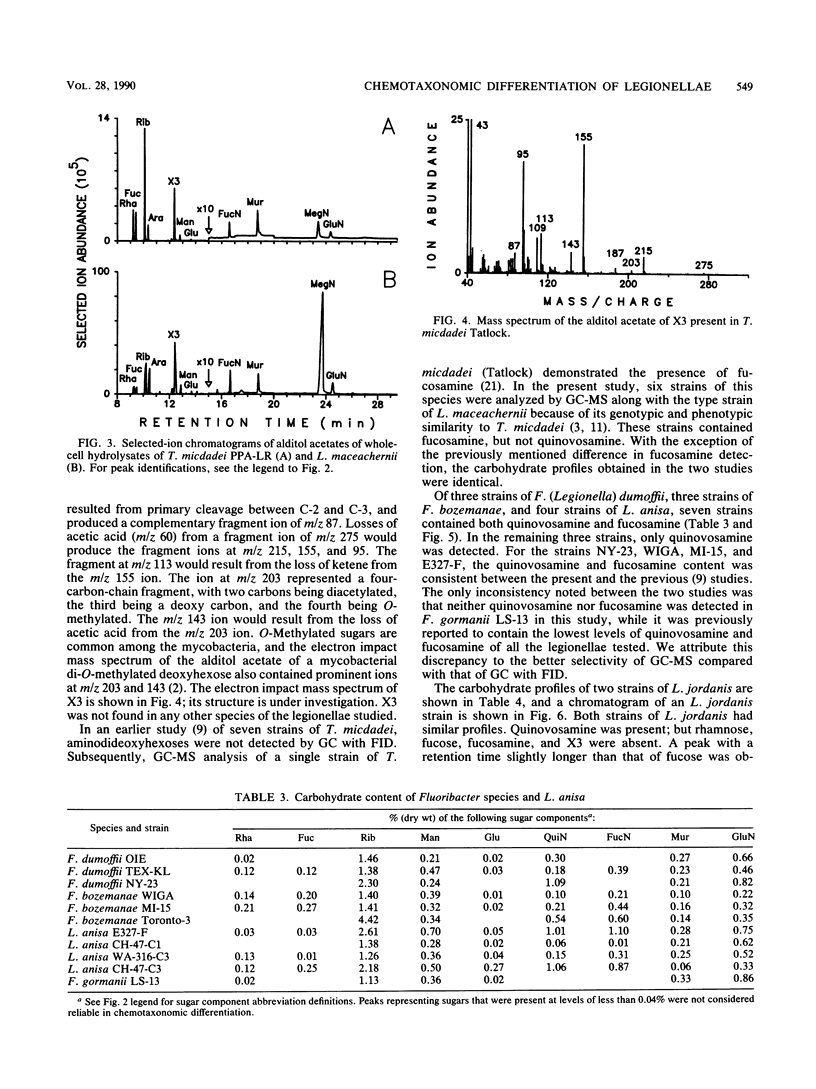
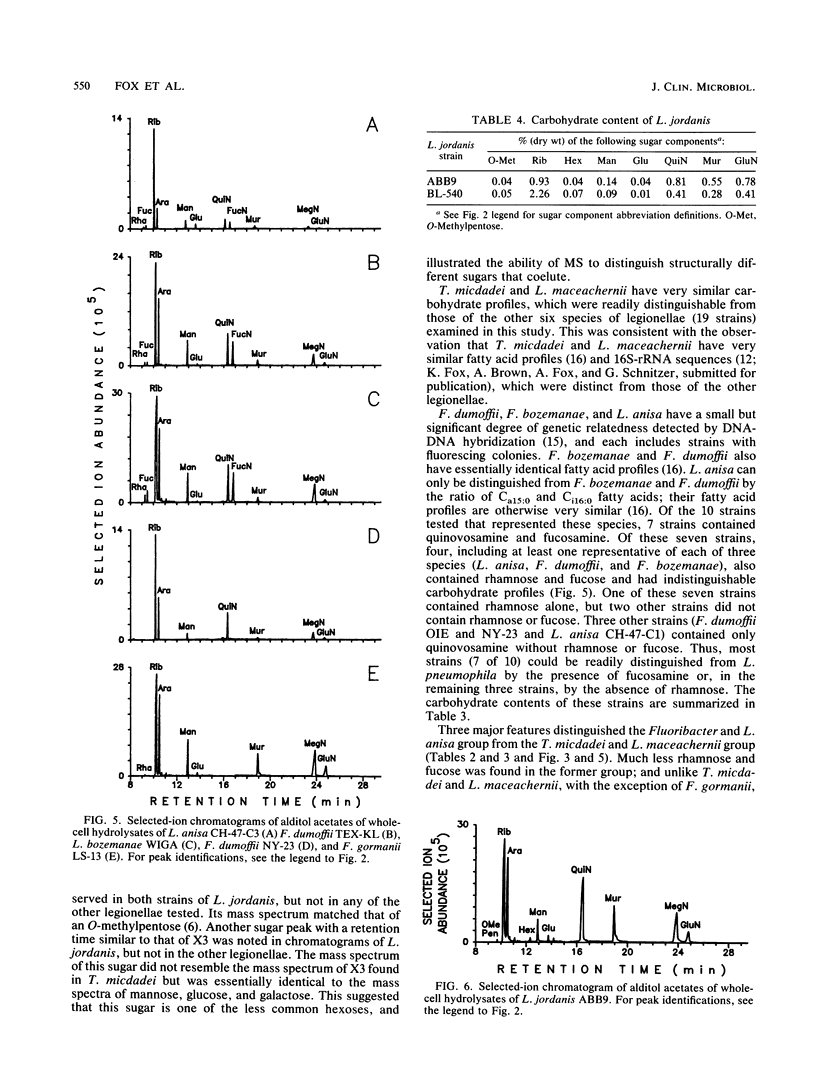
Legionellae have been differentiated previously by analyzing their carbohydrate contents by gas chromatography with flame ionization detection. In the present study, total ion mode gas chromatography-mass spectrometry (GC-MS) was used to detect a number of unusual sugars, including one that is structurally related to O-methyldideoxyheptoses. Increased sensitivity and selectivity for carbohydrate detection was achieved by selected ion-monitoring GC-MS. Two of the uncommon sugars previously discovered in the legionellae (X1 and X2) were identified as quinovosamine and fucosamine, respectively. Legionella pneumophila contained rhamnose and quinovosamine but not the quinovosamine isomer fucosamine. Tatlockia micdadei and Legionella maceachernii contained large amounts of rhamnose, fucose, and fucosamine but not quinovosamine. These two species were the only legionellae studied that contained another unusual sugar that is referred to as X3, pending determination of its structure. Fluoribacter dumoffi, Fluoribacter bozemanae, and Legionella anisa were varied in their carbohydrate contents, both within and between species, but could be distinguished from L. pneumophila and the T. micdadei and L. maceachernii group. Fluoribacter gormanii was unique among the legionellae in that it lacked both quinovosamine and fucosamine. Legionella jordanis contained other unusual carbohydrates in addition to quinovosamine. GC-MS may have wide application in the differentiation of bacterial species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono R., Uramoto M. Presence of fucosamine in teichuronic acid of the alkalophilic Bacillus strain C-125. Biochem J. 1986 Jan 1;233(1):291–294. doi: 10.1042/bj2330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Mayer H., Aspinall G. O., Nam Shin J. E. Structures of the glycopeptidolipid antigens from serovars in the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. Eur J Biochem. 1981 Mar 16;115(1):7–15. doi: 10.1111/j.1432-1033.1981.tb06190.x. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Steigerwalt A. G., Epple P., Bibb W. F., McKinney R. M., Starnes R. W., Colville J. M., Selander R. K., Edelstein P. H., Moss C. W. Legionella pneumophila serogroup Lansing 3 isolated from a patient with fatal pneumonia, and descriptions of L. pneumophila subsp. pneumophila subsp. nov., L. pneumophila subsp. fraseri subsp. nov., and L. pneumophila subsp. pascullei subsp. nov. J Clin Microbiol. 1988 Sep;26(9):1695–1703. doi: 10.1128/jcm.26.9.1695-1703.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry W. B., Gorman G. W., Orrison L. H., Moss C. W., Steigerwalt A. G., Wilkinson H. W., Johnson S. E., McKinney R. M., Brenner D. J. Legionella jordanis: a new species of Legionella isolated from water and sewage. J Clin Microbiol. 1982 Feb;15(2):290–297. doi: 10.1128/jcm.15.2.290-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G. D., Yu V. L., Vickers R. M. Disease due to the Legionellaceae (other than Legionella pneumophila). Historical, microbiological, clinical, and epidemiological review. Medicine (Baltimore) 1989 Mar;68(2):116–132. doi: 10.1097/00005792-198903000-00005. [DOI] [PubMed] [Google Scholar]
- Fox A., Lau P. Y., Brown A., Morgan S. L., Zhu Z. T., Lema M., Walla M. D. Capillary gas chromatographic analysis of carbohydrates of Legionella pneumophila and other members of the family Legionellaceae. J Clin Microbiol. 1984 Mar;19(3):326–332. doi: 10.1128/jcm.19.3.326-332.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. F., Brown A. Application of numerical systematics to the phenotypic differentiation of legionellae. J Clin Microbiol. 1989 Sep;27(9):1952–1955. doi: 10.1128/jcm.27.9.1952-1955.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbart J., Fox A., Morgan S. L. Carbohydrate profiling of bacteria by gas chromatography-mass spectrometry: chemical derivatization and analytical pyrolysis. Eur J Clin Microbiol. 1987 Dec;6(6):715–723. doi: 10.1007/BF02013084. [DOI] [PubMed] [Google Scholar]
- Lambert M. A., Moss C. W. Cellular fatty acid compositions and isoprenoid quinone contents of 23 Legionella species. J Clin Microbiol. 1989 Mar;27(3):465–473. doi: 10.1128/jcm.27.3.465-473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry W. R. Dihydroxy and monohydroxy fatty acids in Legionella pneumophila. J Bacteriol. 1981 Aug;147(2):373–381. doi: 10.1128/jb.147.2.373-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten S., Iyer S., Johnson W., Montgomery R. Serospecific antigens of Legionella pneumophila. J Bacteriol. 1986 Sep;167(3):893–904. doi: 10.1128/jb.167.3.893-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker W. L., Benson R. F., Schifman R. B., Pugh E., Steigerwalt A. G., Mayberry W. R., Brenner D. J., Wilkinson H. W. Legionella tucsonensis sp. nov. isolated from a renal transplant recipient. J Clin Microbiol. 1989 Aug;27(8):1831–1834. doi: 10.1128/jcm.27.8.1831-1834.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walla M. D., Lau P. Y., Morgan S. L., Fox A., Brown A. Capillary gas chromatography-mass spectrometry of carbohydrate components of legionellae and other bacteria. J Chromatogr. 1984 Apr 24;288(2):399–413. doi: 10.1016/s0021-9673(01)93716-1. [DOI] [PubMed] [Google Scholar]


