Abstract
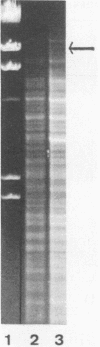
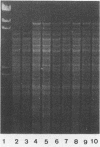
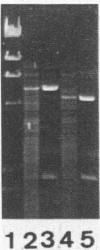
In a previous study, the recurrence of the Campylobacter pylori infection after apparently successful antibacterial therapy was determined to be due to recrudescence rather than reinfection. Although the DNA patterns of pre- and posttreatment isolates were very similar, we detected minor differences between the two patterns in about one third of the patients. These differences were not artifacts, but originated in the coexistence in the stomach of (sub)populations of bacteria with slightly different chromosomal DNAs, plasmids, or both. The presence of such (sub)populations was probably caused by mutation in vivo, as mutation in vitro was demonstrated in one patient after the original isolate was subcultured 10 times. Minor differences were not correlated with a difference in susceptibility to the antibiotic(s) that was used. An additional conclusion of this investigation was that the results of plasmid analysis should be interpreted very carefully when this method is used as an epidemiologic marker in the investigation of C. pylori infections.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowicz J., Lee A. Person-to-person transmission of Campylobacter pylori. Lancet. 1987 Sep 19;2(8560):680–681. doi: 10.1016/s0140-6736(87)92458-5. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B., Lund V., Kristiansen B. E., Korsnes L., Spanne O., Lindqvist B. Applications of restriction endonuclease fingerprinting of chromosomal DNA of Neisseria meningitidis. J Clin Microbiol. 1984 Jun;19(6):763–765. doi: 10.1128/jcm.19.6.763-765.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury W. C., Marko M. A., Hennessy J. N., Penner J. L. Occurrence of plasmid DNA in serologically defined strains of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1983 May;40(2):460–463. doi: 10.1128/iai.40.2.460-463.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoyiannis C. K., Winter P. J., Marshall R. B. Identification of Campylobacter coli isolates from animals and humans by bacterial restriction endonuclease DNA analysis. Appl Environ Microbiol. 1984 Sep;48(3):545–549. doi: 10.1128/aem.48.3.545-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabbaz R. F., Kaper J. B., Moody M. R., Schimpff S. C., Tenney J. H. Molecular epidemiology of group JK Corynebacterium on a cancer ward: lack of evidence for patient-to-patient transmission. J Infect Dis. 1986 Jul;154(1):95–99. doi: 10.1093/infdis/154.1.95. [DOI] [PubMed] [Google Scholar]
- Kuijper E. J., Oudbier J. H., Stuifbergen W. N., Jansz A., Zanen H. C. Application of whole-cell DNA restriction endonuclease profiles to the epidemiology of Clostridium difficile-induced diarrhea. J Clin Microbiol. 1987 Apr;25(4):751–753. doi: 10.1128/jcm.25.4.751-753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Houthoff H. J., Oudbier J. H., van Bohemen C. G., Tytgat G. N., Rietra P. J. Follow-up study of individuals with untreated Campylobacter pylori-associated gastritis and of noninfected persons with non-ulcer dyspepsia. J Infect Dis. 1988 Jun;157(6):1245–1249. doi: 10.1093/infdis/157.6.1245. [DOI] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S. I., Goodwin C. S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988 Mar;157(3):465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Lin F. Y., Morrison C. B., Gross R. J., Khabbaz R., Maher K. O., Rowe B., Israel E., Libonati J. P. Molecular epidemiology of neonatal meningitis due to Citrobacter diversus: a study of isolates from hospitals in Maryland. J Infect Dis. 1986 Sep;154(3):409–414. doi: 10.1093/infdis/154.3.409. [DOI] [PubMed] [Google Scholar]
- Penfold S. S., Lastovica A. J., Elisha B. G. Demonstration of plasmids in Campylobacter pylori. J Infect Dis. 1988 Apr;157(4):850–851. doi: 10.1093/infdis/157.4.850. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Mills S. D., Bradbury W. C. Application of serotyping and chromosomal restriction endonuclease digest analysis in investigating a laboratory-acquired case of Campylobacter jejuni enteritis. J Clin Microbiol. 1983 Dec;18(6):1427–1428. doi: 10.1128/jcm.18.6.1427-1428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Skjold S. A., Quie P. G., Fries L. A., Barnham M., Cleary P. P. DNA fingerprinting of Streptococcus zooepidemicus (Lancefield group C) as an aid to epidemiological study. J Infect Dis. 1987 Jun;155(6):1145–1150. doi: 10.1093/infdis/155.6.1145. [DOI] [PubMed] [Google Scholar]
- Vaira D., D'Anastasio C., Holton J., Dowsett J. F., Londei M., Bertoni F., Beltrandi E., Grauenfels P., Salmon P. R., Gandolfi L. Campylobacter pylori in abattoir workers: is it a zoonosis? Lancet. 1988 Sep 24;2(8613):725–726. doi: 10.1016/s0140-6736(88)90196-1. [DOI] [PubMed] [Google Scholar]
- van Ketel R. J., ter Schegget J., Zanen H. C. Molecular epidemiology of Legionella pneumophila serogroup 1. J Clin Microbiol. 1984 Sep;20(3):362–364. doi: 10.1128/jcm.20.3.362-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]