Abstract
This paper describes a diamination process using di-tert-butyldiaziridinone as nitrogen source and CuCl as catalyst. A wide variety of disubstituted terminal olefins can be efficiently diaminated in good yields under mild condition. This diamination process was used to synthesize potent NK1 antagonist Sch 425078.
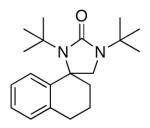
Diamination of olefins provides an effective approach to vicinal diamines, which are biologically and chemically important functional moieties.1 Various metal-mediated2,3 and metal-catalyzed4-6 diaminations have been developed. Recently, we have reported the Pd(0)- and Cu(I)-catalyzed regioselective diamination of conjugated dienes and trienes7,8,9 as well as the dehydrogenative diamination of terminal olefins10,11 using di-tert-butyldiaziridinone,13,14 diaziridinimine,15 or di-tert-butylthiadiaziridine 1,1-dioxide16 as the nitrogen sources. The Cu(I)-catalyzed diamination process has also been extended to activated mono-substituted terminal olefins such as styrenes, enynes, enol ether etc.9,12 Considering the fact that 4,4-disubstituted-2-imidazolidinones (Figure 1) have been shown to be potent NK1 antagonists,17 and in conjunction with our efforts to expand the diamination scope, we have investigated the diamination of disubstituted simple terminal olefins using di-tert-butyldiaziridinone (2) as the nitrogen source (Scheme 1).8 Herein, we wish to report our studies on this subject.
Figure 1.
NK1 antagonists
Scheme 1.
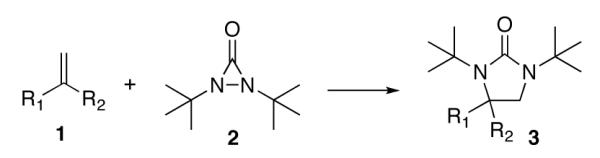
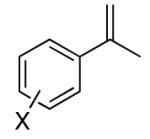
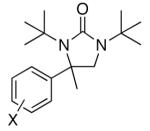
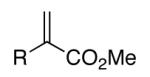
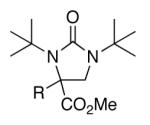
Initial studies were carried out using 2-phenylpropene as the substrate and di-tert-butyldiaziridinone (2) as the nitrogen source. When Pd(PPh3)4 was used as catalyst, only a trace amount of diamination product was observed. However, >95% conversion was obtained when the reaction was carried out with 5 mol % of CuCl-PPh3 (1:1) in CDCl3 at 65 °C.18 As shown in Table 1, various 2-phenylpropenes with different substituents on the phenyl ring can be successfully diaminated in moderate to good yield (Table 1, entries 1-9).19 2-Isopropenylnaphthalene 1j is also an effective substrate (Table 1, entry 10). Substrates with different alkyl substituents (such as ethyl, benzyl, and methoxymethyl groups) can also be diaminated in 55-71% yield (Table 1, entries 11, 12, 13, and 14). The diamination process can also be extended to α,β-unsaturated esters (Table 1, entries 15 and 16). However, dialkyl terminal olefins, such as 2-methyl-3-phenyl-1-propene, are not effective substrates for this diamination.
Table 1.
Catalytic Diamination of Disubstituted Terminal Olefinsa
 | |||
|---|---|---|---|
| entry | substrate 1 | product 3 | yield (%)d |
 |
 |
||
| 1 | 1a, X = H | 91 | |
| 2 | 1b, X = o-F | 47 | |
| 3b | 1c, X = o-OMe | 50 | |
| 4 | 1d, X = m-Br | 86 | |
| 5 | 1e, X = m-OMe | 74 | |
| 6 | 1f, X = m-Me | 83 | |
| 7 | 1g, X = m-CN | 80 | |
| 8 | 1h, X = p-Me | 64 | |
| 9 | 1i, X = p- Cl | 90 | |
| 10c |
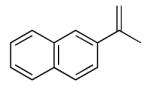 lj |
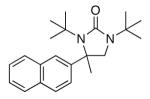 |
86 |
 |
 |
||
| 11 | 1k, R = Me | 71 | |
| 12 | 1l, R = Ph | 66 | |
| 13b | 1m, R = OMe | 63 | |
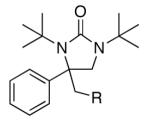
| 14 |
 ln |
 |
55 |
 |
 |
||
| 15 | 1o, R = Ph | 65 | |
| 16 | 1p, R = Me | 54 | |
All reactions were carried out with olefin (1) (0.4 mmol), di-tert-butyldiaziridinone (2) (0.8 mmol) (added by syringe pump over 8 h), CuCl-PPh3 (1:1) (0.02 mmol) in CDCl3 (0.3 mL) at 65 °C unless otherwise stated. Upon complete addition of 2, the reaction mixture was stirred at 65 °C for an additional time period (1 h for entries 1, 4, 5, 6, 8, and 9; 2 h for entries 7, 10, and 15; 4 h for entry 11; 7 h for entries 3, 12, 14, and 16; 10 h for entries 2 and 13).
CuCl-PPh3 (1:1) (0.04 mmol) was used.
The reaction was carried out with olefin (0.2 mmol), di-tert-butyldiaziridinone (2) (0.4 mmol), and CuCl-PPh3 (1:1) (0.02 mmol) in CDCl3 (0.3 mL).
Isolated yield based on olefin.
The deprotection of the diamination products was investigated with compound 3a (Scheme 2). Treating 3a with CH3SO3H in hexane (1:10, v/v) at room temperature gave monodeprotected compound 4a in 99% yield.20 The structure of compound 4a was confirmed by NOE analysis and X-ray structure of related compound 14 (Figure 2, Scheme 4). When the deprotection was carried out at 65 °C, both tert-butyl groups were smoothly removed in 85% yield. Free diamine 6a can be obtained in 87% yield directly from 3a by deprotection with concentrated HCl.7c,10
Scheme 2.
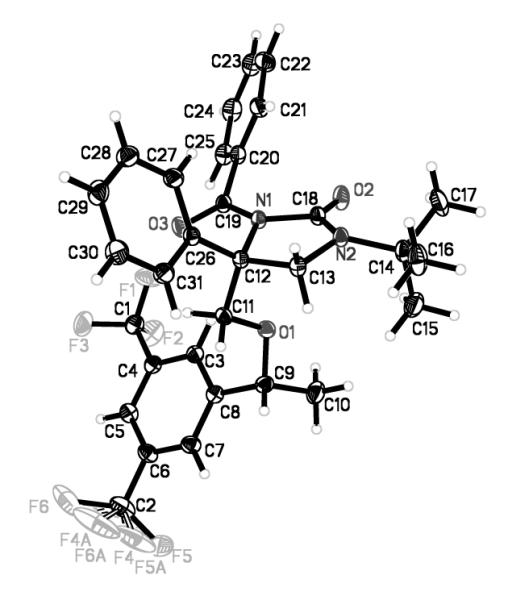
Figure 2.
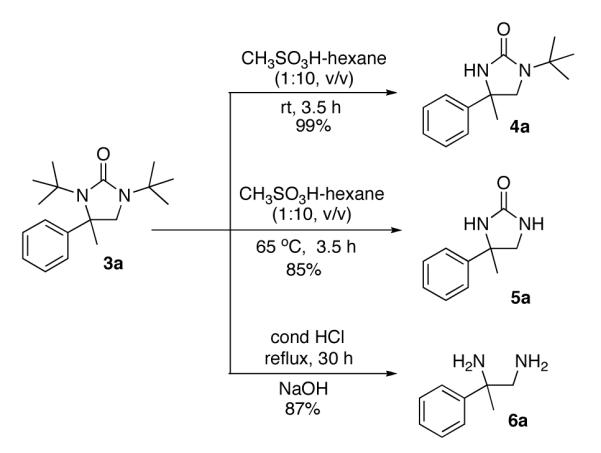
The X-ray structure of compound 14.
Scheme 4.
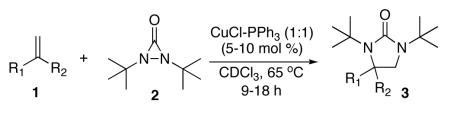
The application of this catalytic diamination to the synthesis of potent NK1 antagonist 4,4-disubstituted 2-imidazolidinone Sch 42507817 is outlined in Scheme 3. Disubstituted terminal olefin 9 was readily prepared in 77% yield by reaction between α-bromomethylstyrene (7) and commercially available (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethanol using NaH as base. Diamination of olefin 9 with CuCl-P(OPh)3 and di-tert-butyldiaziridinone (2) gave 4,4-disubstituted 2-imidazolidinones 10 and 11 in 35% and 30% yield respectively,21 after flash chromatography (the less polar spot on the TLC corresponds to compound 10). Removal of both tert-butyl groups of compound 10 provided compound 12 (Sch 425078) in 74% yield.
Scheme 3.
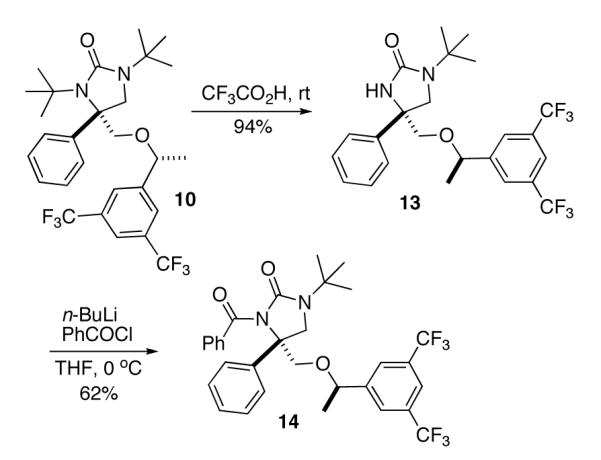
To further confirm the configuration of the diamination product 10, one tert-butyl group was selectively removed using CF3CO2H at room temperature to give compound 13, which was converted to compound 14 with n-BuLi and benzoyl chloride22 (Scheme 4). The structure of compound 14 was determined by X-ray analysis (Figure 2). The determination of the structure of monodeprotected product 13 supports the structure assignment of mono-deprotected compound 4a in Scheme 2.
In summary, a variety of disubstituted terminal olefins have been effectively diaminated using CuCl as catalyst and di-tert-butyldiaziridinone as nitrogen source, which provides a rapid access to various 4,4-disubstituted-2-imidazolidinones.23 In addition, the synthesis of 4,4-disubstituted-2-imidazolidinone Sch 425078 (potent NK1 antagonist) has been achieved in three steps using this diamination. The ability to selectively remove one or two protecting groups would provide opportunities to introduce different substituents on the nitrogens if desired. Future efforts will be devoted to the development of an asymmetric diamination process and its applications.
Supplementary Material
Acknowledgment
We are grateful to the generous financial support from the General Medical Sciences of the National Institutes of Health (GM083944-02).
Footnotes
Supporting Information Available: The experimental procedures, the characterization of diamination products 3, NOE studies of compund 4a, and the 1H and 13C NMR spectra of compounds 3, 4a, 5a, 6a, and 9-14 along with the X-ray data of compound 14 (74 pages). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1) (a).For leading reviews, see: Lucet D, Le Gall T, Mioskowski C. Angew. Chem., Int. Ed. 1998;37:2580. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L.Mortensen MS, O’Doherty GA. Chemtracts: Org. Chem. 2005;18:555.Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug Des. 2006;67:101. doi: 10.1111/j.1747-0285.2006.00347.x.de Figueiredo RM. Angew. Chem., Int. Ed. 2009;48:1190. doi: 10.1002/anie.200804362.
- (2) (a).For examples of metal-mediated diaminations, see: Tl: Aranda VG, Barluenga J, Aznar F. Synthesis. 1974:504.Os: Chong AO, Oshima K, Sharpless KB. J. Am. Chem. Soc. 1977;99:3420.Muñiz K. Eur. J. Org. Chem. 2004:2243.Pd: Bäckvall J-E. Tetrahedron Lett. 1978:163.Hg: Barluenga J, Alonso-Cires L, Asensio G. Synthesis. 1979:962.Co: Becker PN, White MA, Bergman RG. J. Am. Chem. Soc. 1980;102:5676.Mn: Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J. Org. Chem. 1985;50:3647.
- (3) (a).For a recent Cu(II)-mediated intramolecular diamination, see: Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250. doi: 10.1021/ja053335v.Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035. doi: 10.1021/ol0706713.
- (4) (a).For recent Pd(II)- and N(II)-catalyzed intramolecular diamination of olefins, see: Streuff J, Hövelmann CH, Nieger M, Muñiz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y.Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew. Chem., Int. Ed. 2007;46:7125. doi: 10.1002/anie.200702160.Muñiz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f.Muñiz K, Hövelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763. doi: 10.1021/ja075041a.Sibbald PA, Michael FE. Org. Lett. 2009;11:1147. doi: 10.1021/ol9000087.
- (5) (a).For Rh(II)- and Fe(III)-catalyzed diamination with TsNCl2, see: Li G, Wei H-X, Kim SH, Carducci MD. Angew. Chem., Int. Ed. 2001;40:4277. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I.Wei H-X, Kim SH, Li G. J. Org. Chem. 2002;67:4777. doi: 10.1021/jo0200769.
- (6).For a recent Pd(II)-catalyzed intermolecular diamination of conjugated dienes, see: Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308. doi: 10.1021/ja051181d.
- (7) (a).For Pd(0)-catalyzed intermolecular diamination of conjugated dienes and trienes using di-tert-butyldiaziridinone, see: Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762. doi: 10.1021/ja0680562.Xu L, Du H, Shi Y. J. Org. Chem. 2007;72:7038. doi: 10.1021/jo0709394.Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688. doi: 10.1021/ja074698t.
- (8) (a).For Cu(I)-catalyzed intermolecular diamination of conjugated dienes and trienes using di-tert-butyldiaziridinone, see: Yuan W, Du H, Zhao B, Shi Y. Org. Lett. 2007;9:2589. doi: 10.1021/ol071105a.Du H, Zhao B, Yuan W, Shi Y. Org. Lett. 2008;10:4231. doi: 10.1021/ol801605w.
- (9).For Cu(I)-catalyzed intermolecular diamination of conjugated dienes, trienes, and activated terminal olefins using diaziridinimine, see: Zhao B, Du H, Shi Y. Org. Lett. 2008;10:1087. doi: 10.1021/ol702974s.
- (10) (a).For Pd(0)-catalyzed intermolecular allylic and homoallylic C-H diamination of terminal olefins using di-tert-butyldiaziridinone, see: Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:7496. doi: 10.1021/ja072080d.Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394.
- (11).For Pd(0)-catalyzed dehydrogenative diamination of terminal olefins using di-tert-butylthiadiaziridine 1,1-dioxide, see: Wang B, Du H, Shi Y. Angew. Chem., Int. Ed. 2008;47:8224. doi: 10.1002/anie.200803184.
- (12).For Cu(I)-catalyzed intermolecular diamination of activated terminal olefins using di-tert-butylthiadiaziridine 1,1-dioxide, see: Zhao B, Yuan W, Du H, Shi Y. Org. Lett. 2007;9:4943. doi: 10.1021/ol702061s.
- (13).Greene FD, Stowell JC, Bergmark WR. J. Org. Chem. 1969;34:2254. [Google Scholar]
- (14).For a leading review on diaziridinones, see: Heine HW. In: The Chemistry of Heterocyclic Compounds. Hassner A, editor. John Wiley & Sons, Inc.; Hoboken, NJ: 1983. p. 547.
- (15) (a).Quast H, Schmitt E. Angew. Chem. Int. Ed. 1969;8:448. [Google Scholar]; (b) L’abbé G, Verbruggen A, Minami T, Toppet S. J. Org. Chem. 1981;46:4478. [Google Scholar]; (c) Mestres R, Palomo C. Synthesis. 1980:755. [Google Scholar]
- (16).Timberlake JW, Alender J, Garner AW, Hodges ML, Özmeral C, Szilagyi S, Jacobus JO. J. Org. Chem. 1981;46:2082. [Google Scholar]
- (17) (a).For leading references, see: Reichard GA, Stengone C, Paliwal S, Mergelsberg I, Majmundar S, Wang C, Tiberi R, McPhail AT, Piwinski JJ, Shih N-Y. Org. Lett. 2003;23:4249. doi: 10.1021/ol030104p.Shue H-J, Chen X, Shih N-Y, Blythin DJ, Paliwal S, Lin L, Gu D, Schwerdt JH, Shah S, Reichard GA, Piwinski JJ, Duffy RA, Lachowicz JE, Coffin VL, Liu F, Nomeir AA, Morgan CA, Varty GB. Bioorg. Med. Chem. Lett. 2005;15:3896. doi: 10.1016/j.bmcl.2005.05.111.Shue H-J, Chen X, Schwerdt JH, Paliwal S, Blythin DJ, Lin L, Gu D, Wang C, Reichard GA, Wang H, Piwinski JJ, Duffy RA, Lachowicz JE, Coffin VL, Nomeir AA, Morgan CA, Varty GB, Shih N-Y. Bioorg. Med. Chem. Lett. 2006;16:1065. doi: 10.1016/j.bmcl.2005.10.072.
- (18).When the CuCl-catalyzed diamination of 2-phenylpropene was carried out with di-tert-butylthiadiaziridine 1,1-dioxide or 1,2-di-tert-butyl-3-(cyanimino)diaziridine as the nitrogen source under the previously reported conditions (refs. 9 and 12), only a small amount of diamination product or a messy mixtutre was obtained, respectively.
- (19).A representative diamination procedure (Table 1, entry 1): To a 1.5 mL vial equipped with a stir bar was added CuCl (0.002 g, 0.02 mmol), triphenylphosphine (0.0052 g, 0.02 mmol), and CDCl3 (0.3 mL). After the mixture was stirred at room temperature for 10 min, 2-phenylpropene (1a) (0.047 g, 0.4 mmol) was added. The reaction mixture was warmed to 65 °C using an oil bath with stirring, and ditert-butyldiaziridinone (2) (0.136 g, 0.8 mmol) was added by syringe pump over 8 h. The reaction mixture was stirred at this temperature for an additional 1 h and purified by flash chromatography (silica gel, hexane/ether = 10/1) to give diamination product 3a as a white solid (0.105 g, 91%).
- (20).Zhao B, Du H, Shi Y. J. Am. Chem. Soc. 2008;130:7220. doi: 10.1021/ja802242h. [DOI] [PubMed] [Google Scholar]
- (21).Diamination of olefin 9 with CuCl-PPh3 (1:1) (10 mol %) in CDCl3 at 65 °C for 10 h gave compound 10 in 30% yield and 11 in 33% yield. Diamination of 9 with CuCl-(R)-DTBM-SEGPHOS (2:1) (10 mol %)8 in CDCl3 at 65 °C for 10 h gave compound 10 in 32% yield and 11 in 29% yield.
- (22).Rahimizadeh M, Kam K, Jenkins SI, McDonald RS, Harrison PHM. Can. J. Chem. 2002;80:517. [Google Scholar]
- (23) (a).For recent leading references on other approaches to 4,4-disubstituted-2-imidazolidinones, see: Trost BM, Fandrick DR. J. Am. Chem. Soc. 2003;125:11836. doi: 10.1021/ja037450m.Mehrotra MM, Heath JA, Smyth MS, Pandey A, Rose JW, Seroogy JM, Volkots DL, Nannizzi-Alaimo L, Park GL, Lambing JL, Hollenbach SJ, Scarborough RM. J. Med. Chem. 2004;47:2037. doi: 10.1021/jm030354b.Shen D, Zhang F, Brady EJ, Candelore MR, Dallas-Yang Q, Ding VD-H, Dragovic J, Feeney WP, Jiang G, McCann PE, Mock S, Qureshi SA, Saperstein R, Shen X, Tamvakopoulos C, Tong XC, Tota LM, Wright MJ, Yang X, Zheng S, Chapman KT, Zhang B, Tata JR, Parmee ER. Bioorg. Med. Chem. Lett. 2005;15:4564. doi: 10.1016/j.bmcl.2005.06.101.Brackmann F, Es-Sayed M, de Meijere A. Eur. J. Org. Chem. 2005:2250.Nadir UK, Krishna RV, Singh A. Tetrahedron Lett. 2005;46:479.Fritz JA, Nakhla JS, Wolfe JP. Org. Lett. 2006;8:2531. doi: 10.1021/ol060707b.Kim M, Mulcahy JV, Espino CG, Du Bois J. Org. Lett. 2006;8:1073. doi: 10.1021/ol052920y.Lanter JC, Fiordeliso JJ, Allan GF, Musto A, Hahn DW, Sui Z. Bioorg. Med. Chem. Lett. 2006;16:5646. doi: 10.1016/j.bmcl.2006.08.036.Fritz JA, Wolfe JP. Tetrahedron. 2008;64:6838. doi: 10.1016/j.tet.2008.04.015.Park H, Min K, Kwak H-S, Koo KD, Lim D, Seo S-W, Choi J-U, Platt B, Choi D-Y. Bioorg. Med. Chem. Lett. 2008;18:2900. doi: 10.1016/j.bmcl.2008.03.081.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.