Abstract
Context
Modafinil, a wake-promoting drug used to treat narcolepsy, is increasingly being used as a cognitive enhancer. Although initially launched as distinct from stimulants that increase extracellular dopamine by targeting dopamine transporters, recent preclinical studies suggest otherwise.
Objective
To measure the acute effects of modafinil at doses used therapeutically (200 mg and 400 mg given orally) on extracellular dopamine and on dopamine transporters in the male human brain.
Design, Setting, and Participants
Positron emission tomography with [11C]raclopride (D2/D3 radioligand sensitive to changes in endogenous dopamine) and [11C]cocaine (dopamine transporter radioligand) was used to measure the effects of modafinil on extracellular dopamine and on dopamine transporters in 10 healthy male participants. The study took place over an 8-month period (2007–2008) at Brookhaven National Laboratory.
Main Outcome Measures
Primary outcomes were changes in dopamine D2/D3 receptor and dopamine transporter availability (measured by changes in binding potential) after modafinil when compared with after placebo.
Results
Modafinil decreased mean (SD) [11C]raclopride binding potential in caudate (6.1% [6.5%]; 95% confidence interval [CI], 1.5% to 10.8%; P=.02), putamen (6.7% [4.9%]; 95% CI, 3.2% to 10.3%; P=.002), and nucleus accumbens (19.4% [20%]; 95% CI, 5% to 35%; P=.02), reflecting increases in extracellular dopamine. Modafinil also decreased [11C]cocaine binding potential in caudate (53.8% [13.8%]; 95% CI, 43.9% to 63.6%; P<.001), putamen (47.2% [11.4%]; 95% CI, 39.1% to 55.4%; P<.001), and nucleus accumbens (39.3% [10%]; 95% CI, 30% to 49%; P=.001), reflecting occupancy of dopamine transporters.
Conclusions
In this pilot study, modafinil blocked dopamine transporters and increased dopamine in the human brain (including the nucleus accumbens). Because drugs that increase dopamine in the nucleus accumbens have the potential for abuse, and considering the increasing use of modafinil, these results highlight the need for heightened awareness for potential abuse of and dependence on modafinil in vulnerable populations.
Modafinil is a wake-promoting medication used in the treatment of narcolepsy and other sleep disorders. Modafinil may enhance cognition and is used off-label for the treatment of cognitive dysfunction in some psychiatric disorders (ie, schizophrenia, attention-deficit/hyperactivity disorder [ADHD]).1 Moreover, modafinil is increasingly being diverted for nonmedical use by healthy individuals with the expectation that it will improve cognitive performance.2 Although modafinil apparently has very low abuse liability (low reinforcing effects) in non–drug abusing individuals, the Physicians’ Desk Reference cautions that it can produce psychoactive and euphoric effects typical of central nervous system stimulant drugs,3 and there is debate surrounding its potential for abuse.4,5
The mechanisms of action of modafinil are not well understood but are believed to differ from those of stimulant medications (methylphenidate and amphetamine), which increase dopamine in brain by targeting the dopamine transporters.6 It is theorized that modafinil’s effects in the brain involve hypocretin, histamine, epinephrine, γ-aminobutyric acid, and glutamate.7,8 However, there is mounting preclinical evidence that dopamine is involved. For example, mice lacking dopamine transporters do not respond to the wake-promoting effects of modafinil,9 and this is also true for mice lacking D1 and D2 receptors.10 Microdialysis studies have also reported that modafinil increases extracellular dopamine.9,11–13 In addition, a recent imaging study in anesthetized monkeys documented significant occupancy of dopamine transporters by intravenously administered modafinil.14 This latter study also reported significant occupancy of norepinephrine transporters by modafinil, which in conjunction with a recent functional magnetic resonance imaging study showing that modafinil decreased activity in the locus coeruleus,15 highlights a role for nor-epinephrine in modafinil’s effects.
The growing use of modafinil in clinical medicine and as a cognitive enhancing agent and the uncertainties surrounding the mechanisms underlying its pharmacological effects highlight the need to better understand its mechanisms of action. Of particular relevance is the need to resolve the question of whether modafinil at the doses used therapeutically increases dopamine in the human brain. This is relevant because drugs that increase dopamine in brain, particularly those that increase dopamine in the nucleus accumbens, a brain region critical for the rewarding effects of drugs of abuse, have the potential for being diverted, and repeated use by individuals who are vulnerable can result in addiction.16 We tested the hypothesis that modafinil, at therapeutically relevant doses, would elevate extracellular dopamine in the human brain by blocking the dopamine transporter. We tested 2 doses of modafinil: 200 mg, the dose recommended for narcolepsy, and 400 mg, a dose shown to be beneficial for the treatment of ADHD.17
METHODS
Participants
This study was carried out at Brookhaven National Laboratory from 2007 to 2008 and approved by the local institutional review board (Committee on Research Involving Human Subjects, State University of New York at Stony Brook). Written informed consent was obtained from all participants after the study had been fully explained to them. Participants were paid for their participation and received information on potential adverse effects of modafinil during the consenting process. Participants were initially screened by phone and if appropriate were referred for evaluation by a neurologist (F.T.) who ensured that they met study criteria.
Ten healthy men with a mean (SD) age of 34 (7.1) years (range, 23–46 years) who responded to a local newspaper advertisement were selected for the study out of 50 screened participants. Inclusion criteria were male sex, nonsmoking, ability to understand and give informed consent, and age of 18 to 50 years. Excluded were those participants who were urine positive for psychoactive drugs (including phencyclidine, cocaine, amphetamine, opiates, barbiturates, benzodiazepines, and tetrahydrocannabinol); those with clinically significant abnormal laboratory values; those with history of or current medical illness or neurological or psychiatric disease (including mood fluctuations); those who had used psychotropic medications in the past month; those who had experienced head trauma with loss of consciousness longer than 30 minutes; and those with a history of or current substance abuse (including nicotine).
Study Design
[11C]Raclopride (dopamine D2/D3 radiotracer labeled with carbon 11 that competes for binding with endogenous dopamine18) was used to serve as an indicator of changes in extracellular dopamine and [11C]cocaine to measure dopamine transporter availability.18,19 Measures were obtained after a placebo and after an oral dose of modafinil (200 mg or 400 mg) in 10 healthy men. Using [11C]cocaine as a radiotracer for the dopamine transporters also afforded the opportunity to assess whether modafinil binds to the same or a closely associated site as cocaine on dopamine transporter molecules.
Participants were scanned 4 different times over a 2-day period (at least 1 week apart from each other); on one day they underwent 2 scans with [11C]cocaine, and on another day they underwent 2 scans with [11C]raclopride. The order of radiotracers was varied to control for potential ordering effects as follows: for the 200-mg group, 3 participants received the [11C]raclopride first and for the 400-mg group, 2 participants received the [11C]raclopride first, whereas the rest of the participants received the [11C]cocaine first. We completed giving the doses and performing the scans with the 200-mg group before the 400-mg group. On each day, the first scan was done 2 hours after administration of placebo and the second scan was done 2 hours after administration of modafinil, which was given immediately on completion of the first scan.
Participants were blinded to whether they would receive placebo or modafinil or the dose received. Measures of modafinil concentration in plasma were obtained at 2 hours after modafinil (corresponding to the time of scan initiation) and analyzed using high-performance liquid chromatography with spectrophotometric detection (Analytical Psychopharmacology Laboratories, Nathan Kline Institute, Orangeburg, New York). The placebo plasma sample served as a blank for the measurements.
Radiotracer Synthesis and PET Studies
[11C]Cocaine was synthesized from nor-cocaine (National Institute on Drug Abuse Research Technology Branch, Rockville, Maryland) according to the literature method.19 Radiochemical purity was greaterthan98%, mean (SD) specific activity was 61.8 (34.3) MBq/nmol at end of synthesis, and the mean (SD) injected dose was 258 (21.1) MBq. Dynamic positron emission tomographic (PET) imaging was carried out on a Siemens HR+high-resolution, whole-body PET scanner (4.5×4.5×4.8 mm full width at half maximum at center of field of view) in three-dimension acquisition mode in 63planes(Siemens Medical Solutions Inc, Knoxville, Tennessee). For all scans, a transmission scan was obtained with a germanium 68 rotating rod source prior to the emissions can to correct for attenuation. Scanning was carried out for 54 minutes with the following time frames: 1×10 seconds, 12×5 seconds, 1×20 seconds, 1×30 seconds, 4×60 seconds, 4×120 seconds, and 8×300 seconds.
[11C]Raclopride was synthesized by the literature method.20 Radiochemical purity was greater than 98%, mean (SD) specific activity was 67.7 (42.9) MBq/nmol at time of injection, and the mean (SD) injected dose was 235 (29.6) MBq. Scanning was carried out for 60 minutes with the following time frames: 1×10 seconds, 12×5 seconds, 1×20 seconds, 1×30 seconds, 8×60 seconds, and 10×300 seconds. Arterial blood was collected over the course of the study, and plasma was obtained and analyzed for the fraction of carbon 11 present as the parent radiotracer.21
Drug Effect Ratings
Recordings for heart rate and blood pressure were obtained continuously throughout the study. Behavioral effects were evaluated with 2 types of scales. Analog scales assessed self-reports for the descriptors alert, anxious, high, mood, restless, and tired, on a scale of 1 (felt nothing) to 10 (felt intensely).22 The Profile of Mood Scales23 is a scale widely used to assess the effects of drugs on mood states, which were rated on a 10-point scale ranging from 1 (not at all) to 10 (extremely). These measures were obtained prior to and at 2 hours and 3 hours after administration of placebo or of modafinil.
Image Processing and Parameter Estimation
To obtain as high a signal as possible for anatomical region identification, the time frames were summed over the entire scanning period. The summed images were resliced along the anterior commissure–posterior commissure line and planes were summed in groups of 2 for the purpose of placing the regions of interest. For both [11C]cocaine and [11C]raclopride, regions of interest were placed on the caudate, putamen, and cerebellum and then projected onto the dynamic images. For the nucleus accumbens, since it could not be identified clearly on the individual images, the average image with its increased signal-to-noise ratio was used to identify the region and then projected onto the individual (coregistered) images. To minimize misregistration errors, the top 50 pixels from each study were used for quantification. The right and left sides were quantified separately and then averaged after ensuring that there were no significant laterality effects.
Dopamine receptors and dopamine transporters are highly concentrated in caudate, putamen, and nucleus accumbens whereas their concentration in cerebellum is negligible. Thus, the cerebellum serves as a reference region to control for nonspecific binding. Time-activity curves along with the time course of the arterial concentration of the radiotracer were used to calculate K1, the transfer constant from plasma to brain, and the distribution volumes (VT), which correspond to the equilibrium measurement of the ratio of tissue to plasma concentration in the caudate, putamen, nucleus accumbens, and cerebellum using graphical analysis methods for reversible systems.24 The ratio of the VT in the caudate, putamen, and nucleus accumbens to that in the cerebellum is referred to as the distribution volume ratio. The distribution volume ratio minus 1 corresponds to the binding potential (BPND) and is insensitive to changes in blood flow.25 The BPND for the caudate, putamen, and nucleus accumbens was used as an estimate of dopamine transporter availability and D2/D3 receptor availability after placebo and after modafinil. Data are expressed as means and standard deviations. There were no missing data points.
Formation of Averaged Images
Averaging data across participants reduces random noise, which tends to cancel while enhancing the signal, which should be additive. To make images with an improved signal-to-noise ratio, the individual time frame images for all participants in a group were averaged. To average brains of different shapes and sizes, we first normalized them to the SPM template26 so that all brains had corresponding structures in the same space. The blood data from each participant were also averaged for each time frame. Using the mean dynamic image (averaged over all participants in the group) and the average input (blood radioactivity) function, an average distribution volume image was created. The BPND image was created by dividing each voxel in the image by the cerebellum distribution volume and subtracting 1.
Statistics
The sample size was determined based on results from studies that measured dopamine transporter occupancy and dopamine changes with 20-mg oral methylphenidate.27,28 Primary outcomes were changes in dopamine D2/D3 receptor and dopamine transporter availability as measured by changes in BPND after placebo and after modafinil. Significant effects were considered for P<.05 (2-tail). For dopamine transporter occupancy, we predicted a mean (SD) occupancy of 50% (5%); thus, the estimated power of the paired t test at the significance level of .05 (2-sided) with n=10 was 99%. For dopamine changes, we predicted a 6% (6%) change; thus, the statistical power to detect significance at α =.05 (2-sided) with n=10 was 80%. Repeated-measures analysis of variance (ANOVA) was used to examine the modafinil effect (placebo vs modafinil), the dose effect (200 mg vs 400 mg), and the modafinil and dose interaction for K1 and BPND for both [11C]cocaine and [11C]raclopride.
Plasma modafinil levels were compared for the 200-mg and 400-mg dose groups and for the same participant on day 1 and day 2 using repeated-measures ANOVA (fixed effect: dose groups, 200 mg vs 400 mg; repeated measures, day 1 vs day 2). Pearson product moment correlations were used to assess the association between individual plasma modafinil levels and percentage change in BPND for [11C]cocaine and [11C]raclopride. Repeated-measures ANOVA was also used to compare the differences between placebo and modafinil and between the 2 modafinil doses in the cardiovascular and behavioral measures. Statistical analysis was performed with SAS version9.2 (SAS Institute Inc, Cary, North Carolina).29
RESULTS
Modafinil significantly increased heart rate and systolic blood pressure and these effects were significant for both doses. Repeated-measures ANOVA revealed a dose effect that showed higher increases in heart rate for 400-mg than for 200-mg modafinil (P<.05). None of the effects of modafinil on the behavioral measures were significant.
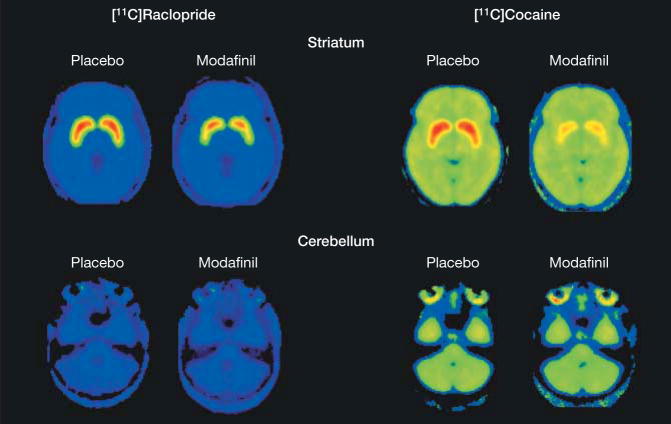
Repeated-measures ANOVA showed no significant dose effect (200 mg vs 400 mg) nor significant dose and drug (placebo vs modafinil) interaction effect for all BPND and K1 measures. Therefore in the subsequent analysis, the 200-mg and 400-mg dose groups were combined. Modafinil produced significant reductions in BPND for [11C]raclopride and for [11C]cocaine (Table), and this can be seen in BPND images after placebo and after modafinil for [11C]raclopride and [11C]cocaine (Figure 1). Modafinil decreased mean (SD)[11C]raclopride BPND in caudate (6.1% [6.5%]; 95% CI, 1.5% to 10.8%; P=.02), putamen (6.7% [4.9%]; 95% CI, 3.2% to 10.3%; P=.002), and nucleus accumbens (19.4% [20%]; 95% CI, 5% to 35%; P=.02), reflecting increases in dopamine. It also decreased [11C]cocaine BPND in caudate (53.8% [13.8%]; 95% CI, 43.9% to 63.6%; P<.001), putamen(47.2% [11.4%]; 95% CI, 39.1% to 55.4%; P<.001), and nucleus accumbens (39.3% [10%]; 95% CI, 30% to 49%; P=.001), reflecting occupancy of the dopamine transporters.
Table.
Model Terms, K1, and Binding Potential for [11C]Raclopride and [11C]Cocaine for the Placebo and Modafinil Conditions
| Mean (SD) |
Mean Difference Between Placebo and Modafinil (95% CI)b | |||
|---|---|---|---|---|
| Model Term | Placebo | Modafinil | P Valuea | |
| [11C]Raclopride | ||||
| K1, mL/cc/min | ||||
| Cerebellum | 0.112 (0.033) | 0.103 (0.015) | .30 | 0.009 (−0.01 to 0.03) |
|
| ||||
| Caudate | 0.101 (0.015) | 0.096 (0.013) | .36 | 0.005 (−0.01 to 0.02) |
|
| ||||
| Putamen | 0.121 (0.018) | 0.116 (0.013) | .38 | 0.005 (−0.01 to 0.02) |
|
| ||||
| BPND | ||||
| Caudate | 2.68 (0.49) | 2.53 (0.56) | .01 | 0.15 (0.04 to 0.26) |
|
| ||||
| Putamen | 3.44 (0.49) | 3.22 (0.55) | .001 | 0.22 (0.11 to 0.33) |
|
| ||||
| Nucleus accumbens | 2.89 (0.46) | 2.31 (0.66) | .02 | 0.58 (0.16 to 1.04) |
|
| ||||
| [11C]Cocaine | ||||
| K1, mL/cc/min | ||||
| Cerebellum | 0.40 (0.07) | 0.44 (0.07) | .01 | −0.04 (−0.07 to −0.01) |
|
| ||||
| Caudate | 0.43 (0.08) | 0.46 (0.08) | .01 | −0.03 (−0.06 to −0.01) |
|
| ||||
| Putamen | 0.49 (0.10) | 0.54 (0.11) | .02 | −0.05 (−0.08 to −0.01) |
|
| ||||
| BPND | ||||
| Caudate | 0.74 (0.11) | 0.34 (0.19) | <.001 | 0.40 (0.31 to 0.49) |
|
| ||||
| Putamen | 0.95 (0.06) | 0.50 (0.09) | <.001 | 0.45 (0.36 to 0.54) |
|
| ||||
| Nucleus accumbens | 0.86 (0.16) | 0.52 (0.11) | .001 | 0.34 (0.26 to 0.43) |
Abbreviations: BPND, binding potential; CI, confidence interval; K1, transfer constant from plasma to brain.
Paired samples t tests are shown with the corresponding 2-sided P values. Independent samples t tests show that there is no significant difference between the 200-mg and 400-mg doses for any of these variables.
Mean differences (placebo - modafinil) and 95% confidence intervals for the combined 200-mg and 400-mg dose groups.
Figure 1.
Averaged Positron Emission Tomography Images for After Placebo and After Modafinil
Averaged [11C]raclopride and [11C]cocaine binding potential (BPND) images at the level of the striatum (top row) and cerebellum (bottom row) after placebo and after modafinil. The color scale is a rainbow scale with red representing the highest value, which corresponds to a BPND of 4.4 in the [11C]raclopride images and a BPND of 1.1 in the [11C]cocaine images.
For the combined group, K1 for [11C]cocaine (but not for [11C]raclopride) was significantly elevated in cerebellum, caudate, and putamen after modafinil, indicating that the transfer of [11C]cocaine from blood to brain was elevated by modafinil (Table). We note K1 is related to blood flow (F) and permeability surface area product (PS) by the equation K1 =F(1−e−(PS/F)).30 Depending on the ratio PS/F, K1 can have values close to F (when PS is much greater than F) or close to PS (when F is much greater than PS). For [11C]raclopride, the latter condition holds because K1 is much less than blood flow (which is ~0.5 mL/min/cc). For [11C]cocaine, the former condition holds because K1 is larger and closer to typical blood flow values. Increases in cerebral blood flow from modafinil, which have been documented previously,31 would therefore be more likely to be seen as increases in K1 for [11C]cocaine but not for [11C]raclopride.
Plasma modafinil concentrations were compared for the 200-mg and 400-mg groups. Although the mean (SD) 400-mg group values tended to be higher (6.2 [2.6] μg/mL vs 4.3 [1.6] μg/mL), repeated-measures ANOVA (fixed effect: dose groups, 200 mg vs 400 mg; repeated measures, day 1 vs day 2) revealed that plasma modafinil concentrations did not differ significantly between doses (F1,8=4.36, P =.07). For the same participant, they also did not differ between day 1 and day 2 (F1,8=2.85, P=.13) (Figure 2).
Figure 2.
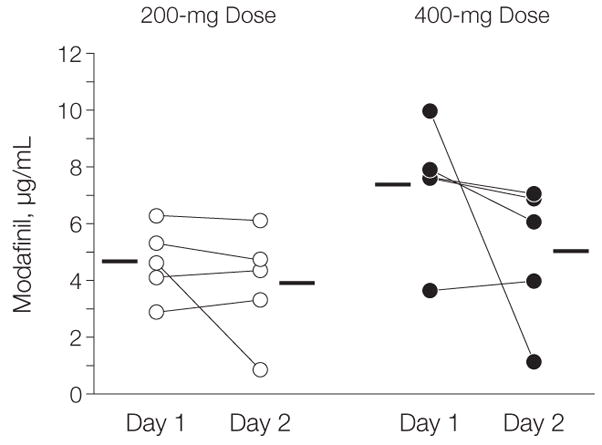
Plasma Modafinil Concentrations in Study Participants Who Received Either 200 mg or 400 mg of Modafinil
Plasma modafinil concentrations were measured 2 hours after either 200 mg or 400 mg of modafinil was administered orally on day 1 and day 2. Lines connect the repeated measures for each participant, and horizontal bars indicate the mean modafinil concentration of the samples for each set.
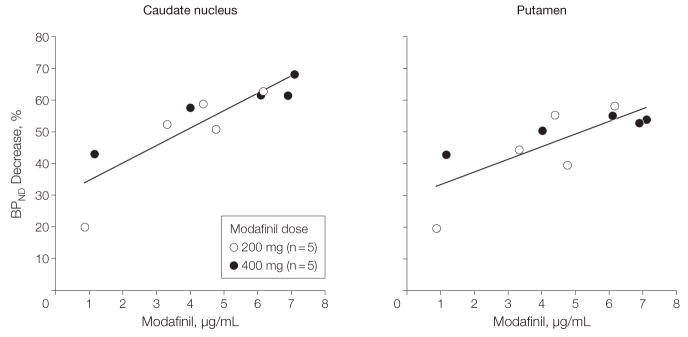
There was a significant correlation between the percentage decrease in [11C]cocaine BPND and plasma modafinil concentration, which corresponded in caudate to R=0.87 (P<.001) and in putamen to R = 0.76 (P = .01) (Figure 3). There were no significant correlations between the percentage decrease in [11C]raclopride BPND and the concentration of modafinil in plasma (R=−0.38, P=.28, 2-sided). The correlation between modafinil-induced changes in [11C]cocaine BPND and changes in [11C]raclopride BPND was not significant for either the caudate (R = 0.37, P = .30) or the putamen (R=−0.11, P=.76, 2-sided).
Figure 3.
Percentage Decrease in [11C]Cocaine Binding Potential vs Plasma Modafinil Concentration
Points on the plot correspond to individual participants where percentage of dopamine transporter decrease was paired with the value of the plasma modafinil concentration measured at the time of the beginning of the positron emission tomographic scan for both the caudate (R=0.87, P<.001) and the putamen (R=0.77, P=.01). BPND indicates binding potential. Curves were fit with linear regression.
COMMENT
At clinically relevant doses, modafinil significantly increases dopamine in the human brain by blocking dopamine transporters. Modafinil’s binding to the dopamine transporter overlaps with the binding site of cocaine because [11C]cocaine binding in striatum was inhibited by modafinil. Along with the mounting evidence from the preclinical literature, this finding provides support for the role of dopamine in modafinil’s pharmacological actions in humans. Thus, the hypothesis of the nondopamine mechanism of action of modafinil needs to be reconsidered.
The mean (SD) reductions in [11C]raclopride and [11C]cocaine BPND after modafinil were similar to those reported for a 20-mg oral dose of methylphenidate in normal volunteers, which corresponded to about 5% (6%) for raclopride28 and to about 54% (5%) for [11C]cocaine.27 This indicates that modafinil at therapeutic doses produces elevations in brain dopamine through blockade of dopamine transporters, which are similar to those produced by therapeutic doses of methylphenidate. Even though modafinil’s affinity for dopamine transporters is low relative to methylphenidate (6.390 μM vs 0.025 μM14), the therapeutic doses are much higher for modafinil (200 mg) than for methylphenidate (20 mg).
Drug Effects
Two doses of modafinil (200 mg and 400 mg) were evaluated, but neither the plasma modafinil concentrations nor the dopamine transporter blockade or dopamine changes differed between the 2 doses, which may reflect the relatively small sample of the study. The lack of a dose effect is also confounded by the large participant variability in modafinil’s plasma concentrations (particularly for 1 participant in the 400-mg dose group) (Figure 2), which predicted the level of dopamine transporter occupancy (Figure 3).
The variability in plasma modafinil concentration is likely to reflect variability in metabolism of modafinil. Neither modafinil’s plasma concentration nor the level of dopamine transporter occupancy correlated with the dopamine changes. We had reported a similar finding with methylphenidate in which a correlation was found between plasma concentration and dopamine transporter occupancy, but neither plasma concentration nor dopamine transporter occupancy correlated with dopamine changes.32 This reflects that although plasma (and presumably brain) modafinil levels proportionally compete with [11C]cocaine for binding to the dopamine transporters, decreases in [11C]raclopride binding are a function of changes in extracellular dopamine, which are determined not only by dopamine transporter blockade but also by dopamine cell firing. For the same level of dopamine transporter blockade, dopamine changes will be greater when the activity of dopamine cells is high than when it is low.
Stimulant medications act as wake-promoting agents by increasing dopamine (as well as norepinephrine) in brain.6 Modafinil was developed with an expectation that a medication could have a nondopaminergic target for its wake-promoting effects. However, the current findings in humans, along with preclinical studies,9,10 documenting the indispensable role of dopamine in the wake-promoting effects of modafinil, support modafinil’s dopamine-enhancing effects as a mechanism for its therapeutic actions.
The dopamine-enhancing effects of modafinil in the nucleus accumbens may help explain reports of its abuse, since this pharmacological effect is considered crucial for drug reinforcement.33 Indeed, modafinil was shown to be self-administered in monkeys previously trained to self-administer cocaine,34 and in humans modafinil can act as a reinforcer under conditions of behavioral demand.35 However, modafinil is much less potent as a reinforcer than stimulant drugs, and reports of modafinil abuse are rare and much less frequent than those for stimulant drugs.36 Nonetheless, considering the broadening use of modafinil and the results in this study showing that it increases dopamine in the nucleus accumbens at therapeutic doses, its potential for abuse should not be disregarded.
In this study, modafinil’s binding to dopamine transporters overlapped with the binding site of cocaine. This could account for the findings that modafinil interfered with the behavioral effects of cocaine.37 Indeed pilot studies have reported some beneficial effects of modafinil in the treatment of cocaine addiction.38
Study Limitations
The [11C]raclopride method does not allow the exclusion of the possibility that decreases reflect down-regulation of D2/D3 receptors and changes in affinity39 rather than dopamine increases. Since microdialysis studies9,11–13 have shown that modafinil increases dopamine in striatum (including nucleus accumbens), this suggests that the findings in this study reflect dopamine increases. The small sample size of the study did not provide sufficient statistical power to detect dose effects. Only healthy young men were tested, which may limit generalizability to other populations. This study did not use a complete placebo design but rather used a placebo to compare the effects of modafinil on each radiotracer, which required that the placebo be given first and the modafinil second, so the possibility of an order effect cannot be ruled out. The order of modafinil doses tested was not randomized; instead the first 5 participants were tested with 200 mg and the subsequent 5 with 400 mg. However, it is unlikely that this affected the results obtained. This study did not measure a clinical outcome, so further studies are necessary to assess this.
CONCLUSION
In this pilot study, modafinil acutely increased dopamine levels and blocked dopamine transporters in the human brain. Because drugs that increase dopamine have the potential for abuse, and considering the increasing use of modafinil for multiple purposes, these results suggest that risk for addiction in vulnerable persons merits heightened awareness.
Acknowledgments
Financial Disclosures: None reported.
Funding/Support: This research was carried out at Brookhaven National Laboratory under contract DE-AC02-98CH10886 with the US Department of Energy with infrastructure support from its Office of Biological and Environmental Research. Support was also provided by grant K05DA020001 (J.S.F.) from the National Institutes of Health, the National Institute on Alcohol Abuse and Alcoholism Intramural research program, grant F32EB997320 (J.M.H.) from the National Institute of Biomedical Imaging and Bioengineering, and grant MO1RR10710 from the General Research Clinical Centers. A Goldhaber distinguished fellowship provided support for Dr Hooker.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Volkow had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. Drs Volkow and Fowler contributed equally to this work.
Study concept and design: Volkow, Fowler, Wang.
Acquisition of data: Telang, Wang, Jayne, Hubbard, Carter, Warner, King, Shea, Xu, Muench, Apelskog-Torres.
Analysis and interpretation of data: Volkow, Fowler, Logan, Alexoff, Zhu, Hooker, Wong.
Drafting of the manuscript: Volkow, Fowler, Alexoff, Telang, Wang, Jayne, Hooker, Wong, Hubbard, Carter, Warner, King, Shea, Xu, Muench, Apelskog-Torres.
Critical revision of the manuscript for important intellectual content: Volkow, Fowler, Logan, Zhu.
Statistical analysis: Zhu.
Obtained funding: Volkow, Fowler.
Administrative, technical, or material support: Telang, Wang, Jayne, Hubbard, Carter, Apelskog-Torres.
Study supervision: Volkow, Fowler, Wang.
Previous Presentation: Presented at the 55th Annual Society of Nuclear Medicine meeting; June 16, 2008; New Orleans, Louisiana.
Additional Contributions: David Schlyer, PhD, and Michael Schueller, PhD, Brookhaven National Laboratory, assisted with cyclotron operations. Joan Terry, and Hai-Dee Lee, MS, Brookhaven National Laboratory, assisted with clinical research center operations. Nikhil Pujari, BS, State University of New York at Stony Brook, helped with the image analysis, and Linda Thomas, BS, National Institutes of Health, provided editorial assistance. None received compensation outside of their salaries. We thank the individuals who volunteered for these studies, who each received a volunteer fee.
References
- 1.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 2.Enhancing not cheating. Nature. 2007;450 (7168):320. doi: 10.1038/450320a. [DOI] [PubMed] [Google Scholar]
- 3.Provigil (modafinil) Physicians’ Desk Reference. 60. Montvale, NJ: Thompson PDR; 2006. pp. 1002–1007. [Google Scholar]
- 4.Kruszewski SP, Klotz SG. Modafinil: mischaracterization. J Clin Psychiatry. 2007;68(6):970–971. doi: 10.4088/jcp.v68n0624b. [DOI] [PubMed] [Google Scholar]
- 5.Kruszewski SP. Euphorigenic and abusive properties of modafinil. Am J Psychiatry. 2006;163(3):549. doi: 10.1176/appi.ajp.163.3.549. [DOI] [PubMed] [Google Scholar]
- 6.Greenhill LL. The science of stimulant abuse. Pediatr Ann. 2006;35(8):552–556. doi: 10.3928/0090-4481-20060801-07. [DOI] [PubMed] [Google Scholar]
- 7.Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate- and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci U S A. 1996;93(24):14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21(5):1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28(34):8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraro L, Antonelli T, O’Connor WT, Tanganelli S, Rambert FA, Fuxe K. Modafinil: an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockers. Biol Psychiatry. 1997;42(12):1181–1183. doi: 10.1016/s0006-3223(97)00353-3. [DOI] [PubMed] [Google Scholar]
- 12.de Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport. 2001;12(16):3533–3537. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- 13.Murillo-Rodríguez E, Haro R, Palomero-Rivero M, Millán-Aldaco D, Drucker-Colín R. Modafinil enhances extracellular levels of dopamine in the nucleus accumbens and increases wakefulness in rats. Behav Brain Res. 2007;176(2):353–357. doi: 10.1016/j.bbr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Madras BK, Xie Z, Lin Z, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319 (2):561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 15.Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low tonic, high-phasic activity during functional MRI. Science. 2008;322(5908):1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- 16.Volkow N, Li TK. The neuroscience of addiction. Nat Neurosci. 2005;8(11):1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- 17.Biederman J, Swanson JM, Wigal SB, Boellner SW, Earl CQ, Lopez FA. Modafinil ADHD Study Group. A comparison of once-daily and divided doses of modafinil in children with attention-deficit/hyperactivity disorder: a randomized, double-blind, and placebo-controlled study. J Clin Psychiatry. 2006;67(5):727–735. doi: 10.4088/jcp.v67n0506. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang GJ, Fowler JS, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16(4):255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- 19.Fowler JS, Volkow ND, Wolf AP, et al. Mapping cocaine binding sites in human and baboon brain in vivo. Synapse. 1989;4(4):371–377. doi: 10.1002/syn.890040412. [DOI] [PubMed] [Google Scholar]
- 20.Ehrin E, Gawell L, Hogberg T, Depaulis T, Strom P. Synthesis of [methoxy-3H]- and [methoxy-11C]-labelled raclopride: specific dopamine-D2 receptor ligands. J Labelled Comp Radiopharm. 1987;24 (8):931–940. [Google Scholar]
- 21.Alexoff DL, Shea C, Fowler JS, et al. Plasma input function determination for PET using a commercial laboratory robot. Nucl Med Biol. 1995;22(7):893–904. doi: 10.1016/0969-8051(95)00042-v. [DOI] [PubMed] [Google Scholar]
- 22.Wang GJ, Volkow ND, Hitzemann RJ, et al. Behavioral and cardiovascular effects of intravenous methylphenidate in normal subjects and cocaine abusers. Eur Addict Res. 1997;3:49–54. [Google Scholar]
- 23.MacNair DM, Lorr M, Droppelman LF. EDITS Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 24.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10(5):740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 25.Logan J, Volkow ND, Fowler SJ, et al. Effects of blood flow on [11C]raclopride binding in the brain: model simulations and kinetic analysis of PET data. J Cereb Blood Flow Metab. 1994;14(6):995–1010. doi: 10.1038/jcbfm.1994.132. [DOI] [PubMed] [Google Scholar]
- 26.SPM: Statistical Parametric Mapping. [Accessed February 18, 2009]; http://www.fil.ion.ucl.ac.uk/spm/
- 27.Volkow ND, Wang GJ, Fowler JS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang GJ, Fowler JS, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44(3):175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute Inc. [Accessed February 18, 2009];SAS/STAT 9.2 user’s guide. http://support.sas.com/documentation/onlinedoc/stat/index.html.
- 30.Crone C. The permeability of capillaries in various organs as determined by the use of the “indicator diffusion” method. Acta Physiol Scand. 1963;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 31.Joo EY, Tae WS, Jung KY, Hong SB. Cerebral blood flow changes in man by wake-promoting drug, modafinil: a randomized double blind study. J Sleep Res. 2008;17(1):82–88. doi: 10.1111/j.1365-2869.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, Wang GJ, Fowler JS, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43(3):181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 33.Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical and post-marketing surveillance: a review of abuse liability issues. Ann Clin Psychiatry. 2004;16(2):101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- 34.Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berl) 1996;126(4):286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- 35.Stoops WW, Lile JA, Fillmore MT, Glaser PEA, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005;182(1):186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 36.Jasinski DR, Kovacevic-Ristanovic R. Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsy. Clin Neuropharmacol. 2000;23(3):149–156. doi: 10.1097/00002826-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33(4):761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- 38.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30(1):205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 39.Gjedde A, Wong DF, Rosa-Neto P, Cumming P. Mapping neuroreceptors at work: on the definition and interpretation of binding potentials after 20 years of progress. Int Rev Neurobiol. 2005;63:1–20. doi: 10.1016/S0074-7742(05)63001-2. [DOI] [PubMed] [Google Scholar]