Abstract
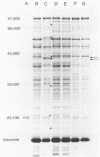
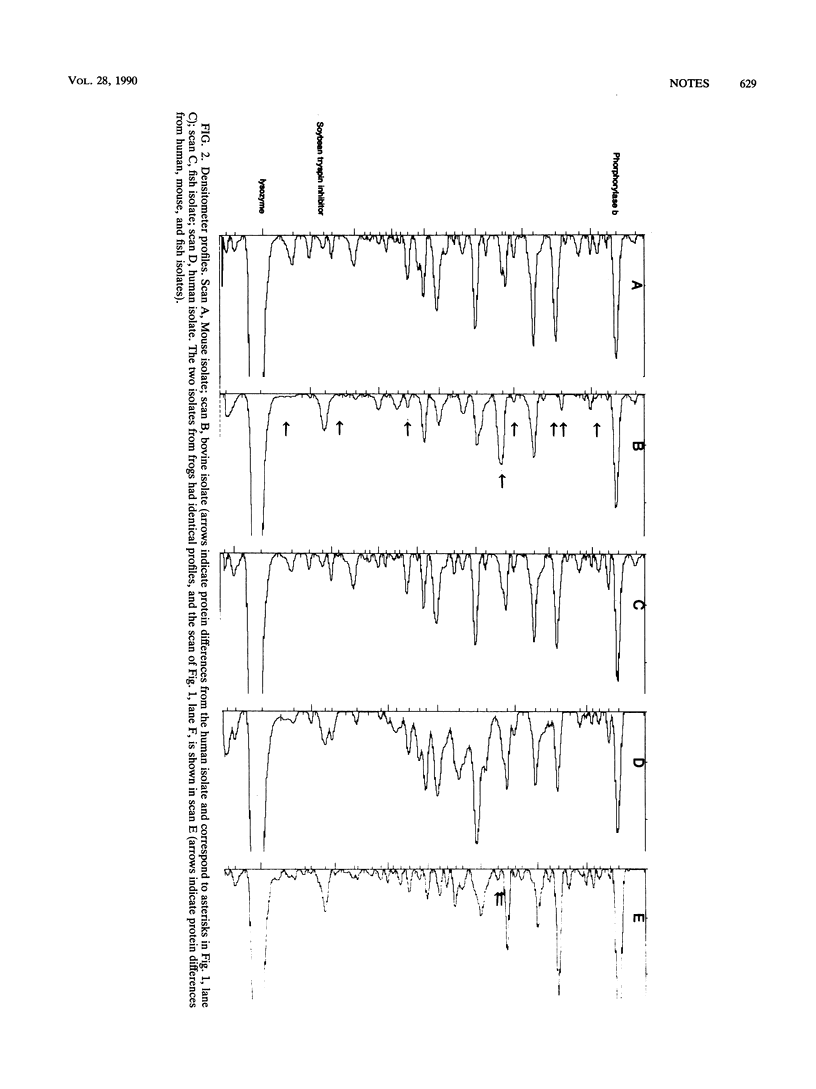
Whole-cell protein and physiological patterns of nonhemolytic group B, type Ib, streptococci isolated from humans, cattle, frogs, fish, and mice were compared. Isolates from humans, fish, and mice were identical. Only minor differences were seen in the isolates from human, bovine, and frog sources. Nonhemolytic group B streptococci from humans, fish, and mice and, to a lesser extent, from cattle and frogs share several characteristics, including a high similarity in proteins (on the basis of molecular weight); this suggests that they may have a common ancestry.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amborski R. L., Snider T. G., 3rd, Thune R. L., Culley D. D., Jr A non-hemolytic, group B Streptococcus infection of cultured bullfrogs, Rana catesbeiana, in Brazil. J Wildl Dis. 1983 Jul;19(3):180–184. doi: 10.7589/0090-3558-19.3.180. [DOI] [PubMed] [Google Scholar]
- Dow S. W., Jones R. L., Thomas T. N., Linn K. A., Hamilton H. B. Group B streptococcal infection in two cats. J Am Vet Med Assoc. 1987 Jan 1;190(1):71–72. [PubMed] [Google Scholar]
- Edelstein R. M., Pegram R. G. Contagious skin necrosis of Somali camels associated with Streptococcus agalactiae. Trop Anim Health Prod. 1974 Nov;6(4):255–256. doi: 10.1007/BF02383286. [DOI] [PubMed] [Google Scholar]
- Henrichsen J. The bacteriology of GBS. Antibiot Chemother (1971) 1985;35:53–56. doi: 10.1159/000410360. [DOI] [PubMed] [Google Scholar]
- Kornblatt A. N., Adams R. L., Barthold S. W., Cameron G. A. Canine neonatal deaths associated with group B streptococcal septicemia. J Am Vet Med Assoc. 1983 Sep 15;183(6):700–701. [PubMed] [Google Scholar]
- Kummeneje K., Nesbakken T., Mikkelsen T. Streptococcus agalactiae infection in a hamster. Acta Vet Scand. 1975;16(4):554–556. doi: 10.1186/BF03546650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Jackman P. J. The similarities between Pseudomonas paucimobilis and allied bacteria derived from analysis of deoxyribonucleic acids and electrophoretic protein patterns. J Gen Microbiol. 1982 Dec;128(12):2945–2954. doi: 10.1099/00221287-128-12-2945. [DOI] [PubMed] [Google Scholar]
- Robinson J. A., Meyer F. P. Streptococcal fish pathogen. J Bacteriol. 1966 Aug;92(2):512–512. doi: 10.1128/jb.92.2.512-512.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger A. R., Dunny G. M. Development of a system for genetic and molecular analysis of Streptococcus agalactiae. Res Vet Sci. 1985 Mar;38(2):202–208. [PubMed] [Google Scholar]
- Wilkinson H. W., Thacker L. G., Facklam R. R. Nonhemolytic group B streptococci of human, bovine, and ichthyic origin. Infect Immun. 1973 Mar;7(3):496–498. doi: 10.1128/iai.7.3.496-498.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]