Abstract
The discoveries of neural (NSCs) and brain tumor stem cells (BTSCs) in the adult human brain and in brain tumors, respectively, have led to a new era in neuroscience research. These cells represent novel approaches to studying normal phenomena such as memory and learning, as well as pathological conditions such as Parkinson’s disease, stroke, and brain tumors. This new paradigm stresses the importance of understanding how these cells behave in vitro and in vivo. It also stresses the need to use human-derived tissue to study human disease because animal models may not necessarily accurately replicate the processes that occur in humans. An important, but often underused, source of human tissue and, consequently, both NSCs and BTSCs, is the operating room. This study describes in detail both current and newly developed laboratory techniques, which in our experience are used to process and study human NSCs and BTSCs from tissue obtained directly from the operating room.
Keywords: neural stem cells, brain tumor stem cells, immunohistochemistry, immunocytochemistry, electron microscopy, neurospheres
1. Introduction
The concept of cells residing within the adult brain capable of self-renewal and multi-potency started in the 1960s with Joseph Altman’s discovery of mitotic activity in the adult rodent brain (Altman 1962; Altman and Das 1965). Since then, several studies have demonstrated neurogenesis in canaries (Goldman and Nottebohm 1983), rodents (Reynolds and Weiss 1992), monkeys (Gould et al. 1998), and humans (Eriksson et al. 1998; Sanai et al. 2004). These discoveries later led to the isolation of a sub-population of cells, so-called neural stem cells (NSCs), from the adult mammalian brain with stem cell-like features of self-renewal and multi-potentiality (Doetsch et al. 1999; Laywell et al. 2000; Reynolds and Weiss 1992). Neurogenesis has been found to primarily occur in two regions, the subventricular zone (SVZ) and the subgranular zone (SGZ) (Eriksson et al. 1998; Johansson et al. 1999; Kukekov et al. 1999; Sanai et al. 2004). Recently, cells have been isolated from brain tumors that have many of the same properties as NSCs, and have been named brain tumor stem cells (BTSCs) (Galli et al. 2004; Hemmati et al. 2003; Ignatova et al. 2002; Singh et al. 2003). These findings have led to a new era in neuroscience research where NSCs and BTSCs are being studied for their potential role in tumorigenesis (Galli et al. 2004; Hemmati et al. 2003; Ignatova et al. 2002; Singh et al. 2003), epilepsy (Parent et al. 2006), stroke (Felling et al. 2006), and demyelinating disorders (Copray et al. 2006) among others.
Underlying these discoveries is a set of important laboratory techniques used to study NSCs and BTSCs. These procedures start when tumor and non-tumor tissue that was part of the planned resection are collected in the operating room (OR) in patients undergoing brain surgery for vascular lesions (i.e. aneurysms and arteriovenous malformations), hydrocephalus, epilepsy, or tumor. This tissue, which would otherwise be discarded, can be used to establish cell cultures, neurosphere assays, and sections for immunohistochemistry (IHC), immunocytochemistry (ICC), and electron microscopy (EM). In essence, the OR becomes an extension of the laboratory.
The purpose of this article was to describe the laboratory methods in studies of NSCs and BTSCs derived from human intra-operative tissue. These techniques can be used to study the organization of the human brain and the putative role of NSCs and BTSCs in normal and pathological conditions. These procedures are also important because NSCs and BTSCs may prove pivotal in the treatment of neurodegenerative diseases, as well as understanding the etiology of devastating brain tumors. Furthermore, since animal models do not accurately represent humans (Quinones-Hinojosa et al. 2006; Sanai et al. 2004), human tissue may be the most relevant way to study human diseases.
2. Materials and Methods
All protocols in this study have been approved by the Johns Hopkins Hospital Institutional Review Board (IRB) (Approval Number B0508080102).
Prior to obtaining and utilizing human brain tissue for experimental purposes, it is important that several guidelines be established, and that protocols are in compliance with governing oversight organizations. The protocols in this study were first written in detail and submitted to our institution’s IRB. After obtaining approval from the IRB, consent was obtained from the patients. This consent was obtained during the patient’s pre-operative clinic visits and re-confirmed on the day of surgery. It is important to emphasize to the patients that the tissue that will be obtained in the operating room will be a part of the planned resection, would otherwise be discarded, and will be used in scientific experiments to better understand human disease. It is also important to both patients and oversight organizations (i.e. HIPAA) that obtained tissue will not be labeled with patient-identifying information. Our tissue is labeled with a unique three digit number (i.e. 187), where the patient information is recorded in a password-locked database only accessible by the primary investigator. Of note, our laboratory typically collects all tissue, regardless of disease, since human tissue is not readily available as compared to animal-derived tissue. This tissue is taken and frozen, as will be described below. The only exceptions are patients who harbor both local and systemic infections. Tissue from these infected patients is discarded to prevent widespread contamination.
Another important point is that all of the procedures detailed in this study were conducted under special precautions that abided to OSHA regulations. All human tissue was handled under sterile conditions. Tissue taken from the operating room was placed in sterile containers and handled only under sterile laminar flow hoods. These hoods were designated for only human use. Likewise, discarded tissue and tissue media were placed into containers designated for human biological waste. It is important that human tissue and experimentation take place in designated animal-free areas to avoid possible contamination.
2.1 Operating Room Setup and Tissue Collection
The patient is placed on the OR table as usual for surgery for arteriovenous malformation, aneurysm, epilepsy, or tumor. The brain is mapped using the BrainSUITE ® iMRI (BrainLAB) at our institution, and the precise location of the tissue to be removed is recorded (Figure 1). All samples were part of the planned resection. Tissue was only collected after appropriate diagnosis was made and sufficient specimen was available for final permanent pathology, as per a senior pathologist. The use of a Penfield #2 dissector is recommended for tissue resection, and special care must be taken in coordination with the surgeon to meticulously collect the tissue without injuring the ependyma (when planning to study the SVZ). The use of bipolar cautery should be avoided, when possible, since it damages tissue architecture.
Figure 1. Intra-operative stereotactic localization of brain tumors in human patients.
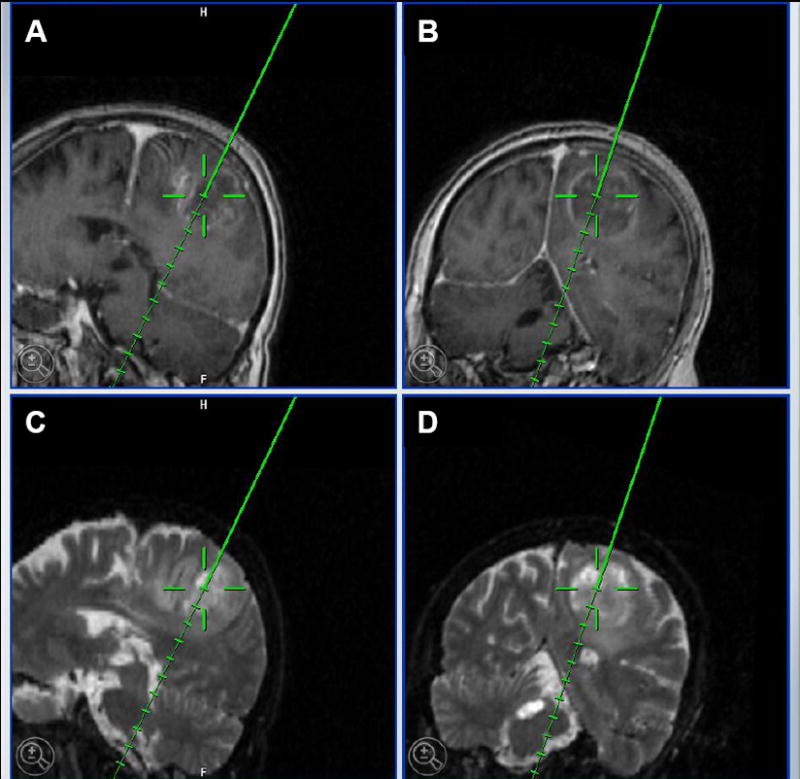
BrainSUITE ® iMRI (BrainLAB) indicating a left parietal glioblastoma multiforme in a 62 year old female patient. A and B, T1 MRI images with contrast. C and D, T2 MRI images. In all images, the tip of the solid line indicates the area that was collected for the purposes of laboratory experiments.
In addition to craniotomies, endoscopic procedures for hydrocephalus are another potential source of human brain tissue for laboratory experiments (Westerlund et al. 2005). Tissue from the lateral ventricular wall, which is part of the planned path, is detached by introduction of an endoscope. The tissue is obtained with biopsy forceps under endoscopic visualization prior to shunt and/or ventricular drain insertion. This tissue can be safely removed without any complications (Westerlund et al. 2005).
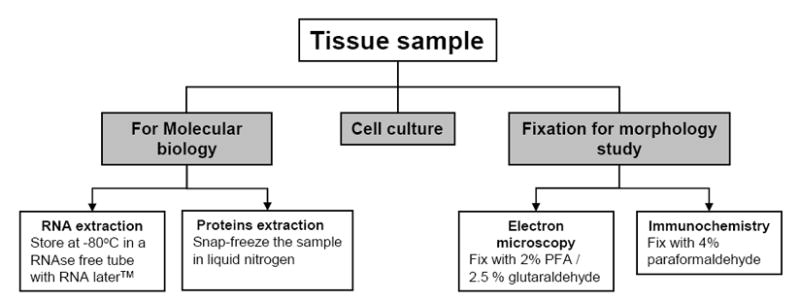
Once the pathological diagnosis is confirmed and a sufficient amount of tissue is reserved for permanent diagnosis, the remainder of the tissue can be used for experimental studies. The pathological diagnoses are established by our senior neuro-pathology staff, and based on well-accepted World Health Organization criteria (Louis et al. 2007). We have an incubator dedicated to human tissue, and special precautions are taken including the use of sodium azide and fungicide in the incubator water to avoid microbial contamination. Under sterile conditions, in a Class II laminar flow hood, the tumor sample is placed in a sterile Petri dish with HBSS +Ca +Mg (Gibco/BRL, Bethesda, MD), and necrotic tissue and blood vessels are removed from the tumor with the aid of a dissecting microscope. The sample is then divided into five pieces for:
Protein extraction, using an appropriate cryovial and snap-freezing the sample in liquid nitrogen
RNA extraction, placing the tumor in a 1.5ml RNase free tube and adding 1mL of RNA later (Qiagen, Valencia, CA) for storage at 4°C
Cell culture for both neurospheres and adherent cells (the constituents of these respective media are listed in detail in Table 1)
4% paraformaldehyde (PFA) for IHC and ICC
2% PFA / 2.5 % glutaraldehyde for EM.
Table 1.
Media components for serum media, Neurobasal media, 4% PFA, and 2% PFA/2.5 % glutaraldehyde. DMEM (Dulbecco’s Modified Eagle Medium), FBS (fetal bovine serum), PFA (paraformaldehyde), PB (phosphate buffer).
| Serum Media | Neurobasal Media | 4% PFA (pH = 7.0; store at 4°C) | 2% PFA/2.5% Glutaraldehyde (pH = 7.4; store at 4°C) |
|---|---|---|---|
| DMEM (Gibco)–88% | Neurobasal media (Gibco) | PFA (Sigma) – 0.04 g/ml | PFA - 0.02 mg/ml |
| FBS (Gibco) –10% | Monobasic sodium phosphate (Sigma) – 2.64 mg/ml | Glutaraldehyde (EMS) (0.4M) – 10% | |
| L-glutamine (Gibco) (0.2M) –1% | Dibasic sodium phosphate (Sigma) – 0.02 g/ml | Monobasic PB (0.2M) (0.28g/ml) – 10% | |
| Antibiotic-antimycotic (Gibco) – 1% | Dibasic PB (0.2M) (0.28 g/ml) – 40% | ||
In addition to the OR, another potential source of tissue for IHC is post-mortem tissue. This tissue must be collected within twelve hours of death to prevent excessive degradation. It is important to mention that some have reported the establishment of cell cultures and neurospheres from post-mortem samples with success (Palmer et al. 2001). In the morgue, the head is perfused to gravity by infusing 4% PFA into the internal carotid arteries and severing the internal jugular veins for drainage. The brain is perfused for approximately 15–20 minutes until there is clear drainage. The brain can be harvested, sectioned, and fixed by submerging the sections in a large container containing 4% PFA in a 1:50 tissue:volume/fixative proportion.
2.2 Cell Culture Preparation
Under sterile conditions, the sample that is going to be used for cell culture is dissociated enzymatically by adding 2ml of Trypsin/EDTA (Gibco/BRL, Bethesda, MD) and mechanically by cutting the tumor into small pieces using microdissecting scissors. The pieces of tissue suspended in 2ml of Trypsin/EDTA are then placed into a 15ml conical tube to be homogenized with a sterile wide-tipped fire-polished Pasteur pipette. Trypsin is then inhibited by adding 3ml of DMEM/F12 + 10% FBS media. The tissue is homogenized further with a sterile narrow-tipped fire polished Pasteur pipette and any remaining non-homogenized pieces of tissue are removed by passing the cell suspension through a 40μm cell strainer. Cells number and viability are determined with a cell viability analyzer (Vi-Cell XR). 1×106 and 1×105 cells per 25-cm2 flask are used for adherent and non-adherent cultures respectively. Cells are centrifuged at 180 × g for 5 minutes at 4° C and resuspended in serum or neurosphere media (Table 3) and plated into adherent (BD Biosciences) or low attachment (Corning 3473) 25cm2 culture flasks. The flasks are placed in an incubator (Forma Series II) at 37°C with a 5% CO2 atmosphere. Water in the incubator is treated with 0.02% w/v of sodium azide and fungicide (Clear Bath algicide 15 drops/gallon). The culture media is changed every three days. The cell cultures are checked daily for viability and density with an inverted microscope.
Table 3.
Neurosphere media components for neurosphere clonal assays, dissociation of neurospheres, and neurosphere differentiation.
| Control Media | Complete Media (approx. 50ml) | Differentiation Media (approx. 30ml) |
|---|---|---|
|
| ||
| DMEM/F12 | Control media | Control media |
| Glucose (30%) – 2% | EGF (Sigma) - 50μl:1ml | FGF – 2μl:3ml |
| Sodium bicarbonate (Gibco) (7.5%)– 2% | FGF (Sigma) - 2μl:5ml | Heparin solution - 10μl:3ml |
| BSA (Sigma) – 200mg | ||
| Glutamine (0.2M) – 1% | Heparin solution – 2μl:1ml | |
| Antibiotic/antimycotic – 2% | Heparin (Sigma) –38,000U | |
| Hormone Mix – 1% | Distilled water - 100 ml | |
|
| ||
| Hormone Mix | Insulin solution | Papain solution |
|
| ||
| DMEM/F12 | Distilled water | Distilled water |
| Glucose (30%) – 2% | Insulin (Sigma) – 5mg:2ml | Papain (Sigma) – 1 μl:250ml |
| Sodium bicarbonate (7.5%) – 1% | ||
| HEPES (1M) – 1% | HCl (Sigma) (0.1N) – 1ml:10ml | Digesting solution – 2μl:125ml |
| Apo-transferrin – 1 mg/ml | ||
| Progesterone (2μM) - 1μl:400ml | PIPES (Sigma) – 2% | |
| Sodium selenite (Sigma) (3mM) - 1μl:10ml | 10X salt solution – 10% | |
| NaCl (Sigma) (1.2M) | ||
| Insulin solution – 10% | ||
| Putrescine solution – 10% | KCl (Sigma) (50mM) | |
| 97% putrescine (Sigma) in distilled water | Glucose (30%) –1ml:50ml | |
| Phenol red (Sigma) –1μl:10ml | ||
| Distilled water | ||
2.3 Astrocyte purification
Astrocytes from the SVZ and SGZ have been proven to have multi-potential and self-renewal capabilities (Doetsch et al. 1999; Eriksson et al. 1998; Galli et al. 2004; Hemmati et al. 2003; Ignatova et al. 2002; Pincus et al. 1997; Sanai et al. 2004). The goal of these cell cultures is to isolate GFAP+ astrocytic cells derived from the subventricular zone (SVZ), subgranular zone (SGZ), and brain tumors, especially glioblastoma multiforme (GBM) (Galli et al. 2004; Hemmati et al. 2003; Ignatova et al. 2002; Singh et al. 2004b), medulloblastoma (Hemmati et al. 2003; Singh et al. 2003), and ependymoma (Taylor et al. 2005). When there is confluent growth, the cell culture is purified by placing the flask on a shaker for 8 hours at 1,000 RPM, so that only astrocytes remain adhered to the flask. This is similar to what has been reported in rodents (Lim and Alvarez-Buylla 1999). The media in the flask is then removed along with detached non-astrocytic cells, and replaced and washed with sterile PBS to eliminate debris. Following the washes, 5 ml of fresh media is placed in the flask, and the flask is returned to the incubator.
2.4 Neurosphere Assays
Cells derived from non-cancer and cancer tissue are placed in neurosphere media, both before and after the purification for astrocytes. The gold standard for clonal analysis entails diluting the cells in neurosphere media, and depositing a single cell per well in several 96-well plates. Another means is to place several cells (~106) in non-adherent flasks. The primary advantage of the single cell per well method is that it minimizes the chance of chimeric neurosphere formation (Singec et al. 2006); however, it is very time-intensive and laborious. The initial formation of neurospheres can be visualized in approximately 1 week with an inverted light microscope.
When the primary neurospheres reach an average diameter of 2mm at approximately three weeks of culturing, they are passaged. In fact, recent studies have found that larger neurospheres (>2mm) are more likely to be derived from NSCs as opposed to progenitor cells with limited proliferative capacity (Louis et al. 2008). The larger neurospheres are obtained and passaged with mechanical trituration. The passage of neurospheres with protocols that involve the use of enzymes is also widely accepted. Some groups, however, have observed a faster neurosphere growth rate when passaged by the use of mechanical trituration instead of enzymatic digestion (Engstrom et al. 2002). To passage the neurospheres and form a single cell suspension without the use of enzymes, centrifuge the neurosphere cell culture for 5 minutes at 180 × g, discard the supernatant, and resuspend the pellet in 200 μl of neurosphere media. The neurospheres are then triturated by repeated pipetting using a 200 μl pipette tip. In our experience, this takes on average 200 times. This process can be repeated indefinitely in tumors (Lee et al. 2006) and non-cancer tissue (Sanai et al. 2004). Once the cells are dissociated, they are placed again in either a 96-well plate (as a single cell per well) or a non-adherent flask. In our experience, the above procedures are repeated at least six times before we consider them neurospheres, as previously published (Reynolds and Rietze 2005). Cultures that peter out before six passages are more likely derived from progenitor cells (Reynolds and Rietze 2005).
Neurospheres were differentiated to test their pluripotential capabilities. Neurospheres are plated on 15mm glass coverslips (Warner Instruments Hamden, CT) coated with laminin (5 micrograms/cm2 diluted in serum free D-Mem F12 culture media) and Poly-D lysine (10 micrograms/μl). This is done after they have been passaged at least six times. We have found that spheres differentiated prior to establishing clonal ability (>6 passages) will typically only display astrocyte characteristics. Furthermore, as previously published (Louis et al. 2008), smaller spheres will also typically only display cells with astrocyte characteristics. These spheres are therefore probably derived from progenitor as opposed to NSC.
2.5 Immunocytochemistry (ICC), Immunohistochemistry (IHC), Electron Microscopy (EM)
The protocol for ICC is described in detail in Supplementary Figure 1. During trypsinization, the cells need to be closely monitored with an inverted light microscope. The purpose of the trypsin is to detach the adherent astrocytes from the flask, but with excessive exposure, antigens can be damaged and the cells are susceptible to degradation and eventual cell death. The IHC procedure is explained in Supplementary Figure 2. The tissue that will be used for IHC must be fixed in 4% PFA within 20 minutes of collection. Also, it is important to not spill the ethanol into the container with the tissue and the embedding medium during the tissue freezing. The ethanol facilitates the freezing of the tissue by lowering the ambient temperature, but if it spills into the container, it will interfere with tissue sectioning. Another significant part of the IHC protocol is to perform both the primary and secondary antibody staining at 4°C for an overnight period to allow for more specific antibody binding.
The protocols for EM preparation of intra-operatively collected tissue, cell cultures, and neurosphere assays are described in detail in Supplementary Figures 3–5. Following EM preparations, the samples are ready to be processed for semi-thin and ultra-thin sectioning for EM analysis. As with IHC, tissue for EM must be fixed within 20 minutes of resection. During the inclusion, the osmium should be checked routinely every 10 minutes for precipitation. This precipitation is evidenced by the solution turning a purple or “wine” color. The osmium solution should be removed and replaced immediately; otherwise the precipitation will damage the tissue. Another point of concern is the transition from100% ethanol to propylene oxide during the tissue dehydration steps. This transition must be done rapidly to prevent the tissue dessication.
Images were obtained with a Nikon C1si confocal and Olympus IX81 inverted epi-fluorescent microscope. The color intensity of the images was edited using the Nikon EZ-C1 viewer and Olympus Slidebook 4.2, respectively.
3. Results
3.1 Cell Culture
By following the procedures described in sections 2.1 and 2.2, we have established neurosphere cell lines from tissue obtained from 12 GBM, 5 fibrillary astrocytomas (WHO grade II), 4 anaplstic astrocytomas (WHO grade III), and 5 non-cancer SVZ. These cell lines have been passaged for at least 6 times. Tissue derived from non-cancer cortex has not formed neurospheres. For the purpose of this report, only information regarding cell culture, immunocytochemistry, and electron microscopy of cells derived from the SVZ and GBM tissue will be described.
3.2 Neurosphere Assays
Several neurosphere cell lines have been established by following the procedures outlined in section 2.3. These neurospheres have been derived from both tumor and non-tumor tissue (Figure 2A–2C). The neurospheres derived from tumor tissue include GBM, medulloblastomas, and low grade gliomas. In addition, neurospheres have been derived from the SVZ from both adult and pediatric non-tumor tissue. These neurospheres, collectively, have been passaged at least 6 times without any complications. The neurospheres contain some GFAP positive cells mainly in the core, while the periphery cells are mostly positive for Nestin (Fig. 2C–2E). After differentiation, neurosphere-derived cells express markers corresponding to the three main cell lineages in the central nervous system: Olig2 for oligodendrocytes, GFAP for astrocytes and Tuj1 for neurons (Figure 2F–2G). Some neurospheres were prepared for electron microscopy as described in Supplement Figure 5.
Figure 2. Neurosphere assay and differentiation from intra-operatively obtained human cells.
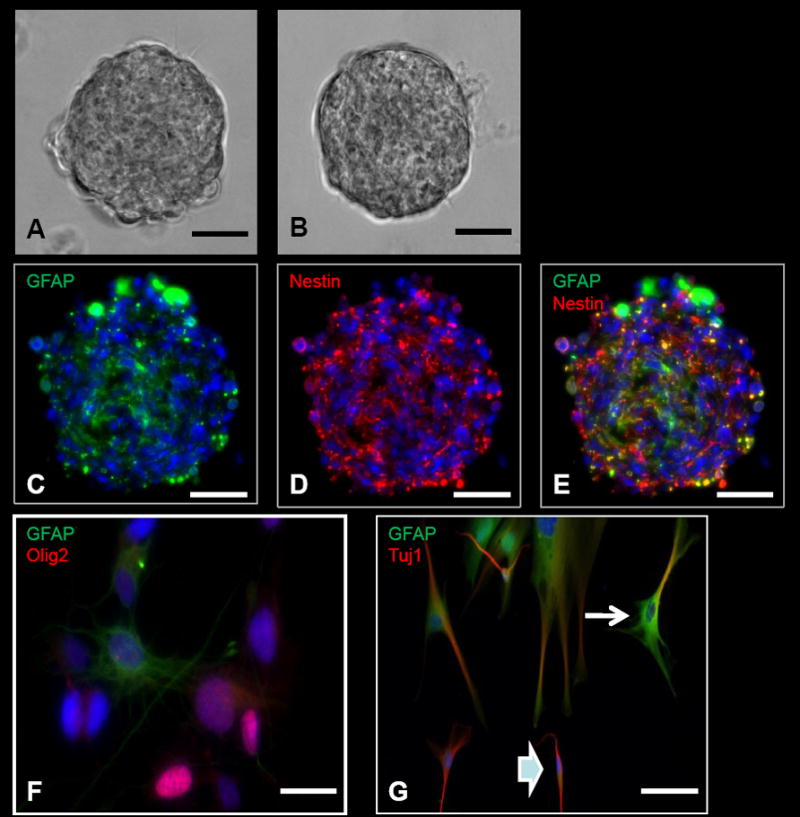
A, Tumor neurosphere derived from a patient with glioblastoma multiforme (GBM). B, Neurosphere derived from the subventricular zone (SVZ) in a patient who underwent a hemispherectomy for seizures. C–E, ICC images of a GBM-derived tumor neurosphere with positive GFAP (C), Nestin (D), and Nestin and GFAP co-stained (E) cells. F, Neurosphere differentiation with cells positive for GFAP (astrocytes) and Olig2 (oligodendrocytes). G, Neurosphere differentiation with cells positive for GFAP (astrocytes) and Tuj1 (neurons). Some of the cells are positive for both GFAP and Tuj1. Some of these cells have morphologies more characteristic of astrocytes with several cytoplasmic projections (arrow), while other cells are more characteristic of neurons with bipolar processes (arrowhead). This co-staining is typically present in differentiated cells from tumor-derived neurospheres, but typically not seen in differentiated cells from SVZ-derived neurospheres. Scale bars: 100μm (A–E), 10μm (F), 30μm (G).
3.3 Immunocytochemistry (ICC)
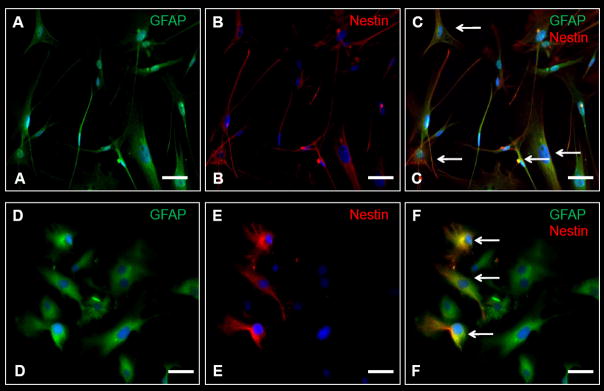
By following the procedures described in Supplementary Figure 1, we have produced several ICC images of astrocytes derived from the germinal niches of the SVZ, as well as cells from different tumor and non-tumor tissue. These cells have been stained for various markers associated with different populations of NSCs and BTSCs (Table 2). GBM-derived cells showed a more heterogeneous morphology, with a wide coexpression of GFAP and nestin while non-cancer SVZ cells were homogeneous and the expression of nestin was restricted only to some of the cells (Figure 3A–3F).
Table 2.
Common primary antibodies used to study different human brain cell populations. A, Cell type and protein markers. Listed are the protein markers characteristic of different cell types. B, Catalogue numbers and dilutions of commonly used primary antibodies to identify these protein markers. C, Secondary antibodies to identify primary antibodies.
| A. | |
|---|---|
| CELL TYPE | PROTEIN MARKERS |
| Type B (Astrocyte) | GFAP, vimentin, nestin, S-100β |
| Type C (Transient Amplifiying Cell) | Nestin, Dlx2 |
| Type A (Migrating Neuroblast) | PSA-NCAM, Type III β-tubulin (Tuj1) |
| Ependymal cell | S-100β |
| Astrocytic lineage | GFAP |
| Neuronal lineage | NeuN, Map2, Tuj1 |
| Oligodendrocytic lineage | Olig-2, O4, S-100β |
| B. | ||||
|---|---|---|---|---|
| TARGET MOLECULE | MANUFACTURER | CATALOGUE NO. | HOST | DILUTION |
| GFAP | Chemicon | MAB3402 | Mouse | 1:500 |
| Dako | Z0334 | Rabbit | 1.500 | |
| Vimentin | Chemicon | MAB3400 | Mouse | 1:1000 |
| Nestin | Chemicon | MAB5326 | Mouse | 1:250 |
| S-100β | Sigma | HPA015768 | Rabbit | 1:500 |
| Dlx-2 | Chemicon | AB5726 | Rabbit | 1:500 |
| Neu-N | Chemicon | MAB377 | Mouse | 1:500 |
| Map-2 | Chemicon | AB5622 | Rabbit | 1:1000 |
| Tuj-1 | Chemicon | MAB1637 | Mouse | 1:250 |
| Olig-2 | Chemicon | AB9610 | Rabbit | 1:500 |
| O4 | Chemicon | MAB345 | Mouse | 1:500 |
| C. | ||||
|---|---|---|---|---|
| Target | Dye | Manufacturer | Catalogue No. | Dilution |
| Mouse | Alexa 488 | Molecular probes | A11001 | 1:500 |
| Mouse | Alexa 594 | Molecular probes | A11005 | 1:500 |
| Rabbit | Alexa 488 | Molecular probes | A11008 | 1:500 |
| Rabbit | Alexa 594 | Molecular probes | A11012 | 1:500 |
Figure 3. Immuocytochemistry (ICC) of human-derived brain tumor and non-tumor cells.
A–C, ICC images of tumor cells obtained from a patient with glioblastoma multiforme (GBM). D–F, ICC images of cells obtained from the subventricular zone (SVZ) in a non-cancer patient. A larger percentage of cells in GBM as opposed to the SVZ co-stain for nestin and GFAP (arrowheads). GFAP – green, Nestin-red, DAPI – blue. Scale Bar: 10 μm.
3.4 Immunohistochemistry (IHC)
We have taken several IHC images of both tumor and-non tumor SVZ tissue by following the protocol described in Supplementary Figure 2. Images derived from tumor tissue include the SVZ (Figure 4A, 4D), non-tumor cortical tissue (Figure 4C), and glioma-derived tissue (Figure 4F).
Figure 4. Immunohistochemistry (IHC) and electron microscopy (EM) of human-derived brain tumor and non-tumor tissue.
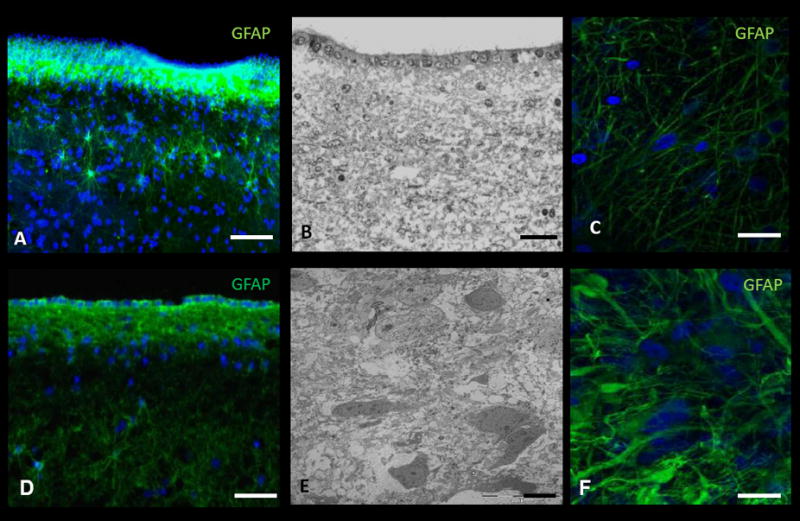
A, IHC and B, EM image of the subventricular zone (SVZ) adjacent to the body of the lateral ventricle in a non-cancer patient. C, IHC of cortical tissue in a non-cancer patient. D, IHC and E, EM image of tumor tissue in a patient with glioblastoma multiforme (GBM). F, IHC of tumor tissue in a patient with GBM. DAPI – blue. Scale Bar: A, 40μm; B, 25μm; C, 20μm; D, 20μm; E, 2μm; F, 5μm.
3.5 Electron Microscopy (EM)
We have created several EM images from cells and tissue derived from both adult and pediatric tumor and non-tumor tissues. This includes tissue derived from the SVZ (Figure 4B), cerebral cortices, and low and high grade gliomas (Figure 4E).
4. Discussion
The “no new neuron” dogma has undergone a major upheaval that started with Altman’s discovery of neurogenesis in rodents (Altman 1962; Altman and Das 1965) and continued with Nottebohm’s study of neurogenesis in female canaries (Goldman and Nottebohm 1983). This eventually led to Reynold and Weiss’ isolation of NSCs from the rodent SVZ, which are cells with self-renewal and multi-potential capabilities (Reynolds and Weiss 1992). These discoveries have opened new avenues to study different aspects of the brain including memory (Aimone et al. 2006), learning (Leuner et al. 2006), and migration (Sawamoto et al. 2006) as well as the pathological processes of tumorigenesis (Galli et al. 2004; Hemmati et al. 2003; Ignatova et al. 2002; Singh et al. 2003), epilepsy (Parent et al. 2006), ischemia (Takemura et al. 2006), and neurodegenerative diseases (Copray et al. 2006; Reif et al. 2006), among others. The idea that NSCs and BTSCs may be involved in all of these facets stresses the importance of having specific methods to study this special population of cells. Also, in a field dominated by animal models, it is important to utilize human tissue to study human disease, especially when animal models do not accurately recapitulate the human brain (Quinones-Hinojosa et al. 2006; Sanai et al. 2004). The purpose of this study, therefore, is to describe the developing laboratory methods of cell culturing and neurosphere assays to study NSCs, as well as the imaging techniques of ICC, IHC, and EM to evaluate the cytoarchitecture and organization of the human brain.
The discovery of a population of stem cell-like astrocytes existing within the SVZ, SGZ, and tumors has made culturing of astrocytes important to the study of NSCs and BTSCs (Doetsch et al. 1999; Galli et al. 2004; Hemmati et al. 2003; Ignatova et al. 2002; Singh et al. 2003). Cell culturing is a process whereby astrocytes are obtained, purified, and grown in either serum or serum-free media, which provide them with the nutrients to divide and proliferate. Once they reach a certain cell density, their protein attachments to the flask are trypsinized and a portion of the cell population is transferred to another media-containing flask to create additional space for continued proliferation. This process is continued indefinitely to provide a constant supply of astrocytes to study. Serum media, however, have recently been shown to alter the characteristics of cells grown from brain tumors by causing them to lose their ability to form neurospheres, and thus their self-renewal and multi-potential capabilities (Lee et al. 2006). This is why we opted to additionally grow our cells in a serum-free media despite the fact we were still able to form neurospheres from serum-passaged tumor and non-tumor astrocytes by trypsinizing the cells and transferring them to neurosphere media.
Neurosphere assays are currently the in vitro standard for determining the presence of NSCs derived from both tumor and non-tumor tissue (Chaichana et al. 2006; Reynolds and Rietze 2005; Vescovi et al. 2006). These assays consist of serum-free media, in which stem-like cells are able to continually divide and form multi-potent clonal spheres called neurospheres, while cells incapable of self-renewal die off with continued passaging (Doetsch et al. 2002; Hemmati et al. 2003; Reynolds and Rietze 2005). These neurospheres, unlike other cells, can also undergo differentiation by the processes described in section 2.4 to generate neurons, astrocytes, and oligodendrocytes. It is important to make sure that the cells derived from the neurosphere assay have stem cell characteristics. These cells should be passaged at least six times to establish clonal ability, as well as differentiate into different neural cell lineages (astrocytes, neurons, oligodendrocytes) (Reynolds and Rietze 2005). Human cells with stem cell characteristics can be used in a variety of ways. These cells can be used to understand the potential role of stem cells in tumorigenesis (Alcantara Llaguno et al. 2009), as well as their potential role in neurodegenerative conditions including ischemic disease, demyelinating disorders, and cerebral atrophy (Chaichana et al. 2006; Daniela et al. 2007).
An important point is that tumor-derived neurospheres often yield differentiated cells that co-stain for markers of different lineages (Hemmati et al. 2003). These cells are positive for a combination of either astrocytic, neuronal, or oligodendrocyte markers. This feature, however, is notably absent in differentiated cells from non-tumor neurospheres (not shown). In fact, this feature is a primary difference between cells differentiated from tumor-derived neurospheres and non-tumor or SVZ-derived neurospheres (Chaichana et al. 2006; Hemmati et al. 2003). One important difficulty we had with our current experimental protocols is the differentiation of neurospheres into oligodendrocyte lineage that stain positive for O4, CNPase, etc. Instead, many of the cells were positive for Olig2. The bHLH transcription factor is essential for the proliferation and differentiation of oligodendrocyte precursors (Lu et al. 2002; Lu et al. 2000; Rowitch et al. 2002; Takebayashi et al. 2002a; Takebayashi et al. 2002b; Zhou et al. 2001; Zhou et al. 2000). In the adult brain, Olig2 expression has been reported in oligodendrocytes precursors and mature oligodendrocytes (Ligon et al. 2004; Rowitch 2004). However, it is not possible based on the expression of Olig2 alone to conclude that precursor cells are committed to an oligodendroglial lineage. The limitation of finding CNPase- and O4-expressing cells may be due to our culture conditions or the intrinsic properties of tumor cells, which may be committed to astrocytic and neuronal lineages.
It is important to use human, as opposed to rodent, cells since human cells more accurately represent human disease and pathology. Rodent cells, while more readily available, may not be the most accurate representation of human conditions. This therefore places an emphasis on using human cells with true stem cell-like characteristics since the use of progenitor cells or cells with non-stem cell characteristics may yield erroneous results. We recommend that all steps be done in extremely sterile conditions and that a set of glassware is designated for the neurosphere assay as these cells are easily contaminated. In addition, we recommend establishing an incubator dedicated to human tissue.
ICC and IHC are important methods to identify the presence of cell-specific proteins through antigen-antibody interactions. This identification is used to study cellular location (Chan et al. 2006; Crespel et al. 2005), migration (Kuhn et al. 1997; Tabar et al. 2005), tissue architecture (Kelley et al. 2005; Quinones-Hinojosa et al. 2006; Sanai et al. 2004), and, more importantly, different NSC populations (Doetsch et al. 1999; Reynolds and Weiss 1992; Vescovi et al. 2006). EM is an equally important imaging technique that allows the visualization of objects too small to be seen with a light microscope. EM, like IHC and ICC, plays a role in elucidating the cytoarchitecture of various locations in the brain, including the two proposed stem cell niches of the SVZ (Ponti et al. 2006; Quinones-Hinojosa et al. 2006; Sanai et al. 2004) and SGZ of the hippocampus (Shapiro and Ribak 2006). EM can also elucidate cell function by depicting cellular structure and characteristic organelles (Rash et al. 1998). We recommend that all EM rinsing be performed at 4°C, and that special care is taken at every step to avoid tissue desiccation. In addition, we have equipment designated strictly for EM to avoid spread of toxic EM materials to other laboratory equipment.
A noteworthy point is that the ability to identify a cell marker unique to NSCs and BTSCs has yet to be achieved. In recent years, CD133 has been a marker that has showed promise in being this unique marker for both NSCs and BTSCs. CD133 was initially discovered in CD34+ hematopoetic stem cells (Miraglia et al. 1997), and later used to isolate NSCs from fetal brain tissue (Uchida et al. 2000). CD133 was then used to identify and isolate a sub-population of BTSCs from gliomas that exhibit stem cell properties in vitro and capable of initiating tumors at low cell concentrations (Bao et al. 2006; Singh et al. 2003; Singh et al. 2004b). However, later studies have found that not all CD133+ cells are capable of forming neurospheres in vitro and initiating tumors in animal models (Singh et al. 2004a). Moreover, CD133− cells have also been found to be able to initiate tumors (Ogden et al. 2008; Wang et al. 2008). These recent studies have therefore suggested that, although selecting for CD133+ cells seems to enrich for BTSC, it is not a specific marker for this particular cell population (Ogden et al. 2008; Wang et al. 2008). Therefore, the procedures and protocols remain the primary standard for identifying NSCs and BTSCs (Reynolds and Rietze 2005; Rietze and Reynolds 2006).
5. Conclusion
The purpose of this paper is to fully describe the primary techniques that are essential for the study of NSCs and BTSCs that are derived from intra-operatively obtained human brain tissue. These techniques include cell culturing, neurosphere assays, ICC, IHC, and EM. This is important because this is the first paper to concisely and collectively describe these techniques as they relate to the study of intra-operatively obtained human tissue. By describing these techniques, it allows other laboratories to use intra-operative human tissue and to join in this new era of studying the role of NSCs and BTSCs in memory, learning, tumorigenesis, and disease such as stroke, multiple sclerosis, and Parkinson’s disease. It also raises awareness among other institutions of the potential value of tissue obtained from the operating room that would otherwise be discarded, with the consent of patients. In addition, it stresses the importance of using human tissue to study human diseases. Most of the NSC and BTSC research have been based on animal models that, in many instances, may have little in common with humans.
Supplementary Material
Figure 5. Electron microscopy (EM) of human-derived brain tumor neurospheres.
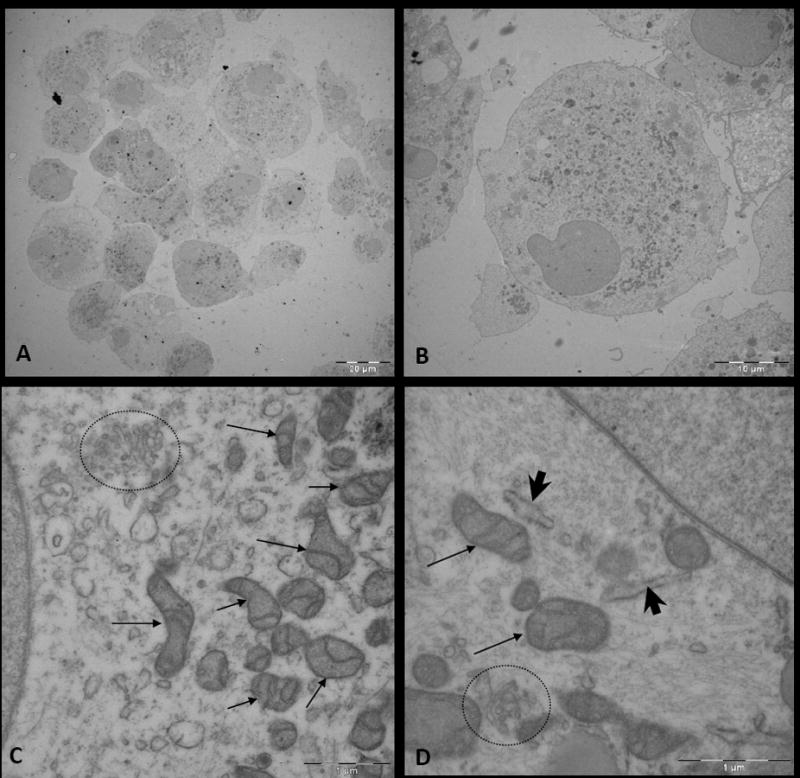
A, Image of cells within a glioblastoma multiforme (GBM)-derived neurosphere. B, Image of a cell within the neurosphere. C and D, Magnified images of the cytoplasm of a cell within a neurosphere, which displays abundant organelles including mitochondria (arrow), Golgi apparatus (circle), and endoplasmic reticulum (arrow head).
Figure 6. Description of tissue processing once human tissue is obtained from the operating room.
Acknowledgments
We would like to thank Roxana Mesias, Ashwini Niranjan, and Alyssa Choi for their help in maintaining cell cultures and taking images. Kaisorn L. Chaichana was funded by the American Brain Tumor Association for some of the work conducted in this paper. This work was funded by NIH Grant K08NS055851 to AQH. AQH, HGC, and GIJ were funded by the Children’s Cancer Foundation. GZB was funded by the HHMI. OGP was partially supported by FRABA Grant 554/08. JMGV was funded by the RETIC grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15(1):45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp Neurol. 1962;5:302–318. doi: 10.1016/0014-4886(62)90040-7. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006 doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Chaichana K, Zamora-Berridi G, Camara-Quintana J, Quinones-Hinojosa A. Neurosphere assays: growth factors and hormone differences in tumor and nontumor studies. Stem Cells. 2006;24(12):2851–2857. doi: 10.1634/stemcells.2006-0399. [DOI] [PubMed] [Google Scholar]
- Chan C, Moore BE, Cotman CW, Okano H, Tavares R, Hovanesian V, Pinar H, Johanson CE, Svendsen CN, Stopa EG. Musashi1 antigen expression in human fetal germinal matrix development. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Copray S, Balasubramaniyan V, Levenga J, de Bruijn J, Liem R, Boddeke E. Olig2 overexpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells. 2006;24(4):1001–1010. doi: 10.1634/stemcells.2005-0239. [DOI] [PubMed] [Google Scholar]
- Crespel A, Rigau V, Coubes P, Rousset MC, de Bock F, Okano H, Baldy-Moulinier M, Bockaert J, Lerner-Natoli M. Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis. 2005;19(3):436–450. doi: 10.1016/j.nbd.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Daniela F, Vescovi AL, Bottai D. The stem cells as a potential treatment for neurodegeneration. Methods Mol Biol. 2007;399:199–213. doi: 10.1007/978-1-59745-504-6_14. [DOI] [PubMed] [Google Scholar]
- Deloulme JC, Raponi E, Gentil BJ, Bertacchi N, Marks A, Labourdette G, Baudier J. Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol Cell Neurosci. 2004;27(4):453–465. doi: 10.1016/j.mcn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36(6):1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Engstrom CM, Demers D, Dooner M, McAuliffe C, Benoit BO, Stencel K, Joly M, Hulspas R, Reilly JL, Savarese T, Recht LD, Ross AH, Quesenberry PJ. A method for clonal analysis of epidermal growth factor-responsive neural progenitors. J Neurosci Methods. 2002;117(2):111–121. doi: 10.1016/s0165-0270(02)00074-2. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z, Givogri MI, Bongarzone ER, Levison SW. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci. 2006;26(16):4359–4369. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80(8):2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95(6):3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem S, Aguirre A, Vives V, Marks A, Gallo V, Legraverend C. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia. 2005;51(2):81–97. doi: 10.1002/glia.20184. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100(25):15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253(2):733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- Kelley TW, Tubbs RR, Prayson RA. Molecular diagnostic techniques for the clinical evaluation of gliomas. Diagn Mol Pathol. 2005;14(1):1–8. doi: 10.1097/01.pdm.0000138207.96718.85. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156(2):333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97(25):13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16(3):216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63(5):499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96(13):7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, Reynolds BA. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008;26(4):988–996. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109(1):75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25(2):317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90(12):5013–5021. [PubMed] [Google Scholar]
- Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, McCormick PC, Canoll P, Bruce JN. Identification of A2B5+CD133− tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62(2):505–514. doi: 10.1227/01.neu.0000316019.28421.95. discussion 514–505. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411(6833):42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- Parent JM, von dem Bussche N, Lowenstein DH. Prolonged seizures recruit caudal subventricular zone glial progenitors into the injured hippocampus. Hippocampus. 2006;16(3):321–328. doi: 10.1002/hipo.20166. [DOI] [PubMed] [Google Scholar]
- Pincus DW, Harrison-Restelli C, Barry J, Goodman RR, Fraser RA, Nedergaard M, Goldman SA. In vitro neurogenesis by adult human epileptic temporal neocortex. Clin Neurosurg. 1997;44:17–25. [PubMed] [Google Scholar]
- Ponti G, Aimar P, Bonfanti L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J Comp Neurol. 2006;498(4):491–507. doi: 10.1002/cne.21043. [DOI] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55(2):165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE. Ultrastructure, histological distribution, and freeze-fracture immunocytochemistry of gap junctions in rat brain and spinal cord. Cell Biol Int. 1998;22(11–12):731–749. doi: 10.1006/cbir.1998.0392. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11(5):514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2(5):333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol. 2006;419:3–23. doi: 10.1016/S0076-6879(06)19001-1. [DOI] [PubMed] [Google Scholar]
- Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5(5):409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Lu QR, Kessaris N, Richardson WD. An ‘oligarchy’ rules neural development. Trends Neurosci. 2002;25(8):417–422. doi: 10.1016/s0166-2236(02)02201-4. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311(5761):629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE. Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res. 2006;69(1):53–66. doi: 10.1016/j.eplepsyres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, Frotscher M, Snyder EY. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3(10):801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004a;23(43):7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004b;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, Studer L. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol. 2005;23(5):601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002a;12(13):1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002b;12(13):1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Takemura S, Kayama T, Kuge A, Ali H, Kokubo Y, Sato S, Kamii H, Goto K, Yoshimoto T. Correlation between copper/zinc superoxide dismutase and the proliferation of neural stem cells in aging and following focal cerebral ischemia. J Neurosurg. 2006;104(1):129–136. doi: 10.3171/jns.2006.104.1.129. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122(4):761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- Westerlund U, Svensson M, Moe MC, Varghese M, Gustavsson B, Wallstedt L, Berg-Johnsen J, Langmoen IA. Endoscopically harvested stem cells: a putative method in future autotransplantation. Neurosurgery. 2005;57(4):779–784. discussion 779–784. [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31(5):791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25(2):331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.