Abstract
The limb blastemal cells of an adult salamander regenerate the structures distal to the level of amputation, and the surface protein Prod 1 is a critical determinant of their proximodistal identity. The Anterior Gradient protein family member nAG is a secreted ligand for Prod 1, and a growth factor for cultured newt blastemal cells. nAG is sequentially expressed after amputation in the regenerating nerve and the wound epidermis, the key tissues of the stem cell niche, and its expression in both locations is abrogated by denervation. The local expression of nAG after electroporation is sufficient to rescue a denervated blastema and regenerate the distal structures. Our analysis brings together the positional identity of the blastema and the classical nerve dependence of limb regeneration.
Limb regeneration occurs in various species of salamander and offers important insights into the possibilities for regenerating a complex structure in adult vertebrates (1). Regeneration proceeds from the limb blastema, a mound of mesenchymal stem cells which arises at the end of the stump. A blastema always regenerates structures distal to its level of origin; a wrist blastema gives rise to the hand, whereas a shoulder blastema gives rise to the arm (2). Distal blastemal cells are converted to more proximal cells by exposure to retinoic acid or other retinoids over a relatively narrow range of concentration (3, 4). This finding led to the identification of Prod 1, a determinant of proximodistal (PD) identity which is expressed at the cell surface as a GPI-anchored protein of the Ly6 superfamily (5). Its expression is graded (P>D) in both normal and regenerating limbs (6), and distal cells of the larval axolotl blastema are converted to more proximal cells following focal electroporation of a plasmid expressing Prod 1 (7). We have suggested that a ligand for Prod 1 could be an important player in PD identity (5).
The stem cell niche for limb regeneration has been studied intensively, and the key tissues are the regenerating peripheral nerves, and the wound epidermis (8). The severed axons retract after amputation, and then regenerate back along the nerve sheath and into the blastema. Axonal regeneration can be prevented or arrested by transecting the spinal nerves at the base of the limb, distant from the amputation level (9). The generation of the intitial cohort of blastemal cells occurs in a denervated limb, but the growth and division of these cells depends on the concomitant regeneration of peripheral axons (10). Both motor and sensory axons have this activity, and it is independent of impulse traffic or transmitter release (11, 12). If a peripheral nerve is cut and deviated into a skin wound, it can even evoke the formation of an ectopic appendage (13, 14). Limb regeneration is abrogated if the blastema is denervated during the initial phase of cellular accumulation, but denervation after the mid-bud stage allows the formation of a regenerate (15). The wound epidermis is not required to support proliferation during the first week of regeneration in an adult newt, but it is critical for subsequent division (16).
It remains unclear which molecules are responsible for the activity of the nerve and wound epidermis (8). The candidates considered to date include neuregulin (17, 18), FGF (19), transferrin (20) and substance P (21). In no case has it been demonstrated that a rigorously denervated blastema can be rescued such that it regenerates to form digits. Here we identify a secreted protein which is a ligand for Prod 1, and is a growth factor for limb blastemal cells. It is induced after amputation as axons regenerate along the nerve sheath, and then appears in the wound epidermis. The expression in both locations is abrogated by denervation. Most notably, the expression of this protein can rescue the denervated limb blastema and support regeneration to the digit stage.
Results
Identification of nAG protein as a ligand of Prod 1
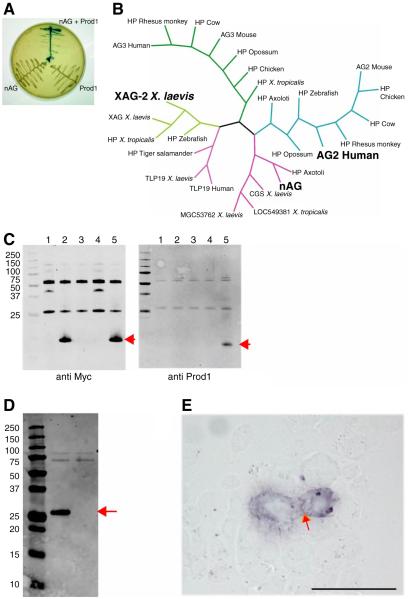
We performed a yeast two hybrid screen with the 69 amino acid newt Prod 1 protein (without N or C terminal signal/anchoring sequences) as bait, and with prey libraries derived from both normal newt limb and limb blastema. In a search for potential extracellular ligands, two secreted proteins were identified as positives from the screen and subsequent control experiments (Fig. 1A). One was a newt member of the family of Anterior Gradient proteins, originally defined by the XAG2 protein which is expressed in the cement gland of the Xenopus tadpole (Fig. 1B) (22). These proteins have a single thioredoxin fold with a secretory signal sequence (23). They are expressed in secretory epithelia and have been identified as elevated in various examples of rodent and human cancer (24, 25).
Fig. 1.
Identification of nAG protein as a ligand for Prod 1. (A) Yeast two hybrid assay illustrating the interaction between nAG and Prod 1. (B) Consensus Bayesian phylogenetic tree of representative members of the AG family of secreted proteins, highlighting nAG, the founder member XAG2, and the human AG2 which is upregulated in several examples of cancer. (C) Pull down assay with epitope-tagged forms of nAG and Prod 1 purified after bacterial expression. Lane 1, CTGF beads; 2, nAG beads; 3, control beads + Prod 1; 4, CTGF beads + Prod 1; 5, nAG beads + Prod 1. Note that Prod 1 is only pulled down in lane 5. (D) Secretion of nAG after transfection of Cos 7 cells. Cos 7 cells were transfected with a plasmid expressing the myc-tagged nAG, or RFP as control. The medium was analysed by Western blotting with anti myc. The central lane is the nAG transfected sample, the right is the RFP, and the left is the molecular weight markers. (E) Reaction of myc-tagged nAG at the surface of Prod 1 transfected mouse PS cells. nAG conditioned medium derived as in D was reacted at 4°C with live PS cells transfected to express Prod 1. Note the purple reaction product at the membrane junction between the two cells (arrowed). Scale bar, 50μm.
Epitope tagged versions of bacterially expressed nAG and Prod 1 were found to complex together in a standard pull down assay (Fig. 1C). When myc-tagged nAG was expressed after transfection of mammalian Cos 7 cells, it was secreted, and detected in immunoblots using two different antibodies directed at non-overlapping sequences (Fig. 1D). The conditioned medium was reacted with live mouse PS cells transfected so as to express the anchored newt Prod 1 on the surface. The binding of nAG to the surface was detected by phosphatase-labelled secondary antibodies (Fig. 1E), and was absent in untransfected PS cells, or after reaction with control medium from mock transfected Cos 7 cells.
Nerve-dependent expression in regeneration
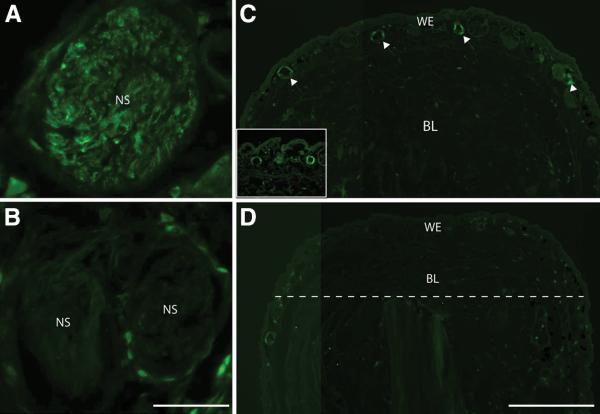
The expression of nAG protein was analysed by reacting sections of the newt limb with the two antibodies, and these gave comparable results (fig. S1). In the normal limb there was weak staining of a subset of glands in the dermis (fig. S2), but after amputation the distal end of the nerve sheath reacted strongly, as illustrated by a longitudinal section at day 5 post amputation (pa), corresponding to the early dedifferentiation stage (26) (Fig. 2A and fig S3). We analysed cross sections of the sheath by staining for both nAG and the Schwann cell marker HNK1, and the nAG was expressed in the Schwann cells and not in axons (Fig. 2B). The wound epidermis was initially negative during regeneration but after day 10 pa it reacted in glandular structures as shown at day 12 pa, corresponding to the early bud stage (26) (Fig. 2C and fig S4).
Fig. 2.
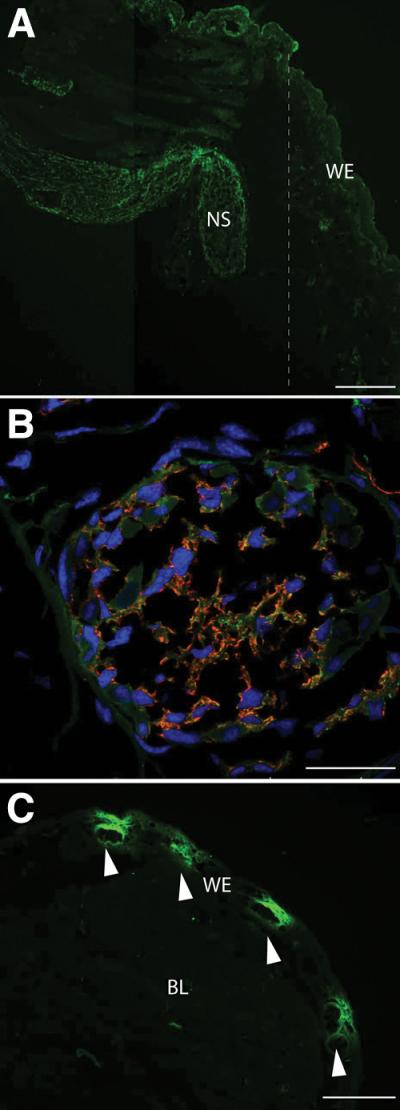
Expression of nAG after amputation of the adult newt. (A) Longitudinal section of a blastema at day 5 pa stained with antibodies to nAG (green). Note the strong reaction of the nerve sheath and lack of reaction in the WE. The dotted line indicates the position of the amputation plane. (B) Cross section of a nerve sheath in a blastema at day 10 pa stained for both nAG (green) and the Schwann cell marker HNK1 (red), and for nuclei (blue). (C) Longitudinal section of a blastema at day 12 pa, stained with antibodies to nAG and showing nAG positive glands (arrowed). NS nerve sheath, BL blastema, WE wound epidermis. Scale bars, A 200 μm, B 50μm and C 250μm.
Newts were denervated by cutting the spinal nerves at the brachial plexus of the right limb, and then amputated on both sides. The nerve sheath in the innervated limb showed strong expression at day 8 pa, whereas the sheath on the denervated side showed no reactivity (Fig. 3, A and B). Interestingly the expression in the wound epidermis was also dependent on the nerve. Figure 3C shows a low power image of the wound epidermis on the innervated side with nAG positive glands clearly visible, whereas the contralateral limb showed no reactivity and no glandular structures (Fig. 3D). We conclude that the nAG protein is expressed in the key niche tissues early in regeneration, and that expression in both locations is abrogated by denervation.
Fig. 3.
Expression of nAG in the early blastema depends on innervation. (A) Cross section of a nerve sheath on the innervated side at day 8 pa, and (B) cross section of a sheath on the contralateral denervated side, both stained in parallel with antibodies to nAG. (C) Longitudinal section of a blastema on the innervated side at day 13 pa, showing nAG positive glands (arrowed). The inset shows two glands at higher magnification. (D) Section of the contralateral denervated epidermis, with the amputation plane (dotted line) and the blastema. Scale bars, A and B 100μm, C and D 500μm.
Activities of nAG on the denervated blastema
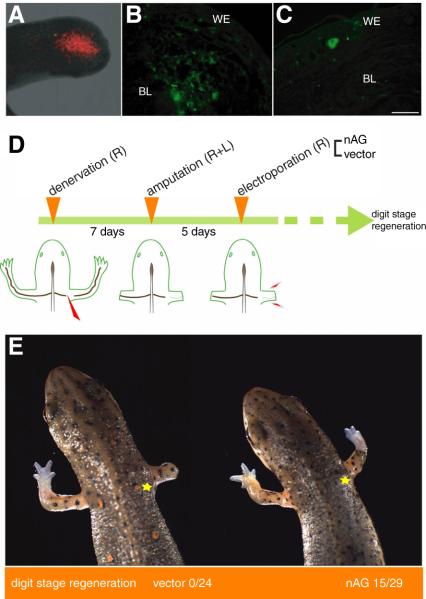
In order to deliver the protein to the adult newt limb, we electroporated plasmid DNA into the distal stump at day 5 pa. In trial experiments, red fluorescent protein (RFP) was strongly expressed in about 30-50% of the mesenchymal cells in this region (Fig. 4A), and persisted for up to 3 weeks. We expressed nAG from a plasmid with the N terminal signal sequence, and the protein was readily detectable both in the electroporated cells (Fig. 4B), and after secretion in the extracellular space of the early regenerate. Since this procedure appeared to deliver the protein effectively, we denervated animals on the right side, amputated both limbs and then electroporated the nAG plasmid or empty vector on the denervated side. At day 8 after electroporation we sectioned the distal limbs on both sides and stained with the nAG antibodies. None of the animals electroporated with the control vector showed the appearance of nAG positive glands in the wound epidermis, but 5 out of 6 animals electroporated with the nAG plasmid showed the induction of nAG positive glands (Fig 4C). Therefore the delivery of this protein to a denervated blastema can induce these elements in the wound epidermis.
Fig. 4.
Delivery of nAG protein to regenerating newt limbs. (A) RFP expression at the end of the limb stump at day 10 pa, following electroporation at day 5 pa. (B) Expression of nAG in cells of the limb after electroporation of nAG plasmid at day 7 pa. The section was stained with antibodies to nAG (green). (C) Section of a nAG positive gland in the WE after electroporation of nAG plasmid into a denervated limb blastema at day 5 pa, and analysis at day 17 pa. (D) Experimental design for assaying activity of nAG on the denervated blastema. Newts were denervated and amputated, prior to electroporation on the denervated side with either vector or nAG plasmid DNA. (E) Representative animals at day 40 pa from the two groups of an experiment outlined in (D). The yellow star indicates the position of the initial denervation. Scale bars, B and C 250μm.
In order to determine if nAG can rescue the nerve dependence of limb regeneration, groups of animals were denervated on the right side, and then amputated bilaterally (Fig 4D). At day 5 pa the right limb was electroporated either with nAG plasmid or with empty vector. The animals were allowed to regenerate and the progress of limb regeneration was monitored up to day 40 pa. Two representative newts are shown at day 40 in Fig. 4E. The position of the initial denervation is marked with a yellow star. The newt on the left has regenerated its control left limb, while the right denervated limb has failed to regenerate. In some animals the axons may subsequently regenerate from the level of the star to the amputation plane, but denervated adult newt blastemas undergo fibrosis and other tissue changes that stop them from making a delayed regenerative response (27). All animals electroporated with the vector resembled the left newt in Fig. 4E. The right animal has also regenerated on the control left side, but strikingly the expression of nAG has rescued the denervated blastema and regeneration has proceeded to the digit stage. We analysed the animals of different batches at day 30-40 pa and half of the nAG-electroporated animals showed digit stage regeneration (Fig. 4E). Some animals regenerated more slowly and were not included as reaching digit stage, while others did not regenerate, possibly because of the variability in the nAG expression level observed after electroporation of plasmids into adult limbs.
Limbs rescued by nAG expression were sectioned and stained with antibodies, along with their contralateral control limbs. After staining with antibody to acetylated tubulin, which stains peripheral nerves, the rescued limb showed few labelled profiles, whereas the control limb was densely innervated (Fig. 5, A and B). This result also indicates that nAG did not rescue the denervated blastema by enhancing the rate of nerve regeneration. These limbs were usually atrophic compared to the contralateral controls (Fig. 4E), and Fig. 5, C and D shows sections stained with anti-myosin antibody. The experimental limbs had less muscle than the innervated controls, and it appears that the dependence of skeletal muscle on its innervation (28) was not satisfied by substituting nAG. It is clear, however, that the nerve requirement for completion of the PD axis was met in these animals.
Fig. 5.
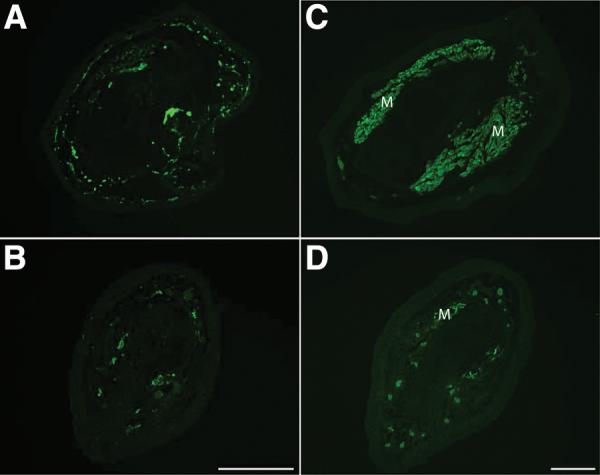
Nerve and skeletal muscle are deficient in nAG-rescued limbs. Rescued limbs were analysed along with the contralateral innervated limbs, generally at mid radius/ulna level. Sections of innervated (A) and rescued (B) regenerate limbs were stained with antibody to acetylated tubulin. Sections of innervated (C) and rescued (D) limbs were stained with antibody to skeletal myosin to label muscle. M, muscle. Scale bars, 200μm.
nAG acts as a growth factor for cultured blastemal cells
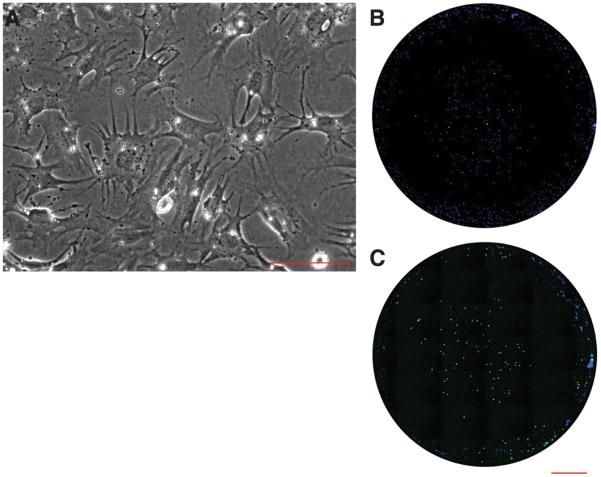
It is difficult to understand the events underlying cell division in limb mesenchyme, due to the complexity of epithelial-mesenchymal interactions in development and regeneration (29, 30). To determine if nAG acts directly on limb blastemal cells to stimulate their proliferation, the wound epidermis was removed from limb blastemas, and the cells were dissociated and allowed to attach to microwells in serum free medium, prior to maintenance in medium containing 1% serum (Fig. 6A). These cultures were reacted under live conditions with antibody to Prod 1, and approximately 70% of the cells were specifically stained on their cell surface. The cells were incubated with medium from Cos 7 cells transfected with a nAG plasmid or with a control plasmid. The nAG protein was detected in the medium after immunoblotting under both reducing and non-reducing conditions as a band at 18kD (fig. S5). The mean stimulation index for S phase entry as determined by BrdU pulse labelling was 8.3 ± 3.3 fold (SD as determined in 8 independent experiments; table S1). All cell preparations were responsive to nAG and an example is shown in Figure 6, B and C. This evidence supports the view that nAG can rescue the denervated blastema by acting directly on blastemal cells to stimulate their proliferation, and therefore mediates the nerve dependent growth of the early regenerate.
Fig. 6.
Activity of nAG on cultured newt limb blastemal cells. (A) Blastemal cells in dissociated culture at 10 days after plating. (B,C) Microwell cultures of blastemal cells that were analysed for S phase entry promoted by (B) control concentrated Cos 7 cell conditioned medium, or (C) nAG-transfected medium processed in parallel. The cells were pulse labelled with BrdU, fixed and stained for nuclei (blue) or BrdU uptake (green). Scale bars, A 200μm; B and C, 1mm.
Discussion
Our identification of nAG as a ligand for the PD determinant Prod 1 has underlined that patterning and cell division are linked at the molecular level. We envisage that PD identity is manifested by the quantitative gradation of Prod 1 (6), and that nAG has no role in specifying that identity but acts through Prod 1 to promote cell division. Blastemal growth is stimulated in experimental confrontations of cells differing in positional identity, for example in PD intercalation where a wrist level blastema is grafted onto a shoulder stump (31, 32), and this is always dependent on the presence of the nerve. In a recent study of supernumerary limb formation in the axolotl, the deflection of the brachial nerve into a skin wound provided a growth stimulus to form an ectopic blastema or ‘bump’; such bumps only progress to form limbs if a piece of skin is grafted from the contralateral skin to the wound site so as to provide dermal fibroblasts of disparate identity (14). It is interesting that dermal fibroblasts express Prod 1 and this expression is upregulated by retinoic acid (6).
Two prior studies on Anterior Gradient proteins are relevant to the present results. First, the human AG2 protein was used as bait in a yeast two hybrid assay and found to complex with a GPI-anchored protein called C4.4 which is associated with metastasis (24, 33). This protein has two Ly6-type domains which are related in sequence to urokinase-type plasminogen activator receptor (uPAR) (34). The degree of relatedness between the three dimensional structures of Prod 1 and C4.4 domains is not yet resolved, but taken together these results suggest that functional interactions between AG proteins and this class of small Cys-rich protein domains may be conserved.
In the second study it was found that overexpression of the XAG2 protein in early cleavage stage Xenopus embryos could induce formation of an ectopic cement gland which expressed XAG2 (22). We find that expression of nAG induces formation of nAG positive glands in the denervated newt wound epidermis. After amputation, nAG appears first in the Schwann cells of the distal nerve sheath and then in glands in the wound epidermis. If axonal regeneration is prevented by denervation, neither the Schwann cell nor the glandular expression is detected. Our results suggest that nAG is released by the distal sheath and induces the formation of glands in the wound epidermis. It appears that the secreted nAG acts directly on the limb blastemal cells. It is unclear how the regenerating axons act on the sheath cells, although the membrane form of neuregulin is a candidate in view of its importance for such interactions (35). The nerve dependence of regeneration offers a distinction between limb development and regeneration, since the outgrowth of the limb bud is not dependent on its innervation (36). Nonetheless the ingrowth of the nerve is critical for establishing the nerve dependence, as shown in elegant transplantation experiments on axolotl larvae (37). The identification of nAG offers a new opportunity to study the mechanisms underlying this switch.
Nerve dependence of regeneration is conserved in phylogeny. It has been studied in regeneration of the fish fin, the taste barbel in catfish, the arms of crinoid and asteroid species in echinoderms, and the body axis in annelids (28, 38). In most vertebrate appendages the density of innervation is lower than in salamanders and Singer suggested that this is a primary determinant for the loss of regenerative ability, for example in mammals (9, 39). This hypothesis now seems unlikely as there are other variables apparently curtailing regeneration (1). It is striking that the expression of a single protein can rescue limb regeneration in an adult animal (Fig. 4E), and this finding underlines that the blastema is an autonomous unit of organisation for which there is no obvious mammalian counterpart. We have suggested that one approach for regenerative medicine would be to understand the specification of the blastema at a level of detail that would allow it to be engineered in mammals (1). The local delivery of permissive regulators like nAG could then evoke formation of the appropriate structures without the need for subsequent intervention.
Supplementary Material
Acknowledgments
We thank P. Driscoll, I. Gout and P. Martin for their help and comments, and M. Larkum for fabrication of the electroporation electrodes. Supported by a Medical Research Council (UK) Research Professorship and Programme Grant to JPB.
References
- 1.Brockes JP, Kumar A. Science. 2005;310:1919. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- 2.Brockes JP. Science. 1997;276:81. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- 3.Maden M. Nature. 1982;295:672. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- 4.Thoms SD, Stocum DL. Dev Biol. 1984;103:319. doi: 10.1016/0012-1606(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 5.Morais da Silva S, Gates PB, Brockes JP. Dev Cell. 2002;3:547. doi: 10.1016/s1534-5807(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Gates PB, Brockes JP. C R Biologies. 2007;330:485. doi: 10.1016/j.crvi.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Echeverri K, Tanaka EM. Dev Biol. 2005;279:391. doi: 10.1016/j.ydbio.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Stocum DL. Curr Top Microbiol Immunol. 2004;280:1. doi: 10.1007/978-3-642-18846-6_1. [DOI] [PubMed] [Google Scholar]
- 9.Singer M. Q Rev Biol. 1952;27:169. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- 10.Brockes JP. Science. 1984;225:1280. doi: 10.1126/science.6474177. [DOI] [PubMed] [Google Scholar]
- 11.Drachman DB, Singer M. Exp Neurol. 1971;32:1. doi: 10.1016/0014-4886(71)90159-2. [DOI] [PubMed] [Google Scholar]
- 12.Sidman RL, Singer M. Am J Physiol. 1951;165:257. doi: 10.1152/ajplegacy.1951.165.1.257. [DOI] [PubMed] [Google Scholar]
- 13.Egar MW. Anat Rec. 1988;221:550. doi: 10.1002/ar.1092210111. [DOI] [PubMed] [Google Scholar]
- 14.Endo T, Bryant SV, Gardiner DM. Dev Biol. 2004;270:135. doi: 10.1016/j.ydbio.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Singer M, Craven L. J Exp Zool. 1948;108:279. doi: 10.1002/jez.1401080207. [DOI] [PubMed] [Google Scholar]
- 16.Mescher AL. J Exp Zool. 1976;195:117. doi: 10.1002/jez.1401950111. [DOI] [PubMed] [Google Scholar]
- 17.Brockes JP, Kintner CR. Cell. 1986;45:301. doi: 10.1016/0092-8674(86)90394-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Marchionni MA, Tassava RA. J Neurobiol. 2000;43:150. doi: 10.1002/(sici)1097-4695(200005)43:2<150::aid-neu5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Mullen LM, Bryant SV, Torok MA, Blumberg B, Gardiner DM. Development. 1996;122:3487. doi: 10.1242/dev.122.11.3487. [DOI] [PubMed] [Google Scholar]
- 20.Mescher AL, Connell E, Hsu C, Patel C, Overton B. Dev Growth Differ. 1997;39:677. doi: 10.1046/j.1440-169x.1997.t01-5-00003.x. [DOI] [PubMed] [Google Scholar]
- 21.Globus M, Smith MJ, Vethamany-Globus S. Ann N Y Acad Sci. 1991;632:396. doi: 10.1111/j.1749-6632.1991.tb33135.x. [DOI] [PubMed] [Google Scholar]
- 22.Aberger F, Weidinger G, Grunz H, Richter K. Mech Dev. 1998;72:115. doi: 10.1016/s0925-4773(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 23.Persson S, et al. Mol Phylogenet Evol. 2005;36:734. doi: 10.1016/j.ympev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Adam PJ, et al. J Biol Chem. 2003;278:6482. doi: 10.1074/jbc.M210184200. [DOI] [PubMed] [Google Scholar]
- 25.Thompson DA, Weigel RJ. Biochem Biophys Res Commun. 1998;251:111. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 26.Iten L, Bryant S. Willhem Roux Arch Dev Biol. 1973;173:263. doi: 10.1007/BF00575834. [DOI] [PubMed] [Google Scholar]
- 27.Salley JD, Tassava RA. J Exp Zool. 1981;215:183. doi: 10.1002/jez.1402150208. [DOI] [PubMed] [Google Scholar]
- 28.Carlson BM. Principles of Regenerative Biology. Elsevier Inc.; London: 2007. [Google Scholar]
- 29.Mariani FV, Martin GR. Nature. 2003;423:319. doi: 10.1038/nature01655. [DOI] [PubMed] [Google Scholar]
- 30.Niswander L. Nat Rev Genet. 2003;4:133. doi: 10.1038/nrg1001. [DOI] [PubMed] [Google Scholar]
- 31.Crawford K, Stocum DL. Development. 1988;102:687. doi: 10.1242/dev.102.4.687. [DOI] [PubMed] [Google Scholar]
- 32.Pecorino LT, Entwistle A, Brockes JP. Curr Biol. 1996;6:563. doi: 10.1016/s0960-9822(02)00542-0. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher GC, et al. Br J Cancer. 2003;88:579. doi: 10.1038/sj.bjc.6600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosel M, Claas C, Seiter S, Herlevsen M, Zoller M. Oncogene. 1998;17:1989. doi: 10.1038/sj.onc.1202079. [DOI] [PubMed] [Google Scholar]
- 35.Lemke G. Sci STKE. 2006;2006:pe11. doi: 10.1126/stke.3252006pe11. [DOI] [PubMed] [Google Scholar]
- 36.Yntema CL. J Exp Zool. 1959;142:423. doi: 10.1002/jez.1401420119. [DOI] [PubMed] [Google Scholar]
- 37.Thornton CS, Thornton MT. J. Exp Zool. 1970;173:293. [Google Scholar]
- 38.Goss RJ. Principles of regeneration. Academic Press; New York: 1969. [Google Scholar]
- 39.Singer M, Rzehak K, Maier CS. J Exp Zool. 1967;166:89. doi: 10.1002/jez.1401660110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.