Abstract
Bmi1 is a Polycomb Group protein that functions as a component of Polycomb Repressive Complex 1 (PRC1) to control axial skeleton development through Hox gene repression. Bmi1 also represses transcription of the Ink4a-Arf locus and is consequently required to maintain the proliferative and self-renewal properties of hematopoietic and neural stem cells. Previously, one E2F family member, E2F6, has been shown to interact with Bmi1 and other known PRC1 components. However, the biological relevance of this interaction is unknown. In this study, we use mouse models to investigate the interplay between E2F6 and Bmi1. This analysis shows that E2f6 and Bmi1 cooperate in the regulation of Hox genes, and consequently axial skeleton development, but not in the repression of the Ink4a-Arf locus. These findings underscore the significance of the E2F6-Bmi1 interaction in vivo and suggest that the Hox and Ink4a-Arf loci are regulated by somewhat different mechanisms.
Keywords: E2F6, Bmi1, p16, p19, ARF, axial skeleton, polycomb, cell cycle, stem cells
INTRODUCTION
The E2F transcription factors are a family of key regulators of cell proliferation and differentiation (Dyson, 1998; Nevins, 1998; Trimarchi and Lees, 2002). They act by controlling the transcription of genes whose expression is essential for cell cycle progression and DNA synthesis. In mammals, eight E2f genes (E2f1–8) have been identified. E2f1–5 encode proteins that function as transcriptional activators or repressors by virtue of their association with the family of pocket proteins, pRB, p107, and p130. E2f6–8 are more distantly related members of the E2F family. In particular, they lack the domain required for pocket protein-binding and are therefore not susceptible to pocket protein regulation (Cartwright et al., 1998; de Bruin et al., 2003; Gaubatz et al., 1998; Logan et al., 2004; Maiti et al., 2005; Morkel et al., 1997; Trimarchi et al., 1998; Trimarchi et al., 2001). E2F6 binds DNA as a heterodimer with DP, in a similar manner to E2F1– 5, but due to the absence of a transactivation domain it does not activate transcription (Cartwright et al., 1998; Gaubatz et al., 1998; Morkel et al., 1997; Trimarchi et al., 1998). Early studies showed that over-expressed E2F6 can repress classic E2F-responsive genes, at least in part, by binding to E2F-responsive promoters and blocking access to other activating E2Fs (Gaubatz et al., 1998; Morkel et al., 1997; Trimarchi et al., 1998). Subsequently, E2F6 was shown to exist in complexes that contain both chromatin remodeling enzymes and members of the mammalian Polycomb Group (PcG), including Bmi1, Ring1, and RYBP (Attwooll et al., 2004; Ogawa et al., 2002; Trimarchi et al., 2001). The PcG proteins, first identified in Drosophila, form large multimeric complexes that are responsible for the repression of the Hox genes, which determine the patterning of the developing embryo (Kennison, 1995; Simon, 1995). The documented interaction between E2F6 and PcG proteins suggested that E2F6 might actively enforce repression of E2F target genes in vivo through recruitment of the PcG complex(es) and might also participate in the regulation of known PcG-responsive targets.
In mammals, there are at least two distinct PcG complexes, called Polycomb Repressive Complexes (Otte and Kwaks, 2003). The Eed-containing PcG complex, PRC2, initiates polycomb-mediated repression, whereas the Bmi-1-containing PcG complex, PRC1, maintains the repression at later stages of development. Loss of proteins of the PcG-PRC2 in mice generally results in early embryonic lethality (Faust et al., 1995; O’Carroll et al., 2001) whereas PcG-PRC1 mutant mice typically survive until birth and display homeotic transformations of the axial skeleton (Akasaka et al., 1996; Akasaka et al., 1997; del Mar Lorente et al., 2000; van der Lugt et al., 1994). Notably, E2F6 has been shown to interact with known components of both PcG-PRC1 and PcG-PRC2 (Attwooll et al., 2004; Ogawa et al., 2002; Trimarchi et al., 2001). Based on our previous association data (Trimarchi et al., 2001), we have focused our attention on understanding the interplay between E2F6 and Bmi1.
Bmi1-deficient mice display defects in axial skeletal patterning, hematopoiesis, the central nervous system, and the peripheral nervous system (Jacobs and van Lohuizen, 2002; van der Lugt et al., 1994). In the hematopoietic system, the stem cell defect results in a loss of mature T and B cells, hypocellularity of the bone marrow, decreased spleen size, and an involuted thymus (Lessard and Sauvageau, 2003; van der Lugt et al., 1994). The neurological defects in the Bmi1 mutant mice include an ataxic gait, seizures, hypocellularity of the molecular and granular layers of the cerebellum, and astrogliosis in the cortex and cerebellum of the brain (Leung et al., 2004; Molofsky et al., 2003; van der Lugt et al., 1994; Zencak et al., 2005). The neurological and hematopoietic defects can be partially attributed to a deficiency in the proliferation and self-renewal capacity of the stem cells in these compartments (Lessard and Sauvageau, 2003; Molofsky et al., 2005; Park et al., 2003; Zencak et al., 2005).
The proliferation defects observed in vivo are consistent with a role for the PcG proteins in the control of the cell cycle. Indeed, mouse embryonic fibroblasts (MEFs) deficient for Bmi1, Mel–18 or M33 have impaired proliferation properties and undergo premature senescence (Core et al., 1997; Jacobs et al., 1999). The cell cycle defects in the Bmi1−/− MEFs result from the derepression of the Ink4a-Arf locus (Jacobs et al., 1999). This locus encodes two proteins, p16INK4a and p19ARF, which regulate cellular proliferation and apoptosis. Only a few PcG proteins have been reported to bind DNA directly including Mel-18 and YY1 (Brown et al., 1998; Kanno et al., 1995). These proteins cannot account for all the Polycomb DNA binding activity suggesting that other DNA binding factors may be facilitate the recruitment of PcG complexes to specific promoters. Notably, several E2F family members have been shown to play a direct role in the transcriptional regulation of p19ARF (Aslanian et al., 2004). Since E2F6 associates with PcG proteins and is able to directly bind DNA in a sequence-specific manner, we hypothesized that E2F6 might act to recruit PcG complexes to target promoters including Arf. To investigate the role of E2F6 in development and cell cycle control, we and others have generated E2f6-deficient mice (this study; Storre et al. 2002). Consistent with our prior observation that E2F6 interacts with Bmi1 and other PRC1 components (Trimarchi et al., 2001), E2f6−/− mice display subtle axial skeletal transformations. It is well established that the combined mutation of two PcG-PRC1 components in flies or mice yields synergistic phenotypic effects (Adler et al., 1991; Akasaka et al., 2001; Bel et al., 1998; Kwon et al., 2003). Thus, to test for a possible genetic interaction between E2F6 and Bmi1, we have generated mice and MEFs that are deficient for both E2f6 and Bmi1 genes. Our data show that E2f6-deficiency increases the severity of the axial skeletal defect in Bmi1 mutant mice but does not modulate the other Bmi1 mutant phenotypes.
RESULTS
E2f6 mutant mice are viable but display axial skeletal defects
In order to study the role of E2F6 in vivo, we generated an E2f6 mutant mouse strain in which we have deleted a large proportion of the E2f6 coding sequences including the exons that encode the DNA binding, leucine zipper and marked box domains (Figure 1A). Western blotting of E2f6−/− MEFs confirms loss of the E2F6 protein (Figure 1B). Consistent with previous studies (Storre et al., 2002) we find that E2f6-deficient mice are born at the expected frequency (120% of expected, n=233) and display no gross morphological defects. Previous studies have not assessed the lifespan in littermates. We generated a cohort of aging animals and found that there was no difference in the percentage of E2f6+/+, E2f6+/− versus E2f6−/− mice that were alive after 530 days (70%, 68% and 75%, respectively; Figure 1C) or in the eventual cause of death of these animals. Thus, we conclude that E2f6-loss has no detectable effect on murine viability.
Figure 1. E2f6 mutant mice are fully viable and display axial skeletal transformations.
(A) Generation of the E2f6−/− mice. The E2f6 genomic locus comprises 8 exons that include non-coding sequences (black boxes) coding sequences (gray boxes) and an alternatively spliced exon 2 (white box). DBD, DNA binding domain; DIM, dimerization domain; MB, marked box domain. The E2F6 mutant allele was generated by replacing coding sequences of exons 4 through 8 with a PGK-neo cassette that includes a STOP codon at the beginning. PGK-neo, neomycin resistance gene under the regulation of the PGK promoter for positive selection. Disruption of the E2f6 locus was confirmed by (B) Western of lysate from Wild-type and E2f6−/− MEFs. (C) Survival curve of E2f6 wild-type, heterozygote and mutant mice followed for over 500 days. (D) Ventral view of axial skeletons of newborn E2f+/+ and E2f6−/− mice stained with alcian blue (cartilage) and alzarin red (bone). E2f6−/− mice display two axial skeletal transformations, the thirteenth thoracic vertebra (T13) is transformed into the first lumbar vertebra (L1) as shown by the degeneration of the thirteenth ribs and the sixth lumbar vertebra (L6) is transformed into the first sacral vertebra (S1) as evidenced by the formation of the sacraliliac joints.
We have previously shown that E2F6 associates with Bmi1 and other components of the PcG-PRC1 complex in vivo. Since loss of Bmi1 in mice results in posterior transformations along the entire axial skeleton, (van der Lugt et al., 1994) we analyzed the skeletons of E2f6+/+, E2f6+/− and E2f6−/− mice at post-natal day 3 (P3) by staining with Alcian blue and Alzarin red, which stain the cartilage in blue and the bone in red (Figure 1D). Similar to Bmi1 and other PcG knockout mice (Akasaka et al., 1996; van der Lugt et al., 1994), E2f6 mutant mice displayed posterior transformations. First, the thoracic vertebra T13 is transformed into a lumbar vertebra L1 as evidenced by the lack of ribs in 0% of E2f6+/+, 9% of E2f6+/−, and 67% of E2f6−/− animals. Second, the lumbar vertebra L6 is transformed into the sacral vertebra S1 as shown by its association with the iliac bones in 0% of E2f6+/+, 35% of E2f6+/−, and 80% of E2f6−/− mice (Figure 1D). These observations are consistent with those of Storre et al., 2002 who previously reported T13 to L1 and L6 to S1 transformations in a distinct E2f6 mutant mouse model. Together, our data suggest that E2f6 mutation results in dosage-dependent posterior transformations of the axial skeleton that are reminiscent of axial skeletal transformations seen in Bmi1 and other PcG-PRC1 knockout mice.
Viability of E2f6−/−Bmi1−/− mice
Compound mutants of PcG-PRC1 proteins have been generated in flies and mice resulting in dramatic synergistic effects (Adler et al., 1991; Akasaka et al., 2001; Bel et al., 1998; Kwon et al., 2003). Thus, to determine the biological relevance of the interaction between E2F6 and Bmi1, we have generated and analyzed E2f6−/−Bmi1−/− mice. E2f6 deletion alone does not affect the viability and survival of mice. In contrast, while Bmi1−/− mice are born at the expected frequency, they are selectively cannibalized by their mothers shortly after birth and only approximately 50% survive into adulthood (van der Lugt et al., 1994). These animals are significantly smaller than their wild-type littermates and display poor health that results in early lethality (3 to 20 weeks). In good agreement with this prior analysis, examination of the progeny from E2f6+/−;Bmi1+/− intercrosses showed that Bmi1−/− single mutant mice were underrepresented at 3 weeks of age (51% of expected: Table 1) but were present at near expected frequencies at embryonic day 18.5 (122% of expected: Table 2). In contrast, E2f6−/−Bmi1−/− mice arising from E2f6+/−;Bmi1+/− intercrosses seemed to be underrepresented at both three weeks (38% of expected; Table 1) and also at E18.5 (46% of expected; Table 2). To better assess the relatively viability of E2f6−/−Bmi1−/− versus Bmi1−/− embryos, we conducted E2f6−/−Bmi1+/− intercrosses to generate the test genotypes at a much higher frequency. Analysis of the resulting progeny showed that there was no significant difference in the viability of E2f6−/−Bmi1−/− versus Bmi1−/− embryos at E18.5. (Table 3; p=0.87). Therefore, loss of E2F6 has no effect on the viability of Bmi1 mutant mice. In addition, the E2f6−/−Bmi1−/− mice showed a similar degree of growth retardation as their Bmi1−/− littermates and these two genotypes both developed severe anemia and had to be sacrificed in a similar time window (data not shown).
Table 1.
Viability of E2f6;Bmi1 compound mutant mice at three weeks of age
| n=250 | E2f6+/+ | E2f6+/+ | E2f6+/− | E2f6−/− |
|---|---|---|---|---|
| Bmi1+/+ | Bmi1−/− | Bmi1−/− | Bmi1−/− | |
| Expected | 15.63 | 15.63 | 31.25 | 15.63 |
| Observed | 16 | 8 | 24 | 6 |
| % of expected | 102 | 51 | 77 | 38 |
Mice generated from E2f6+/−:Bmi1+/− intercrosses and genotyped at three weeks of age.
Table 2.
Viability of E2f6:Bmi1 compound mutant mice at E18.5
| n=105 | E2f6+/+ | E2f6+/+ | E2f6+/− | E2f6−/− |
|---|---|---|---|---|
| Bmi1+/+ | Bmi1−/− | Bmi1−/− | Bmi1−/− | |
| Expected | 6.56 | 6.65 | 13.13 | 6.56 |
| Observed | 5 | 8 | 7 | 3 |
| % of expected | 76 | 122 | 53 | 46 |
Mice generated from E2f6+/−:Bmi1+/− intercrosses and genotyped at embryonic day 18.5.
Table 3.
Viability of E2f6:Bmi1 compound mutant mice at E18.5
| n=27 | E2f6−/− | E2f6−/− | E2f6−/− |
|---|---|---|---|
| Bmi1+/+ | Bmi1+/− | Bmi1−/− | |
| Expected | 6.75 | 13.6 | 6.75 |
| Observed | 6 | 13 | 8 |
| % of expected | 89 | 96 | 118 |
Mice generated from E2f6−/−:Bmi1+/− intercrosses and genotyped at embryonic day 18.5.
E2F6 does not cooperate with Bmi1 in the regulation of the INK4a-ARF locus
To determine whether E2F6 and Bmi1 play cooperating roles, we conducted a careful analysis of the cells and tissues that are known to be affected by Bmi1-loss. The lethal anemia of Bmi1−/− mice results from a progressive decrease in the number of hematopoietic cells (van der Lugt et al., 1994). Thus, we first compared the levels of various hematopoietic lineages in wildtype, E2f6−/−, Bmi1−/− and E2f6−/−Bmi1−/− mice at eight weeks of age. The mutation of E2f6 alone had no detectable effect on the levels of hematopoietic cells or the distribution of the various white blood cell lineages (Figure 2). In contrast, the Bmi1−/− and E2f6−/−Bmi1−/− mice both showed a profound hematopoietic defect. First, we observed a significant reduction in the level of hematopoietic cells in the bone marrow, spleen, and thymus (Figure 2A). Second, there was a clear shift in the distribution of immature versus mature cells in various lineages. For example, in the thymus, there was a significant depletion of double positive (CD4+/CD8+) thymocytes, whereas immature (CD4−/IL2−R+, CD8−/IL2−R+, and CD4−and mature (CD4+/CD8−4−cells are still present (Figure 2B). Moreover, in the bone marrow there was a significant shift in the distribution of myeloid (Gr-1+/Mac-1+) versus B lymphoid cells in both the Bmi1−/− and E2f6−/−Bmi1−/− mice. In each case, this lead to a higher percentage of myeloid cells, although the absolute number of myeloid cells is still reduced relative to wildtype (Figure 2C). Within the B cell population, we also observed a greater deletion of the immature B cells (B220+/HSA+; seven fold decrease in the percentage of cells) than the mature B cells (B220+/IgM+; three fold decrease in the percentage of cells) when compared with wildtype (Figure 2C). Similar results were seen in the spleen (data not shown). These changes are all consistent with the known defect in the maintenance and self-renewal capacity of the Bmi1−/− hematopoietic stem cells (van der Lugt et al., 1994). Importantly, there was no significant difference in the degree of these defects in the E2f6−/−Bmi1−/− versus the Bmi1−/− mice, indicating that E2F6-loss does not modulate the effect of Bmi1-deficiency on the hematopoietic compartment.
Figure 2. E2F6-loss does not modulate the hematopoietic defect within Bmi1 mutant mice.
(A) Cell counts of single cell preparations made from the bone marrow, spleen, and thymus of eight week old mice. E2f6+/+;Bmi1+/+ n=2; E2f6−/−;Bmi1+/+ n=2; E2f6+/+;Bmi1−/− n=3; E2f6+/−;Bmi1−/− n=1; E2f6−/−;Bmi1−/− n=3. FACS analysis of single cell preparations made from the thymus (B) and bone marrow (C) and immunostained with the indicated antibodies. Results are presented as percentage of cells. E2f6+/+;Bmi1+/+ n=2; E2f6−/−;Bmi1+/+ n=2 for part B, n=3 for part C; E2f6+/+;Bmi1−/− n=3; E2f6+/−;Bmi1−/− n=1 for part B, n=2 for part C; E2f6−/−;Bmi1−/− n=3.
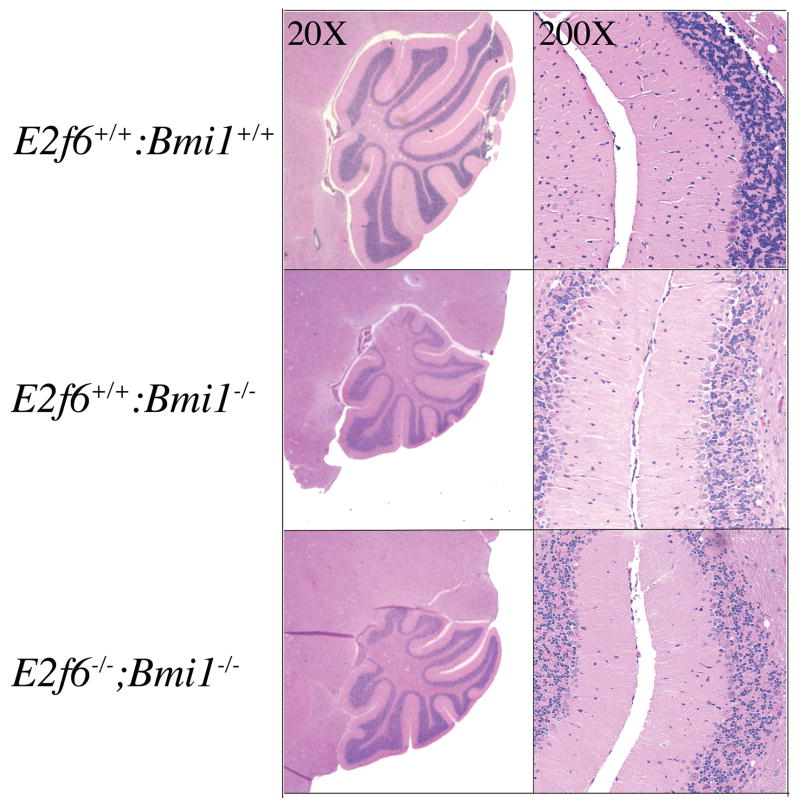
Bmi1 deficient mice exhibit hypocellularity of various layers of the cerebellum and develop ataxia at the age of 2– weeks (Leung et al., 2004; van der Lugt et al., 1994). Thus, we next tested whether the loss of E2F6 modulates these neuronal defects. First, we screened animals from E2f6+/− intercrosses that survived to 2 months of age for ataxia (data not shown). This defect was completely absent in animals that were either wildtype or Bmi1+/−, irrespective of their E2f6 status. In contrast, we observed ataxia in a similarly high proportion of the Bmi1−/− (60%; n=4), E2f6+/−;Bmi1−/− (65%; n=13) and E2f6−/−Bmi1−/− (70%; n=7). We did observe a general correlation between the degree of growth retardation and the likelihood that an animal would develop ataxia. It is unclear whether these defects are causally linked or whether they simply reflect some variation in the penetrance of Bmi1 mutation in the mixed (C57/BL6 x 129S/v) genetic background of our animals. However, our data clearly show that E2f6 status did not alter either the penetrance or the time of onset of the ataxia. To complement this analysis, we also performed a histological analysis of brain sections of single and double mutant animals (Figure 3). Consistent with previous results, the Bmi1−/− cerebellums were significantly smaller than wild-type with all three layers of the cerebellum affected. We observed a similar level of cerebellum hypocellularity in the E2f6−/−Bmi1−/− mice. Given these observations, we conclude that E2F6-loss does not modulate either the defective cerebellar development or the consequent ataxia of the Bmi1-deficient mice.
Figure 3. E2f6−/−;Bmi1−/− and Bmi1−/− mice display defects in gross cerebellar structure.
Hematoxylin and eosin staining of cerebellum sections from the midline of 8 week old wild-type, Bmi1 mutant, and E2f6;Bmi1 compound mutant mice.
It is well established that Bmi1-loss leads to the derepression of the Ink4-Arf locus and the resulting upregulation of p16INK4a and p19ARF. This upregulation is a key determinant of the hematopoietic and neural defects of the Bmi1−/− mice via impairment of the self-renewal capacity of hematopoietic and neural stem cells (Lessard and Sauvageau, 2003; Molofsky et al., 2005; Molofsky et al., 2003; Park et al., 2003; Zencak et al., 2005). The Ink4-Arf derepression was first observed, and is best characterized, in Bmi1−/− MEFs where it causes impaired proliferation and premature senescence (Jacobs et al., 1999). Given this fact, and the documented role of other E2F family members in the regulation of Arf in MEFs (Aslanian et al., 2004), we also compared the properties of wild-type, Bmi1−/−, E2f6−/− and E2f6−/−Bmi1−/− MEFs. E2f6−/− MEFs displayed no obvious proliferation defects (data not shown). In contrast, Bmi1−/− MEFs showed a defect in asynchronous proliferation (Figure 4A) and underwent premature senescence (Figure 4B) as previously reported (Jacobs et al., 1999). In addition, we found that serum deprived Bmi1−/− MEFs were impaired in their ability to re-enter the cell cycle following the re-addition of serum (Figure 4C). There was some variation in the degree of these defects from one cell line to the next, likely because of the mixed genetic background. We also derived five E2f6−/−Bmi1−/− MEF lines from four different litters and compared their properties with those of MEFs derived from Bmi1−/− (n=5) and wild-type (n=4) littermates. The presented data are from cell lines derived from one representative litter (Figure 4). The E2f6−/−Bmi1−/− MEFs showed defects in asynchronous proliferation, cellular senescence and cell cycle re-entry that were comparable to those of the Bmi1−/− MEFs (Figure 4A–C). Consistent with this finding, real-time PCR analysis of RNA collected from these MEFs revealed the E2F6 status did not change the level of derepression of p16INK4a and p19ARF in the Bmi1−/− MEFs (Figure 4D). Similarly, the levels of two E2F6 repressed genes, STAG3 and SMC1s, were not further derepressed in compound mutant MEFs (data not shown). These results suggest that E2F6 does not play a role in repression of INK4a-ARF.
Figure 4. Cell cycle properties of E2f6 and Bmi1 mutant MEFs.
Mouse embryonic fibroblasts of wild-type (υ), Bmi1 mutant (σ, 5), and E2f6;Bmi1 compound mutant (Σ, λ) mice were assayed for (A) asynchronous proliferation rate, (B) senescence properties, (C) S-phase re-entry following serum withdrawal and re-addition, and (D) p16INK4a and p19ARF expression by RT-PCR from serum-starved cells.
Bmi1 and E2F6 synergize in axial skeleton development and co-regulate Hox genes
To determine whether E2f6 and Bmi1 synergize in axial skeletal development, we examined the axial skeletons of all genotypes arising from a double heterozygous cross. Since 50% of the Bmi1−/− mice die perinatally, we conducted this analysis using E18.5 embryos to ensure good representation of Bmi1−/− and E2f6−/−Bmi1−/− animals (Figure 5). Bmi1−/− mice are known to display morphological abnormalities along the anteroposterior axis that indicate posterior transformations of vertebra identity (van der Lugt et al., 1994). These include (1) an extra piece of bone rostral to the cervical vertebra C1, (2) a C1 to C2 conversion, (3) a partial C7 to T1 (thoracic vertebra) conversion evidenced by the presence of ribs at C7 which then fuse on the ventral side with the ribs associated with T1 (in some cases, the transformation was full, that is, the C7 rib connected to the sternum instead of fusing with the T1 rib), (4) a T7 to T8 conversion resulting in only six instead of seven vertebrosternal ribs, (5) a T13 to L1 (lumbar vertebra) conversion shown by the absence of ribs at T13, and (6) a L6 to S1 (sacral vertebra) conversion evidenced by the joints between L6 and the iliac bones. Consistent with prior studies, we observed all of these transformations in the Bmi1−/− embryos with partial penetrance (Figure 5). Analysis of the compound mutants showed that E2f6 mutation increased the severity of the Bmi1−/− skeletal defects in a dose dependent manner (Figure 5B). Specifically, deletion of only one allele of E2F6 was sufficient to increase the penetrance of the C1 to C2, C5 to C6, partial C7 to T1, T13 to L1, and L6 to S1 transformations. Further deletion of the remaining E2f6 allele led to an even higher penetrance, indicating that E2F6 and Bmi1 synergistically contribute to these abnormalities. No synergy was found for the extra, C7 to T1 (full), and T7 to T8 transformations, suggesting that these defects are specific to Bmi1-loss. However, in the case of the extra piece of bone (E) we observed an increase in the E2f6+/−;Bmi1−/− but not in the E2f6−/−Bmi1−/− mice. As this piece of bone is very small and weakly attached, we cannot rule out the possibility that it was broken off during manipulation of the axial skeletons. Finally, we have identified a novel transformation, the C5 to C6 conversion that occurs in 10% of E2f6−/− embryos and 23% of E2f6−/−Bmi1−/− embryos but was never detected in the Bmi1−/− embryos. The increase in penetrance of some, but not all, posterior transformations in the E2f6−/−Bmi1−/− embryos underscores the conclusion that E2F6 influences the regulation of a subset of Bmi1 target genes.
Figure 5. E2f6 and Bmi1 show a genetic interaction in the development of the axial skeleton.
(A) Alcian blue (cartilage) and alzarin red (bone) stainings of wild-type, Bmi1 mutant and E2f6;Bmi1 compound mutant mice at E18.5. The following skeletal transformations are depicted in the Bmi1 and E2f6:Bmi1 compound mutant mice: An extra piece of bone anterior to the first cervical vertebra (C1); the C1 vertebra is transformed into the second cervical vertebra (C2) as evidenced by the altered morphology; the seventh cervical vertebra (C7) is transformed into the first thoracic vertebra (T1) as shown by the presence of vertebrosternal ribs. The right panel shows the novel C5 to C6 transformation present in the E2f6 mutant and E2f6;Bmi1 compound mutant mice. This is evidenced by the presence of a piece of cartlidge on C5 instead of on C6. (B) Penetrance of axial skeletal transformations in E2f6;Bmi1 compound mutant embryos.
It is well established the axial skeletal defects in the Bmi1 mutants results from the derepression of Hox genes that are essential for embryonic patterning. The observed synergy between E2f6 and Bmi1 in axial skeletal suggests that E2F6 and Bmi1 might co-regulate Hox genes. To address this question, we first used real-time PCR analysis to compare Hox mRNA levels genes in the wild-type (n=2), Bmi−/− (n=2), and E2f6;Bmi1 DKO (n=3) MEFs used above to assess Ink4a-Arf expression. Although E2F6-loss had no affect the level of derepression of p16INK4a and p19ARF in the Bmi1−/− MEFs (Figure 4D), it did modulate the Bmi1 mutant phenotype with regard to the Hox genes (Figure 6A). The Bmi1−/− MEFs had increased mRNA levels of HoxC10 (both lines analyzed), HoxA9 (both lines analyzed) and HoxB6 (1 out of 2 lines analyzed). Loss of E2F6 in the Bmi1 mutant cells led to further deregulation of HoxB6 and HoxC10, but not HoxA9, in every E2f6;Bmi1 mutant cell line analyzed (Figure 6A and data not shown). The mRNA levels of HoxB6, HoxC10, or HoxA9 were unaffected by the mutation of E2f6 alone (data not shown) indicating that this reflects a synergistic effect of E2f6 and Bmi1 in Hox gene regulation.
Figure 6. E2f6 and Bmi1 co-regulate Hox genes.

(A) Real-time PCR analysis of Hox genes in MEFs. (B) Chromatin immunoprecipitation analysis of mouse embryonic stem cells. Sonicated, cross-linked chromatin was immunoprecipitated with a Bmi1, E2F6, or control IgG antibody and the purified DNA was analyzed by PCR with primers specific for the promoter of HoxA7, HoxA10, HoxA11, Arf, or a control sequence (1kb upstream of the E2F1 promoter).
The MEF analysis supports our genetic evidence that E2f6 and Bmi1 act together to regulate Hox genes but not Arf. However, these experiments do not establish whether E2F6 is directly, or indirectly, involved in the transcriptional regulation of the Hox genes. To address this question, we wished to evaluate the promoter occupancy of the Hox genes. It has previously been shown that repression of Hox genes and other key developmental regulators is established in embryonic stem (ES) cells (Boyer et al., 2006). Thus, we used murine ES cells to perform chromatin immunoprecipitation on four genes, HoxA7, HoxA10, HoxA11, and Arf, which have previously been shown to be directly regulated by Bmi1 (Bracken et al., 2007; Cao et al., 2005; Kotake et al., 2007; Xi et al., 2007). We found that Bmi1 was directly bound to the promoters of HoxA7, HoxA10, HoxAll, and Arf in mouse embryonic stem cells (Figure 6B). In contrast, E2F6 bound specifically to the promoters of HoxA10 and HoxA11, but did not associate with either HoxA7 or Arf (Figure 6B). These results provide in vivo biochemical evidence of the co-regulation of a subset of Hox genes by E2F6 and Bmi1 and reinforce the conclusion that E2F6 is not required for repression of Arf.
DISCUSSION
Bmi1 is a key component of the PRC1 repressor complex. Bmi1 mutant mice have axial skeletal transformations that reflect a key role for Bmi1 in the appropriate repression of the Hox genes (Akasaka et al., 1996; Akasaka et al., 1997; del Mar Lorente et al., 2000; van der Lugt et al., 1994). Additionally, Bmi1 deficient mice have impaired proliferation and premature senescence due to the up-regulation of Ink4a-Arf (Jacobs et al., 1999). Bmi1 loss and up-regulation of p16INK4a and p19ARF compromises the proliferative and self-renewal capacity of stem cells in the developing mouse (Lessard and Sauvageau, 2003; Molofsky et al., 2003; Park et al., 2003). This causes impaired development of both the hematopoietic compartment and nervous system of Bmi1 mutant mice. Consequently, these mice display reduced newborn survival, ataxia, anemia, and a reduction of cell populations in the hematopoietic compartment (van der Lugt et al., 1994). The neural stem cell defect can be partially rescued by disruption of the Ink4a-Arf locus (Molofsky et al., 2005). Although Bmi1 has been shown by chromatin immunoprecipitation to be present at the promoters of p16INK4a and p19ARF (Bracken et al., 2007; Kotake et al., 2007), Bmi1 is unable to directly bind to DNA in a sequence-specific manner (Alkema et al., 1997; Tagawa et al., 1990).
We have previously established that E2F6 associates with Bmi1, and other known components of the PRC1 complex in vivo (Trimarchi et al., 2001). Since other members of the E2F family are known to regulate Arf, we hypothesized that E2F6 might cooperate with Bmi1 in the regulation of the Ink4a-Arf locus, and perhaps also in axial skeletal development. To address this question, we have generated E2f6 mutant mice and subsequently E2f6;Bmi1 compound mutants. Despite the documented role of various E2F family members in the regulation of Arf (Aslanian et al., 2004), we did not detect any evidence that E2F6 contributes to the regulation of the Ink4a-Arf locus. First, E2F6-loss had no detectable effect on the proliferation properties of MEFs and there was no detectable de-repression of p19ARF. Second, we observed no further derepression of p16INK4a and p19ARF in E2f6−/−Bmi1−/− versus Bmi1−/− MEFs and no significant difference in the proliferative or senescent properties of these two genotypes. Finally, E2F6 was not detected at the promoter of Arf in ES cells. We did find that Bmi1 deficient cells have a cell cycle re-entry defect that has not been previously reported. However, the compound mutant cells do not differ significantly in their ability to re-enter the cell cycle. Previously, E2F4 had been shown to compensate for loss of E2F6 at the promoters of cell cycle genes (Zhu et al., 2004), and one hypothesis was that a lack of a genetic interaction between E2F6 and Bmi1 could be due to E2F4 or another E2F family member compensating for the loss of E2F6 at Arf. However, the promoter occupancy analysis presented here strongly suggests that E2F6 plays little or no role in the direct regulation of Ink4a-Arf. Certainly, our data show that E2F6 is fully dispensable for the appropriate regulation of this locus. Consistent with this finding, the loss of E2F6 did not exacerbate the defects in either the hematopoietic compartment or the cerebellar development of the Bmi1 mutant mice that largely result from the derepression of Ink4a-Arf. Thus, these data suggest that E2F6 does not influence the regulation of Ink4a-Arf by Bmi1 by either direct or indirect mechanisms.
In stark contrast to the regulation of Ink4a-Arf, our data show a role for E2f6 in axial skeletal development. First, data from both this and previous (Storre et al., 2002) studies show that, like other PcG proteins, E2F6-loss results in axial skeletal transformations. Second, we find that E2f6 mutation acts in a dosage-dependent manner to increase the penetrance of skeletal transformations in the Bmi1 deficient background. This genetic analysis suggests that E2F6 plays a vital role in the regulation of a subset of Bmi1 target genes, presumably the Hox genes. To further this study, we took a more biochemical approach to analyze if the increased penetrance of the skeletal transformations in the E2f6;Bmi1 mutant mice is due to enhanced deregulation and loss of direct transcriptional control of the Hox genes. Indeed, we did find enhanced deregulation of a subset of Bmi1 target Hox genes and a direct association of E2F6 with a subset of Bmi1-responsive Hox gene promoters. This biochemical analysis clearly provides insight into how E2F6 loss in Bmi1 mutant mice leads to an increase in the penetrance of skeletal transformations. Furthermore, it underscores the diversity of the Bmi1 complexes and the mechanisms by which it regulates target genes. Finally, we have established that E2F6 directly regulates Hox genes in vivo and have reported a novel C5 to C6 transformation which is present in E2f6 and E2f6;Bmi1 mutant embryos. Thus, we conclude that E2F6 and Bmi1 act synergistically in development of the axial skeleton. Taken together, our data show that E2F6 plays a vital role in the regulation of a subset of Bmi1 target Hox genes that govern the anteroposterior patterning of the developing embryo but is not required for the regulation of Ink4a-Arf and the control of cellular proliferation.
EXPERIMENTAL PROCEDURES
Generation and genotyping of E2f6−/− mice
The BAC clone b39J22 (Research Genetics) known to contain the E2f6 genomic locus (Peterfy et al., 1999) was mapped and cloned to obtain the sequences necessary for designing the targeting strategy. The targeting vector described above was introduced into 129Sv J1 ES cells by electroporation and the cells were selected with G418 and Gancyclovir. 96 resistant clones were picked for genotyping. E2f6 +/− cells were detected by Southern blot using external 5′ and 3′ probes as well as a neomycin probe. Once the heterozygous clones were identified and verified to contain a diploid genome by karyotyping, they were injected into C57/BL6 3.5 d.p.c. blastocysts. The injected blastocysts were subsequently implanted into pseudo-pregnant females and the chimeric progeny were identified by coat color. Mice with a high contribution of agouti cells were mated to pure C57/BL6 mice. The agouti progeny of these mice were genotyped by PCR of DNA obtained from ear or tail pieces using the common primer 5′-ATCTCTGTCTGGTCTGATCC-3′, the wild-type E2f6-specific primer 5-GATGCCATCCAAGACATTGG-3′, and the mutant targeting vector specific primer 5-GCCGCATAACTTCGTATAGC-3′. The E2f6+/− mice were then interbred to produce E2f6−/− mice.
Histological and skeletal analysis
Euthanized animals were dissected and processed for histological analysis. Soft tissues were fixed in 10% formalin and hard tissues were fixed in Bouin’s fixative. Paraffin sections were prepared and stained with hematoxylin and eosin. Skeletal analysis was performed on 3-day old mice and 18.5 d.p.c. embryos. After removing the skin and viscera, the skeletons were fixed in acetone and stained with cartilage-specific Alcian Blue and bone-specific Alzarin Red. Soft tissue was cleared with KOH.
Mouse embryonic fibroblasts and cell cycle assays
MEFs were prepared from 13.5 d.p.c. embryos as previously described (Humbert et al., 2000) and genotyped by PCR of DNA obtained from yolk sacs. Proliferation curves were obtained by plating 2×104 MEFs in triplicate in 24-well plates. At the indicated time points, MEFs were trypsinized and counted. For cell cycle re-entry assays, 2×105 MEFs were plated in triplicate in 6-well plates. After 2 days of growth in media containing 10% serum, cells were incubated in media containing 0.1% serum for 3–4 days. Re-entry into the cell cycle was induced by incubation in media containing 10% serum and at the indicated time points, 5 μCi of 3H-thymidine was added to the cells for 1 hour. Cells were then scraped from the plates and cell pellets were analyzed for 3H-thymidine incorporation using a scintillation counter. A 3T3 protocol was followed to monitor senescence. 3×105 MEFs were plated in duplicates in 6-cm plates and re-fed 2 days later. On the third day, they were trypsinized, counted and replated. The fold replication was determined by dividing the number of cells obtained at day 3 by 3×105. Western blots were performed as described previously (Moberg et al., 1996) with 50–100 μg of whole cell lysates using primary antibodies against E2F6 (mouse monoclonal, clone 2E10, J.A. Lees) and p19ARF (rat monoclonal, sc-32748, Santa Cruz Biotechnology).
Real-Time PCR Analysis
For RT-PCR analysis, RNA was collected from asynchronously proliferating cell pellets. RNA was processed using the Rneasy MinElute Cleanup Kit (Qiagen, 74204). cDNA was made from the RNA using SuperScript First-Strand Synthesis System (Invitrogen, 11904-018). 2μl of cDNA (diluted 1:100), 0.9μM primer pair, and 10μl of SybrGreen Master Mix (Applied Biosystems, 4309155) was used for each PCR reaction. RT-PCR signals were normalized to an ubiquitin internal control. Primer sequences are available upon request.
Analysis of the hematopoietic compartment
Single-cell preparations were made from the bone marrow, thymus, and spleen of eight-week old mice by mincing the tissue and then pressing through a nylon mesh. Cells were then counted with a hemocytometer. For flow cytometry analysis, cells were resuspended in a 96-well plate at 3×105 cells/well in FACS buffer (PBS, 0.5% BSA, 0.1%NaN3). Cells were washed with FACS buffer, blocked with Fc Block (1/4000 in FACS buffer, BD Biosciences), and incubated with saturating amounts of monoclonal antibodies conjugated to FITC, PE, or Biotin at 4°C for 30 minutes in the dark. Cells were then washed 2 times with FACS buffer and, if required, incubated with a secondary antibody (Streptavidin-APC) at 4°C for 30 minutes in the dark. Cells were then washed 2 times with FACS buffer and resuspended in 400μl PI-containing FACS buffer (1μg/ml). Cells were then analyzed on a FACScan. The following antibodies were used: Anti-mouse IgM-Biot (IB4ABI, Southern Biotechnology Associates), PE-Anti-Mouse CD8a (553032, BD Pharmingen), Biotin Anti-Mouse CD4 (553044, BD Pharmingen), PE Anti-mouse B220 (553089, BD Pharmingen), PE Anti-mouse Ly-6G (Gr-1) (553128, BD Pharmingen), Biotin Anti-mouse CD11b (Mac-1) (557395, BD Pharmingen), Streptavidin-APC (554067, BD Pharmingen), FITC Anti-mouse CD28 (IL2-R) (553071, BD Pharmingen), Biotin Anti-mouse CD24 (HSA) (555296, BD Pharmingen).
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed as previously described (Aslanian et al., 2004). Oligonucleotide primers (Integrated DNA Technologies) used for PCR analysis are available upon request. Antibodies used were E2f6 (sc-8366, Santa Cruz Biotechnlogy) and Bmi1 (Bmi1-1, J.A. Lees).
Acknowledgments
We are grateful to Marteen van Lohuizen for the generous gift of the Bmi1−/− mice. We also thank Alicia Caron and Roderick Bronson for the generation and analysis of histological sections. We would also like to thank the members of the Lees lab for helpful discussions. This work was funded by NIH grants awarded to J.A.L. (GM53204, CA121921, CA42063). J.A.L. is a Ludwig Scholar.
BIBLIOGRAPHY
- Adler PN, Martin EC, Charlton J, Jones K. Phenotypic consequences and genetic interactions of a null mutation in the Drosophila Posterior Sex Combs gene. Dev Genet. 1991;12:349–361. doi: 10.1002/dvg.1020120504. [DOI] [PubMed] [Google Scholar]
- Akasaka T, Kanno M, Balling R, Mieza MA, Taniguchi M, Koseki H. A role for mel-18, a Polycomb group-related vertebrate gene, during theanteroposterior specification of the axial skeleton. Development. 1996;122:1513–1522. doi: 10.1242/dev.122.5.1513. [DOI] [PubMed] [Google Scholar]
- Akasaka T, Tsuji K, Kawahira H, Kanno M, Harigaya K, Hu L, Ebihara Y, Nakahata T, Tetsu O, Taniguchi M, Koseki H. The role of mel-18, a mammalian Polycomb group gene, during IL-7-dependent proliferation of lymphocyte precursors. Immunity. 1997;7:135–146. doi: 10.1016/s1074-7613(00)80516-6. [DOI] [PubMed] [Google Scholar]
- Akasaka T, van Lohuizen M, van der Lugt N, Mizutani-Koseki Y, Kanno M, Taniguchi M, Vidal M, Alkema M, Berns A, Koseki H. Mice doubly deficient for the Polycomb Group genes Mel18 and Bmi1 reveal synergy and requirement for maintenance but not initiation of Hox gene expression. Development. 2001;128:1587–1597. doi: 10.1242/dev.128.9.1587. [DOI] [PubMed] [Google Scholar]
- Alkema MJ, Bronk M, Verhoeven E, Otte A, van ’t Veer LJ, Berns A, van Lohuizen M. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian polycomb complex. Genes Dev. 1997;11:226–240. doi: 10.1101/gad.11.2.226. [DOI] [PubMed] [Google Scholar]
- Aslanian A, Iaquinta PJ, Verona R, Lees JA. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 2004;18:1413–1422. doi: 10.1101/gad.1196704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwooll C, Denchi EL, Helin K. The E2F family: specific functions and overlapping interests. Embo J. 2004;23:4709–4716. doi: 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel S, Core N, Djabali M, Kieboom K, Van der Lugt N, Alkema MJ, Van Lohuizen M. Genetic interactions and dosage effects of Polycomb group genes in mice. Development. 1998;125:3543–3551. doi: 10.1242/dev.125.18.3543. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Monch K, Minucci S, Porse BT, Marine JC, Hansen KH, Helin K. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Mucci D, Whiteley M, Dirksen ML, Kassis JA. The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol Cell. 1998;1:1057–1064. doi: 10.1016/s1097-2765(00)80106-9. [DOI] [PubMed] [Google Scholar]
- Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20:845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Cartwright P, Muller H, Wagener C, Holm K, Helin K. E2F-6: a novel member of the E2F family is an inhibitor of E2F-dependent transcription. Oncogene. 1998;17:611–623. doi: 10.1038/sj.onc.1201975. [DOI] [PubMed] [Google Scholar]
- Core N, Bel S, Gaunt SJ, Aurrand-Lions M, Pearce J, Fisher A, Djabali M. Altered cellular proliferation and mesoderm patterning in Polycomb-M33-deficient mice. Development. 1997;124:721–729. doi: 10.1242/dev.124.3.721. [DOI] [PubMed] [Google Scholar]
- de Bruin A, Maiti B, Jakoi L, Timmers C, Buerki R, Leone G. Identification and characterization of E2F7, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem. 2003;278:42041–42049. doi: 10.1074/jbc.M308105200. [DOI] [PubMed] [Google Scholar]
- del Mar Lorente M, Marcos-Gutierrez C, Perez C, Schoorlemmer J, Ramirez A, Magin T, Vidal M. Loss- and gain-of-function mutations show a polycomb group function for Ring1A in mice. Development. 2000;127:5093–5100. doi: 10.1242/dev.127.23.5093. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Faust C, Schumacher A, Holdener B, Magnuson T. The eed mutation disrupts anterior mesoderm production in mice. Development. 1995;121:273–285. doi: 10.1242/dev.121.2.273. [DOI] [PubMed] [Google Scholar]
- Gaubatz S, Wood JG, Livingston DM. Unusual proliferation arrest and transcriptional control properties of a newly discovered E2F family member, E2F-6. Proc Natl Acad Sci U S A. 1998;95:9190–9195. doi: 10.1073/pnas.95.16.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert PO, Verona R, Trimarchi JM, Rogers C, Dandapani S, Lees JA. E2f3 is critical for normal cellular proliferation. Genes Dev. 2000;14:690–703. [PMC free article] [PubMed] [Google Scholar]
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, van Lohuizen M. Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta. 2002;1602:151–161. doi: 10.1016/s0304-419x(02)00052-5. [DOI] [PubMed] [Google Scholar]
- Kanno M, Hasegawa M, Ishida A, Isono K, Taniguchi M. mel-18, a Polycomb group-related mammalian gene, encodes a transcriptional negative regulator with tumor suppressive activity. Embo J. 1995;14:5672–5678. doi: 10.1002/j.1460-2075.1995.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison JA. The Polycomb and trithorax group proteins of Drosophila: trans-regulators of homeotic gene function. Annu Rev Genet. 1995;29:289–303. doi: 10.1146/annurev.ge.29.120195.001445. [DOI] [PubMed] [Google Scholar]
- Kotake Y, Cao R, Viatour P, Sage J, Zhang Y, Xiong Y. pRB family proteins are required for H3K27 trimethylation and Polycomb repression complexes binding to and silencing p16INK4alpha tumor suppressor gene. Genes Dev. 2007;21:49–54. doi: 10.1101/gad.1499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Kim SH, Chung HM, Girton JR, Jeon SH. The Drosophila pleiohomeotic mutation enhances the Polycomblike and Polycomb mutant phenotypes during embryogenesis and in the adult. Int J Dev Biol. 2003;47:389–395. [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. Polycomb group genes as epigenetic regulators of normal and leukemic hemopoiesis. Exp Hematol. 2003;31:567–585. doi: 10.1016/s0301-472x(03)00081-x. [DOI] [PubMed] [Google Scholar]
- Leung C, Lingbeek M, Shakhova O, Liu J, Tanger E, Saremaslani P, Van Lohuizen M, Marino S. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428:337–341. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- Logan N, Delavaine L, Graham A, Reilly C, Wilson J, Brummelkamp TR, Hijmans EM, Bernards R, La Thangue NB. E2F-7: a distinctive E2F family member with an unusual organization of DNA-binding domains. Oncogene. 2004;23:5138–5150. doi: 10.1038/sj.onc.1207649. [DOI] [PubMed] [Google Scholar]
- Maiti B, Li J, de Bruin A, Gordon F, Timmers C, Opavsky R, Patil K, Tuttle J, Cleghorn W, Leone G. Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem. 2005 doi: 10.1074/jbc.M501410200. [DOI] [PubMed] [Google Scholar]
- Moberg K, Starz MA, Lees JA. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkel M, Wenkel J, Bannister AJ, Kouzarides T, Hagemeier C. An E2F-like repressor of transcription. Nature. 1997;390:567–568. doi: 10.1038/37507. [DOI] [PubMed] [Google Scholar]
- Nevins JR. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296:1132–1136. doi: 10.1126/science.1069861. [DOI] [PubMed] [Google Scholar]
- Otte AP, Kwaks TH. Gene repression by Polycomb group protein complexes: a distinct complex for every occasion? Curr Opin Genet Dev. 2003;13:448–454. doi: 10.1016/s0959-437x(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Peterfy M, Phan J, Oswell GM, Xu P, Reue K. Genetic, physical, and transcript map of the fld region on mouse chromosome 12. Genomics. 1999;62:436–444. doi: 10.1006/geno.1999.6023. [DOI] [PubMed] [Google Scholar]
- Simon J. Locking in stable states of gene expression: transcriptional control during Drosophila development. Curr Opin Cell Biol. 1995;7:376–385. doi: 10.1016/0955-0674(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Storre J, Elsasser HP, Fuchs M, Ullmann D, Livingston DM, Gaubatz S. Homeotic transformations of the axial skeleton that accompany a targeted deletion of E2f6. EMBO Rep. 2002;3:695–700. doi: 10.1093/embo-reports/kvf141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa M, Sakamoto T, Shigemoto K, Matsubara H, Tamura Y, Ito T, Nakamura I, Okitsu A, Imai K, Taniguchi M. Expression of novel DNA-binding protein with zinc finger structure in various tumor cells. J Biol Chem. 1990;265:20021–20026. [PubMed] [Google Scholar]
- Trimarchi JM, Fairchild B, Verona R, Moberg K, Andon N, Lees JA. E2F-6, a member of the E2F family that can behave as a transcriptional repressor. Proc Natl Acad Sci U S A. 1998;95:2850–2855. doi: 10.1073/pnas.95.6.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Fairchild B, Wen J, Lees JA. The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc Natl Acad Sci U S A. 2001;98:1519–1524. doi: 10.1073/pnas.041597698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nature Reviews Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M, et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- Xi S, Zhu H, Xu H, Schmidtmann A, Geiman TM, Muegge K. Lsh controls Hox gene silencing during development. Proc Natl Acad Sci U S A. 2007;104:14366–14371. doi: 10.1073/pnas.0703669104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zencak D, Lingbeek M, Kostic C, Tekaya M, Tanger E, Hornfeld D, Jaquet M, Munier FL, Schorderet DF, van Lohuizen M, Arsenijevic Y. Bmi1 loss produces an increase in astroglial cells and a decrease in neural stem cell population and proliferation. J Neurosci. 2005;25:5774–5783. doi: 10.1523/JNEUROSCI.3452-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Giangrande PH, Nevins JR. E2Fs link the control of G1/S and G2/M transcription. Embo J. 2004;23:4615–4626. doi: 10.1038/sj.emboj.7600459. [DOI] [PMC free article] [PubMed] [Google Scholar]