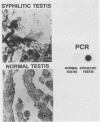
Abstract
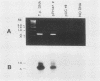
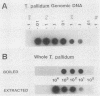
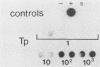
We have developed a sensitive assay for Treponema pallidum subsp. pallidum (T. pallidum), the agent of veneral syphilis, based upon the polymerase chain reaction (PCR). A 658-bp portion of the gene encoding the 47-kDa membrane immunogen was amplified, and the PCR products were probed by DNA-DNA hybridization with a 496-bp fragment internal to the amplitifed DNA. The assay detected approximately 0.01 pg of purified T. pallidum DNA, and positive results were obtained routinely from suspensions of treponemes calculated to contain 10 or more organism and from some suspensions calculated to contain a single organism. Specific PCR products were obtained for the closely related agent of yaws, Treponema pallidum subsp. pertenue, but not with human DNA or DNAs from other spirochetes (including Borrelia burgdoferi), skin microorganisms, sexually transmitted disease pathogens, and central nervous system pathogens. T. pallidum DNA was detected in serum, cerebrospinal fluids, and amniotic fluids from syphilis patients but not in in nonsyphilitic controls. T. pallidum DNA was also amplified from paraffin-embedded tissue. The diagnosis of syphillis by using PCR may become a significant addition to the diagnostic armamentarium and a valuable technique for the investigation of syphilis pathogenesis.
Full text
PDF



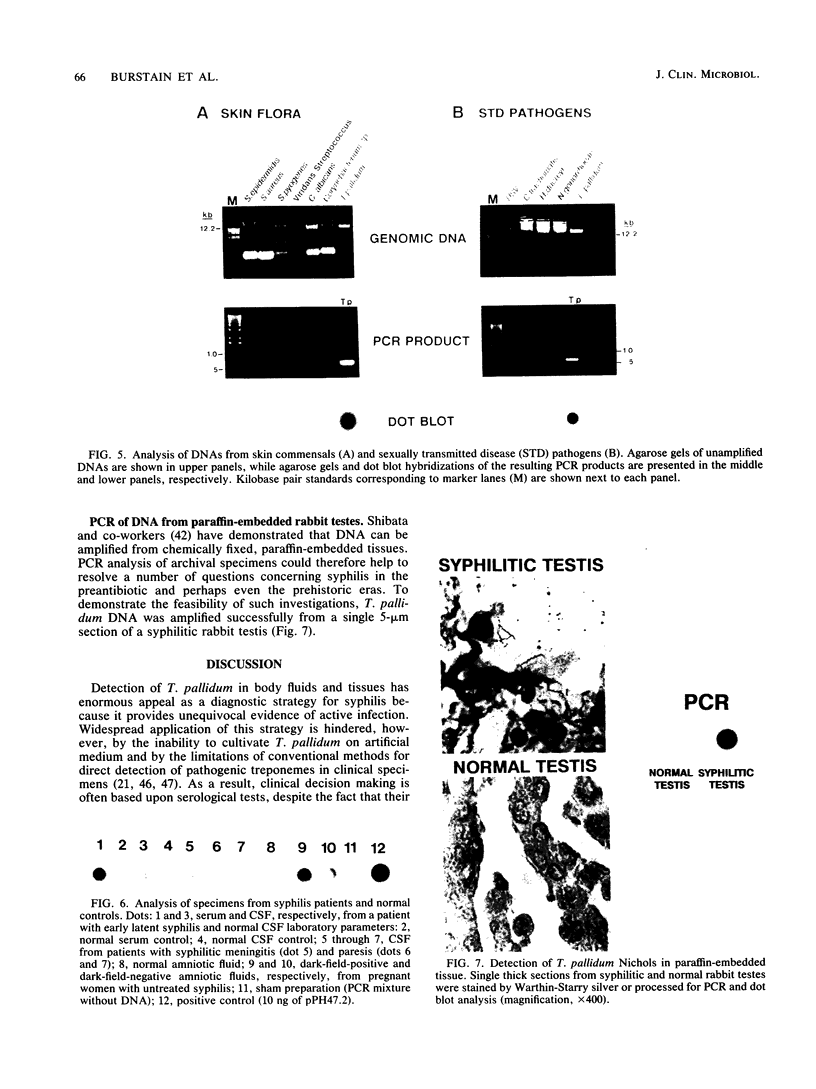



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernet C., Garret M., de Barbeyrac B., Bebear C., Bonnet J. Detection of Mycoplasma pneumoniae by using the polymerase chain reaction. J Clin Microbiol. 1989 Nov;27(11):2492–2496. doi: 10.1128/jcm.27.11.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. D., Hooton T. M., Collier A. C., Lukehart S. A. Neurologic relapse after benzathine penicillin therapy for secondary syphilis in a patient with HIV infection. N Engl J Med. 1987 Jun 18;316(25):1587–1589. doi: 10.1056/NEJM198706183162507. [DOI] [PubMed] [Google Scholar]
- Burg J. L., Grover C. M., Pouletty P., Boothroyd J. C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattwyler R. J., Luft B. J. Immunodiagnosis of Lyme borreliosis. Rheum Dis Clin North Am. 1989 Nov;15(4):727–734. [PubMed] [Google Scholar]
- Eisenstein B. I. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med. 1990 Jan 18;322(3):178–183. doi: 10.1056/NEJM199001183220307. [DOI] [PubMed] [Google Scholar]
- Fohn M. J., Wignall S., Baker-Zander S. A., Lukehart S. A. Specificity of antibodies from patients with pinta for antigens of Treponema pallidum subspecies pallidum. J Infect Dis. 1988 Jan;157(1):32–37. doi: 10.1093/infdis/157.1.32. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Grandchamp B., Lévy-Frébault V., Lecossier D., Rauzier J., Bocart D., Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989 Jul;3(7):843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hart G. Syphilis tests in diagnostic and therapeutic decision making. Ann Intern Med. 1986 Mar;104(3):368–376. doi: 10.7326/0003-4819-104-3-368. [DOI] [PubMed] [Google Scholar]
- Hay P. E., Clarke J. R., Strugnell R. A., Taylor-Robinson D., Goldmeier D. Use of the polymerase chain reaction to detect DNA sequences specific to pathogenic treponemes in cerebrospinal fluid. FEMS Microbiol Lett. 1990 Mar 15;56(3):233–238. doi: 10.1111/j.1574-6968.1990.tb13943.x. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Roddy R. E., Lukehart S. A., Hom J., Holmes K. K., Tam M. R. Detection of Treponema pallidum in lesion exudate with a pathogen-specific monoclonal antibody. J Clin Microbiol. 1985 Aug;22(2):241–244. doi: 10.1128/jcm.22.2.241-244.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. W., 3rd Syphilis and HIV infection. J Infect Dis. 1989 Sep;160(3):530–534. doi: 10.1093/infdis/160.3.530. [DOI] [PubMed] [Google Scholar]
- Hsu P. L., Chamberlain N. R., Orth K., Moomaw C. R., Zhang L. Q., Slaughter C. A., Radolf J. D., Sell S., Norgard M. V. Sequence analysis of the 47-kilodalton major integral membrane immunogen of Treponema pallidum. Infect Immun. 1989 Jan;57(1):196–203. doi: 10.1128/iai.57.1.196-203.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E. F., Russell H., Farshy C. E., Sampson J. S., Larsen S. A. Evaluation of sera from patients with Lyme disease in the fluorescent treponemal antibody-absorption test for syphilis. Sex Transm Dis. 1986 Oct-Dec;13(4):232–236. doi: 10.1097/00007435-198610000-00005. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Tierney M., Felsenstein D. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med. 1987 Jun 18;316(25):1569–1572. doi: 10.1056/NEJM198706183162503. [DOI] [PubMed] [Google Scholar]
- Jordan K. G. Modern neurosyphilis--a critical analysis. West J Med. 1988 Jul;149(1):47–57. [PMC free article] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Hook E. W., 3rd, Baker-Zander S. A., Collier A. C., Critchlow C. W., Handsfield H. H. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988 Dec 1;109(11):855–862. doi: 10.7326/0003-4819-109-11-855. [DOI] [PubMed] [Google Scholar]
- Mahbubani M. H., Bej A. K., Miller R., Haff L., DiCesare J., Atlas R. M. Detection of Legionella with polymerase chain reaction and gene probe methods. Mol Cell Probes. 1990 Jun;4(3):175–187. doi: 10.1016/0890-8508(90)90051-z. [DOI] [PubMed] [Google Scholar]
- Marchitto K. S., Jones S. A., Schell R. F., Holmans P. L., Norgard M. V. Monoclonal antibody analysis of specific antigenic similarities among pathogenic Treponema pallidum subspecies. Infect Immun. 1984 Sep;45(3):660–666. doi: 10.1128/iai.45.3.660-666.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitto K. S., Selland-Grossling C. K., Norgard M. V. Molecular specificities of monoclonal antibodies directed against virulent Treponema pallidum. Infect Immun. 1986 Jan;51(1):168–176. doi: 10.1128/iai.51.1.168-176.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol. 1980 Jan;141(1):427–429. doi: 10.1128/jb.141.1.427-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser D. R., Kirchhoff L. V., Donelson J. E. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol. 1989 Jul;27(7):1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. L., Young K. K., Barbour A. G. Detection of Borrelia burgdorferi DNA by the polymerase chain reaction. Mol Cell Probes. 1990 Feb;4(1):73–79. doi: 10.1016/0890-8508(90)90041-w. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Chamberlain N. R., Swancutt M. A., Goldberg M. S. Cloning and expression of the major 47-kilodalton surface immunogen of Treponema pallidum in Escherichia coli. Infect Immun. 1986 Nov;54(2):500–506. doi: 10.1128/iai.54.2.500-506.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Kaplan R. P. Unusual manifestations of secondary syphilis and abnormal humoral immune response to Treponema pallidum antigens in a homosexual man with asymptomatic human immunodeficiency virus infection. J Am Acad Dermatol. 1988 Feb;18(2 Pt 2):423–428. doi: 10.1016/s0190-9622(88)70062-6. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V. Pathogen specificity of Treponema pallidum subsp. pallidum integral membrane proteins identified by phase partitioning with Triton X-114. Infect Immun. 1988 Jul;56(7):1825–1828. doi: 10.1128/iai.56.7.1825-1828.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T. G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989 Dec;160(6):1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- Rothschild B. M., Turnbull W. Treponemal infection in a Pleistocene bear. Nature. 1987 Sep 3;329(6134):61–62. doi: 10.1038/329061a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saito I., Servenius B., Compton T., Fox R. I. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989 Jun 1;169(6):2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan G., Ramamurthy R. S., Bharathi A., Voora S., Pildes R. S. Congenital syphilis: a diagnostic and therapeutic dilemma. Pediatr Infect Dis. 1983 Nov-Dec;2(6):436–441. [PubMed] [Google Scholar]
- Starnbach M. N., Falkow S., Tompkins L. S. Species-specific detection of Legionella pneumophila in water by DNA amplification and hybridization. J Clin Microbiol. 1989 Jun;27(6):1257–1261. doi: 10.1128/jcm.27.6.1257-1261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg R. W., Baseman J. B. Comparison of major protein antigens and protein profiles of Treponema pallidum and Treponema pertenue. Infect Immun. 1983 Nov;42(2):623–627. doi: 10.1128/iai.42.2.623-627.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. B., Hardy P. H., Newman B. Infectivity tests in syphilis. Br J Vener Dis. 1969 Sep;45(3):183–195. doi: 10.1136/sti.45.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]