Abstract
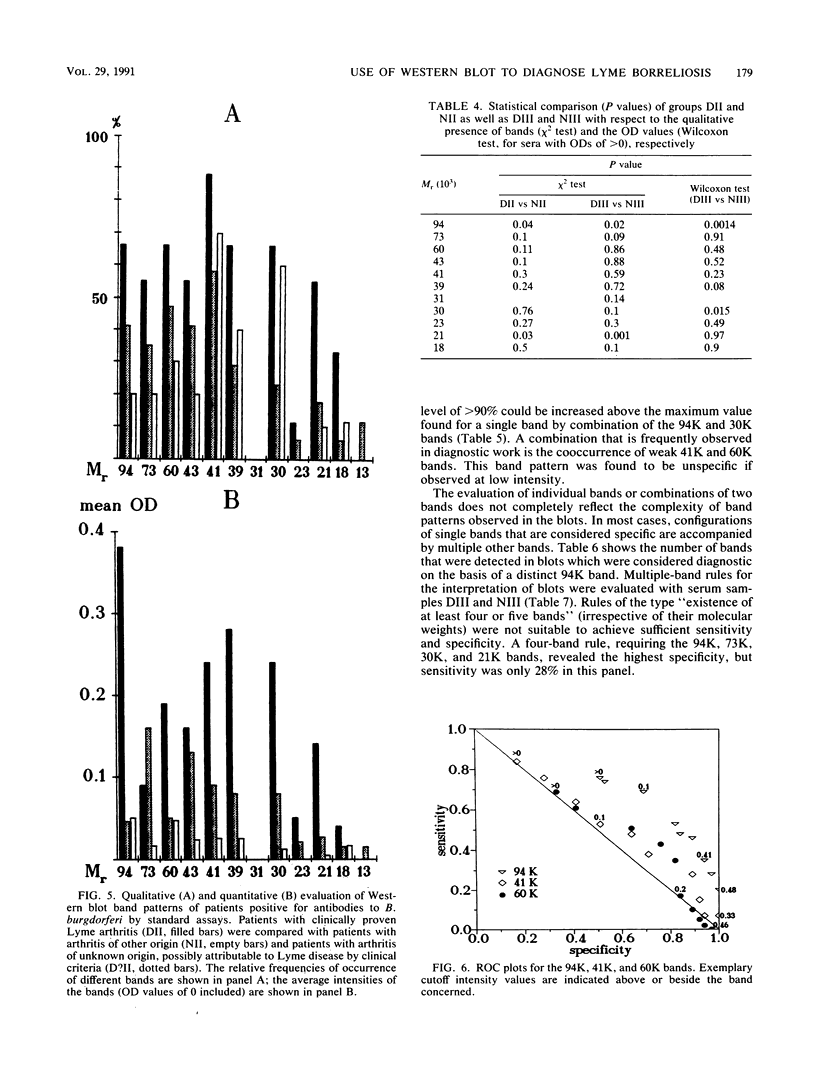
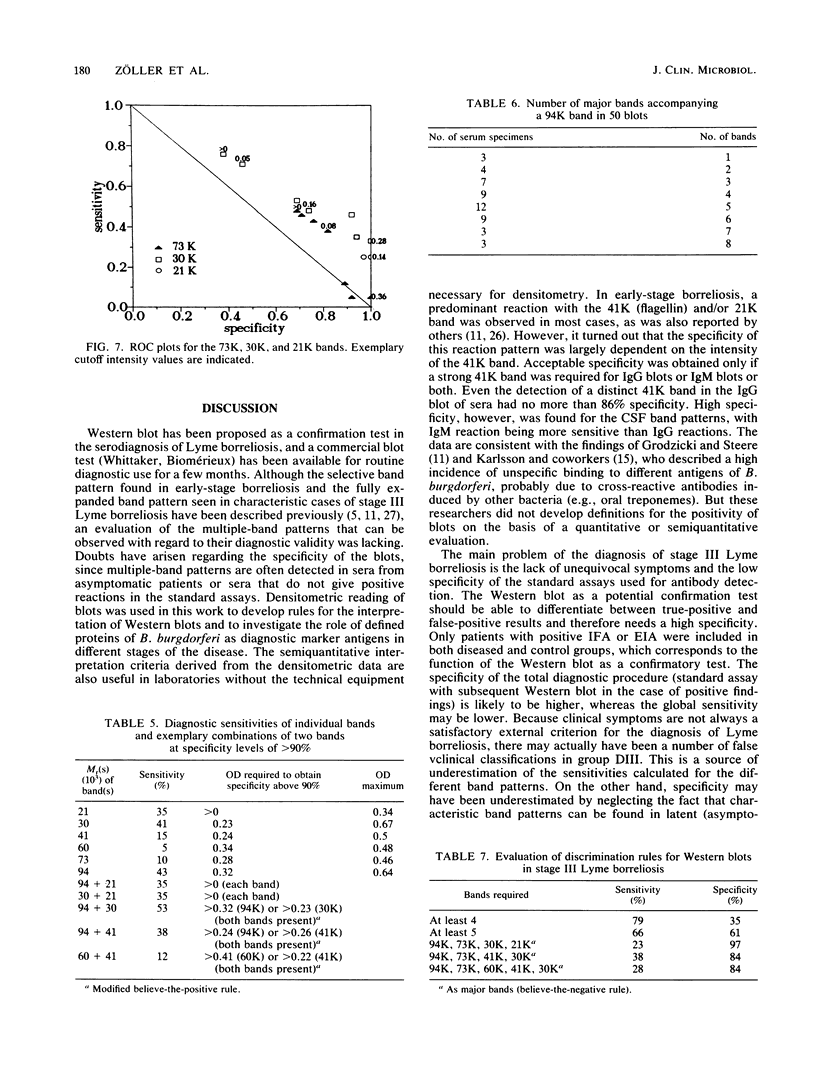
Serodiagnosis of Lyme disease is hampered by low specificity of the standard assays currently used. The Western immunoblot has therefore been proposed as a potential confirmatory test. For the present report, the method was evaluated by testing sera from patients with clinically defined early- and late-stage borreliosis. In early-stage borreliosis, the 41,000-molecular-weight flagellin protein (41K) of Borrelia burgdorferi was the major antigen detected by antibodies in sera, but the specificity of the reaction pattern was dependent on the intensity of the band. The evaluation of different interpretation rules based on a semiquantitative record of band intensities showed the highest specificity (96%) and a corresponding sensitivity of 78% if there was at least one distinct (optical density range, 0.2 to 0.4) immunoglobulin G and immunoglobulin M reaction with the 41K band. Blots of B. burgdorferi proteins were also probed with sera from patients who were diagnosed by clinical criteria as having stage III Lyme borreliosis and with a control group of sera from asymptomatic persons with positive antibody titers against B. burgdorferi in the standard assays. Reaction patterns were recorded densitometrically. Statistical analysis and graphical marker analysis revealed significant discriminating capacities and relatively high specificities, respectively, for the 94K, 30K, and 21K bands, whereas the 41K and 60K bands were not discriminative between the symptomatic and asymptomatic groups and were specific only at high intensity values. Different multiple-band rules were evaluated, revealing a low specificity for positivity definitions of the type "four or five bands present" if the rules were not confined to known major bands.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann R., Kabatzki J., Boisten H. P., Steere A. C., Grodzicki R. L., Hartung S., Runne U. Spirochäten-Atiologie der Erythema-chronicum-migrans-Krankheit. Dtsch Med Wochenschr. 1984 Jan 20;109(3):92–97. doi: 10.1055/s-2008-1069145. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Laboratory aspects of Lyme borreliosis. Clin Microbiol Rev. 1988 Oct;1(4):399–414. doi: 10.1128/cmr.1.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J. E., Grodzicki R. L., Steere A. C. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984 May;149(5):789–795. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- Fister R. D., Weymouth L. A., McLaughlin J. C., Ryan R. W., Tilton R. C. Comparative evaluation of three products for the detection of Borrelia burgdorferi antibody in human serum. J Clin Microbiol. 1989 Dec;27(12):2834–2837. doi: 10.1128/jcm.27.12.2834-2837.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann G. S., Deutzmann R., Vogt A., Göbel U. B. N-terminal amino acid sequence of the Borrelia burgdorferi flagellin. FEMS Microbiol Lett. 1989 Jul 1;51(1):101–105. doi: 10.1016/0378-1097(89)90085-2. [DOI] [PubMed] [Google Scholar]
- Greene R. T., Walker R. L., Burgess E. C., Levine J. F. Heterogeneity in immunoblot patterns obtained by using four strains of Borrelia burgdorferi and sera from naturally exposed dogs. J Clin Microbiol. 1988 Nov;26(11):2287–2291. doi: 10.1128/jcm.26.11.2287-2291.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicki R. L., Steere A. C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis. 1988 Apr;157(4):790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988 Feb;26(2):338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler D., Zöller L., Haude M., Hufnagel H. D., Heinrich F., Sonntag H. G. Cefotaxime versus penicillin in the late stage of Lyme disease--prospective, randomized therapeutic study. Infection. 1990 Jan-Feb;18(1):16–20. doi: 10.1007/BF01644175. [DOI] [PubMed] [Google Scholar]
- Karlsson M., Möllegård I., Stiernstedt G., Wretlind B. Comparison of Western blot and enzyme-linked immunosorbent assay for diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):871–877. doi: 10.1007/BF01963773. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Jiang W., Munoz P., Dattwyler R. J., Gorevic P. D. Biochemical and immunological characterization of the surface proteins of Borrelia burgdorferi. Infect Immun. 1989 Nov;57(11):3637–3645. doi: 10.1128/iai.57.11.3637-3645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Barbour A. G. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J Infect Dis. 1989 Jan;159(1):43–49. doi: 10.1093/infdis/159.1.43. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Meegan J. M., Anderson J. F., Chappell W. A. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J Clin Microbiol. 1984 Aug;20(2):181–184. doi: 10.1128/jcm.20.2.181-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Miller J. N., Anderson J. F., Riviere G. R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990 Jun;28(6):1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Schierz G. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Immune responses to Borrelia burgdorferi in patients with reactive arthritis. Arthritis Rheum. 1989 Sep;32(9):1057–1064. doi: 10.1002/anr.1780320902. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Busch K. V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Gueye W., Herzer P., Weber K. Immunochemische Analyse der Immunantwort bei Spätmanifestationen der Lyme Borreliose. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Mar;267(4):549–558. [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]