Abstract
Expression of α6 integrin, a laminin receptor, on tumor cell surfaces is associated with reduced patient survival and increased metastasis in a variety of tumors. In prostate cancer, tumor extra capsular escape occurs in part via laminin coated nerves and vascular dissemination, resulting in clinically significant bone metastases. We previously identified a novel form of α6 integrin, called α6p, generated by urokinase (uPA) dependent cleavage of the laminin binding domain from the tumor cell surface. Cleavage increased laminin dependent migration. Currently, we used the known conformation sensitivity of integrin function to determine if engagement of the extracellular domain inhibited integrin cleavage and the extravasation step of metastasis. We show that α6 integrin was present on prostate carcinoma escaping the gland via nerves. Both endogenous and inducible levels of α6p were inhibited by engaging the extracellular domain of α6 with monoclonal antibody J8H. J8H inhibited tumor cell invasion through Matrigel. A SCID mouse model of extravasation and bone metastasis produced detectable, progressive osteolytic lesions within three weeks of intracardiac injections. Injection of tumor cells, pre-treated with J8H, delayed the appearance of metastases. Validation of the α6 cleavage effect on extravasation was confirmed through a genetic approach using tumor cells transfected with uncleavable α6 integrin. Uncleavable α6 integrin significantly delayed the onset and progression of osseous metastases out to 6 weeks post injection. The results suggest that α6 integrin cleavage permits extravasation of human prostate cancer cells from circulation to bone and can be manipulated to prevent metastasis.
Keywords: integrin, bone, metastasis, cleavage, uPA
Introduction
The α6 integrin, a laminin receptor, is expressed on tumor cell surfaces and is associated with poor patient prognosis, reduced survival and increased metastasis in a variety of tumors (1-4). Integrins are type I transmembrane heterodimers composed of α and β subunits. The heterodimer confers ligand binding specificity to a particular extracellular matrix substrate (5). Integrins α6β1 or α6β4 are receptors for laminin 111 (laminin 1), laminin 511 (laminin 10) or laminin 332 (laminin 5), respectively. In human prostate cancer escape from the gland occurs in part via laminin 511 coated nerves (6, 7) followed by dissemination and subsequent escape from circulation, resulting in clinically significant bone metastasis (8, 9). Additionally, mouse and human bone marrow have both been shown to be rich laminin environments (10-12).
The two most persistently expressed integrin heterodimer pairs in human prostate cancer are the laminin binding α6β1 and α3β1 receptors (13-15). In addition, the basement membrane component, Laminin 332 is not expressed while laminin 511 persists, creating an environment selective for α6β1 function (16). We previously reported a novel form of α6 integrin, called α6p, generated by cleavage of the laminin binding domain from the tumor cell surface by urokinase (uPA), a pro-metastatic factor (17, 18). Expression levels of both uPA and its cognate receptor were shown to be negatively correlated with prostate cancer patient survival (19, 20). The uPA dependent cleavage of α6 integrin increased cellular migration in vitro and was proposed as a mechanism for tumor cell release from adhesion to laminin (21). We initiated the current study to determine whether inhibiting cleavage of the α6β1 integrin would alter the ability of tumor cells to reach the bone from the circulation.
Integrins provide linkage from the extracellular environment to intracellular cytoskeletal components that focally interact at the internal portion of the receptor. This integrated function is necessary for cellular adhesion, migration, survival and differentiation (13). Integrin function is dictated in part by changes in receptor conformation that results in the alteration of ligand affinity and “outside-in” signaling (22, 23). This was initially inferred by the generation of monoclonal antibodies that could bind extracellular domains and alter integrin conformation and activity. Experimentally, integrins can be activated, or functionally blocked from adhesion by externally applied antibodies (24). Circulating levels of immunoglobulins that engage and block adhesion function of the α6β1 heterodimer, in patients with oral pemphigoid, results in formation of blisters and erosive lesions in the oral mucosa (25, 26).
We reasoned that engagement of extracellular epitopes on the receptor with α6 integrin antibodies would block uPA mediated cleavage. We report here the activity of the previously characterized J8H antibody that does not affect cellular adhesion on laminin (27), does block integrin cleavage. J8H was used to test the influence of blocking integrin cleavage on the appearance of bone metastasis. A separate genetic approach, i.e. tumor cells expressing an uncleavable α6 integrin mutant (21, 28) was used as an independent technique to determine if extravasation required integrin cleavage.
Materials and Methods
Antibodies and Reagents
J8H, a mouse mAb, recognizes an extracellular epitope of α6 integrin and was a generous gift from Dr. Arnoud Sonnenberg (27). The integrin α6 rat mAb J1B5 was generated by Dr. Caroline Damsky (29). AA6NT a rabbit pAb, was generated against a recombinant fragment of the N-terminal integrin α6 β-barrel domain and was used for immunohistochemistry (IHC) analysis of archival material. In contrast, AA6A is a rabbit pAb, recognizing the intracellular C-terminal domain of α6 integrin was previously characterized by us and used for western blot analysis (21). Donkey anti-mouse Alexa conjugated antibodies and anti-Rabbit HRP antibodies were obtained from Invitrogen (Carlsbad, CA). Human, single chain, activated, urokinase was obtained from Millipore (Temecula, CA). Growth Factor Reduced Matrigel was from BD Biosciences (Bedford, MA).
Cell Culture
Cells were maintained in Iscoves Dulbecco's modified Eagle's medium (IMDM) (Gibco BRL, MD) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Hyclone Laboratories Inc., Logan, UT) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA) at 37 °C in a 5% CO2 atmosphere at constant humidity. PC3 and DU145 cells were obtained from ATCC (Manassas, VA) and PC3N cells were previously characterized by us (30). Cell line identities were verified using genomic probes reported by others (31). The PC3B1 cells were isolated from the bone marrow of SCID mice that had been injected six weeks previously with the PC3 cell line. The bone marrow containing the tumor cells was retrieved with phosphate-buffered-saline (PBS) and the PC3B1 cells were propagated in tissue culture. PC3B1 α6 WT and PC3B1 α6 RR cell lines were grown under Blasticidin (Invitrogen, Carlsbad, CA) selection pressure (3μg/mL). 293FT cells, used for generation of lentivirus, were grown in minimal essential media (MEM) supplemented with 10% FBS and Geneticin (500 ug/mL) (Invitrogen, Carlsbad, CA). For antibody blocking experiments, cells were grown under optimal growth conditions for 24 hours, followed by replacement of media with 5mL of complete IMDM containing J8H (20μL/mL). J8H/media replacement was performed every 24 hours.
Human Prostate Tissue Immunohistochemistry
Prostate tissues were harvested, fixed in 10% neutral buffered formalin for 24 hours, processed and embedded in paraffin using the Tissue Acquisition and Cellular/Molecular Analysis Shared Service (TACMASS) of The Arizona Cancer Center. IHC was performed using the affinity purified AA6NT rabbit pAb diluted to 1:700 and stained on a Discovery XT Automated Immunostainer (Ventana Medical Systems, Inc. (VMSI), Tucson, AZ). Antigen retrieval was performed using a borate-EDTA buffer at 100°C.
uPA mediated cleavage of α6 integrin
In immunoprecipitation (IP) experiments (21), α6 integrin was retrieved from PC3N lysate using antibodies J1B5 or J8H for 3 hours at 4°C and the resulting Sepharose G beads were re-suspended in 500μL of Dulbecco's phosphate buffered saline (PBS) (Invitrogen, Carlsbad, CA) with 20ng of activated single chain uPA. The mixture was incubated overnight at 4°C with rotation, centrifuged, resuspended in non-reducing gel sample buffer and analyzed by SDS polyacrylamide gel electrophoresis. In cell surface experiments, PC3N cells were harvested with PBS containing 5mM EDTA and resuspended in 500μL of serum free IMDM media. Cells were incubated for 30 minutes with or without J8H antibody at 4°C. Activated uPA (25μg) was added to the cells and incubated for 3 hrs at 37°C. Cells (5×106) were washed once in PBS and lysed in RIPA buffer (50mM Tris, 150 nM NaCl, Triton 1%, SDS 0.10%, 1% Deoxycholate, 1mM PMSF, 1mM Leupeptin, 1mM Aprotinin). Following the IP, samples were analyzed by SDS polyacrylamide gel electrophoresis.
Flow Cytometry
Cells were harvested using PBS containing 5mM EDTA, washed in PBS and resuspended in 200μL of PBS with 0.02% bovine serum albumin (BSA) with J8H hybridoma supernatant (1:20). All antibody incubations were carried out on ice for 30 min. Antibody binding was detected by Alexa 488 anti-mouse secondary antibody (1:1000) and analyzed using the Flow Cytometry Service of the Arizona Cancer Center.
Invasion Assay
Growth factor reduced Matrigel (50 μl) diluted (1:3) with serum free IMDM media was placed in 8.0 micron cell culture inserts (BD Falcon, Franklin Lakes, NJ ) and allowed to solidify for 1 hr at 37°C. Inserts were placed into a 24 well plate. PC3B1 (1×105) cells were placed in the upper insert chamber with 200 μL of serum free IMDM. IMDM (600 μL), supplemented with 10% FBS was pipetted into the bottom well below the insert. After a 20 hr incubation, inserts were washed in PBS and Matrigel was removed with a cotton swab. Cells on the underside of the insert were fixed/permeablized in MeOH/acetone and stained with DAPI (1μg/mL) for nuclei detection. Cell numbers were counted using a Zeiss Axiophot inverted microscope. Three images were collected per insert and experiments were performed in triplicate.
SCID Mouse Model of Extravasation
Mice experiments were conducted with animal care and use committee approval and performed using the facilities and staff of the Experimental Mouse Shared Service at the Arizona Cancer Center. Left ventricle injections of single cell suspensions of approximately 0.5 million cells in 0.2 ml of PBS were performed with a 27g needle as previously described (32, 33). Mice were anesthetized with isoflurane (2−3% delivered through a nose cone). Twelve mice were used in each treatment group as dictated by statistical power analysis software (http://www.cs.uiowa.edu/~rlenth/Power/). One-way ANOVA between two treatment groups was used as the model with a 80% chance of detecting a difference and no more than a 5% chance of error. Mice receiving incorrectly placed injections or containing chest tumors at necropsy were removed from the study. Animals were terminated by CO2 inhalation if micro-fractures were detected by radiographic images or if they showed signs of pain/suffering as specified by protocol.
Radiographic Imaging
Animals were anesthetized with IP injections of ketamine-HCl (50mg/kg) and xylazine (15−20mg/kg). Digital radiographs were collected on live animals 4, 5 and 6 weeks after tumor cell injection using a Faxitron MX-20 machine at 7μΜ nominal resolution with an X-ray current of 300 μA and a voltage of 26kV (Faxitron X-ray Corp., Wheeling, IL). Each digital image required 10 seconds. Animals were allowed to recover from anesthesia and returned to the animal care facility. Images were read and interpreted by G.D.P. (board certified radiologist) without knowledge of the treatment groups.
Generation of PC3B1 Integrin α6 RR and WT mutant cell lines
The α6 integrin cDNA was amplified with primers (5’ CACCCGACTCACTATAGGGAGACCCAAGC, 3’ CTATGCATCAGAAGTAAGCCTCTCTTTATCAGATG), and directionally cloned into the pENTR/D-TOPO vector (Invitrogen Carlsbad, CA. The Quik Change II XL site-directed mutagenesis kit (Stratagene Cedar Creek, TX) was used to introduce alanine mutations at arginine residues 594, 595 (RR), with primers previously characterized (21). Using the Gateway recombination cloning method (34) (Invitrogen Carlsbad, CA), α6 integrin wild type (WT) and RR mutant pENTR/D-TOPO vectors were recombined into the pLenti/UbC/V5-DEST expression vector. Generation of replication-incompetent lentiviral stocks was performed by transfecting the pLenti/UbC/α6 integrin vector in combination with ViraPower Packing Mix and Lipofectamine 2000(Invitrogen, Carlsbad, CA) into 293FT cells. Virus was harvested after 72 hours, centrifuged at high speed for 20 minutes at 4°C and frozen at −80°C. Lentivirus was used to infect PC3B1 cells. Three days following transfection, cells were placed under Blasticidin (3ug/mL) selection. Expression of UbC driven, α6 integrin RR mutant expression was confirmed by RT-PCR as previously described (28).
Results
Expression of α6 integrin on tumor cell surface during escape from human prostate gland
Previous work has shown α6 integrin expression in human normal prostate, prostatic intraepithelial neoplasia (PIN) and invasive cancer using frozen tissues and indirect immunofluorescence microscopy. Here, using an alternative method with human FFPE archival prostate tissue, we demonstrate simultaneous detection of tumor cell antigens and cell types (fibroblast, Schwann, and endothelial) or structures (i.e. nerves and vessels). Detection of α6 integrin using FFPE tissues confirmed that normal prostate epithelial cells display polarized expression of α6 integrin at the basal cell/stromal interface (Fig. 1A, Normal) as shown previously by us and others [14].
Figure 1.

Integrin α6 expression in human prostate cancer progression and antibody specificity. A, Normal glands (Normal), prostatic intraepithelial neoplasia (PIN), Gleason grade 3+3 invasive carcinoma (Cancer), perineural and endoneural invasion (NI) are shown. FFPE tissue was reacted with primary antibody (AA6NT). Arrows indicate vessels detected by AA6NT. Black bar in top two panels is 40 microns and in bottom two panels is 80 microns. B, Immunoblot (IB) analysis α6 integrin retrieved from DU145 cell lysate by immunoprecipitation with anti-α6 integrin antibody (J1B5). AA6A pAb detects both full length α6 and α6p forms under non-reducing (NR) conditions and the 25kDa light chain (arrow) under reducing (R) conditions. AA6NT detects full length integrin under NR conditions and the N-terminal cleavage product α6N (arrowhead) under NR and R conditions. C, Schematic of the relative location of α6 integrin epitopes and cleavage site (RR).
We observed loss of α6 integrin polarity during progression from PIN (Fig. 1A, PIN) to invasive cancer (Fig. 1A, Cancer). α6 Integrin was expressed by vessels (Fig. 1A, PIN, arrowhead), as previously reported. We also demonstrate a new finding that α6 integrin was expressed on the tumor cell surface during neural invasion. (Fig. 1A, NI). Neural invasion by the tumor includes invasion both around and within the nerve. Perineural and endoneural invasion is characteristic of tumor present in the peripheral zone of the prostate gland (6, 35). The presence of α6 integrin on perineural fibroblasts and Schwann cells of the nerve were observed, consistent with previous reports (36-38). Both antibodies (AA6A and AA6NT) used in this study recognize the full length α6 integrin by western blot (Fig. 1B). The AA6A antibody was generated against the C-terminal cytoplasmic domain of the α6 integrin and thus will recognize the cleaved integrin receptor, α6p under non-reduced conditions (NR). Under reducing conditions (R), AA6A recognizes the α6 light chain that is shifted to approximately 25kD. In contrast, the AA6NT antibody, raised against the N-terminal domain of the α6 integrin, recognizes the N-terminal fragment, called α6N. The α6N heavy chain shifts to an apparent larger MW upon reduction (R), as expected. A schematic illustrating the relative location of the epitopes on integrin α6 recognized by the four antibodies (AA6NT, J8H, J1B5 and AA6A) used in this study and the uPA cleavage site (RR) is shown (Fig. 1C).
SCID Mouse Model of Prostate Cancer Extravasation
Injection of human tumor cells into the left ventricle of the mouse heart ensured broad dissemination of tumor cells via the circulation. The model is relevant to human prostate cancer progression since metastases develop from the arterial distribution of tumor emboli in circulation (39). We developed a model for generating reproducible and aggressive bone metastases by comparing the effectiveness of PC3 cells versus PC3B1 cells (Fig. 2A). Human tumor within the bone expressed α6 integrin on the cell surface (Fig. 2B). Radiograph images of the entire skeleton were surveyed on all animals and a metastasis in any bone resulted in a positive score. All bone metastases detected were lytic lesions located primarily within the metaphysis and abutting the epiphyseal plate (Fig. 2C, arrows), and all were progressive (data not shown). This model system enabled testing of how tumor cell properties influence extravasation and development of bone metastases.
Figure 2.
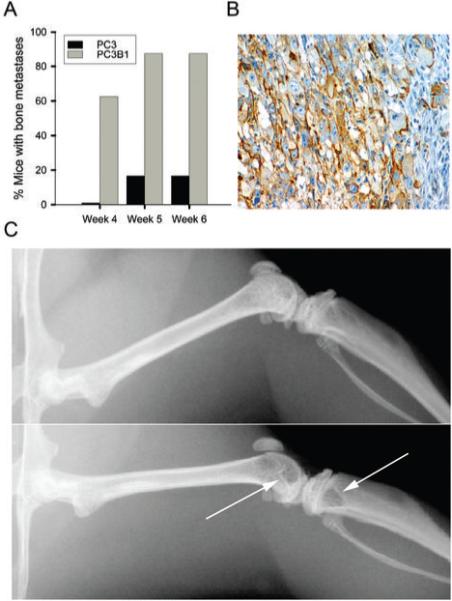
Characterization of a SCID mouse model of extravasation and bone metastasis. A, Comparison of extravasation ability of PC3 cells and PC3B1 cells. B, Immunohistochemistry analysis of α6 integrin expression on PC3 cells within the mouse trabecular bone using AA6NT antibody. C, Representative digital radiographs of mouse bone. Top panel displays normal bone, bottom panel indicates presence of osteolytic metastases in the distal femur and proximal tibia (arrows) at week 4.
The α6 Integrin Antibody, J8H inhibited uPA mediated cleavage of α6 Integrin
Prostate cancer cell lines PC3, PC3N, DU145 and PC3B1 produced varying amounts of α6p under normal growth conditions (Fig 3A). The PC3N cell line was chosen for further study since α6 integrin expressed on the cell surface was primarily in the full length form. Α6 integrin was retrieved via immunoprecipitation using either J1B5 or J8H antibody and its ability to be cleaved was tested by addition of uPA. α6 integrin retrieved by J1B5 was converted to α6p integrin in the presence of uPA, as shown by the decrease in the full length form (α6) and a corresponding increase in α6p form (Fig. 3B). In contrast, α6 integrin retrieved by J8H remained in the full length form (α6) in the presence of uPA (Fig. 3B).
Figure 3.
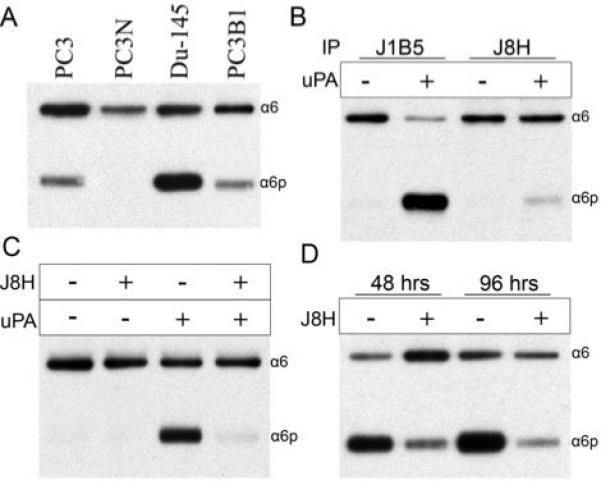
J8H engagement of α6 integrin blocked uPA mediated cleavage. In all panels, α6 integrin was retrieved from cell lysates by immunoprecipitation (IP) with anti-α6 integrin antibody followed by immunoblot (IB) detection of full length α6 (α6) or cleaved α6 (α6p) using AA6A antibody. A, IB analysis of constitutive levels of α6 and α6p from prostate cancer cell lines. B, IP of α6 integrin from PC3N lysate using J1B5 or J8H antibodies and treatment of the IP with activated uPA (20ng/500μL) for 18 hours. C, PC3N cells were pre-treated with or without the antibody J8H before being incubated with uPA (25μg/500μL) for a period of 3 h. D, DU-145 cells received daily treatments of J8H for periods up to 96 h.
We next tested if J8H antibody blocked integrin cleavage on the cell surface. PC3N cells were pre-treated with or without J8H before incubation with uPA. In the absence of J8H and uPA or the absence of uPA alone, the α6 integrin remained in the full length form on the cells (Fig. 3C). The addition of uPA without the J8H antibody resulted in α6 integrin converted to α6p, as shown by the decrease in the full length form (α6) and a corresponding increase in the cleavage product, α6p (Fig. 3C). Importantly, pretreatment of cells with J8H antibody prevented the production of the cleaved form (α6p) via uPA (Fig. 3C). These results indicated that induction of α6p by the exogenous addition of uPA can be blocked by either engaging the α6 integrin in an immunoprecipitation reaction or by engaging α6 integrin on the cell surface with the antibody, J8H.
We next tested if J8H could block the production of α6p in DU145 cells. DU145 cells were selected for this experiment because they do not require exogenous addition of uPA to generate α6p integrin (Fig. 3A). Previously published data indicated that the biological half life of α6p on the cell surface was approximately 72 hrs (40). Therefore, experiments were designed to test the ability of J8H to block endogenous α6p production over several days. J8H treatment of DU145 cells for 48 hrs dramatically decreased the endogenous production of α6p (Fig. 3D). Inhibition of α6p production was also observed after 96 hrs.
J8H diminished the invasive potential of PC3B1 cells
The data thus far indicated that J8H prevented α6p production. Previous work suggested that preventing α6p production would decrease cell migration on laminin (21, 28). Since tumor invasion of laminin coated nerves was observed (Fig. 1A), we next determined whether J8H altered cancer cell invasion on laminin. We utilized a Matrigel invasion assay in the presence of purified laminin 111, a ligand of α6 integrin. PC3B1 cells were selected for this experiment due to their aggressive nature in the mouse metastasis model and because they produce α6p (Fig. 2A). We first confirmed, through flow cytometry, that PC3B1 cells have surface expressed α6 integrin recognized by the J8H antibody (Fig. 4A). Cells were applied to Matrigel coated inserts in the presence of J8H to determine if invasion was altered. After 20 hours of incubation, the ability of PC3B1 cells to invade (control) was inhibited significantly in the presence of J8H antibody (Fig. 4B). The image results were quantitated and approximately 80% of the cells were inhibited from reaching the underside of the Matrigel coated insert in the presence of J8H (Fig. 4C).
Figure 4.
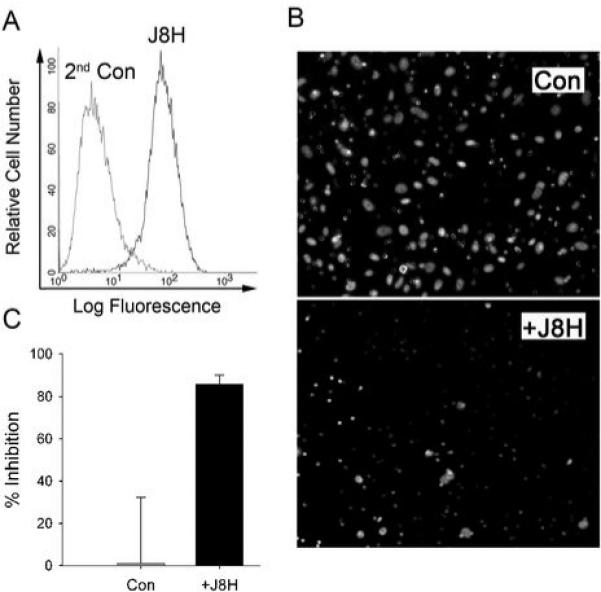
J8H inhibited the invasive potential of PC3B1 cells through Matrigel. A, Flow cytometric analysis of α6 integrin surface expression on PC3B1 using J8H antibody. B, Matrigel invasion assay detected cells that invaded to the underside of the insert by DAPI staining. Top panel, untreated PC3B1 cells (Con); bottom panel, PC3B1 cells in the presence of J8H antibody (+ J8H) (1mg/ml). C, The inhibition of invasion by J8H antibody. Results are expressed as the mean of three independent experiments. Percent inhibition of J8H treated cells was based on PC3B1 control untreated data. Error bars calculated as standard error about the mean.
Pre-treatment of PC3B1 cells with J8H significantly delayed bone metastasis
Utilizing the SCID mouse model of extravasation, we tested whether engagement of the α6 integrin with J8H, the cleavage blocking antibody, would inhibit bone metastasis. Previous work by others showed that tumor cells within the circulation can extravasate to bone within 1−2 hrs of injection (41-43). Titration analysis of the J8H antibody was performed by flow cytometry on PC3B1 cells to determine maximal surface labeling (data not shown). PC3B1 cells alone or J8H treated cells were introduced into the circulation of SCID mice. The percentage of mice containing bone metastases was determined by digital radiographs of live animals 3, 4, 5 and 6 weeks later (Fig. 5A). Injection of PC3B1 cells resulted in approximately 40% of the animals containing bone metastases within 3 weeks and by 4 weeks 80% of the animals contained bone metastasis (Fig. 5A). By week 5, 80% of the animals required termination (Table 1). In contrast, injection of PC3B1 cells pretreated with J8H resulted in no metastases within 3 weeks and at 4 weeks, 80% of the animals were free of bone metastasis (Fig 5A). Interestingly, by week 5, 80% of the animals displayed bone metastases. By week 6, 80% of the animals required termination (Table 1). The lesions detected in both groups of animals were osteolytic, progressive and arose primarily within the distal femur or proximal tibia (Table 1). Of particular note, no lesions were detected in the vertebral column, the pelvic girdle, mandible or skull (data not shown).
Figure 5.
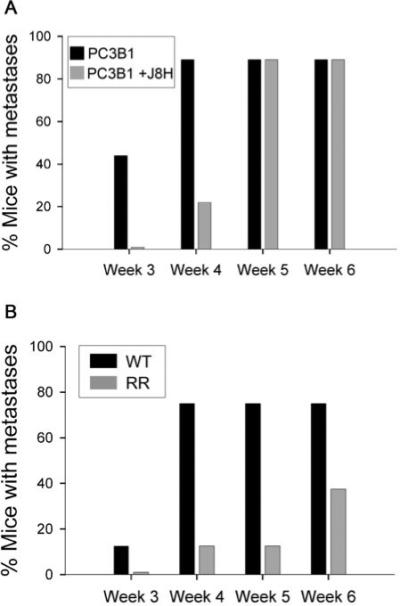
J8H Antibody or genetic inhibition of α6 integrin cleavage inhibited bone metastases. A. SCID mice were injected with untreated PC3B1 cells (PC3B1) or cells containing surface bound J8H (PC3B1 + J8H). B. SCID mice were injected with PC3B1 cells expressing a cleavable α6 integrin (WT) or an uncleavable α6 integrin mutant (RR). In both panels, the entire skeleton of the mouse was inspected for metastases using digital radiographs collected at 3, 4, 5, and 6 weeks after injection. The presence of an osteolytic lesion in any bone was scored as a positive metastasis and all metastatic lesions were progressive (data not shown). The analysis was done without knowledge of the treatment groups. Sample size contained twelve mice per treatment group.
Table 1.
Radiographic Detection of Bone Metastases
| Mouse/PC3B1 | Week 3 | Week 4 | Week 5 | Week 6 |
|---|---|---|---|---|
| 1 | Normal | RDF | RDF, LDF | Terminated |
| 2 | Normal | LDF, LPT | Terminated | Terminated |
| 3 | RPT | RDF, RPT, LPT | Terminated | Terminated |
| 4 | Normal | RF, LT, LF | Terminated | Terminated |
| 5 | Normal | Normal | Normal | Terminated |
| 6 | RDF | RDF, RPT | Terminated | Terminated |
| 7 | RPT | RPT, LPT | Terminated | Terminated |
| 8 | RPT | RPT | Terminated | Terminated |
| 9 | Normal | RPT | Terminated | Terminated |
| Mouse/PC3B1 +J8H | ||||
| 10 | Normal | Normal | RPT | Terminated |
| 11 | Normal | Normal | LPT, RPF | Terminated |
| 12 | Normal | LDF, LPT | RT, LDF, LPT | Terminated |
| 13 | Normal | LDF, LDT | LDF, LPT, | Terminated |
| 14 | Normal | Normal | Normal | Terminated |
| 15 | Normal | Normal | RDF, LDF, LPT | Terminated |
| 16 | Normal | Normal | RT, LT | Terminated |
| 17 | Normal | Normal | LDF | Terminated |
| 18 | Normal | Normal | PRF | Terminated |
| Mouse/PC3B1-WT | ||||
| 1 | Normal | Normal | Normal | Normal |
| 2 | Positive LDF | Progressive LDF | Progressive LDF | Progressive LDF |
| 3 | Normal | Positive RDF | Progressive RDF | Progressive RDF |
| 4 | Normal | Positive RDF | Progressive RDF | Progressive RDF |
| 5 | Normal | Normal | Normal | Normal |
| 6 | Normal | Positive RDF | Progressive RDF | Progressive RDF |
| 7 | Normal | Positive LDF | Progressive LDF | Progressive LDF |
| 8 | Normal | Positive LDF, R Fibula thin | Progressive LDF R Fibula thinner | Progressive LDF R Fibula gone |
| Mouse/PC3B1-RR | ||||
| 9 | Normal | Normal | Normal | Normal |
| 10 | Normal | Positive RT | Progressive RT | Progressive RT |
| 11 | Normal | Normal | Normal | Normal |
| 12 | Normal | Normal | Normal | Normal |
| 13 | Normal | Normal | Normal | Positive RDF |
| 14 | Normal | Normal | Normal | Normal |
| 15 | Normal | Normal | Normal | Normal |
| 16 | Normal | Normal | Normal | Positive RDF |
Location of detected metastatic bone lesions: RDF, right distal femur; LDF, left distal femur; LPT, left proximal tibia; RPT, right proximal tibia; RF, right femur; LT, left tibia; RT, right tibia; LF, left femur; RPF, right proximal femur.
Mutation of α6 integrin cleavage site prevented PC3B1 bone metastasis
Our next step was to validate the J8H blocking results and determine if expression of an uncleavable α6 integrin in tumor cells would prevent extravasation to bone. We expressed the mutant form of α6 integrin, called RR, in PC3B1 cells. Endogenous levels of α6 integrin were not altered in this experiment. We had previously shown cellular expression of the integrin RR mutant results in a fully functional receptor expressed on the cell surface, laminin dependent adhesion, and viable tumor xenografts in a mouse model (21, 28). PC3B1 cells were transfected with either wild type α6 integrin (PC3B1-WT) or α6 integrin containing alanine substitutions for arginine at amino acid positions 594 and 595 (PC3B1-RR). The expression level of the α6 integrin on the cell surface was comparable between the groups as determined by FACS analysis (data not shown). Injection of PC3B1-WT cells resulted in detectable bone metastasis in approximately 10% of the animals within 3 weeks and 80% of the animals by weeks 4, 5 and 6 (Fig. 5B, WT). In contrast, injection of the PC3B1-RR cells resulted in no lesions within 3 weeks, and only 10% of the animals demonstrated lesions by weeks 4 and 5 (Fig. 5B, RR). By week 6, less than half of the animals had detectable metastatic lesions (Table 1). Radiographically, lesions that developed in the PC3B1-RR group were sharply circumscribed and not strikingly expansile as compared to the PC3B1-WT. Of particular note, no lesions were detected in the vertebral column, the pelvic girdle, mandible or skull (data not shown). Necropsy analysis detected no lesions in the lung, liver or adrenal gland (data not shown).
Discussion
In this study, we show that inhibiting α6 integrin cleavage on the tumor cell surface, either through antibody engagement or integrin mutation, will substantially delay the appearance of osseous metastases in a mouse xenograft model. The results reported here support the hypothesis that α6 integrin cleavage permits extravasation of tumor cells from the circulation since subcutaneous injection or direct injection of PC3N-RR mutant cells into bone has no affect on tumor growth at either location (28, 44). This is significant in the course of the human disease since extravasation from the circulation is a critical factor of metastatic spread (8, 39).
The influence of antibody engagement to delay the metastatic phenotype suggests that providing circulating levels of the integrin specific antibody may be beneficial in preventing bone metastasis. The ability of J8H to reversibly delay the appearance of metastases by one week is significant since this corresponds to the expected half life of therapeutic type antibodies in the SCID mouse (45). Toxicity of J8H in the normal tissue is not expected since this antibody does not block cell adhesion to laminin (27). Previous work has shown that inhibiting the A6 adhesion function will block experimental metastasis to the lung (14). However, the utility of this approach as a therapeutic strategy appears limited. Circulating levels of immunoglobulin specific for blocking α6 integrin adhesion function in humans results in the formation of blisters and erosive lesions in the oral mucosa (25, 26). This underscores the potential therapeutic benefits of the J8H antibody, a reagent that inhibits α6 cleavage and not α6 dependent adhesion.
A6 integrin is utilized by hematopoetic stem cells to target the bone (46). The ability of the α6 integrin RR mutation to reduce the metastatic potential of tumor cells homing to bone occurs in the presence of endogenous, wild type α6 integrin. This leads to speculation that cleavage of the receptor has a dominant role in the process. The results indicate that both the time to metastasis and the number of mice developing bone lesions were substantially reduced in the PC3B1-RR group. In contrast to the antibody blocking experiments, the majority of the animals did not develop bone lesions over the 6 week course of the study. Necropsies of the animals receiving tumor cell injections did not reveal other common sites of metastasis (i.e. lung, liver or adrenal gland), suggesting that circulating tumor cells were either eliminated from the mouse or achieved a level of dormancy (47, 48) in the animal. Further experiments utilizing labeled cells and sensitive imaging technology as developed by other groups (49), could distinguish these possibilities.
It is also interesting to note that after week 6, approximately 40% of the animals injected with PC3B1-RR mutant cells developed a detectable metastatic lesion in bone. Although these lesions were progressive, the rate of progression compared to PC3B1-WT was slow as determined by the radiographic features of observed lesions. Termination of these animals was not required because metastases did not produce aggressive lesions leading to pathologic fractures. This suggests that tumor cells that possess uncleavable α6 integrin may eventually adapt to and colonize an osseous microenvironment to produce lytic lesions, but remain less aggressive in nature. This result is consistent with the reported less aggressive phenotype of the RR mutant tumor cells directly injected into the distal end of a mouse femur (28).
We note that endogenous levels of α6p observed for cell lines in culture do not correlate with secreted levels of uPA and uPA activity. PC3 cells express minimal levels of α6p, while DU145 cells convert a majority of α6 integrin into the cleaved product (Fig. 3A). However, PC3 cells secrete at least 2 fold more active uPA when compared to DU145 (50). This suggests that uPA concentration is not the limiting factor in the regulation of integrin α6 cleavage. The ability to block α6 integrin cleavage by extracellular engagement of the receptor points to the possibility that lateral membrane associations with surface expressed proteins like tetraspanins (51, 52) and urokinase receptor (uPAR) (53, 54), could influence uPA mediated integrin cleavage in a physiologically relevant manner. Current work investigating this possibility may reveal other potential cell surface targets for disruption of extravasation of prostate cancer to bone.
The amount of α6p in vitro is not prognostic for bone metastasis in vivo. However, the inability to produce α6p will significantly hinder bone metastasis development in mice. It will be important to have a method to detect cleaved α6p in vivo to determine if the cleaved integrin could serve as a prognostic factor. We are currently developing an ELISA to determine if the released extracellular fragment of α6 integrin is detectable in blood. We consider it likely that α6 cleavage may add to the multiple molecular features required to reliably detect tumor cells with metastatic potential.
Acknowledgments
Grant Support: NIH T32CA009213, P30 CA23074, PO1 CA56666.
We thank the dedicated staff of the Tissue Acquisition Core Service, the Experimental Mouse Shared Service and the Flow Cytometry Service located in the Arizona Cancer Center.
Footnotes
No Conflicts of Interest
References
- 1.Hangan D, Morris VL, Boeters L, von Ballestrem C, Uniyal S, Chan BM. An epitope on VLA-6 (alpha6beta1) integrin involved in migration but not adhesion is required for extravasation of murine melanoma B16F1 cells in liver. Cancer Res. 1997;57:3812–3817. [PubMed] [Google Scholar]
- 2.Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;55:901–906. [PubMed] [Google Scholar]
- 3.Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–410. [PubMed] [Google Scholar]
- 4.Danen EH, van Muijen GN, van de Wiel-van Kemenade E, Jansen KF, Ruiter DJ, Figdor CG. Regulation of integrin-mediated adhesion to laminin and collagen in human melanocytes and in non-metastatic and highly metastatic human melanoma cells. Int J Cancer. 1993;54:315–321. doi: 10.1002/ijc.2910540225. [DOI] [PubMed] [Google Scholar]
- 5.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 6.Ayala GE, Dai H, Powell M, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 7.Villers A, McNeal JE, Redwine EA, Freiha FS, Stamey TA. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol. 1989;142:763–768. doi: 10.1016/s0022-5347(17)38881-x. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs SC. Spread of prostatic cancer to bone. Urology. 1983;21:337–344. doi: 10.1016/0090-4295(83)90147-4. [DOI] [PubMed] [Google Scholar]
- 9.Singh G, Orr FW. Bone metastasis and molecular mechanisms : Pathophysiology. Kluwer Academic; Dordrecht ; Boston: 2004. p. xvi.p. 308. [Google Scholar]
- 10.Siler U, Seiffert M, Puch S, et al. Characterization and functional analysis of laminin isoforms in human bone marrow. Blood. 2000;96:4194–4203. [PubMed] [Google Scholar]
- 11.Gu Y, Sorokin L, Durbeej M, Hjalt T, Jonsson JI, Ekblom M. Characterization of bone marrow laminins and identification of alpha5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-Mix cells. Blood. 1999;93:2533–2542. [PubMed] [Google Scholar]
- 12.Gu YC, Kortesmaa J, Tryggvason K, et al. Laminin isoform-specific promotion of adhesion and migration of human bone marrow progenitor cells. Blood. 2003;101:877–885. doi: 10.1182/blood-2002-03-0796. [DOI] [PubMed] [Google Scholar]
- 13.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 14.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 15.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–1507. [PMC free article] [PubMed] [Google Scholar]
- 16.Hao J, Yang Y, McDaniel KM, Dalkin BL, Cress AE, Nagle RB. Differential expression of laminin 5 (alpha 3 beta 3 gamma 2) by human malignant and normal prostate. Am J Pathol. 1996;149:1341–1349. [PMC free article] [PubMed] [Google Scholar]
- 17.Demetriou MC, Cress AE. Integrin clipping: a novel adhesion switch? J Cell Biochem. 2004;91:26–35. doi: 10.1002/jcb.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demetriou MC, Pennington ME, Nagle RB, Cress AE. Extracellular alpha 6 integrin cleavage by urokinase-type plasminogen activator in human prostate cancer. Exp Cell Res. 2004;294:550–558. doi: 10.1016/j.yexcr.2003.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng S. The urokinase-type plasminogen activator system in prostate cancer metastasis. Cancer Metastasis Rev. 2001;20:287–296. doi: 10.1023/a:1015539612576. [DOI] [PubMed] [Google Scholar]
- 20.Shariat SF, Roehrborn CG, McConnell JD, et al. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J Clin Oncol. 2007;25:349–355. doi: 10.1200/JCO.2006.05.6853. [DOI] [PubMed] [Google Scholar]
- 21.Pawar SC, Demetriou MC, Nagle RB, Bowden GT, Cress AE. Integrin alpha6 cleavage: a novel modification to modulate cell migration. Exp Cell Res. 2007;313:1080–1089. doi: 10.1016/j.yexcr.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 24.Tsuchida J, Ueki S, Saito Y, Takagi J. Classification of ‘activation’ antibodies against integrin beta1 chain. FEBS Lett. 1997;416:212–216. doi: 10.1016/s0014-5793(97)01206-4. [DOI] [PubMed] [Google Scholar]
- 25.Rashid KA, Stern JN, Ahmed AR. Identification of an epitope within human integrin alpha 6 subunit for the binding of autoantibody and its role in basement membrane separation in oral pemphigoid. J Immunol. 2006;176:1968–1977. doi: 10.4049/jimmunol.176.3.1968. [DOI] [PubMed] [Google Scholar]
- 26.Mignogna M, Lanza A, Rossiello L, Ruocco V, Ahmed AR. Comparison of reactivity and epitope recognition between sera from American and Italian patients with oral pemphigoid. Clin Exp Immunol. 2006;145:28–35. doi: 10.1111/j.1365-2249.2006.03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogervorst F, Kuikman I, Noteboom E, Sonnenberg A. The role of phosphorylation in activation of the alpha 6A beta 1 laminin receptor. J Biol Chem. 1993;268:18427–18430. [PubMed] [Google Scholar]
- 28.King TE, Pawar SC, Majuta L, et al. The role of alpha 6 integrin in prostate cancer migration and bone pain in a novel xenograft model. PLoS ONE. 2008;3:3535. doi: 10.1371/journal.pone.0003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damsky CH, Librach C, Lim KH, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 30.Tran NL, Nagle RB, Cress AE, Heimark RL. N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion with stromal cells. Am J Pathol. 1999;155:787–798. doi: 10.1016/S0002-9440(10)65177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Bokhoven A, Varella-Garcia M, Korch C, Hessels D, Miller GJ. Widely used prostate carcinoma cell lines share common origins. Prostate. 2001;47:36–51. doi: 10.1002/pros.1045. [DOI] [PubMed] [Google Scholar]
- 32.Arguello F, Baggs RB, Frantz CN. A murine model of experimental metastasis to bone and bone marrow. Cancer Res. 1988;48:6876–6881. [PubMed] [Google Scholar]
- 33.Arguello F, Furlanetto RW, Baggs RB, et al. Incidence and distribution of experimental metastases in mutant mice with defective organ microenvironments (genotypes Sl/Sld and W/Wv). Cancer Res. 1992;52:2304–2309. [PubMed] [Google Scholar]
- 34.Ohara O, Temple G. Directional cDNA library construction assisted by the in vitro recombination reaction. Nucleic Acids Res. 2001;29:22. doi: 10.1093/nar/29.4.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SB, Hong SK, Choe G, Lee SE. Periprostatic distribution of nerves in specimens from non-nerve-sparing radical retropubic prostatectomy. Urology. 2008;72:878–881. doi: 10.1016/j.urology.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 36.Niessen CM, Cremona O, Daams H, Ferraresi S, Sonnenberg A, Marchisio PC. Expression of the integrin alpha 6 beta 4 in peripheral nerves: localization in Schwann and perineural cells and different variants of the beta 4 subunit. J Cell Sci. 1994;107:543–552. doi: 10.1242/jcs.107.2.543. [DOI] [PubMed] [Google Scholar]
- 37.Sonnenberg A, Linders CJ, Daams JH, Kennel SJ. The alpha 6 beta 1 (VLA-6) and alpha 6 beta 4 protein complexes: tissue distribution and biochemical properties. J Cell Sci. 1990;96:207–217. doi: 10.1242/jcs.96.2.207. [DOI] [PubMed] [Google Scholar]
- 38.Nodari A, Zambroni D, Quattrini A, et al. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coman DR, de LR, Mcc UM. Studies on the mechanisms of metastasis; the distribution of tumors in various organs in relation to the distribution of arterial emboli. Cancer Res. 1951;11:648–651. [PubMed] [Google Scholar]
- 40.Davis TL, Rabinovitz I, Futscher BW, et al. Identification of a novel structural variant of the alpha 6 integrin. J Biol Chem. 2001;276:26099–26106. doi: 10.1074/jbc.M102811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harms JF, Welch DR. MDA-MB-435 human breast carcinoma metastasis to bone. Clin Exp Metastasis. 2003;20:327–334. doi: 10.1023/a:1024062911144. [DOI] [PubMed] [Google Scholar]
- 42.Phadke PA, Mercer RR, Harms JF, et al. Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res. 2006;12:1431–1440. doi: 10.1158/1078-0432.CCR-05-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wetterwald A, van der Pluijm G, Que I, et al. Optical imaging of cancer metastasis to bone marrow: a mouse model of minimal residual disease. Am J Pathol. 2002;160:1143–1153. doi: 10.1016/S0002-9440(10)64934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawar SC, Dougherty S, Pennington ME, Demetriou MC, Stea BD, Dorr RT, Cress AE. alpha6 integrin cleavage: sensitizing human prostate cancer to ionizing radiation. Int J Radiat Biol. 2007;83:761–767. doi: 10.1080/09553000701633135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaufmann R, Hainzl A, Sterry W, Alberti S, Klein CE. In vivo targeting of integrin receptors in human skin xenografts by intravenously applied antibodies. Arch Dermatol Res. 1994;286:6–11. doi: 10.1007/BF00375836. [DOI] [PubMed] [Google Scholar]
- 46.Qian H, Georges-Labouesse E, Nystrom A, et al. Distinct roles of integrins alpha6 and alpha4 in homing of fetal liver hematopoietic stem and progenitor cells. Blood. 2007;110:2399–2407. doi: 10.1182/blood-2006-10-051276. [DOI] [PubMed] [Google Scholar]
- 47.Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumour dormancy. Apmis. 2008;116:754–770. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 48.Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87–98. doi: 10.3233/bd-2007-26108. [DOI] [PubMed] [Google Scholar]
- 49.Kaijzel EL, Snoeks TJ, Buijs JT, van der Pluijm G, Lowik CW. Multimodal imaging and treatment of bone metastasis. Clin Exp Metastasis. 2009;26:371–379. doi: 10.1007/s10585-008-9217-8. [DOI] [PubMed] [Google Scholar]
- 50.Dondi D, Festuccia C, Piccolella M, Bologna M, Motta M. GnRH agonists and antagonists decrease the metastatic progression of human prostate cancer cell lines by inhibiting the plasminogen activator system. Oncol Rep. 2006;15:393–400. doi: 10.3892/or.15.2.393. [DOI] [PubMed] [Google Scholar]
- 51.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 52.Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- 53.Kugler MC, Wei Y, Chapman HA. Urokinase receptor and integrin interactions. Curr Pharm Des. 2003;9:1565–1574. doi: 10.2174/1381612033454658. [DOI] [PubMed] [Google Scholar]
- 54.Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001;276:3983–3990. doi: 10.1074/jbc.M008220200. [DOI] [PubMed] [Google Scholar]


