Abstract
Objective
To develop a noninvasive method of studying brain mechanisms involved in energy homeostasis and appetite regulation in humans by using visual food cues that are relevant to individuals attempting weight loss.
Design
Functional magnetic resonance imaging (fMRI) was used to compare brain activation in regions of interest between groups of food photographs.
Participants
10 healthy, nonobese women who were not dieting for weight loss.
Measurements
Independent raters viewed food photographs and evaluated whether the foods depicted should be eaten by individuals attempting a calorically-restricted diet. Based on their responses, we categorized photographs into “non-fattening” and “fattening” food groups, the latter characterized by obviously high caloric content and usually also high fat or high sugar content. Blood oxygen level-dependent (BOLD) response was measured by fMRI while participants viewed photographs of “fattening” foods, “non-fattening” foods, and non-food objects.
Results
Viewing photographs of fattening food compared to non-food objects resulted in significantly greater activation in the brainstem; hypothalamus; left amygdala; left dorsolateral prefrontal cortex; left orbitofrontal cortex; right insular cortex; bilateral striatum, including the nucleus accumbens, caudate nucleus, and putamen; bilateral thalamus; and occipital lobe. By comparison, only the occipital region had greater activation by non-fattening food than by object photographs. Combining responses to all food types resulted in attenuation of activation in the brainstem, hypothalamus, and striatum.
Conclusion
These findings suggest that, in nonobese women, neural circuits engaged in energy homeostasis and reward processing are selectively attuned to representations of high-calorie foods that are perceived as fattening. Studies to investigate hormonal action or manipulation of energy balance may benefit from fMRI protocols that contrast energy-rich food stimuli with non-food or low-calorie food stimuli.
Introduction
Food intake is influenced by a complex regulatory system involving the integration of a wide variety of sensory inputs by multiple brain areas. Peripheral signals, such as the adipocyte hormone leptin, reflect overall energy balance and have primary sites of action in the hypothalamus.1 In contrast, most short-term meal-related “satiety” signals that originate in the gut, including autonomic inputs conveyed by vagal afferent fibers and hormones such as cholecystokinin (CCK), have sites of action in the hindbrain, including the nucleus of the solitary tract.1 Food, of course, has pleasurable qualities. Mesolimbic dopaminergic and opioid signaling pathways from the midbrain ventral tegmental area and the nucleus accumbens play a key role in establishing these rewarding properties.2 Finally, cognitive factors such as social situation, emotional state, or intentional efforts to control consumption can also influence food intake. Much of what we know about these regulatory systems derives from animal models, leaving large gaps in our understanding of the control of eating behavior in humans.
Consistent with the biological imperative to identify and partake of food, neuroimaging studies have begun to document the responsiveness of the human brain to food cues such as odors and/or taste samples of food,3, 4 videos of people with food,5 photographs of food,6-15 and visual presentation of actual food.16 Food cues have been contrasted directly with non-food stimuli8, 13, 16 or chosen to represent foods with high hedonic value and energy content.7, 11, 14, 15 However, little is known about the relative advantages or disadvantages of these methods—a consideration that is critical to designing or interpreting the results of studies that use visual food cues.
To elucidate the neural basis of human eating behavior, we have developed a method that, like several others,10, 11, 17 measures brain response to visual food cues. Using functional magnetic resonance imaging (fMRI), we can observe brain response to viewing photographs of food and non-food objects. More specifically, we evaluated brain response in a group of nonobese women to food photographs selected on the basis of whether the food was perceived to be compatible with efforts at weight loss. We looked specifically at brain regions important to the regulation of human appetite and food intake, and directly compared results obtained with different choices of visual stimuli. Thus, we hope to illuminate promising methods that use visual food cues to investigate mechanisms of human eating behavior, and to facilitate a more unified and reproducible approach to neuroimaging studies of eating behavior.
Methods
Participants
Participants were 10 females aged 18-65 with no history of weight loss surgery or current dieting. Seven of the participants were recruited from the University of Washington Twin Registry, a community-based registry of twin pairs derived from applications for drivers' licenses in Washington State.18 However, only one member of a given twin pair was included in this research. Regardless of twin or non-twin status, participants were excluded if they: 1) had a body mass index (BMI) < 20 or > 29.9 kg/m2; 2) smoked > 1 cigarette/day; 3) drank > 2 alcoholic beverages per day or used recreational drugs; 4) had a lifetime history of eating disorders; 5) were pregnant or had a major medical problem such as diabetes or were taking medications known to alter appetite or body weight; or 6) were currently in a formal weight loss program. Age, weight, and height were self-reported for screening purposes. Weight measured at the time of the visit was used for BMI calculations (weight/height2). All participants completed informed consent procedures, as approved by the University of Washington Institutional Research Board, and all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Procedures
Blood oxygen level-dependent (BOLD) response was measured by fMRI while participants viewed photographs of “fattening” food, “non-fattening” food, and non-food objects. On the day of the study, subjects were instructed to eat what they considered a normal meal according to their appetite at their habitual mealtimes. Scans were scheduled for 2-4 hours after a meal. To distract attention from the grouping of the stimuli, participants were told that they were participating in a study of taste. Participants viewed a brief sample slideshow on a computer to become accustomed to the tempo at which photographs would appear. No images from the sample slide show were included in the fMRI protocol, and all sample photographs were of natural objects (flowers) instead of foods to avoid habituation. Before and during the fMRI scan, participants were advised to focus carefully on each image, as they would later be tested to determine which photographs they remembered. After all procedures were complete, we performed a brief interview in which participants were asked about their experience of the scans and whether or not they noticed any grouping of the photographs.
Stimuli
The stimuli were individual photographs depicting either food or non-food objects. To standardize our study images, 214 food photographs were evaluated by 13 independent raters who did not participate in the fMRI study. Volunteers were male and female staff, faculty, and students at the University of Washington. For each photograph, raters responded to the following instruction: “If someone was dieting in order to lose weight, rate how acceptable each food would be for them to eat.” Photographs were rated on a Likert scale from 1 to 7. Categories 1-3 were defined as “definitely”, “probably”, and “maybe” should not eat when dieting. Category 4 was defined as “unsure/can't tell from picture”. Categories 5-7 were defined as “maybe,” “probably,” and “definitely” OK to eat when dieting. Photographs with mean scores of ≤ 2 or ≥ 6 were selected.
We chose this approach because we were interested in assessing foods that individuals would try to avoid if they were attempting weight loss. Foods judged to be unacceptable when dieting were universally characterized by high caloric content and were usually high in fat and/or sugar content. They included candy, desserts, pastries, and high-fat savory foods such as pizza, hamburgers, chicken wings, and other fried foods. We named this category the “fattening” food group, a term chosen to indicate that such foods are typically regarded as incompatible with achieving weight loss. We chose the term “non-fattening” to describe food photographs that were deemed compatible with weight loss. These depicted low-calorie foods, including fruits, vegetables, salads, low-fat meats (e.g. chicken breast and turkey), and seafood. Food photographs eliminated because of indeterminate scores tended to depict either carbohydrate-rich foods, such as bread and meatless pasta, or multiple food types, such as turkey with mashed potatoes and gravy or salad with cheese and thick dressing.
Non-food images consisted of common, recognizable large and small objects such as furniture, tools, toiletries, sundries, electronics, and household items. Food-related objects such as utensils, plates, or cooking implements were not used. All food and object images were commercial-quality stock photographs obtained from Web sites or donated for research use. All photographs were matched for size (600 X 400), quality, and visual interest, and were group-matched for luminosity (F(2, 257) = 0.00, P = 0.99). Quality and visual interest were assessed by 3 authors (ES, NK, KM), and the independent raters provided feedback on food pictures. Finally, during debriefing sessions, subjects were asked if any pictures stood out or were unclear.
fMRI Acquisition
fMRI uses the blood oxygen level-dependent (BOLD) response as an indirect measure of increased neural activity in response to a stimulus. Structural and fMRI exams were performed on a 3T Philips Achieva MR System (version 1.5, Philips Medical Systems, Best, The Netherlands) with dual Quasar gradients (80 mT/m with a slew rate of 110 mT/m/s or 40 mT/m at a slew rate of 220 mT/m/s) using an 8-channel SENSE head coil. Functional whole-brain T2*-weighted images were acquired by using a single-shot gradient-recalled echo-planer imaging (EPI) sequence (TR = 2000 ms; TE = 30 ms; flip angle = 76°; FOV = 240 mm) with a matrix size of 64 × 64 (reconstructed in-plane resolution = 3 × 3 mm). Thirty-two axial slices covering the entire brain (slice thickness = 4 mm, 0.5 mm gap) were acquired during each volume by using interleaved slice acquisition. The total scan duration was 5 minutes and 28 seconds; 156 dynamic volumes and 5 dummy scans were acquired during that time period. A B0 field map was collected to correct for distortions in the EPI data due to magnetic field inhomogeneities. The B0 field map was acquired by using a fast field echo sequence (TR = 15 ms; TE = 6.9 ms; flip angle = 90°; FOV = 240 mm) with a matrix size of 64 × 64 (in-plane resolution = 3.75 × 3.75 mm). Thirty-two axial slices covering the entire brain (slice thickness = 5 mm, 0 mm gap) were acquired for each image. A 4-minute 3D T1-weighted volume was acquired in the sagittal plane (0.98 × 0.98 × 0.75 mm3 spatial resolution, reconstructed matrix = 256 × 256, FOV = 240, TE = 6.8 ms, TR = 3.1 ms, and θ = 8°, SENSE factor = 1). This high-resolution anatomical image was used for image registration and anatomical localization.
fMRI Task and Behavioral Posttest
The paradigm consists of 3 blocks of images of fattening foods, 3 blocks of non-fattening foods, and 7 blocks of non-food objects. Ten photographs were presented per block. Each photograph was projected for 2.4 seconds onto a screen easily viewed in a mirror by the participant. Non-food object blocks alternated with fattening and non-fattening food blocks. Immediately after the scan, participants were given a behavioral posttest (memory task) consisting of images viewed in the scanner mixed with distracter images not previously seen. They were asked to state whether or not they saw each image while in the scanner. Thirty-two pictures were included (16 non-food, 8 fattening food, 8 non-fattening food) of which 50% in each category were distracter images.
fMRI Processing and Statistical Analysis
fMRI data analyses were performed by using the Oxford Centre for Functional MRI of the Brain (FMRIB) Software Library version 3.3 (FSL; http://www.fmrib.ox.ac.uk/fsl/). The following preprocessing steps were applied: 1) motion correction was conducted with Motion Correction using FMRIB's Linear Image Registration Tool (MCFLIRT); 2) nonbrain structures were removed by using the Brain Extraction Tool; and 3) data were spatially smoothed by using a Gaussian kernel of full width at half maximum = 5 mm and temporally smoothed with a high-pass filter of sigma = 72 s. Time series statistical analyses were carried out by using FMRIB's Improved Linear Model with local autocorrelation correction. Condition effects were estimated at each voxel for the following contrasts: 1) fattening food > object; 2) non-fattening food > object; 3) all food > object; 4) fattening food > non-fattening food; 5) non-fattening food > fattening food; and 6) object > all food. The “all food” category was created by combining all images obtained during viewing of fattening food and non-fattening food blocks into one condition (fattening food + non-fattening food). Individual fMRI data were registered to the high-resolution scan by using fieldmap corrections, warped with an affine transformation to the MNI152 standard image by using FMRIB's Linear Image Registration Tool, and resampled to 2 mm3 voxels.
Using a region-of-interest approach, analyses of group-wise effects were conducted by using FMRIB's Local Analysis of Mixed Effects (FLAME), a method for modeling and estimating the random-effects component of the measured inter-session mixed-effects variance. This method allows inferences to be made about the wider population from which the participants were drawn. Anatomical masks were either hand-drawn on the MNI152 standard brain or defined by Anatomical Automatic Labeling, an in-house package made by Neurofunctional Imaging Group (GIN, UMR6095, CYCERON, Caen, France). The following 13 brain regions were tested separately: brainstem, hypothalamus, left/right amygdala, left/right inferior frontal lobe, left/right insula, left/right striatum, left/right thalamus, and occipital lobe. Brain regions of interest for these analyses were chosen a priori based on previous studies and anatomical regions known to be involved in energy homeostasis and appetite regulation. Clusters with a P value < 0.05 after correction for multiple comparisons were considered significant. Statistical corrections for multiple comparisons were conducted by using cluster-thresholding based on Markov Chain Monte Carlo sampling for each region of interest. All fMRI results were corrected for multiple comparisons with this method. Figures represent group means for all participants.
Results
Participant Characteristics
Participants were 10 females, nonsmokers and nondieters. Most were within the normal weight range (Table 1). Participants demonstrated greater than 90% accuracy on the behavioral posttest completed immediately after the scan, indicating that they had seen and retained information relevant to the images shown. No participant reported noticing the grouping of foods by fattening and non-fattening categories.
Table 1.
Participant characteristics.
| Characteristic | Mean | SD | Range |
|---|---|---|---|
| Age, years | 29.4 | 12.1 | 20.4-49.5 |
| BMI, kg/m2 | 23.2 | 2.0 | 20.4-26.8 |
| Time from last meal, hours:minutes | 3:13 | 0:46 | 1:44 – 4:02 |
| Post-test accuracy, % | 91 | 6 | 81-100 |
SD = standard deviation.
fMRI Results
All Food > Object
When the fattening and non-fattening food images were combined into an all-food condition and compared to object blocks, we found significant clusters of activation in the brainstem, hypothalamus, left dorsolateral prefrontal cortex, right insular cortex, left putamen and nucleus accumbens, left thalamus, and occipital lobe (Table 2).
Table 2.
Size, significance level (z-score), and Talaraich coordinates of clusters with greater activation in the all foods combined condition than in the object condition in regions of interest analyses (P < 0.05, corrected for multiple comparisons).
| All food > Object | No. of voxels | Maximum z-score | x (mm) | y (mm) | z (mm) |
|---|---|---|---|---|---|
| Brainstem (midbrain) | 546 | 3.41 | 8 | -12 | -16 |
| Left hypothalamus | 7 | 3.24 | -8 | -10 | -10 |
| Left hypothalamus | 5 | 2.79 | -8 | 2 | -6 |
| Right hypothalamus | 6 | 2.76 | 6 | -10 | -14 |
| Left amygdala | none | ||||
| Right amygdala | none | ||||
| Left inferior frontal (BA 9) | 304 | 4.09 | -40 | 18 | 30 |
| Right inferior frontal | none | ||||
| Left insula | none | ||||
| Right insula | 164 | 3.29 | 40 | -2 | 2 |
| Left striatum (putamen, nucleus accumbens) | 161 | 3.64 | -18 | 8 | 6 |
| Right striatum | none | ||||
| Left thalamus | 265 | 3.35 | -10 | -2 | 4 |
| Right thalamus | none | ||||
| Occipital lobe (BA 17,18) | 934 | 3.87 | -12 | -90 | -6 |
BA = Brodmann area.
Fattening Food > Object
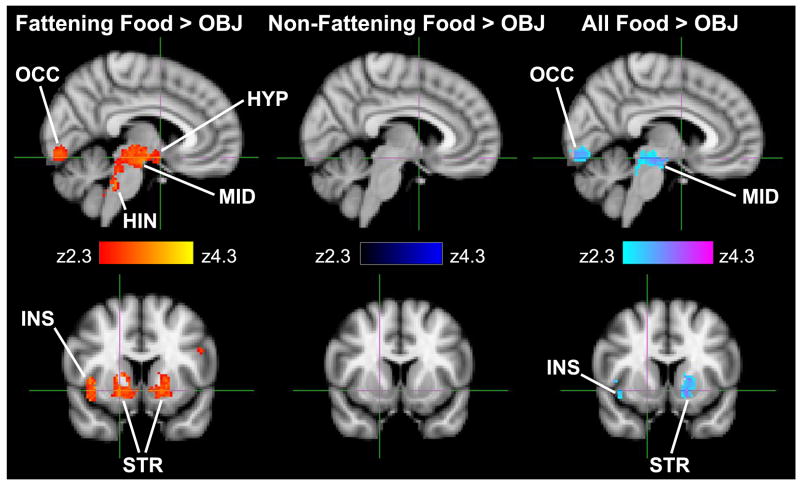
Significant clusters of activation were found throughout the selected brain regions involved in energy homeostasis, satiety and reward processing, and higher cortical function (Table 3). Viewing fattening food photographs compared to non-food object photographs resulted in significantly greater activation in the brainstem (midbrain including the ventral tegmental area and hindbrain adjacent to the 4th ventricle; Figure 1); hypothalamus; left amygdala; left dorsolateral prefrontal cortex; left orbitofrontal cortex; right insular cortex; bilateral striatum, including the nucleus accumbens, caudate nucleus, and putamen; bilateral thalamus; and Brodmann areas 17 and 18 of the occipital lobe.
Table 3.
Size, significance level (z-score), and Talaraich coordinates of clusters with greater activation in the fattening food photograph condition than in the object condition in regions of interest analyses (P < 0.05, corrected for multiple comparisons).
| Fattening Food > Object | No. of voxels | Maximum z-score | x (mm) | y (mm) | z (mm) |
|---|---|---|---|---|---|
| Brainstem (midbrain) | 1025 | 3.64 | -6 | -28 | -8 |
| Brainstem (near 4th ventricle) | 3.39 | 4 | -36 | -34 | |
| Right hypothalamus | 55 | 3.43 | 8 | -4 | -12 |
| Left hypothalamus | 33 | 3.27 | -6 | -8 | -10 |
| Left amygdala | 47 | 3.18 | -24 | -6 | -24 |
| Right amygdala | none | ||||
| Left inferior frontal (BA 9) | 382 | 3.45 | -38 | 32 | 18 |
| Left inferior frontal (OFC) | 218 | 3.44 | -36 | 28 | -10 |
| Right inferior frontal | none | ||||
| Left insula | none | ||||
| Right insula | 340 | 3.54 | 44 | 12 | -8 |
| Left striatum (putamen, nucleus accumbens, caudate) | 328 | 3.55 | -20 | 8 | 6 |
| Right striatum (putamen, nucleus accumbens, caudate) | 378 | 3.67 | 18 | 10 | -6 |
| Right striatum (putamen) | 139 | 3.43 | 32 | 0 | -6 |
| Left thalamus | 613 | 3.46 | -8 | -12 | 8 |
| Right thalamus | 331 | 3.54 | 8 | -10 | -6 |
| Occipital lobe | 855 | 3.93 | -12 | -92 | -4 |
OFC = orbitofrontal cortex; BA = Brodmann area.
Figure 1. Patterns of brain activation differ in regions of interest based on the type of food depicted.
Top row: Sagittal section through the hypothalamus (Talairach coordinate 6, 2, -12) for the contrasts of fattening food vs. objects, non-fattening food vs. objects, and all food vs. objects. Significant clusters (P < 0.05 after correction for multiple comparisons) are presented by z-score (range 2.3 - 4.3). Bottom row: coronal section through the striatum (18, 10, -6). OCC = occipital lobe; HYP = hypothalamus; MID = brainstem (midbrain); HIN = brainstem (hindbrain); INS = insula; STR = striatum.
Non-Fattening Food > Object
The only brain region that showed significantly more activation by non-fattening foods compared to non-food objects was the occipital lobe (Brodmann area 17 and 18). The cluster of activation (355 voxels; z-score = 3.57) was centered in the left primary visual cortex (Talaraich coordinates = -10, -96, -6) and extended to visual association areas. Thus, when results from the non-fattening food blocks were combined with those from the fattening food blocks into the all-foods condition, activation was attenuated in brain regions important to energy homeostasis, satiety, and reward processing, as compared to the condition including only fattening food images (Figure 1).
Fattening Food > Non-Fattening Food
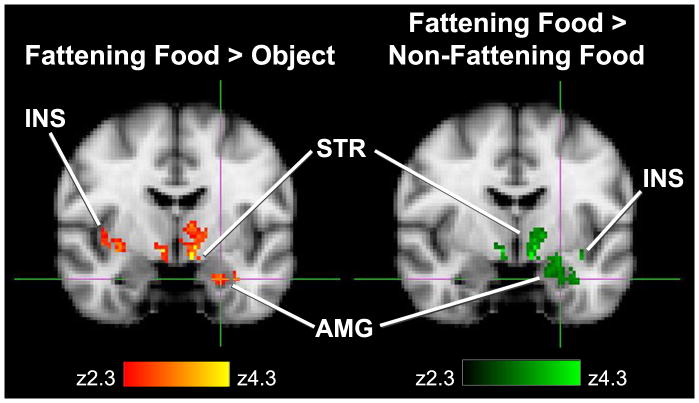
We found significantly increased activation in the brainstem, right hypothalamus, left amygdala, right inferior frontal gyrus, insular cortex bilaterally, bilateral striatum, and bilateral thalamus while viewing foods perceived as fattening compared to viewing those perceived as non-fattening (Table 4). No significant differences in activation were found in the occipital lobe visual cortex and association areas. For the contrast of fattening vs. non-fattening food, regions of interest with significant activation clusters were very similar, but not identical, to the contrast of fattening food vs. non-food objects (Figure 2).
Table 4.
Size, significance level (z-score), and Talaraich coordinates of clusters with greater activation in the fattening food than in the non-fattening food photograph condition in regions of interest analyses (P < 0.05, corrected for multiple comparisons).
| Fattening Food > Non-Fattening Food | No. of voxels | Maximum z-score | x (mm) | y (mm) | z (mm) |
|---|---|---|---|---|---|
| Brainstem (midbrain) | 538 | 3.48 | 8 | -22 | -14 |
| Right hypothalamus | 94 | 3.62 | 6 | 2 | -12 |
| Left amygdala | 123 | 3.38 | -22 | -10 | -10 |
| Right amygdala | none | ||||
| Left inferior frontal | none | ||||
| Right inferior frontal (BA 47, inferior frontal gyrus) | 300 | 3.64 | 56 | 24 | -6 |
| Left insula | 107 | 3.26 | -36 | -6 | -12 |
| Right insula | 153 | 3.45 | 30 | 18 | -20 |
| Left striatum (caudate, nucleus accumbens) | 143 | 3.53 | -18 | 20 | -6 |
| Right striatum (putamen, nucleus accumbens, caudate) | 380 | 3.62 | 18 | 12 | -4 |
| Left thalamus | 483 | 3.44 | -18 | -22 | 8 |
| Right thalamus | 245 | 3.36 | 12 | -16 | 0 |
| Occipital lobe | none |
BA = Brodmann area.
Figure 2. A comparison of brain activation in regions of interest based on choice of control condition.
Coronal sections through the amygdala (Talairach coordinates -24, -8, -24) for the contrasts of fattening food vs. objects and fattening food vs. non-fattening food. Significant clusters (P < 0.05 after correction for multiple comparisons) are presented by z-score (range 2.3 - 4.3). INS = insula; STR = striatum; AMG = amygdala
Non-Fattening Foods > Fattening Foods
No brain region showed significantly greater activation by fattening foods than by non-fattening foods.
Object > All Foods
Only the right lateral occipital lobe had a significant cluster where viewing objects had greater activation than all foods. This cluster (578 voxels; z-score = 3.5) included Brodmann areas 18 & 19 (Talaraich coordinates = 46, -80, 10).
Discussion
Our findings present clear evidence that, among nonobese women, the brain responds differently to visual food cues based on how acceptable the foods are to eat while attempting weight loss. Specifically, viewing photographs of “fattening” foods, as compared to photographs of non-food objects, resulted in significantly greater activation in brain regions involved in many different aspects of food intake regulation, including the hypothalamus, caudate, putamen, nucleus accumbens, thalamus, midbrain, right insula, left amygdala, prefrontal cortex, and occipital lobe. By comparison, the occipital lobe was the only brain region in which activation in response to images of foods perceived as non-fattening was greater than the response to non-food images. When we combined the fattening and non-fattening food photographs to create a category of all foods, clusters of activity were almost universally smaller in size than we observed in response to fattening food images, and some no longer met threshold levels for significance. From this observation, we conclude that neural circuits involved in energy homeostasis, satiety perception, reward processing, and cognitive control of behavior are selectively attuned to representations of foods perceived as fattening.
By contrasting the BOLD response to fattening food images with the response to non-food object images in non-fasted female subjects, we observed activation in the hypothalamus as well as a cluster in the hindbrain abutting the fourth ventricle, where the nucleus of the solitary tract is located. The medial hypothalamus and the nucleus of the solitary tract are crucial sites for sensing and processing inputs relevant to energy homeostasis and satiety, respectively. These sites integrate information on the status of body energy stores with signals generated by the presence of nutrients in the gut.1 The activation in these brain regions suggests that photographs of fattening foods stimulate brain areas critical to our subjective experience of appetite. As with other brain regions, these areas were not activated by images of non-fattening foods. Thus, when we examined the brain's response to all foods combined, the significant clusters in the hypothalamus were smaller, falling below 10 voxels—a cutoff for cluster size used in some fMRI studies—and the hindbrain cluster was no longer detected. Data from reward pathways revealed that fattening food photographs induce significantly greater activation than non-food object photographs in the left and right striatum (ventral striatum, putamen, caudate), as well as the midbrain (including the ventral tegmental area), left amygdala, and left orbitofrontal cortex. These pathways integrate aspects of motivation for feeding with hypothalamic inputs on the state of energy balance2 and are also targets for circulating appetite-regulating hormones.19, 20 Furthermore, reward pathways showed more activation by fattening than by non-fattening food images when these groups were compared directly. Because the latter photographs were relatively ineffective in activating these brain areas, reward system activation was not consistently greater in the combined all-food condition than in the object condition.
We interpret these findings as evidence that the neural circuitry primarily engaged in energy homeostasis (hypothalamus), satiety perception (hindbrain), reward processing (midbrain ventral tegmental area, ventral striatum), and cognitive control of behavior (orbitofrontal cortex and prefrontal cortex) is selectively attuned to representations of foods perceived as fattening. There is precedent for such neural distinctions, as preference for highly palatable foods is mediated through a striatal opioid system that selectively increases feeding of high-reward foods.2 Thus, it is plausible that attention, motivation, and cognitive areas have developed to selectively attend to environmental food cues in conjunction with a reward system that reinforces their ingestion. We therefore propose that investigations of hormonal action or manipulation of energy balance may benefit from fMRI study designs in which analyses contrast stimuli grouped by actual or perceived caloric content or by perceived fattening vs. non-fattening foods. Such designs are directly relevant to understanding the pathogenesis of obesity in the current environment of ample, available, energy-rich foods. A corollary of this proposition is that experimental paradigms that collapse food categories may yield inconsistent or negative results depending on the relative proportions of high- and low-calorie foods depicted.
Although our data are consistent with other reports, methodologic differences resulted in outcomes and interpretations that warrant comment. Because of our interest in eating behavior and volitional control of food intake for the purposes of weight loss, we selected photographs on the basis of their acceptability to individuals attempting a calorically-restricted diet. Photographs in the fattening food group depicted foods with high caloric content and usually also high fat or high sugar content, as compared to those deemed non-fattening, which depicted low-calorie foods. Although this approach has not been applied previously, we believe it reveals meaningful differences in how the brain responds to visual food cues. One study using caloric content to categorize foods also found, among other areas, left amygdala, brainstem, and left thalamic activation in a high-calorie food vs. objects comparison,10 but found no hypothalamic or striatal activation. Others have also found reward pathway activation in response to images of high-calorie foods vs. objects, but the effect was observed only in obese participants.12, 15 Another published method, which used hedonic valuations to create comparison groups, found greater hypothalamic activation (6, 0, -12) while viewing images of high hedonic valuation foods than neutral foods.6 These responses were attenuated by overfeeding, implying that the state of energy balance altered brain response to highly hedonic food cues.
In summary, our findings confirm other fMRI studies that used contrasts of high calorie or high hedonic valuation images vs. non-food or low-calorie images to stimulate neural activity in reward and homeostatic centers. We also find that refining the stimuli to select for perceived fattening and non-fattening food may capture additional salience of food cues to energy homeostasis and reward areas.
Our findings contextualize the results of studies that use protocols combining high- and low-calorie foods into a food vs. non-food design. Food vs. non-food studies have consistently detected insular activation8, 13, 21 and support a role for the insula—which serves as the primary taste cortex22—in distinguishing food from inedible objects. Other brain areas that may also be responsive to food vs. non-food comparisons in adults include the thalamus,8 extrastriate cortex,13 and orbitofrontal cortex21—a secondary taste cortex thought to be crucial in regulating food intake.22 One study in children and adolescents found greater activation in response to food vs. non-food images in the orbitofrontal cortex, medial frontal cortex, fusiform, superior parietal cortex, and cerebellum.9 None of these studies found differential activation by food vs. non-food stimuli in reward pathways, the hypothalamus, or the brainstem. In genetically leptin-deficient individuals who are markedly hyperphagic, a food vs. non-food design did, however, document attenuation of the ventral striatum with leptin replacement.7 In sum, both prior studies and our own results suggest that study designs that combine all foods into a single category may not consistently detect brain activity in regions involved in energy homeostasis and perception of food reward in healthy populations. By looking specifically at brain regions important to the regulation of human appetite and food intake, and by directly comparing results obtained with different choices of visual stimuli, we provide new and detailed data on the advantages and disadvantages of various fMRI techniques.
Another methodologic consideration in interpreting our results is our choice of the control condition. Because BOLD response is a relative rather than an absolute measure of the hemodynamic response to neural activity, both our study design and others necessarily utilize a control stimulus group. We examined 2 different control groups and demonstrated that results differ somewhat when fattening food photographs are contrasted to non-food objects rather than to non-fattening food photographs. Specifically, regions of interest in which clusters were present in the contrast of fattening foods to objects, but not in the contrast of fattening foods to non-fattening foods, include the left hypothalamus, left inferior frontal cortex, left insula, and occipital lobe. Prior studies have used control stimuli that include tools,13 animals,9 buildings,11 cars,15 eating utensils,10, 12 natural objects (e.g., rocks, flowers),10 and other common objects.16 Such diversity, however, limits reproducibility between studies and makes direct comparisons problematic. Standardization of stimulus and control conditions across studies, or repeated use of a paradigm in different populations, would facilitate more definitive conclusions about neural pathways that respond to visual food cues.
Our study has several important limitations. We cannot ascertain whether the response to the fattening food group was mediated through the caloric content of the foods depicted, their hedonic valuation, or their cultural significance, because food has tremendous social meaning as well. In addition, because of the relatively small sample size, we used conservative analytic techniques and limited our investigation to regions of interest. Thus, our results do not represent a complete picture of all brain regions that are responsive to visual food stimuli. Additional whole-brain analyses in a larger cohort would be required to accomplish this goal. Our small sample size and inclusion only of women who were nonobese and not dieting further limits the generalizablility of our findings. Several studies have documented differences in cortical, limbic, and/or striatal responses by gender,23, 24 obesity status,3, 12, 15 and weight-reduced status.16, 25 It is also possible that brain response to fattening foods differs during the menstrual cycle, so that additional variance in our findings may have been introduced by the inclusion of women at all phases of their menstrual cycle. Because the importance of handedness outside of language and motor control is uncertain,26 we did not control for handedness, which may have introduced further variability. Furthermore, due to susceptibility artifacts related to air in local sinuses, measurement errors may occur in the hypothalamus and inferior frontal cortex with fMRI.27 Finally, as with all fMRI studies, whether the areas of activation represent excitatory or inhibitory inputs cannot be determined.
Offsetting these limitations are several advantages inherent in our methods. Our participants were successfully blinded to the underlying study hypotheses to distract their attention from the blocking of food groups. Whether our study would yield similar results if only fattening food blocks were shown with non-food blocks, or if the participants had been informed about the study's objective, are questions for future investigations. For example, the presence of the non-fattening food blocks may be crucial to our protocol through their effects on maintaining attention, on motivation, or on the salience of the fattening food. In addition, we tested subjects outside the immediate post-prandial period, but within their usual meal patterns. Although we do not have data on brain response according to the caloric content of the previous meal, we anticipate that results could vary in fasted or highly satiated states.28
The process of identifying and procuring food to meet our metabolic needs is central to survival.29 Our findings demonstrate that, in nonobese women, brain regions involved in energy homeostasis, reward, and cognitive processing are selectively responsive to visual food cues depicting high-calorie foods that are perceived as fattening. We also propose that study designs using comparisons of fattening foods vs. non-food objects or non-fattening foods (as opposed to foods vs. non-foods) may best exploit fMRI techniques to illuminate the central regulation of energy balance. Noninvasive experiments in humans are urgently needed to unravel the complexity of human eating behavior, investigate the hedonic aspects of food intake, and test potential interventions for obesity management. fMRI is an important tool for gaining insight into brain function,30 and attention to detail in study design will be vital to maximizing its utility.
Acknowledgments
This work was supported by NIH grant K23 DK070826-01 (PI: Schur). We also acknowledge the donation of a number of photographs used for this study by Great American Stock (Brookfield, WI). We thank our participants for their contribution and the twins in the University of Washington Twin Registry for their dedication.
References
- 1.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 2.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 3.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GJ, Volkow ND, Telang F, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21(4):1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Meltzoff AN, Decety J. Motivation modulates the activity of the human mirror-neuron system. Cereb Cortex. 2007;17(8):1979–1986. doi: 10.1093/cercor/bhl107. [DOI] [PubMed] [Google Scholar]
- 6.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. American Journal of Clinical Nutrition. 2007;86(4):965–971. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 7.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity. 2008;16(5):945–950. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 9.Holsen LM, Zarcone JR, Thompson TI, et al. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27(3):669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19(4):1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 11.Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15(10):1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 12.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 13.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 14.Baicy K, London ED, Monterosso J, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104(46):18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118(7):2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169(1):111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Afari N, Noonan C, Goldberg J, et al. University of Washington Twin Registry: construction and characteristics of a community-based twin registry. Twin Res Hum Genet. 2006;9(6):1023–1029. doi: 10.1375/183242706779462543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964(1):107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 20.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11):2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: An fMRI study. Neuroimage. 2006;32(3):1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 22.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85(1):45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Del Parigi A, Chen K, Gautier JF, et al. Sex differences in the human brain's response to hunger and satiation. Am J Clin Nutr. 2002;75(6):1017–1022. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- 24.Wang GJ, Volkow ND, Telang F, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106(4):1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DelParigi A, Chen K, Salbe AD, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31(3):440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 26.Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7(8):655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- 27.Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 28.Del Parigi A, Gautier JF, Chen K, et al. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci. 2002;967:389–397. [PubMed] [Google Scholar]
- 29.Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37(4):811–823. doi: 10.1016/j.ecl.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logothetis N. What we can and cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]