Abstract
As a major inhibitory neurotransmitter, GABA plays a vital role in the brain by controlling the extent of neuronal excitation. This widespread role is reflected by the ubiquitous distribution of GABAA receptors throughout the central nervous system. To regulate the level of neuronal inhibition requires some endogenous control over the release of GABA and/or its postsynaptic response. In this context, Ca2+ ions are often used as primary or secondary messengers frequently resulting in the activation of protein kinases and phosphatases. One such kinase, Ca2+/calmodulin-dependent protein kinase II (CaMKII), can target the GABAA receptor to cause its phosphorylation. Evidence is now emerging, which is reviewed here, that GABAA receptors are indeed substrates for CaMKII and that this covalent modification alters the expression of cell surface receptors and their function. This type of regulation can also feature at inhibitory synapses leading to long-term inhibitory synaptic plasticity. Most recently, CaMKII has now been proposed to differentially phosphorylate particular isoforms of GABAA receptors in a synapse-specific context.
Type A γ-aminobutyric acid (GABAA) receptors form part of the Cys-loop ligand-gated ion channel family and are activated by GABA, which is released from inhibitory axon terminals (Fritschy & Brunig, 2003; Jacob et al. 2008). They are responsible for controlling neuronal excitability, which they achieve by mediating two forms of inhibition, termed synaptic and tonic (Farrant & Nusser, 2005). The former relies on the rapid activation of GABAA receptors at inhibitory synapses following the phasic release of GABA, whereas tonic inhibition requires the persistent activation of extrasynaptic GABAA receptors by low ambient levels of GABA.
The coding and integration of information is a major function of the mammalian central nervous system and this relies heavily on the interplay between synaptic excitation and inhibition (Silberberg et al. 2005). Therefore, regulating the efficacy of GABA mediated inhibition, by affecting GABA current amplitude and/or its duration, will have significant consequences for the input-output relationships of neurons.
There are modulators that can change synaptic efficacy by targeting pre- and/or postsynaptic sites. For synaptic inhibition, the action potential driven release of GABA often attains quite high concentrations, sufficient to fully occupy the postsynaptic GABAA receptors (Grabauskas, 2005; Nusser et al. 2001). Thus, under normal physiological conditions, inhibitory postsynaptic current (IPSC) amplitudes may not be increased by modulators unless the number of postsynaptic receptors is also increased. Modulators that affect GABAA receptor kinetics are more likely to affect the IPSC duration. By contrast, for tonic inhibition, manipulating the current duration is less meaningful due to the persistent nature of the GABAA receptor activation; however, modulating the tonic current amplitude is feasible as the basal concentrations of GABA activating extrasynaptic receptors will be insufficient to attain full receptor occupancy (Mody, 2001; Farrant & Nusser, 2005; Mortensen & Smart, 2006).
In addition to short-term changes to synaptic inhibition, normal function in the nervous system also requires synaptic transmission to be dynamically regulated over longer periods. Such synaptic plasticity, often involving receptor trafficking, is thought to be the cellular and molecular correlate of learning and memory (Lisman et al. 2002; Neves et al. 2008) as well as being important for the appropriate establishment of synaptic connections during development (Ben Ari et al. 2007).
One of the most important groups of modulators that affect GABAA receptor mediated inhibition are the protein kinases (Moss & Smart, 1996; Brandon et al. 2002; Kittler & Moss, 2003). They catalyse phosphorylation by transferring a charged phosphate group from ATP to the substrate protein. Activating protein kinases by intracellular signalling pathways has the ability to directly affect receptor function and/or alter the trafficking of receptors. Here, we concentrate on reviewing the evidence underlying the roles that Ca2+/calmodulin-dependent protein kinase II (CaMKII) has in the modulation of GABAA receptor function.
The GABAA receptor: a substrate for CaMKII?
Calcium/calmodulin-dependent protein kinase II is a serine/threonine second messenger-dependent protein kinase that responds to increases in intracellular Ca2+ concentrations (Soderling et al. 2002; Hudmon & Schulman, 2002; Fink & Meyer, 2002). It is a multifunctional protein that phosphorylates a broad range of substrates many of them involved in synaptic transmission (Colbran, 2004; Schulman, 2004). It is activated by the binding of Ca2+/Calmodulin, which disrupts the association of an auto-inhibitory domain with the catalytic domain thereby enabling the latter to bind to substrates. The regulation of CaMKII activity is complex and is determined by a number of autophosphorylation sites, one of which (Thr286) also acts to disrupt the auto-inhibitory domain and allows Ca2+-independent kinase activity (Hudmon & Schulman, 2002).
CaMKII is well known as an important part of the postsynaptic density of excitatory synapses (Sheng & Hoogenraad, 2007). It can associate with a number of different synaptic scaffolding proteins (Colbran, 2004), which can potentially alter its activity (Strack et al. 2000; Robison et al. 2005). CaMKII is known to translocate to the postsynaptic density after its activation (Shen & Meyer, 1999; Shen et al. 2000; Merrill et al. 2005). The ability of CaMKII to regulate excitatory synaptic transmission and also to sustain a persistent level of kinase activity after undergoing autophosphorylation at Thr286 has led to its recognition as a key molecule underlying the synaptic plasticity phenomenon of long-term potentiation (LTP) and the cellular mechanisms that underlie learning and memory (Lisman et al. 2002; Merrill et al. 2005).
By comparison, the potential involvement of CaMKII in regulating inhibitory synaptic transmission has received less attention. Such an interaction was evident from early studies that introduced pre-activated CaMKII into neurons via a patch or intracellular recording pipette (Table 1). Pre-activation of the kinase with Ca2+ and calmodulin removes the normal reliance of activation on Ca2+ influx and Ca2+ stores. For both GABA-evoked whole-cell currents in isolated spinal dorsal horn neurons and GABA-mediated evoked IPSPs in CA1 hippocampal pyramidal cells, supplementing the intracellular solution with the α isoform of CaMKII led to the enhancement of current amplitudes (Wang et al. 1995). A similar application of pre-activated CaMKII to cerebellar Purkinje cells was also found to enhance GABA-induced whole-cell currents and sIPSCs (Kano et al. 1996). In both the hippocampal and cerebellar studies, heat-inactivated CaMKII was ineffective. Whilst demonstrating that the modulation of GABAA receptors by CaMKII was clearly possible, these early studies did not address whether CaMKII was directly phosphorylating the receptor, or if its effect was mediated by intermediary proteins. It was therefore quite important to know whether the GABAA receptor was a substrate for CaMKII and this could only be established using biochemical methods.
Table 1.
Effect of CaMKII on native and recombinant GABAA receptors
| Cells | Receptors | CaMKII prep | Phosphatase inhibitors | Kinase inhibitors | Plasticity | Other effects | Ref |
|---|---|---|---|---|---|---|---|
| Cerebellar Purkinje neurons | Native | Native, depol. stim. | Calyculin-A (PP1/PP2A) | Staurosporine, KN-62, calmodulin binding domain peptide (CBD), calmidazolium, heat inactivation | sIPSCs, IGABA | Amplitude enhanced | Kano et al. 1996 |
| Preactivated CaMKI | IGABA | Amplitude enhanced | |||||
| Dorsal horn spinal neurones | Native | Preactivated CaMKII | Calyculin-A (PP1, PP2A) | Heat inactivation | IGABA | Amplitude increased, Desensitisation reduced | Wang et al. 1995 |
| Hippocampal CA1 | eIPSPs | Enhanced amplitude | |||||
| HEK cells | Recombinant α1β2, α1β2γ2 | Preactivated CaMKII | — | — | IGABA | No modulation | Houston and Smart 2006 |
| α1β3, α1β3γ2 | IGABA | No modulation | |||||
| NG108-15 cells | Recombinant α1β2, α1β2γ2 | Preactivated CaMKII | — | — | IGABA | No modulation | Houston and Smart 2006 |
| α1β3, α1β3γ2 | IGABA | Enhanced | |||||
| Cerebellar granule neurons | Recombinant α1β2, α1β2γ2 | Preactivated CaMKII | — | — | IGABA | No modulation | Houston and Smart 2006 |
| α1β3, α1β3γ2 | IGABA | Enhanced | |||||
| NG108-15 cells | Recombinant α1β3S383A | Preactivated CaMKII | — | — | IGABA | No modulation | Houston et al. 2007 |
| α1β3S383Aγ2 | IGABA | Reduced modulation | |||||
| α1β3S383A+γ2Y365F, Y367F | IGABA | No modulation | |||||
| Cerebellar granule neurons | Native | Preactivated CaMKII | — | — | IGABA | Enhanced | Houston and Smart 2006 |
| Cerebellar granule neurons | Native β2−/− | Preactivated CaMKII | — | — | IGABA | Enhanced | Houston and Smart 2006 |
| Cerebellar granule neurons | Native | Preactivated CaMKII | — | — | sIPSCs | Amplitude enhanced, Duration prolonged | Houston et al. 2008 |
| Cerebellar granule neurons | Native β2−/− | Preactivated CaMKII | — | — | sIPSCs | Only duration prolonged | Houston et al. 2008 |
| Hippocampal CA1 | Native | Native +Ca2+/calmodulin | CaMKII autoinhib. pep. (281–301), BAPTA, Vincristine, Cytochalasin D, Colchicine | eIPSCs sIPSCs | Amplitude enhanced, Amplitude & Frequency enhanced | Wei et al. 2004 |
Summary of the effects of CaMKII on native neuronal GABAA receptors (wild-type and a β2 knock-out), and on recombinant GABAA receptors. The CaMKII preparations used are either: native, which is then activated by depolarising stimulation (depol. stim.) together in one study, with the intracellular addition of Ca2+ and calmodulin, or it is a preactivated form of CaMKII that is perfused internally into the cells. The effect of CaMKII was studied on whole-cell GABA-activated currents (IGABA) or spontaneous (s) or evoked (e) IPSCs, with one study using IPSPs.
Biochemical studies of CaMKII phosphorylation of GABAA receptors
To ascertain whether protein kinases are directly affecting the function and/or trafficking of neurotransmitter receptors requires an unequivocal demonstration that the receptors are themselves phosphorylated by the kinases. Studies of many other proteins have enabled a consensus site for CaMKII to be proposed as: R-X-X-S/T, where the phosphorylated serine or threonine residues are N-terminally flanked by two ‘recognition neutral’ residues (X) that could be any amino acid, and preceded by an arginine (R), with its positively charged side-chain (Kennelly & Krebs, 1991; Pearson & Kemp, 1991; Moss & Smart, 1996). For GABAA receptors, such consensus sites have been identified within the large intracellular domain residing between transmembrane (M) domains 3 and 4 of receptor β and γ subunits. To determine whether these putative consensus sites were actually phosphorylated by CaMKII, purified glutathione-S-transferase (GST) fusion proteins of the intracellular domains for GABA receptor β and γ2 subunits were generated (McDonald & Moss, 1994; Brandon et al. 2002). These GST fusion proteins were exposed to CaMKII in the presence of 32P-orthophosphoric acid and then subject to two-dimensional phosphopeptide mapping and finally phosphoamino acid analysis (Moss et al. 1992). This revealed sites of phosphorylation for CaMKII on the β1 fusion protein at Ser409 (RRRASQLK) and Ser384 (RKPLSSRE) (McDonald & Moss, 1994). The latter site is interesting because it departs from the typical consensus site for CaMKII and is also a unique site of phosphorylation on GABAA receptors that is reserved for CaMKII. By contrast, Ser409 is an established site of phosphorylation for other serine/threonine kinases, such as cAMP-dependent protein kinase (PKA), cGMP-dependent protein kinase (PKG) and Ca2+-dependent protein kinase (PKC) (Moss & Smart, 1996; Brandon et al. 2002). Similar analyses, using fusion proteins based on the large intracellular domains of β2 and β3 subunits, identified only Ser410 (RRRASQLK) as the major site of CaMKII phosphorylation in β2 subunits, whilst Ser383 (RKQSMPK) and Ser409 (RRRSSQLK) were substrates for CaMKII in β3 subunits (McDonald & Moss, 1997). As for S409 in β1, the homologous Ser410 in β2 and Ser409 in β3 are also substrates for other serine/threonine kinases (Moss & Smart, 1996; Brandon et al. 2000), but Ser383 in β3 subunits, just like Ser384 in β1, is a unique site for CaMKII (Table 2).
Table 2.
Sites of phosphorylation for CaMKII on GABAA receptor β and γ2 subunits
| Receptor subunit | CaMKII substrate residues | Reference | ||
|---|---|---|---|---|
| α1 | n/i | — | — | Churn et al. 2002 |
| β1 | S384 | S409 | — | McDonald & Moss, 1994 |
| β2 | — | S410 | — | |
| β3 | S383 | S409 | — | |
| γ2S | — | S348 | T350 | Houston et al. 2007 |
| γ2L | S343 | S348 | T350 | |
The sites of CaMKII phosphorylation on GABAA receptor subunits are shown. The residues (S-serine; T-threonine) are located between the 3rd and 4th transmembrane domains of β and γ2 subunits and have been identified using biochemical analyses of GSK fusion proteins. n/i- residue(s) not identified.
Biochemical analysis of phosphorylation also revealed that a γ2 fusion protein was phosphorylated by CaMKII at Ser348 (IRPRSATIQ) and Thr350 (PRSATIQMN) (McDonald & Moss, 1994). The γ2 subunit can be expressed in two forms (short (S) or long (L)) following alternative RNA splicing (Whiting et al. 1990). The extra insert of eight amino acids (LLRMFSFK) in γ2L contains another consensus sequence for phosphorylation (Moss & Smart, 1996). Indeed, Ser343 proved to be phosphorylated by CaMKII in the γ2L form (McDonald & Moss, 1994; Machu et al. 1993) and it is also a substrate for PKC (Krishek et al. 1994).
Although it is considered that GABAA receptor β and γ subunits are the major substrates for protein kinase phosphorylation, there is evidence that other subunits may also be phosphorylated. Activation of endogenous CaMKII in a synaptosomal membrane preparation has been shown to increase agonist binding by increasing the number of GABA receptors (Churn & DeLorenzo, 1998). Notably, this group reported that the α1 subunit from rat forebrain can be phosphorylated by CaMKII, but the residue(s) that form the substrate site have yet to be identified (Churn et al. 2002). Thus, the major outcome from the biochemical studies is a clear demonstration that the GABAA receptor is a substrate for CaMKII, and that phosphorylation occurs principally on β and γ2 subunits, and most likely also on the α1 subunit. The identification of these phosphorylated residues posed the inevitable question – what are the effect(s) of CaMKII phosphorylation on GABAA receptor function and trafficking?
CaMKII regulation of GABAA receptor function
The effects of covalent modification of GABAA receptor structure by protein kinase phosphorylation can range from altered receptor function to the regulation of receptor trafficking (Moss & Smart, 1996; Smart, 1997; Brandon et al. 2002; Kittler & Moss, 2003). To understand how phosphorylation affects GABAA receptors and to determine the importance of the receptor's substrate sites usually requires the use of recombinant receptors expressed in heterologous cell lines. The usual cellular vehicle for such studies is the human embryonic kidney (HEK) cell line, but for CaMKII this proved a poor choice as α1β2 and α1β3 GABAA receptors were unaffected by CaMKII (Houston & Smart, 2006) (Table 1). By contrast, the undifferentiated NG108-15 neuroblastoma cell line, which also lacks endogenous GABAA receptors (Searles & Singer, 1988; Ishizawa et al. 1997; Houston & Smart, 2006), but retains a neuronal lineage unlike HEK cells, supported CaMKII modulation of recombinant GABAA receptors. In these cells, CaMKII potentiated GABA-activated currents for α1β3 and α1β3γ2S receptors, but not for α1β2 and α1β2γ2S GABAA receptors (Houston & Smart, 2006). The reasons why CaMKII was only able to modulate GABAA receptors in NG108-15 cells, is unknown, but it could reflect a limited availability of anchoring proteins for CaMKII to bind to GABAA receptors in HEK cells. To date, little is known about the anchoring of CaMKII to GABAA receptors.
Interestingly, demonstrating that PKA could modulate α1β2γ2 receptors in HEK cells also proved difficult (McDonald et al. 1998) despite PKA modulating IPSCs in neurons that express high levels of the β2 subunit (Poisbeau et al. 1999; Nusser et al. 1999). In comparison, phosphorylation of β2 subunits at Ser410, the major substrate in this subunit for all Ser/Thr kinases, has been shown to mediate an increase in cell surface receptor number when phosphorylated by PKB in HEK cells and neurons (Wang et al. 2003).
A differential regulation of GABAA receptors by CaMKII was also evident after over-expressing α1/β3 or α1/β2 subunits in cultured cerebellar granule cells (Houston & Smart, 2006). The potentiation of GABA currents by CaMKII, apparent with β3 over-expressing neurons, was significantly reduced when there was a surplus of β2 subunit-containing receptors. Although β2 subunits are known to be phosphorylated by CaMKII, the lack of any functional effect suggested that β2 subunit phosphorylation may have a distinct role. Indeed, the potentiation of GABA currents by CaMKII is unaffected when recording from neurons taken from β2−/− mice (Houston & Smart, 2006). Thus, in terms of functional regulation, the major GABAA receptor target for CaMKII appears to be the β3 subunit, as β1 subunits are poorly expressed in these neurons (Laurie et al. 1992; Pirker et al. 2000).
Sites of action for CaMKII on GABAA receptors
Identifying the sites for CaMKII phosphorylation on GABAA receptors does not necessarily indicate that phosphorylation at each of these sites is able to modulate GABA-activated currents. By expressing α1β3 receptors in NG108-15 cells, and using site-directed mutagenesis of GABAA receptor β3 subunits, Ser383 was identified as the major site for CaMKII modulation of GABA currents, as phosphorylation of Ser409 had no functional consequences (Houston et al. 2007). This might explain the lack of effect of CaMKII in modulating GABAA receptors containing the β2 subunit, since the homologous residue to Ser383 in the β3 subunit is not phosphorylated by CaMKII.
Mutating both the CaMKII substrates in the γ2S subunit, Ser348 and Thr350, did not affect the modulation of the α1β3γ2S receptor. However, co-expressing the mutant β3 subunit (β3S383A), which abolished potentiation by CaMKII on α1β3 receptors, with α1 and γ2SS348A,T350A, reduced, but did not ablate the functional effects of CaMKII (Houston et al. 2007). This strongly suggested that other residues must be involved, yet the biochemical analyses had not identified any further CaMKII substrates. This implied that another kinase may be involved. The prime candidate was a tyrosine kinase as this had previously been shown to potentiate GABA currents at αβγ subunit-containing receptors (Moss et al. 1995; Valenzuela et al. 1995). Indeed, genistein, a tyrosine kinase inhibitor, reduced CaMKII potentiation of GABA currents at α1β3γ2S receptors and abolished it at α1β3S383Aγ2S. Mutating the sites for tyrosine phosphorylation on the γ2S subunits, Y365 and Y367, together with the CaMKII site on the β3 subunit, S383, was entirely sufficient to ablate the effects of CaMKII on GABA-activated currents (Houston et al. 2007). These results clearly implicated the tyrosine residues in the regulation of GABAA receptor function after CaMKII activation. This was emphasised by biochemical analyses demonstrating increased phosphorylation of tyrosine residues 365 and 367 on the γ2 subunit after CaMKII was activated. Thus tyrosine kinases and CaMKII may interact to modulate GABAA receptors, with CaMKII possibly activating a tyrosine kinase (Zwick et al. 1999; Guo et al. 2004; Fan et al. 2005).
Regulation of inhibitory synaptic receptors by CaMKII
Although preceding studies revealed that CaMKII phosphorylation depended upon the GABAA receptor subunit composition, and also identified which sites on the receptors were actually phosphorylated by CaMKII, we had little idea as to whether the location of the receptor in the cell membrane (e.g. synaptic or extrasynaptic) was important. This was most simply studied by monitoring GABA-mediated IPSCs in the presence of CaMKII. Any modulation would immediately implicate synaptic receptors as targets for this Ca2+-activated kinase. In cerebellar granule neurons, CaMKII increased the amplitude and prolonged the duration of sIPSCs (Houston et al. 2008). By using a β2 knock-out line, these effects were attributed to specific receptor isoforms with an increased IPSC amplitude associated with β2 subunit receptors and the prolongation of the sIPSC duration reflecting phosphorylation of β3 subunit-containing GABAA receptors (Houston et al. 2008). Immunofluorescence of β2 and β3 subunits in cerebellar granule neurons suggested that some inhibitory synapses are likely to contain GABAA receptors with only one or other of the β subunits. This differential localisation of β subunits is also apparent in dentate gyrus granule cells where β2 subunits are mostly extrasynaptic and β3 subunits primarily synaptic (Herd et al. 2008). Thus CaMKII modulation of GABAA receptors is not only subtype selective but also appears to be inhibitory synapse specific.
The surprising result from this study was that β2 subunit-containing receptors were modulated by CaMKII. This was unexpected considering that β2 subunit receptors expressed in NG108-15 cells remained unaffected by CaMKII. This observation implies that the cytoskeleton, scaffolding proteins and signalling pathways present at inhibitory synapses may be important for CaMKII to modulate GABAA receptors. It further suggests that β2 subunit-containing receptors expressed at extrasynaptic locations are not modulated by CaMKII, probably due to a lack of kinase anchoring proteins.
Using non-stationary noise analysis of the sIPSC decays suggested that CaMKII enhanced IPSC amplitudes by increasing the number of open β2 subunit-containing channels at the peak of the IPSC. This could have occurred by simply increasing receptor number in the synapse, and/or by increasing the channel open probability. By contrast, for β3 subunit-containing receptors, the change in sIPSC decay rate was a consequence of altered channel kinetics following phosphorylation of the receptor (Houston et al. 2008). Recruitment of GABAA receptors to the cell surface membrane has been suggested in the hippocampus, where IPSC amplitudes were enhanced by promoting internal Ca2+ release, or by internally perfusing neurons with Ca2+/calmodulin (Wei et al. 2004). The effectiveness of CaMKII inhibitor peptide (281–301) and the blockers of tubulin and actin polymerization in preventing the potentiation of IPSCs implicated CaMKII in the trafficking of synaptic GABAA receptors.
Ca2+/calmodulin dependent regulation of GABAA receptors
In addition to CaMKII regulating GABAA receptors, several studies have also noted that these receptors can be modulated by just changing intracellular Ca2+ and calmodulin concentrations (Inoue et al. 1986; Taleb et al. 1990; Chen et al. 1990; Mouginot et al. 1991; Stelzer & Shi, 1994; Chen & Wong, 1995; Aguayo et al. 1998; Akopian et al. 1998; Lu et al. 2000; Yu et al. 2006), possibly by altering the phosphorylation status of the GABAA receptor. It is therefore not surprising that many effects have been reported, presumably depending on whether the elevation of internal Ca2+ concentration resulted in kinase or phosphatase activation, or simply from a direct action on the GABAA receptor. Changes to GABA current amplitudes can occur by affecting the entry/exit rates of the receptor into/out of various conformations, such as desensitised states, e.g. high internal Ca2+ reduced whole-cell GABA current amplitudes and increased their desensitisation in hippocampal neurons (Mozrzymas & Cherubini, 1998). The potential for Ca2+ and calmodulin to act independently of CaMKII can cause difficulties when interpreting the effect of this kinase on GABAA receptors. Such difficulties can be obviated by pre-activating CaMKII prior to its use. Thus any regulation of synaptic GABAA receptors is likely to be a function of CaMKII activity, rather than being caused by Ca2+ or calmodulin. Furthermore, a direct effect of CaMKII on the phosphorylation status and function of GABAA receptors can also be inferred by establishing the effectiveness of mutating substrate sites on the receptor in ablating the actions of the kinase (Houston et al. 2007).
CaMKII and inhibitory synaptic plasticity
Changes to inhibitory transmission that last longer than just a few minutes can be indicative of underlying structural changes at synapses. These could be manifest in the formation of new synapses or long-term changes in the number of receptors expressed at existing synapses; both could also be coupled with chronic alterations to receptor function. All of these changes typically characterise synaptic plasticity (Bliss et al. 2003). For inhibitory synapses, there is now increasing evidence of numerous forms of synaptic plasticity (Gaiarsa et al. 2002), but one of the earliest recorded examples that involves CaMKII concerns the phenomenon of rebound potentiation, which is expressed at the interneuron–Purkinje cell synapse in the cerebellum and involves an increase in the mean IPSC amplitude (Kano et al. 1992).
Rebound potentiation can be induced by stimulating the climbing fibre (CF) input (Kano et al. 1992) or by directly depolarizing the Purkinje neuron (Hashimoto et al. 1996) to cause internal Ca2+ to rise. Notably, whole-cell GABA-activated currents are also enhanced by CF stimulation. The increase in the IPSC amplitude can be blocked by inhibiting the internal Ca2+ rise or by inhibiting CaMKII (Kano et al. 1996; Hashimoto et al. 1996; Kawaguchi & Hirano, 2002). A similar enhancement was observed after just internally perfusing unstimulated Purkinje neurons with CaMKII, and calyculin-A, a protein phosphatase 1 and 2A inhibitor (Kano et al. 1996). A variety of inhibitors ranging from KN-62 (CaMKII inhibitor) and staurosproine (non-specific kinase inhibitor) to calmidazolium (calmodulin antagonist) and calmodulin binding domain peptide (dominant-negative peptide), all resulted in a reduced rebound potentiation providing compelling evidence of a role for CaMKII in this form of synaptic plasticity. Delaying the application of KN-62 or calmidazolium until 5 min after the CF stimulation was sufficient to neutralise their inhibitory activity (Kano et al. 1996). This suggested that activation of CaMKII was necessary to induce or trigger rebound potentiation, but that its continued activation was unnecessary for its maintenance. However, KN-62 does not block CaMKII autophosphorylation, which may be critical for the maintenance phase of rebound potentiation. In comparison, the regulation of IPSCs by CaMKII recorded in cerebellar granule cells required the binding of both Ca2+ and calmodulin to the kinase, but did not require the enzyme to be autophosphorylated (Houston et al. 2008).
Rebound potentiation has recently been shown to be dependent on GABAA receptor binding to GABAA receptor-associated protein (GABARAP) (Kawaguchi & Hirano, 2007). Tyrosine kinases have also been implicated in the induction of this plasticity (Boxall, 2000; Kawaguchi & Hirano, 2006). As an interaction between CaMKII and tyrosine kinase signalling pathways leading to phosphorylation of GABAA receptors in NG108-15 cells has been demonstrated (Houston et al. 2007), it seems plausible that these two kinases also interact to regulate the induction of rebound potentiation. Interestingly, the predominant β subunit in Purkinje neurons is the β2 subunit (Laurie et al. 1992; Pirker et al. 2000), which is in accord with the β2-dependent CaMKII mediated increase in IPSC amplitudes observed in cerebellar granule cells (Houston et al. 2008).
There is growing evidence that inhibitory synaptic plasticity may be important in many different physiological conditions. In hippocampal neurons, it has been reported that NMDA receptor activation, which drives excitatory LTP, simultaneously drives inhibitory potentiation in a form of homeostatic plasticity. This process was dependent on CaMKII, and the trafficking proteins GABARAP and NSF, causing an increase in the number of functional receptors at the cell surface (Marsden et al. 2007). Furthermore, a Ca2+-dependent plasticity mechanism involving alterations to GABAA receptor trafficking is involved in the transition from awake to sleep states in cortical pyramidal neurons (Kurotani et al. 2008). It has also been demonstrated that frequency-dependent plasticity of developing inhibitory synapses is dependent on GABAB receptor activation, which stimulates postsynaptic CaMKII activation (Xu et al. 2008). Thus, taken overall, CaMKII not only produces relatively acute changes to inhibitory synaptic transmission, but can also underwrite longer-term inhibitory synaptic plasticity.
Discussion and conclusion
CaMKII is considered to be an important kinase that is associated with the postsynaptic density of excitatory synapses, as well as being a vital component of LTP. The realisation that CaMKII can also modulate the efficacy of synaptic inhibition increases its significance, not least because it can act as a point of convergence for numerous signalling pathways that involve changes to intracellular Ca2+. Thus, CaMKII can affect the function of synaptic GABAA receptors and may play a key role in homeostatic forms of inhibitory synaptic plasticity.
Many studies have investigated the effects of internal Ca2+ on GABAA receptor function yielding a spectrum of results ranging from potentiation to inhibition of GABA-activated currents. This variation could well be related to the delicate balance that is struck between the activities of kinases and phosphatases in neurons. Although many studies did not investigate whether or not GABAA receptors are directly phosphorylated, it is conceivable that CaMKII and calcineurin are prominent key proteins that affect GABAA receptor function when internal Ca2+ levels are perturbed.
When CaMKII has been preactivated and perfused into neurons, both the subunit composition and the location of the GABAA receptors appear to be important in determining the functional outcome (Fig. 1). For example, it seemed quite clear that β2 subunit-containing recombinant receptors were unaffected by CaMKII, in contrast to β3 subunit receptors, where GABA currents were potentiated. This distinction was apparent even when recombinant receptors were over-expressed in neurons. Nevertheless, by focusing on synaptic GABAA receptors the regulation by CaMKII revealed some interesting differences. Firstly, IPSCs mediated by synaptic receptors that contained β2 subunits exhibited large amplitude enhancements, whilst IPSCs mediated by β3 subunits showed only prolonged durations. It is therefore important to note the cellular context in which the role of CaMKII is studied. The inability of β2 subunit containing receptors to be modulated by CaMKII in heterologous expression systems, or in extrasynaptic domains when over-expressed in neurons, suggested that CaMKII can only phosphorylate these receptors at inhibitory synapses. This might indicate that CaMKII can only bind to β2 subunit-containing GABAA receptors at inhibitory synapses, possibly because of the density of anchoring proteins. Although CaMKII can phosphorylate GABAA receptors, it is also likely that there may be other targets in the postsynaptic density such as anchoring proteins, e.g. gephyrin. How these proteins will be affected by phosphorylation and what the consequences will be for inhibitory synaptic transmission is at present unknown.
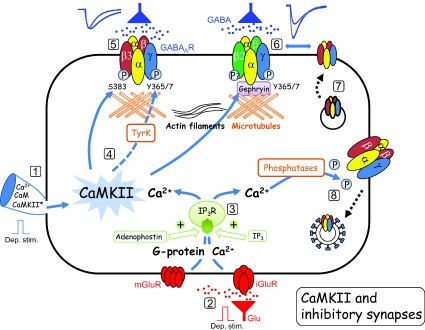
Figure 1. Modulation of GABAA receptors by CaMKII: a working model.
This schematic diagram is based on a number of studies (see main text) that have investigated the actions of CaMKII at inhibitory GABAergic synapses and at recombinant GABAA receptors in heterologous expression systems. It shows a hypothetical model that integrates the main observations to explain how CaMKII may regulate GABAA receptors in neurons. Specifically, direct stimulation of the neuron via voltage steps delivered by the patch electrode (1) or by stimulating a glutamatergic afferent input (red, 2), can cause an increase in internal Ca2+, either directly via ionotropic glutamate receptors (iGluR), or by metabotropic glutamate receptors (mGluR) resulting in the release of Ca2+ from internal stores (3). This release can also be achieved by using IP3 receptor agonists, adenophostin or IP3 (3). The rise in internal Ca2+, with calmodulin, activates CaMKII (4). CaMKII activation can also be achieved by internal delivery of Ca2+ and calmodulin via the patch pipette (1) or native CaMKII activation can simply be by-passed by internally perfusing with pre-activated CaMKII (CaMKII*) (1). The activation of CaMKII has two major effects on synaptic GABAA receptors (blue, 5, 6). It is proposed that by phosphorylating the receptor β and γ subunits at specific residues that also involves a tyrosine kinase (TryK; dashed arrow as mechanism is unclear), GABAergic IPSCs are either simply prolonged in duration at β3 subunit-containing receptors (5) or increased in amplitude with unchanged kinetics at β2 subunit-containing receptors (6). The simplest explanation for the increased amplitude of IPSCs at the latter synapses is an increase in trafficking of receptors from secretory vesicles to the cell surface involving both actin filament and microtubule polymerization (7). Several studies also report that a rise in internal Ca2+ can cause a reduction in GABA synaptic and whole-cell currents. This may conceivably occur by the activation of Ca2+ dependent phosphatases (calcineurin) perhaps causing clarithin-dependent endocytosis of GABAA receptors (8) and/or by direct effects on receptor kinetics.
A notable feature of CaMKII regulation of synaptic GABAA receptor function is that the mechanism by which it potentiates IPSCs varies depending on the inhibitory synapse and the isoforms of GABAA receptor β subunits expressed therein (Fig. 1). At β2 subunit-containing synapses, CaMKII increased IPSC amplitudes, which, at synapses where GABA reaches saturating concentrations, is more likely to be mediated through changes to cell surface receptor numbers, whilst at β3 subunit-containing synapses, the regulation of IPSCs is mediated by a change to their decay times, probably by receptor phosphorylation changing the kinetic properties of the GABA channels.
Potentially, a change in IPSC decay times at a specific set of inhibitory synapses, compared to a change of amplitude at other synapses, could have diverse consequences for integration of excitatory inputs and thus the overall effect on neuronal excitability. Across the whole brain it is increasingly evident that different GABAA receptor subtypes have specific physiological roles. Indeed, in keeping with their localisation at specific subsets of synapses, β subunits are thought to play different physiological roles in mice in mediating the sedative and anaesthetic actions of the anaesthetic etomidate (Reynolds et al. 2003).
The role of CaMKII in regulating synaptic inhibition by modulating IPSCs in a synapse-specific manner is likely to be important in mature synapses by determining the extent of inhibitory synaptic plasticity, such as rebound potentiation (Kano et al. 1996; Kawaguchi & Hirano, 2002). CaMKII regulation of synaptic GABAA receptors could also play a significant role during activity-dependent formation of inhibitory synapses, which occurs throughout development (Ben Ari et al. 2007). The significance of a signalling mechanism is often best appreciated when it suffers dysfunction. There are several examples where CaMKII signalling is implicated in neurological disorders that affect inhibitory transmission. Notably, the neurodevelopmental disorder, Angelman's syndrome, is associated with low expression of GABAA receptor β3 subunits and it is also linked to disrupted CaMKII signalling (DeLorey et al. 1998; Weeber et al. 2003). Also of significance is the link between dysfunctional CaMKII signalling and epilepsy, which may be mediated by an interaction with GABAA receptors (Churn et al. 2000; Singleton et al. 2005).
The implications of CaMKII differentially regulating inhibitory transmission by phosphorylation of GABAA receptor subtypes are potentially of some significance, particularly given that these receptors are targeted to specific locations within neurons, where they can undertake different functional roles (Nyíri et al. 2001; Brünig et al. 2002; Reynolds et al. 2003; Herd et al. 2008). Finally, CaMKII is notable in having the capacity to act as a point of signalling convergence for homeostatic synaptic plasticity whereby excitatory transmission, which involves Ca2+ influx via either AMPA or NMDA receptors, can enable glutamate to exert some control over the postsynaptic sensitivity of neurons to GABA and thus the efficacy of synaptic inhibition.
Acknowledgments
This work was supported by the MRC and BBSRC. Q.H. was supported by an MRC PhD studentship.
References
- Aguayo LG, Espinoza F, Kunos G, Satin LS. Effects of intracellular calcium on GABAA receptors in mouse cortical neurons. Pflugers Arch. 1998;435:382–387. doi: 10.1007/s004240050527. [DOI] [PubMed] [Google Scholar]
- Akopian A, Gabriel R, Witkovsky P. Calcium released from intracellular stores inhibits GABAA-mediated currents in ganglion cells of the turtle retina. J Neurophysiol. 1998;80:1105–1115. doi: 10.1152/jn.1998.80.3.1105. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Morris RG. Introduction. Long-term potentiation and structure of the issue. Philos Trans R Soc Lond B Biol Sci. 2003;358:607–611. doi: 10.1098/rstb.2003.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall AR. GABAergic mIPSCs in rat cerebellar purkinje cells are modulated by TrkB and mGluR1-mediated stimulation of Src. J Physiol. 2000;524:677–684. doi: 10.1111/j.1469-7793.2000.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of γ-aminobutyric acidA receptor function and cell surface expression. Pharmacol Ther. 2002;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brünig I, Scotti E, Sidler C, Fritschy J-M. Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Chen QX, Stelzer A, Kay AR, Wong RK. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QX, Wong RK. Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J Physiol. 1995;482:353–362. doi: 10.1113/jphysiol.1995.sp020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, Sombati S, Jakoi ER, Severt L, DeLorenzo RJ. Inhibition of calcium/calmodulin kinase II α subunit expression results in epileptiform activity in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2000;97:5604–5609. doi: 10.1073/pnas.080071697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, DeLorenzo RJ. Modulation of GABAergic receptor binding by activation of calcium and calmodulin-dependent kinase II membrane phosphorylation. Brain Res. 1998;809:68–76. doi: 10.1016/s0006-8993(98)00834-8. [DOI] [PubMed] [Google Scholar]
- Churn SB, Rana A, Lee K, Parsons JT, De Blas A, DeLorenzo RJ. Calcium/calmodulin-dependent kinase II phosphorylation of the GABAA receptor α1 subunit modulates benzodiazepine binding. J Neurochem. 2002;82:1065–1076. doi: 10.1046/j.1471-4159.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the β3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioural characteristics of angelman syndrome. J Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan RS, Jacamo RO, Jiang X, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor activation rapidly stimulates focal adhesion kinase phosphorylation at Ser-843. Mediation by Ca2+, calmodulin, and Ca2+/calmodulin-dependent kinase II. J Biol Chem. 2005;280:24212–24220. doi: 10.1074/jbc.M500716200. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fink CC, Meyer T. Molecular mechanisms of CaMK-II activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12:293–299. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Caillard O, Ben Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- Grabauskas G. Time course of GABA in the synaptic clefts of inhibitory synapses in the rostral nucleus of the solitary tract. Neurosci Lett. 2005;373:10–15. doi: 10.1016/j.neulet.2004.09.051. [DOI] [PubMed] [Google Scholar]
- Guo J, Meng F, Fu X, Song B, Yan X, Zhang G. N-methyl-D-aspartate receptor and L-type voltage-gated Ca2+ channel activation mediate proline-rich tyrosine kinase 2 phosphorylation during cerebral ischemia in rats. Neurosci Lett. 2004;355:177–180. doi: 10.1016/j.neulet.2003.10.076. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Ishii T, Ohmori H. Release of Ca2+ is the crucial step for the potentiation of IPSCs in the cultured cerebellar Purjinke cells of the rat. J Physiol. 1996;497:611–627. doi: 10.1113/jphysiol.1996.sp021794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAAβ subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, Smart TG. CaMK-II modulation of GABAA receptors expressed in HEK293, NG108-15 and rat cerebellar granule neurons. Eur J Neurosci. 2006;24:2504–2514. doi: 10.1111/j.1460-9568.2006.05145.x. [DOI] [PubMed] [Google Scholar]
- Houston CM, Hosie AM, Smart TG. Distinct regulation of β2 and β3 subunit-containing cerebellar synaptic GABAA receptors by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2008;28:7574–7584. doi: 10.1523/JNEUROSCI.5531-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, Lee HHC, Hosie AM, Moss SJ, Smart TG. Identification of the sites for CaMK-II-dependent phosphorylation of GABAA receptors. J Biol Chem. 2007;282:17855–17865. doi: 10.1074/jbc.M611533200. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal CaMK-II:The role of structure and autoregulatioon in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Inoue M, Oomura Y, Yakushiji T, Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986;324:156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Ishizawa Y, Furuya K, Yamagishi S, Dohi S. Non-GABAergic effects of midazolam, diazepam and flumazenil on voltage-dependent ion currents in NG108-15 cells. Neuroreport. 1997;8:2635–2638. doi: 10.1097/00001756-199707280-00042. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Kano M, Fukunaga K, Konnerth A. Ca2+-induced rebound potentiation of γ-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc Natl Acad Sci U S A. 1996;93:13351–13356. doi: 10.1073/pnas.93.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi S-Y, Hirano T. Signaling cascade regulating long-term potentiation of GABAA receptor responsiveness in cerebellar Purkinje neurons. J Neurosci. 2002;22:3969–3976. doi: 10.1523/JNEUROSCI.22-10-03969.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. Integrin α3β1 suppresses long-term potentiation at inhibitory synapses on the cerebellar Purkinje neuron. Mol Cell Neurosci. 2006;31:416–426. doi: 10.1016/j.mcn.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. Sustained structural change of GABAA receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J Neurosci. 2007;27:6788–6799. doi: 10.1523/JNEUROSCI.1981-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Kurotani T, Yamada K, Yoshimura Y, Crair MC, Komatsu Y. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron. 2008;57:905–916. doi: 10.1016/j.neuron.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Machu TK, Firestone JA, Browning MD. Ca2+/calmodulin-dependent protein kinase II and protein kinase C phosphorylate a synthetic peptide corresponding to a sequence that is specific for the γ2L subunit of the GABAA receptor. J Neurochem. 1993;61:375–377. doi: 10.1111/j.1471-4159.1993.tb03582.x. [DOI] [PubMed] [Google Scholar]
- Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABAA receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Differential phosphorylation of intracellular domains of γ-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J Biol Chem. 1994;269:18111–18117. [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Conserved phosphorylation of the intracellular domains of GABAA receptor β2 and β3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology. 1997;36:1377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Gorrie GH, Amato A, Smart TG. Modulation of GABAA receptors by tyrosine phosphorylation. Nature. 1995;377:344–348. doi: 10.1038/377344a0. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Modulation of amino acid-gated ion channels by protein phosphorylation. Int Rev Neurobiol. 1996;39:1–52. doi: 10.1016/s0074-7742(08)60662-5. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science. 1992;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- Mouginot D, Feltz P, Schlichter R. modulation of GABA-gated chloride currents by intracellular Ca2+ in cultured porcine melanotrophs. J Physiol. 1991;437:109–132. doi: 10.1113/jphysiol.1991.sp018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozrzymas JW, Cherubini E. Changes in intracellular calcium concentration affect desensitization of GABAA receptors in acutely dissociated P2-P6 rat hippocampal neurons. J Neurophysiol. 1998;79:1321–1328. doi: 10.1152/jn.1998.79.3.1321. [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TVP. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Naylor D, Mody I. Synapse-specific contribution of the variation of transmitter concentration to the decay of inhibitory postsynaptic currents. Biophys J. 2001;80:1251–1261. doi: 10.1016/S0006-3495(01)76101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Mody I. Differential regulation of synaptic GABAA receptors by cAMP-dependent protein kinase in mouse cerebellar and olfactory bulb neurones. J Physiol. 1999;521:421–435. doi: 10.1111/j.1469-7793.1999.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyíri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of α2-subunit-containing GABAA receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J Neurosci. 2003;23:8608–8617. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Bass MA, Jiao Y, MacMillan LB, Carmody LC, Bartlett RK, Colbran RJ. Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and α-actinin-2. J Biol Chem. 2005;280:35329–35336. doi: 10.1074/jbc.M502191200. [DOI] [PubMed] [Google Scholar]
- Schulman H. Activity-dependent regulation of calcium/calmodulin dependent protein kinase II localization. J Neurosci. 2004;24:8399–8403. doi: 10.1523/JNEUROSCI.3606-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles CD, Singer HS. The identification and characterization of a GABAergic system in the cholinergic neuroblastoma x glioma hybrid clone NG108-15. Brain Res. 1988;448:373–376. doi: 10.1016/0006-8993(88)91280-2. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Grillner S, LeBeau FEN, Maex R, Markram H. Synaptic pathways in neural microcircuits. Trends Neurosci. 2005;28:541–551. doi: 10.1016/j.tins.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Singleton MW, Holbert WH, II, Ryan ML, Lee AT, Kurz JE, Churn SB. Age dependence of pilocarpine-induced status epilepticus and inhibition of CaM Kinase II activity in the rat. Dev Brain Res. 2005;156:67–77. doi: 10.1016/j.devbrainres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Smart TG. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr Opin Neurobiol. 1997;7:358–367. doi: 10.1016/s0959-4388(97)80063-3. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Chang B, Brickey D. Cellular signaling through multifunctional Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2002;276:3719–3722. doi: 10.1074/jbc.R000013200. [DOI] [PubMed] [Google Scholar]
- Stelzer A, Shi H. Impairment of GABAA receptor function by N-methyl-D-aspartate-mediated calcium influx in isolated CA1 pyramidal cells. Neuroscience. 1994;62:813–828. doi: 10.1016/0306-4522(94)90479-0. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- Taleb O, Feltz P, Bossu J-L, Feltz A. In: Sensitivity of Chloride Channels to Changes in Intracellular Calcium: Investigations on Spontaneous and GABA-evoked Activity. Avanzini G, Engel J Jr, Fariello RG, Heinemann S, editors. New York: Demos; 1990. pp. 69–81. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Machu TK, McKernan RM, Whiting P, VanRenterghem BB, McManaman JL, Brozowski SJ, Smith GB, Olsen RW, Harris RA. Tyrosine kinase phosphorylation of GABAA receptors. Brain Res Mol Brain Res. 1995;31:165–172. doi: 10.1016/0169-328x(95)00048-w. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wang RA, Cheng G, Kolaj M, Randic M. α-Subunit of calcium/calmodulin-dependent protein kinase II enhances γ-aminobutyric acid and inhibitory synaptic responses of rat neurons in vitro. J Neurophysiol. 1995;73:2099–2106. doi: 10.1152/jn.1995.73.5.2099. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Jiang Y-H, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, Christian JM, Mirnikjoo B, Silva A, Beaudet AL, Sweatt JD. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Zhang M, Zhu Y, Wang JH. Ca2+-calmodulin signalling pathway up-regulates GABA synaptic transmission through cytoskeleton-mediated mechanisms. Neuroscience. 2004;127:637–647. doi: 10.1016/j.neuroscience.2004.05.056. [DOI] [PubMed] [Google Scholar]
- Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in γ-aminobutyrate type A receptors: RNA splicing directs expression of two forms of γ2 subunit, one of which contains a protein kinase C phosphorylation site. Proc Natl Acad Sci U S A. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zhao MX, Poo MM, Zhang XH. GABAB receptor activation mediates frequency-dependent plasticity of developing GABAergic synapses. Nat Neurosci. 2008;11:1410–1418. doi: 10.1038/nn.2215. [DOI] [PubMed] [Google Scholar]
- Yu YC, Cao LH, Yang XL. Modulation by brain natriuretic peptide of GABA receptors on rat retinal ON-type bipolar cells. J Neurosci. 2006;26:696–707. doi: 10.1523/JNEUROSCI.3653-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick E, Wallasch C, Daub H, Ullrich A. Distinct calcium-dependent pathways of epidermal growth factor receptor transactivation and PYK2 tyrosine phosphorylation in PC12 cells. J Biol Chem. 1999;274:20989–20996. doi: 10.1074/jbc.274.30.20989. [DOI] [PubMed] [Google Scholar]