Abstract
Humans can stand using sensory information solely from the ankle muscles. Muscle length and tension in the calf muscles (gastrocnemius and soleus) are unlikely to signal postural sways on account of balance-related modulation in agonist activity. These facts pose two questions: (1) Which ankle muscles provide the proprioceptive information? (2) Which peripheral mechanism could modulate agonist activity? To address these issues, subjects were asked to stand normally on two force plates. Ultrasound and surface EMG were recorded from the calf and tibialis anterior (TA) muscles. For all nine subjects, changes in muscle length of TA were mainly (84 ± 9% whole trial duration) orthodoxly correlated with bodily sway (centre of gravity, CoG), i.e. in accordance with passive ankle rotation. When orthodox, TA had the highest correlation with CoG (−0.66 ± 0.07, deep compartment, P < 0.001). For five subjects, the superficial TA compartment showed counter-intuitive changes in muscle length with CoG, probably due to the flattening of the foot and proximal attachment geometry. Gastrocnemius and soleus were usually (duration 71 ± 23 and 81 ± 16%, respectively) active agonists (paradoxically correlated with CoG) but, for short periods of time, they could be orthodox and then presented a moderate correlation (0.38 ± 0.16 and 0.28 ± 0.09, respectively) with CoG. Considering the duration and extent to which muscle length is orthodox and correlated with CoG, TA may be a better source of proprioceptive information than the active agonists (soleus and gastrocnemius). Therefore, if a peripheral feedback mechanism modulates agonist activity then reciprocal inhibition acted by TA on the calf muscles is more likely to be effective than the autogenic pathway.
It is well known that human standing is successfully maintained using visual, vestibular and somatosensory information. Authors have discussed the role of visual perception (Sasaki et al. 2002), of tactile receptors on the soles of the foot (Kavounoudias et al. 2001) and of the vestibular system (Paloski et al. 2006); but a group of researchers has demonstrated that normal subjects can stand in a stable manner when receptors in the ankle muscles are the only source of information about postural sway (Fitzpatrick et al. 1994). The experiment involved anaesthetising the feet and ankles using pneumatic cuffs placed above the ankles and below soleus, inflated to 350 mmHg for approximately 1 h, in order to prevent the influence of any muscular receptors in the muscles of the foot, any tactile receptors of the soles and any ankle joint receptors. These researchers also prevented influence of the vestibular system by asking the subjects to balance an equivalent inverted pendulum, and of the visual input by asking the participants to keep their eyes shut. Therefore, the subjects could stand, or balance the equivalent load, relying only on the propriceptors from muscles crossing the ankle joint. This fact leads to an interesting question. From which muscle or muscles crossing the ankle joint can the subject derive sensory information of standing sway?
It is known that during quiet standing, sway of the entire body is correlated highly with ankle joint rotation and this explains why muscles crossing the ankle joint are able to provide sensory information necessary to maintain upright standing (Gatev et al. 1999; Loram et al. 2005a). Restricting our attention to muscle proprioceptors only, the muscles crossing the ankle joint which might provide this sensory information include soleus, gastrocnemius and tibialis anterior. It is normally accepted that soleus and gastrocnemius, plantar flexors of the ankle, act as active agonists and, because the foot is constrained on the ground, these muscles prevent forward toppling of the body whose centre of gravity (CoG) is maintained in front of the ankle joint (Basmajian & De Luca, 1964, p. 257). The principal antagonist, tibialis anterior, dorsi flexor of the ankle, is usually considered to be un-modulated (i.e. showing no change in activity) but its changes in muscle length have not been investigated closely during standing.
In line with the expectation that autogenic reflexes are fundamental to postural control, it has been assumed in the literature that soleus is one of the principal sources of proprioceptive information during standing (Roberts, 1978; Rothwell, 1986; Lakie et al. 2003). In fact, soleus and gastrocnemius, often considered together, have traditionally been considered the source of muscle proprioceptive information signalling changes in body position (Nashner, 1976; Fitzpatrick et al. 1992b; Schieppati & Nardone, 1999; Loram & Lakie, 2002; Lakie et al. 2003; Loram et al. 2005a).
It is well known that muscle sensory organs, i.e. spindles and Golgi tendon organs, play a key role in the proprioception of movement. The tendon organs are sensitive to changes in muscle tension while the spindles are sensitive to changes in muscle fibre length. The mainstream view is that muscle spindles are the main sensors of joint rotation (Matthews, 1981; Gandevia, 1996, pp. 128–172; Proske, 2006). If changes in muscle length of the soleus and gastrocnemius muscles result predominantly from joint rotation, which throughout the paper we call orthodox behaviour, then when the body sways forward the muscle fibres would lengthen, muscle length would be positively correlated with bodily sway and the brain would be able to acquire from these muscles the proprioceptive information necessary to stabilise the body.
However, it has been seen that this traditional assumption is not correct in standing. During normal standing, the Achilles tendon has been shown to be very compliant in relation to the gastrocnemius and soleus muscles (Loram et al. 2007) and to be less stiff than the load stiffness (Loram et al. 2005a,b, 2007) where load stiffness is the ratio of gravitational moment acting on the body to angle of the CoG from the vertical (Fitzpatrick et al. 1992b). This compliance of the Achilles tendon has two main implications. (i) The body is unstable which means that appropriate modulation of muscle activity is necessary to maintain balance. (ii) The changes in muscle length of the calf muscles are mechanically decoupled from bodily sway (Lakie et al. 2003; Loram et al. 2004, 2005a,b, 2007). As a consequence, generating sufficient tension in the calf muscles to maintain balance results in changes in muscle length which are paradoxical with respect to bodily sway i.e. when the body sways forward the muscle fibres are actively shortened to maintain balance. When viewed on a graph showing changes in calf muscle length versus body position relative to the ankle joint, these paradoxical changes are shown by a negative slope while orthodox changes are shown by a positive slope. Throughout the paper, we use the term paradoxical to mean changes in muscle length which move in the opposite direction to orthodox changes and they can only be produced by changing (modulated) muscle activity.
To summarise, agonist activity in the calf muscles is identified by two facts: (i) muscle length is negatively correlated with position of the CoG and (ii) modulation of muscle activity associated with balancing an unstable bodily load. Considering these two facts we can know when a muscle acts as an active agonist but we cannot assume that the active agonist can also provide proprioceptive information regarding bodily sway. In fact, the main source of muscle proprioceptive information is changes in muscle length. Muscle activity affects these changes in muscle length, and thus modulation of muscle activity interferes with the proprioceptive role of the muscle. During normal standing, the changes in muscle length of the agonist are almost entirely determined by fluctuations in muscle activity that are required for maintaining balance (Fig. 2, Loram et al. 2005a). These active fluctuations hide the changes in muscle length which result mechanically from bodily sway and ankle rotation. It is possible that the nervous system can extract those changes in muscle length that result mechanically from ankle rotation from the active changes in muscle length. However, the high tendon compliance combined with the high short range muscle stiffness, predicts that the hidden signal is far too small to be extracted from the much larger changes resulting from active modulation (authors’ unpublished observations). Whether or not the CNS can extract this small signal, it is indisputable that modulations in muscle activity complicate the proprioceptive function of the agonist muscle. Thus, muscles crossing the ankle joint which are un-modulated in activity are likely to be better sources of proprioceptive information.
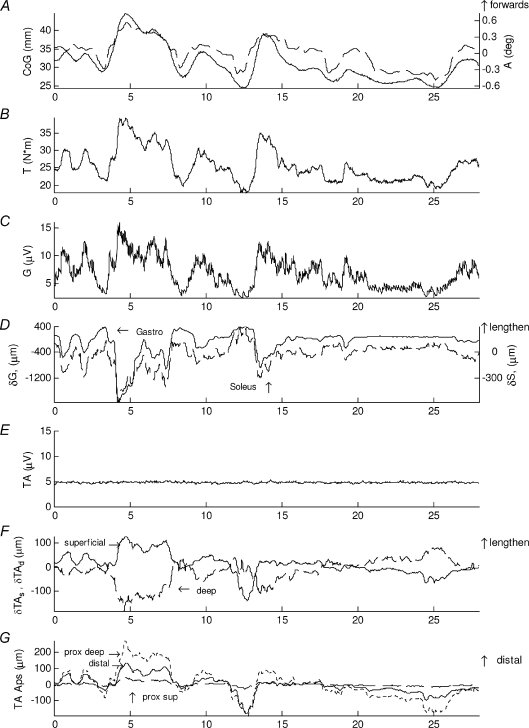
Figure 2. Normal standing.
Normal standing typical sways of representative subject showing A, sagittal centre of gravity relative to the ankle joint centre (CoG) (continuous line, left scale) and ankle flexion angle (dashed line, right scale), B, left ankle plantarflexion torque (T). C, low-pass filtered EMG of gastrocnemius medialis (G). D, change in muscle length of gastrocnemius medialis (δG) (continuous line, left scale) and soleus (δS) (dashed line, right scale). E, tibialis anterior EMG (TA). F, change in muscle length of superficial (δTAs) (continuous line) and deep (δTAd) (dashed line) compartment of tibialis anterior, G, displacement of the aponeuroses of tibialis anterior (TA Aps), distal aponeuroses of the two compartments (continuous line), proximal aponeurosis of the superficial one (dashed) and proximal aponeurosis of the deep compartment (dotted line). The forward sway corresponds to an increase in ankle angle and torque. Positive muscular change in muscle length means lengthening and positive aponeuroses displacement means distal movement. Surprisingly, it is clear that the two compartments of tibialis anterior show opposite behaviours (F) and this is explained by the different behaviour of the aponeuroses of this muscle (G) which are all moving distally but to a different extent during forward sway (e.g. at 4–5 s).
Balancing an unstable body requires variable adjustments in muscle activity: however, it is possible that the activity in soleus and gastrocnemius is not modulated all the time; there may be periods of un-modulated activity in each of these muscles. Moreover, the other muscles crossing the ankle joint such as tibialis anterior may also be involved in maintaining balance and may have periods in which their activity is modulated. Each muscle might have periods of un-modulated activity in which it can temporarily provide uncomplicated information regarding changes of ankle rotation.
If one muscle was always modulated and another muscle was always un-modulated in activity, then the CNS could simply listen to a predefined muscle for its sensory information. However, if there is frequent change in which muscles are modulated and un-modulated, then it might make sense for the CNS to adopt a reciprocal mechanism for modulating the activity of the agonist. Either way, effective peripheral modulation of the agonist should derive from the muscle whose changes in length and tension best signal standing sway. Reciprocal pathways may provide a better sensory source than the assumed autogenic pathway.
The approach of this paper is to use ultrasound to observe changes in muscle length of the soleus, gastrocnemius and tibialis anterior muscles and by combining this information with EMG, to infer when each muscle is acting as an active agonist and when each muscle is un-modulated in activity. Ultrasound allows one to observe lengthening and shortening of a whole muscle. By comparing with sway of the body and with EMG, one can observe when the muscle is shortening paradoxically and actively modulated and thus acting as an agonist, and when the muscle is lengthening orthodoxly and un-modulated in activity and thus an ideal source of proprioceptive information concerning sway.
We do not measure muscle tension. However, in an un-modulated muscle, tension changes in parallel with muscle length. While in a modulated muscle changes in tension are complicated by active modulation just as are changes in muscle length.
The aim of this paper is to answer the following questions for normal standing: (1) Tibialis anterior: is muscle length mechanically (orthodoxly) correlated with body position? If so, what is the duration and extent of this correlation? Do both compartments (superficial and deep) behave in the same way? (2) Gastrocnemius and soleus: are they solely agonists (paradoxical), or are there periods in which muscle length is mechanically correlated with body position? Do these two muscles show the same behaviour? (3) Which muscle has orthodox changes in length best correlated with body position? (4) When orthodox, does muscle length provide an absolute measure of body position or is there ambiguity in the relationship? (5) Which muscle proprioceptive, peripheral feedback mechanism is most likely to modulate agonist activity?
Methods
Ethical approval
These experiments were approved by the ethics committee of the Institute for Biomedical Research into Human Movement and Health, Manchester Metropolitan University. Participants gave written, informed consent to these experiments which conformed to the standards set by the latest revision of the Declaration of Helsinki.
Subjects and set-up
Nine healthy participants, aged 35 ± 11 years (mean ±s.d.), two women and seven men, stood quietly with feet at a habitual distance apart with neither foot in front of the other. For each trial they were asked to stand normally for 30–40 s each (five subjects performed trials of 40 s, the remaining subjects of 30 s). Recording started after the request to stand normally and when the participant was standing quietly. All the subjects performed at least four trials of normal standing. Half of the trials were performed with eyes open and the other half with eyes closed: because no difference was observed, the two conditions were collapsed and averaged together.
Apparatus and measurements
The subjects stood with each foot on an adjacent force platform (AMTI, OR6-7, Watertown, MA, USA). The force plates were separated by a 3 mm gap, allowing normal symmetrical standing. The ground reaction force (GRF) and its point of application (PoA) were recorded. A ten-camera motion analysis system (VICON 612, Oxford Metrics, UK) was used to measure the body kinematics: retroreflective markers placed on the second metatarsal head, the lateral malleolus, the heel, the tibia and the lateral femoral epicondyle were used to calculate ankle joint centre and sagittal ankle angle. Ankle torque was calculated from the vertical component of the ground reaction force and distance of the point of application from the ankle joint (Winter et al. 2001). Both kinematic and force plate data were sampled at 60 Hz.
Having shaved and cleaned the skin, surface EMG (Delsys, Boston, MA, USA) data were recorded from gastrocnemius and tibialis anterior of the left and right leg (sampling rate 1 kHz), amplified (×1000) and band-pass filtered (at 20–450 Hz). The EMG signals were digitally rectified and low-pass filtered using the first order transfer function
where s is the Laplace variable and the time constant τ is 0.2 s and down-sampled to 100 Hz.
Two ultrasound probes were used; one was fixed along the left calf and the other along the left tibialis anterior muscle to provide a parasagittal-plane view of the underlying muscles (Fig. 1A). Images from the ultrasound scanner were digitized at 25 frames s−1 using frame grabbers (Data Translation Inc., 3120, 3130, Marlboro, MA, USA) and recorded on computer using MATLAB software (The MathWorks Inc., Natick, MA, USA).
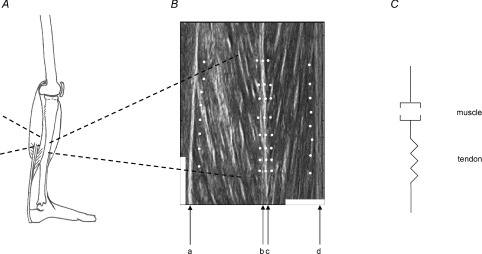
Figure 1. Anatomy and model of human standing.
A, the calf muscles (soleus and gastrocnemius) connect the Achilles tendon to the back of the lower leg bones and the back of the knee respectively. Tibialis anterior is situated anterior to the tibia and attached to the upper part of this bone. Its tendon traverses the front of the tibia and ankle joint and passes along the medial side of the foot to the base of the first metatarsal bone. B, sonograph of the superficial and deep compartment of tibialis anterior. a, proximal aponeurosis of the deep compartment; b, distal aponeurosis of the deep compartment; c, distal aponeurosis of the superficial compartment; d, proximal aponeurosis of the superficial compartment. b and c are morphologically distinct but moved as a unit and were tracked using a triple set of markers. A 1 cm white scale is shown horizontally (perpendicular to the aponeuroses) and a 2 cm white scale is shown vertically (parallel to the aponeuroses). C, model of the muscle tendon unit. The muscle is modelled as a contractile element in series with the tendon spring. The changes in muscle length based on the sonograph and calculated through our method, are representative of shortening and lengthening of the whole muscle as a series component in this muscle–tendon unit.
A common synchronisation signal supplied to all recording computers was used to synchronise all kinematic, forceplate, low-pass filtered EMG and ultrasound data. All data were converted to a 0.01 s time step using cubic interpolation.
The method for tracking and calculating the changes in muscle length has been described previously (Loram et al. 2006). From the sonographs, the fascicles and the aponeuroses were visible for soleus, gastrocnemius and the deep and superficial compartments of tibialis anterior. On the sonographs recorded, for each muscle we placed eight dots close to the proximal (Fig. 1B, ‘a’ and ‘d’) and distal aponeuroses (Fig. 1B, ‘b’ and ‘c’). The software is based on the fact that for small movements of the muscle the dots are anchored to the feature on which they were placed and follow the movement of that feature. The movement of each aponeurosis relative to the probe and along the line of the muscle is given by the group of eight dots. The average movement was calculated for each group of eight dots. The change in muscle length we refer to in this paper is the difference in movement along the line of the muscle between the distal and proximal aponeuroses. In this paper, the muscle tendon unit is regarded as a muscle in series with a tendon (Fig. 1C). Functionally, the calculated change in muscle length is representative of shortening and lengthening of the whole muscle as a series component in this muscle–tendon unit.
Data analysis
The following quantities were calculated: (i) centre of gravity (CoG); for this analysis, the CoG is considered in the sagittal plane only. It was calculated by lowpass filtering the PoA (sagittal component) with a frequency cut-off of 0.5 Hz (Loram & Lakie, 2002). It is calculated relative to the ankle joint (0 mm represents the ankle joint centre); (ii) ankle flexion angle, the changes in the angle of the tibia relative to the foot; (iii) ankle plantarflexion torque; (iv) standard deviation of EMG (modulated activity) for left gastrocnemius and tibialis anterior; (v) mean EMG (tonic activity) for left gastrocnemius and tibialis anterior; (vi) changes in muscle length of left gastrocnemius, soleus and tibialis anterior (deep and superficial compartments); (vii) changes in position of tibialis anterior aponeuroses.
Trial partition analysis
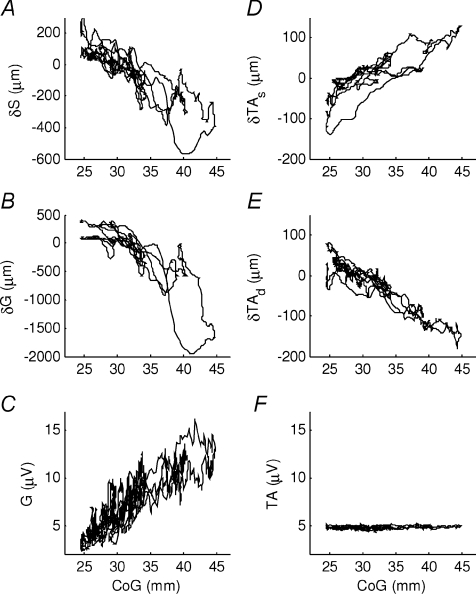
Preliminary observations showed that for each muscle there are periods when it appears to be un-modulated, orthodox and mechanically correlated with bodily sways (Fig. 2C and D, 21–24s; Fig. 2E and F, Fig. 3D and F, Fig. 3B 24–27 mm), and others when it is modulated in activity and paradoxically correlated with body position (Figs 2C and D, and 3A and B after 28 mm). For each muscle, our aim was to establish the extent of orthodox and paradoxical behaviour and to quantify key parameters during the orthodox and paradoxical periods. For the entire trial, the orthodox and the paradoxical period, we quantified the following: (i) correlation between muscle length and CoG, (ii) EMG modulation (s.d.), (iii) tonic EMG activity and (iv) percentage duration of the whole trial.
Figure 3. Muscular behaviour during normal standing.
Muscular change in muscle length and EMG during normal standing sways of representative subject (same trial as Fig. 2). The horizontal axis is the centre of gravity relative to the ankle joint centre position (CoG), 0 mm is the ankle joint centre position. The following panels show the muscular change in muscle length of A, soleus; B, gastrocnemius; D, superficial compartment of tibialis anterior; E, deep compartment of tibialis anterior. The following panels show the low-pass filtered superficial EMG of C, gastrocnemius and F, tibialis anterior. Negative displacement means shortening relative to the initial length. The two compartments of tibialis anterior show an opposite correlation (D vs. E) and the EMG does not appear to be modulated (F), but also soleus and gastrocnemius show different behaviour, in particular when the CoG is further backwards (A vs. B).
For each trial the data were analysed in time windows of 3 s. The above quantities of interest were calculated for the first 3 s portion of data. They were then calculated repeatedly for successive overlapping portions using a step of 0.1 s until the end of the trial. For a 30 s trial, this produced 269 measures of each quantity.
For each muscle and for each trial, the 269 measures were classified into three categories: ‘whole trial’, ‘negatively correlated’ and ‘positively correlated’ (Figs 6–9). The whole trial includes all 269 measures. The ‘negatively correlated’ category includes those measures from the 269 in which the correlation between muscle length and CoG is negative. The ‘positively correlated’ category includes those measures in which the correlation between muscle length and CoG is positive. For each trial, for each muscle, for each category, the mean value of all the quantities above was calculated.
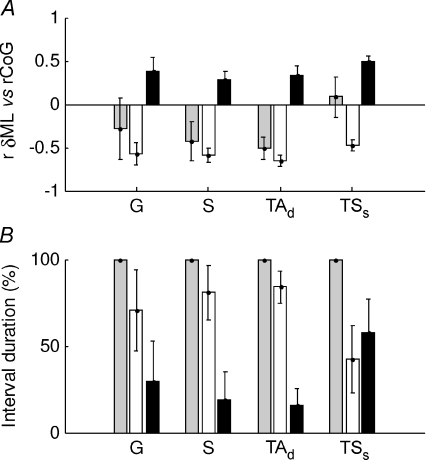
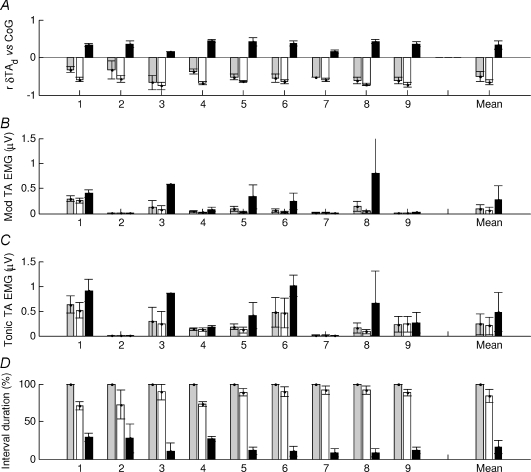
Figure 6. All muscle behaviour during normal standing.
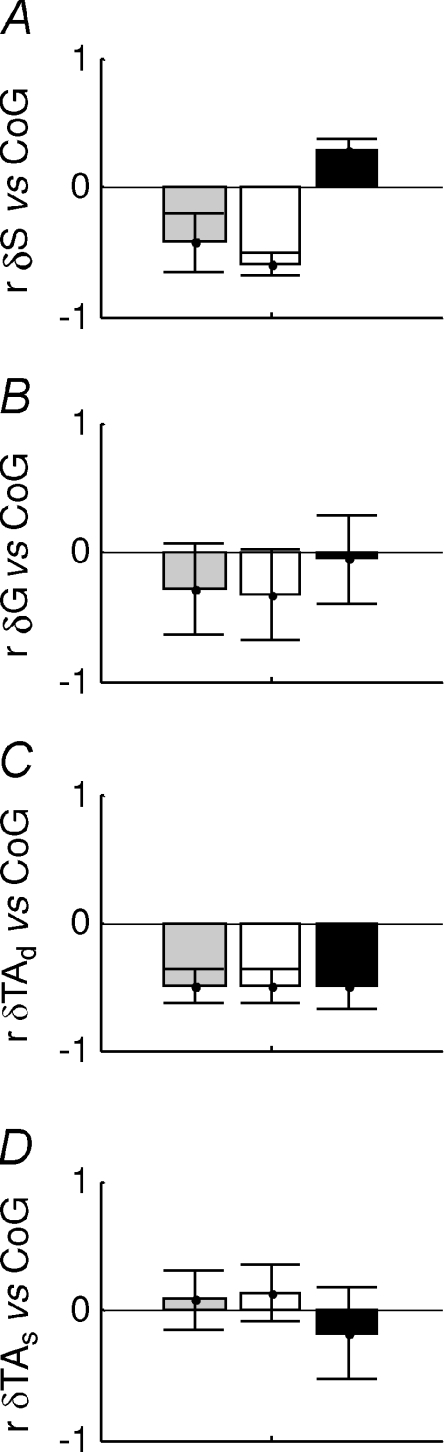
For each 3 s interval of a whole trial the correlation between each muscle and the CoG was calculated (G, gastrocnemius: S, soleus, TAd, deep compartment of tibialis anterior and TAs, superficial compartment of tibialis anterior). The intervals were classified into negatively (white bars) and positively correlated intervals (black bars) and whole trial (grey bars). For these groups the following quantities are shown: A, correlation between the change in muscle length and the CoG, B, percentage duration in each condition. The values shown are the mean and s.e.m. for each subject. On average, gastrocnemius is negatively correlated with CoG (G, white bars), though this is not very consistent among the subjects. Soleus behaviour is similar but more consistent among the subjects. The deep compartment of tibialis anterior is overall negatively correlated.
Figure 9. Soleus interlinked behaviour during normal standing.
For each 3 s interval of a whole trial the correlation between soleus and body position was calculated. The intervals were classified into negatively (white bars) and positively correlated intervals (black bars) and whole trial (grey bars). For these groups the following quantities are shown: A, correlation between the soleus change in muscle length and CoG; B, correlation between the gastrocnemius change in muscle length and CoG, based on the trial partition obtained from soleus correlation; C, correlation between the deep compartment of tibialis anterior change in muscle length and CoG, based on the trial partition obtained from soleus correlation; D, correlation between the superficial compartment of tibialis anterior change in muscle length and CoG, based on the trial partition obtained from soleus correlation. The average values from the nine subjects are presented and error bars show the standard error of the mean. On average soleus is negatively correlated with body position (A) and when soleus changes from a negative to positive correlation (A), gastrocnemius’ correlation with CoG becomes less negative (or more positive) (B), and the superficial compartment of tibialis anterior shows a more negative correlation (D). The deep compartment of the same muscle does not show any difference in behaviour related to soleus (C). The association between soleus and the superficial compartment of tibialis anterior is evidence that the counter-intuitive behaviour in the superficial compartment of tibialis anterior has an anatomical or physiological explanation.
To test for appropriateness of the window duration, several time window intervals were tried (2 s, 2.5 s, 3 s, 3.5 s and 4 s). The results were very repeatable. These interval lengths were chosen because if the interval was too long, different behaviours would have been present in the same period of time. If the interval was too short the correlation between bodily sway and change in muscle length would not be meaningful because the main period of the sway in normal standing is 3 s (Loram et al. 2005b).
Statistical analysis
For each muscle, for key quantities a two-way analysis of variance was used to test for differences between subjects and category. There were nine subjects and three levels of category (‘whole trial’, ‘negatively correlated’, ‘positively correlated’). Each subject performed four or five trials giving 38 trials in total. Thus there were 114 samples (38 × 3).
Unless stated otherwise, results generally are quoted as a mean ± standard deviation (s.d.).
Results
Representative behaviour in normal standing
During normal standing, the subject's CoG swayed forwards and backwards involuntarily by 20 mm (Fig. 2A, continuous line, left scale) and this was in good agreement with ankle dorsiflexion angle (Fig. 2A, dotted line, right scale) and torque (Fig. 2B).
When gastrocnemius activity was modulated (Figs 2C and 3C), its peaks had a clear correspondence with the change in muscle length (Fig. 2D, continuous line, left scale) and the correlation between muscle length and CoG was high, negative, paradoxical and consistent with its expected agonist role (Fig. 3B, 28–43 mm). More surprisingly, when the CoG was closer to the ankles the changes in muscle length, if present, were not correlated with CoG movement and the muscle showed little modulation in activity (Fig. 3B, C, 25–28 mm; Fig. 2C, 23–25 s). Soleus was actively modulated and the changes in muscle length were paradoxical, negatively correlated with CoG, consistent with agonist behaviour (Fig. 3A). During normal standing, tibialis anterior was not modulated in activity (Figs 2E and 3F). Surprisingly, the two compartments of this muscle presented opposite behaviours: the changes in length of the superficial compartment were highly positively correlated with CoG (Fig. 2F, continuous line, and Fig. 3E); at the same time for the deeper compartment, changes in length were highly, negatively correlated with CoG (Fig. 2F, dashed line and Fig. 3D). Among all the muscles, the deep compartment of tibialis anterior showed the longest duration in which the muscle activity was un-modulated and the changes in muscle length were related to changes in CoG with minimum variability (Fig. 3).
To further explore the differences seen in the two compartments of tibialis anterior (Fig. 2F), we also investigated the behaviour of the aponeuroses. In the absence of any modulation in surface EMG activity (Fig. 2E), when the body was swaying forwards (Fig. 2A, 4–8.5 s) the proximal aponeurosis of the deep compartment moved distally relative to the probe (Fig. 2G, 4–8.5 s, dotted line), the distal aponeurosis of both compartments moved distally, but by a smaller amount (Fig. 2G, 4 s–8.5 s, continuous line) and as a result, as expected for dorsiflexion, the deep compartment shortened; the proximal aponeurosis of the superficial compartments moved very little and as a result, surprisingly for dorsiflexion, the superficial compartment lengthened (Fig. 2G, 4–8 s, dashed line). Still while un-modulated in activity, when the body was swaying backwards (Fig. 2A, 24–25.5 s), the pattern was reversed. This surprising difference in behaviour in the two compartments of tibialis anterior occurred during sways typical of normal standing while the muscle was not modulated in its activity.
Gastrocnemius and soleus difference
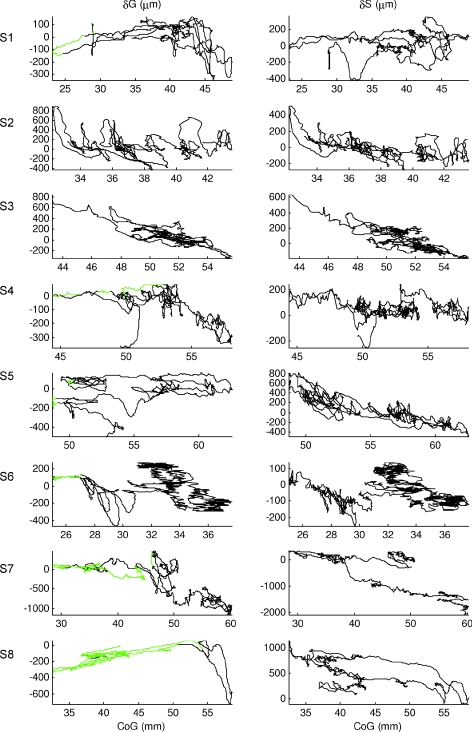
Figure 4 shows important differences in behaviour between gastrocnemius and soleus. Soleus showed changes in muscle length that were almost always negatively correlated with CoG. The changes in length were actively modulated, consistent with a continuous agonist role in regulating balance (Fig. 4, right column, δS). For one subject only, S1, soleus was un-modulated for a substantial extent of the trial, and the muscle either had constant length or was positively correlated with CoG when the CoG was close to the ankle (S1, 22–27 mm). Two subjects (S7, S8) showed clear examples of ‘parallel lines’: the muscle length was not uniquely related to CoG position, but changes in muscle length were temporarily well related to changes in CoG position.
Figure 4. Calf muscles change in muscle length.
Gastrocnemius (left column) and soleus (right column) change in muscle length relative to CoG, during normal standing for all the other subjects (representative subject of Fig. 3 not shown). The green portions were manually identified from the surface EMG of gastrocnemius recognizing periods in which the activity was un-modulated. It is very clear that for several subjects the two calf muscles present a different behaviour: while soleus shows almost always a negative correlation with CoG, gastrocnemius, when CoG is nearer the ankle joint, shows also periods where the correlation with CoG is positive in some participants (S1, S4, S6, S8). Both muscles presented parallel lines when the muscles were un-modulated in their activity; their similar slope is an indication that even if the overall correlation is quite low, for short periods of time it might be high and for those durations the changes in muscle length appeared to be a good source of proprioceptive information. That is also the reason why we performed a trial partition analysis using intervals of 3 s.
On the other hand, gastrocnemius had extended periods when the gradient of the change in muscle length versus CoG was clearly positive and others when it was clearly negative. The periods of positive slope (positive correlation) were shown when the CoG was closer to the ankles and the modulation in activity was low (green portions). In the regions of negative slope the muscle was actively modulated.
When the muscle is un-modulated in activity, i.e. the regions of positive slope or constant muscle length, the changes in muscle length per millimetre are much smaller compared with the negative slope regions (S1, S4, S6, S7 and S8). When un-modulated, gastrocnemius showed ‘parallel lines’ (S1, S4, S5, S7 and S8) such that the muscle length does not uniquely specify CoG position but in which the change in muscle length is temporarily well related to change in CoG position.
The main difference between soleus and gastrocnemius is that soleus was almost always modulated in activity as an active agonist, while gastrocnemius also had periods of un-modulated activity where changes in muscle length were passively driven by ankle rotation.
Tibialis anterior compartments difference
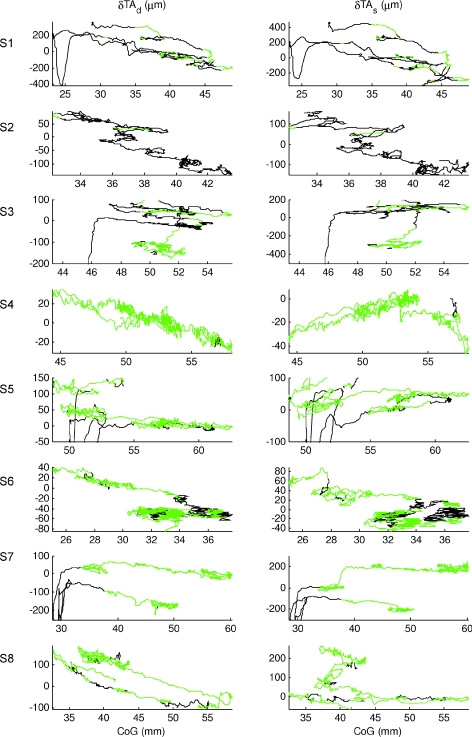
The behaviour of the two compartments of tibialis anterior is shown in Fig. 5. Both compartments were predominantly un-modulated in activity. Some subjects (S1, S3, S5 and S7) showed modulation in activity when the CoG was closer to the ankles. These regions of modulated activity were associated with a positive slope between muscle length and CoG, and large changes in muscle length and were consistent with an agonist role in regulating balance. The regions of un-modulated activity (green portions) were associated with smaller changes in muscle length per millimetre of CoG movement. For the deep compartments, these changes in muscle length were always negatively correlated with CoG, consistent with a passive response to joint rotation. Rather surprisingly, for the superficial compartment, in some subjects (S3, S4 and S5) and the representative subject (Fig. 3), the un-modulated changes in muscle length were positively correlated with CoG position. This fact means that, for the superficial compartment, a positive correlation between muscle changes and CoG is not indicative of modulation of muscular activity.
Figure 5. Two compartments of tibialis anterior change in muscle length.
Deep compartment (TAd; left column) and superficial compartment (TAs; right column) of tibialis anterior change in muscle length relative to CoG, during normal standing for all the other subjects (representative subject of Fig. 3 not shown). The green portions were manually identified from the surface EMG of tibialis anterior recognizing periods in which the activity was un-modulated. The two compartments of tibialis anterior show different behaviour: the change in muscle length of the deep compartment is for all the participants negatively correlated with body position (CoG) while, for the superficial compartment, when the participants are standing with the CoG backwards, still in absence of modulation of EMG activity (green portions), some of them show also intervals positively correlated with CoG. Both compartments, when the person was standing away from the ankle, showed parallel lines when the muscles were un-modulated in their activity. Again their slope is locally very similar which indicates high local correlation.
In both compartments, there were many examples of ‘parallel lines’ in which muscle length is not uniquely related to CoG position, but changes in muscle length were temporarily well related to changes in CoG position.
Summary of all subjects for normal standing
We wished to establish which muscle had orthodox changes in length that correlated most highly with movement of the centre of gravity (CoG).
For all subjects and for each muscle, Fig. 6 shows the mean correlation between changes in muscle length and CoG for each of the categories –‘whole trial’, ‘negatively correlated’ and ‘positively correlated’ periods. Overall, gastrocnemius muscle length (Fig. 6A bars labelled G) was negatively correlated with bodily sway (−0.29 ± 0.35) and thus paradoxical. The large standard deviation shows that this correlation was not very consistent among the nine subjects. In fact, the mean correlation is comprised of periods when gastrocnemius is negatively correlated (−0.57 ± 0.13), but also when it is positively correlated (0.38 ± 0.16). For these negatively and positively correlated periods, the standard deviations and hence the variability between subjects is relatively low. The nine subjects’ muscle length were negatively correlated with CoG for 71 ± 24% and also unexpectedly, positively correlated for 29 ± 24% of the total duration (Fig. 6B).
Soleus muscle length (bars labelled S) followed the same trend and for the ‘whole trial’ it was negatively correlated with CoG (−0.42 ± 0.22). This overall negative correlation was more consistent between subjects than for gastrocnemius. The correlations between muscle length and CoG for the ‘negative’ and ‘positive’ periods, respectively, were −0.59 ± 0.08 and +0.28 ± 0.09. The total duration of the paradoxical ‘negatively correlated’ intervals was 80.78 ± 15.82% (Fig. 6B).
For the ‘whole trial’, the deep compartment of tibialis anterior (bars labelled TAd) was negatively correlated with CoG (−0.50 ± 0.13) revealing high consistency between subjects. For the orthodox, ‘negatively correlated periods’ the correlation between changes in muscle length and CoG was −0.66 ± 0.07 and for the paradoxical, ‘positively correlated’ periods it was +0.33 ± 0.11. The deep compartment was almost always negatively correlated with CoG occupying 84 ± 9% of the ‘whole trial’ duration (Fig. 6B).
The superficial compartment of tibialis anterior (bars labelled TAs) behaved differently from the deep compartment. Overall the correlation between muscle length and CoG was low and positive 0.08 ± 0.23. This low value reflects a mixture of orthodox, ‘negatively correlated’ periods (–0.47 ± 0.06) and ambiguous ‘positively correlated’ periods (0.49 ± 0.07). The duration of these two categories was approximately equal at 58 ± 19.26% for the positively correlated periods (Fig. 6B).
Comparing the absolute values of the orthodox correlation between muscle length and CoG for gastrocnemius, soleus and the deep compartment of tibialis anterior (Fig. 6A), tibialis anterior correlated most highly with the centre of gravity (P < 0.001, using Tukey–Kramer for post hoc comparison).
Since gastrocnemius showed a wide variation between subjects, we wished to account for this variation. We also wanted to quantify whether orthodox and paradoxical changes in muscle length were modulated in muscle activity and whether there were changes in the tonic level of activity. These questions are answered in Fig. 7.
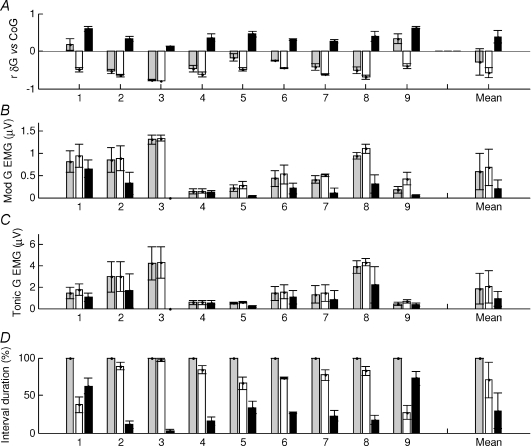
Figure 7. Gastrocnemius muscular behaviour during normal standing.
For each 3 s interval of a whole trial the correlation between gastrocnemius and the CoG was calculated. The intervals were classified into negatively (white bars) and positively correlated intervals (black bars) and whole trial (grey bars). For these groups the following quantities are shown: A, correlation between the gastrocnemius change in muscle length and the CoG; B, gastrocnemius standard deviation of EMG levels (modulation of activity); C, mean gastrocnemius EMG levels (tonic activity); D, percentage duration in each condition. The horizontal axis represents each subject tested (1–9); values are the mean for each subject and error bars show the standard error of the mean. The ‘Mean’ column is the average for all the individuals. On average gastrocnemius is negatively correlated with CoG (A) and the negatively correlated portion are associated with a higher level of modulation of EMG (B) and tonic activity (C). The negative correlated portions have the longest duration (D). The muscle lengths of S1 and S9 are overall positively correlated with CoG (A) and for longer duration (D) when the muscle activity is not modulated (B).
For gastrocnemius, the average paradoxical changes in muscle length were made by seven subjects whose changes in muscle length versus CoG were predominantly paradoxical and two (S1 and S9) whose changes were predominantly orthodox.
We tested whether the modulation of the EMG (standard deviation of the signal) differed between the orthodox and paradoxical periods of change in muscle length. We found that the intra-individual difference between orthodox and paradoxical periods was significant for the modulation in EMG (P < 0.001) (Fig. 7B). The positive correlated (orthodox) portions were associated with less modulation in EMG activity. By comparison, for the tonic EMG (mean of the signal) the intra-individual difference was only slightly statistically significant (P < 0.05) (Fig. 7C).
These results confirm that the orthodox, positively correlated portions are relatively un-modulated (0.2 ± 0.2 μV) and low in activity (0.9 ± 0.7 μV).
For the deep compartment of tibialis anterior, we wanted to examine the consistency of behaviour between subjects and also to assess the relationship between orthodox and paradoxical periods in the modulation of activity for each subject (Fig. 8). For each subject the overall correlation between changes in muscle length and CoG was negative and orthodox (Fig. 8A) and, without exception for any individual, the trial duration was almost entirely orthodox (Fig. 8D).
Figure 8. Behaviour of the deep compartment of tibialis anterior during normal standing.
For each 3 s interval of a whole trial the correlation between the deep compartment of tibialis anterior and CoG was calculated. The intervals were classified into negatively (white bars) and positively correlated intervals (black bars) and whole trial (grey bars). For these groups the following quantities are shown: A, correlation between the deep compartment of tibialis anterior change in muscle length and CoG; B, tibialis anterior standard deviation of EMG levels (modulation of activity); C, mean tibialis anterior EMG levels (tonic activity); D, percentage duration in each condition. The horizontal axis represents each subject tested (1–9); values are the mean for each subject and error bars show the standard error of the mean. The ‘Mean’ column is the average and s.e.m. for all the individuals. For all the subjects, the deep compartment of tibialis anterior is mainly negatively correlated with CoG (A and D) and its negative correlation portions are associated with a low modulation in the EMG activity (B) and in tonic activity (C).
We tested whether the modulation of the EMG (s.d.) differed between the orthodox and paradoxical periods of change in muscle length. We found that differences in modulation in EMG between paradoxical and orthodox periods were statistically significant (P= 0.0012). The ‘positively correlated’ portions were associated with more modulation in EMG activity (Fig. 8B). Likewise, for tonic EMG in the deep compartment, the activation was higher in paradoxical rather than orthodox periods, but the statistical significance was lower (P < 0.05) (Fig. 8C).
Thus, orthodox behaviour, with low activity and modulation is highly normative for all participants.
Soleus behaviour was consistent between subjects, but there were clear relationships between the behaviour of soleus, gastrocnemius and the superficial compartment of tibialis anterior (Fig. 9). When changes in soleus muscle length were orthodox (positive) rather than paradoxical, gastrocnemius changes in muscle length were less negatively correlated with CoG (Fig. 9A vs B) (P < 0.01). As expected, soleus and gastrocnemius both progress from orthodox to paradoxical behaviour, but as shown in Figs 3 and 4, the transition points are different.
When soleus changed from paradoxical to orthodox periods, there was no difference in the behaviour of the deep compartment of tibialis anterior (P > 0.05), while changes in muscle length of the superficial compartment changed from positively to negatively correlated with CoG (P < 0.001) (Fig. 9D). This shows that the counter-intuitive, un-modulated, ‘positively correlated’ changes in muscle length of the superficial tibialis anterior are associated with paradoxical activity in the soleus muscle.
Discussion
Using ultrasound and surface EMG observation of the gastrocnemius, soleus and tibialis anterior muscles during quiet standing, we have established the following facts. For convenience, we reiterate our terminology. Orthodox refers to changes in muscle length that accord mechanically with joint rotation and paradoxical refers to changes in muscle length which are in the opposite sense. For tibialis anterior and the calf muscles, orthodox changes in muscle length are negatively and positively correlated with sway, respectively. (1) Soleus behaviour is consistent between subjects and the changes in muscle length are almost always (81 ± 16% of the trial duration) paradoxical as a result of modulating activity to successfully maintain balance. (2) Gastrocnemius behaviour varies considerably between subjects and is also variable for each individual. While it is predominantly paradoxically, for 29 ± 23% of the duration its changes in muscle length are orthodox, showing a moderate positive correlation with sway (0.38 ± 0.16). For two subjects out of nine, gastrocnemius was predominantly orthodox and passive. (3) The deep compartment of tibialis anterior was very consistent between subjects and during trials. Changes in muscle length were predominantly (84 ± 9%) orthodox and un-modulated in activity. These orthodox changes in muscle length were highly correlated with standing sway (−0.66 ± 0.07). (4) Among all the muscles, the deep compartment of tibialis anterior showed the highest duration and highest correlation between orthodox changes in muscle length and standing sway. (5) The superficial compartment of tibialis anterior showed a counter-intuitive behaviour. In some subjects, changes in muscle length were positively correlated with sway (i.e. the muscle lengthens while the subject sways forwards) while the activity was un-modulated. (6) When soleus was orthodox rather than paradoxically correlated with sway, this was related to changes in gastrocnemius and the superficial but not the deep compartment of tibialis anterior.
On the basis of these results, four major issues need to be discussed: (i) the agonist and proprioceptive role of the three investigated muscles in maintaining standing balance, (ii) the muscle proprioceptive, peripheral feedback mechanism that could modulate the activity in the active agonist, (iii) the differences shown by soleus and gastrocnemius and (iv) the differences between the two compartments of tibialis anterior.
(i)The agonist and proprioceptive role of the three muscles in maintaining standing balance
Previously, soleus has been considered the main agonist and proprioceptor during upright standing (Basmajian & De Luca, 1964, p. 257), but the proprioceptive role is less certain now. The compliance of the Achilles tendon and the high short range stiffness of the muscle (Loram et al. 2007) means the body is intrinsically unstable. The changes in muscle length of the calf muscles are mechanically decoupled from bodily sway and as a consequence of successfully maintaining balance the calf muscles move paradoxically in standing (Lakie et al. 2003; Loram et al. 2007). Thus, the paradoxical correlation between muscle length and CoG is possible because the central nervous system knows the sway of the body and does not imply that the calf muscle is the source of that information. How can we identify the most likely source of information from among the ankle muscles? Muscle proprioceptive information of small standing sways is most likely to be received from muscles which are un-modulated in activity (authors’ unpublished observations). The changes in muscle length of un-modulated muscles are orthodox since they are mechanically driven by joint rotation.
The agonist role is identified when the changes in muscle length are paradoxical and the muscle is actively modulated to maintain balance.
Active agonist in balance
From the principles above, and from the correlation between changes in muscle length and CoG, we confirm, in line with expectation (Basmajian & De Luca, 1964, p. 257), that soleus is the main agonist regulating quiet standing. However, contrary to expectation, some subjects had substantial periods when soleus was not an agonist and these periods occurred when the CoG was closer to the ankle (Fig. 4). Also, in accord with established EMG knowledge (Carlsoo, 1964), the orthodox changes in muscle length with CoG show that tibialis anterior is predominantly not an active agonist. However, when the CoG was close to the ankles, tibialis anterior showed a paradoxical, agonist role although the distance from the ankles at which this occurs, if at all, varied considerably between subjects (Fig. 5). The main surprise came with gastrocnemius which is often regarded as a whole with soleus (Fitzpatrick et al. 1992a; Loram & Lakie, 2002; Lakie et al. 2003; Loram et al. 2005a). Gastrocnemius is not always an agonist and in two subjects the agonist behaviour was the exception (Fig. 7); Fig. 4 (S1, S4, S6, S7 and S8) suggests that recruitment of agonist behaviour may depend on how far forward from the ankles an individual maintains the CoG.
One might expect alternation of agonist activity between the soleus and tibialis anterior associated with forwards and backwards sway of the body. However, changes between agonist (paradoxical) and orthodox behaviour in soleus were associated with similar changes in gastrocnemius but were not associated with any change towards agonist behaviour in the deep or superficial compartments of tibialis anterior (Fig. 9). This implies that agonist behaviour in soleus and gastrocnemius is replaced by no alternative agonist (i.e. entirely passive balance) rather than agonist action from tibialis anterior (antagonist muscle).
Proprioception of balance
As it has been demonstrated that standing relying only on the proprioceptors from the muscles across the ankle joint is possible (Fitzpatrick et al. 1994), our aim is to find the best source of proprioceptive information among these muscles.
The reader may question why we discount the proprioceptive role of the agonist muscles in quiet standing. Surely the nervous system is sophisticated enough to register joint rotation through an active muscle? Surely, increased γ drive increases the ‘bias’ of spindles to compensate for muscle shortening in dynamic tasks?
It is true that β and γ fusimotor activity are usually increased to compensate for active shortening of muscle and consequent shortening of the sensory regions of the embedded spindles. These adjustments of spindle ‘bias’ allow spindles to register joint rotations when muscle is active and the joint rotations are relatively large. However, the problem is not the activity of the muscle, it is the dynamic modulation of activity. When muscular activity is modulated, the spindle ‘bias’ must be modulated synchronously with the fluctuation in extrafusal activity. However, it is not known whether there is an effective rigid link between the activation of intrafusal (β, γ) and extrafusal (α) motoneurons that would allow a dynamic subtraction of extrafusal muscular modulation from the signal of joint rotation at the spindles level. This subtraction, if it occurred, would not be perfect and a ‘noise’ would be introduced. Thus, the proprioception of joint rotation in a modulated muscle would be degraded by this ‘noise’. To overcome this limitation, the changes in muscle length, reflective of joint rotation at the ankle, need to be bigger to be seen at mechanical input to the spindle. However, in normal standing, which is within the regime of short range muscle stiffness, the muscle is ten times stiffer than the tendon (Loram et al. 2007) and the changes in muscle length of the muscle are very small in relation to the larger changes due to muscular activity (Loram et al. 2009) (authors’ unpublished observations). The signal of muscle length is then obscured and it is most likely that spindles would register active shortening. Close inspection of Figs 3C and 7 in the study published by Aniss and colleagues in 1990 (Aniss et al. 1990), in which tibialis anterior was the active agonist behaving paradoxically, would appear to support this reasoning. It has also been found that in the presence of modulation in muscular activity (co-contraction) there is a rise in the threshold sensitivity of muscle spindles (Wise et al. 1999).
In an un-modulated muscle, the ‘noise’ due to modulation of activity is not present and the signal of muscle length determined by joint rotation is not affected by active fluctuation of EMG and can be more easily registered by muscle spindles. Therefore, the least complicated source of proprioceptive information is orthodox changes in muscle length in an un-modulated muscle across the ankle joint. The orthodox correlation was observed most consistently and for the longest duration (84 ± 9%) in the deep compartment of tibialis anterior (Figs 5, 6 and 8). Also soleus and gastrocnemius presented periods in which their correlation with CoG was orthodox. This was observed in gastrocnemius more often than soleus; surprisingly, when gastrocnemius was orthodox, soleus was usually paradoxical (Figs 4, 6 and 7). The ultrasound recordings show clearly that changes in muscle length in the deep compartment of tibialis anterior best predict body sway (Fig. 6). Thus, from the muscles we have observed, we predict that the muscle proprioception of balance from the ankle muscles is mainly performed by the deep compartment of tibialis anterior.
These observations imply that all un-modulated muscles across the ankle and foot may be a source of muscle proprioceptive information when stretched by body sway: such muscles may include tibialis posterior, peroneus muscles, extensor longus digitorum and the intrinsic muscles of the foot. For example, the changes in muscle length of the un-modulated, superficial compartment of tibialis anterior were still highly correlated with body position (Fig. 6A), even though the geometry of this compartment is complicated, resulting in counter-intuitive changes in muscle length with forward and backward sway. We predict that, surprisingly, all these un-modulated muscles and other innervated structures may register standing sway, but not the modulated soleus and gastrocnemius.
However, it should not be understood that tibialis anterior is always the best register of body sway and the calf muscles are always the agonist among the ankle muscles. The roles are dynamic. It is likely that the roles may reverse according to the position of the CoG relative to the ankle joints.
The reader will have noticed that orthodox changes in muscle length consist of ‘parallel lines’ (Figs 4 and 5) linked by a relatively sudden change in muscle length, rather than a single line relating muscle length to CoG position. There are several causes of these parallel lines. One cause is a change in whole-body configuration which alters the length of the muscle at that CoG position. We know this because plotting muscle length versus ankle angle collapses some of these parallel lines (not shown). A second cause is an intermittent burst in muscle activity that changes the muscle length between periods of un-modulated behaviour. A third possible cause is hysteresis and dead-zones resulting from short range stiffness (Fig. 3, Loram et al. 2007): if the muscle becomes ‘stuck’ due to short range muscle stiffness, then muscle length becomes insensitive to changes in CoG position (e.g. Fig. 5, S1, TAd, CoG at 35 mm). The ‘parallel lines’ imply that the correlation between muscle length and CoG may be high temporarily even though this high correlation may not be sustained for a long duration.
As the lines were parallel to each other, the changes in muscle length and not the absolute value of muscle length are reliably related to body position. Change in muscle length with CoG position conveys velocity rather than position. These ultrasound observations support clearly the observation that, from the calf muscles alone, body sway velocity is perceived more accurately than body position (Kiemel et al. 2002; Jeka et al. 2004).
The reader may expect that tension rather than velocity information is crucial for registering sway. The Golgi tendon organs are sensitive to muscle tension (Pierrot-Deseilligny & Burke, 2005, p. 245). In the modulated muscle, this system has no mechanism for subtracting active modulation and is thus a more complicated source than un-modulated muscle. In an analogous experiment, balancing a pendulum via the hand and a spring without actually seeing the pendulum, thus testing whether an inverted pendulum can be balanced using muscle–tendon information alone, the pendulum can be balanced for up to 10 s (Lakie & Loram, 2006). This implies that the muscle–tendon tension information from the active agonist alone is inadequate to sustain balance.
In the un-modulated muscle, tension and muscle length change together in a similar manner; hence muscle length observed through ultrasound will effectively represent the signal registered by spindles and tendon organs. It has recently been shown that Golgi–tendon organs can register passive muscle tension effectively (Proske & Gregory, 2002) and thus they may well register joint rotation via the un-modulated muscle (authors’ unpublished observations).
(ii) What peripheral muscle proprioceptive, feedback mechanism could modulate the activity in the active agonist?
It has traditionally been expected that activity in the agonist is modulated by autogenic mechanisms and in particular the stretch reflex, the archetypal postural mechanism (Rothwell, 1986; Fitzpatrick et al. 1992a,b, 1994; Gatev et al. 1999). Arguably, it is unlikely that a peripheral mechanism modulates the calf muscles activity (Loram et al. 2005a,b). However, if there is a peripheral contribution to a central mechanism, it is more likely to come from reciprocal inhibition (played by tibialis anterior on soleus), because this pathway has access to better correlated information with body position (Figs 6A and 8A). Recent research using decerebrated cats has shown that reciprocal inhibition is very effective in providing a local and specific persistent inward current control system and it may provide a focused, local inhibitory pathway (Hyngstrom et al. 2008). For quiet standing, reciprocal inhibition may be more important than the autogenic stretch reflex.
(iii) Difference between gastrocnemius and soleus
It is often assumed that the calf muscles act as a whole structure but this assumption does not take into account our observed differences between gastrocnemius and soleus during standing. Gastrocnemius had periods (predominant periods in two subjects) of orthodox behaviour while soleus was almost always an active agonist (Fig. 4, Fig. 6 and Fig. 7, S1 and S9). Previously, the explanation of the orthodox behaviour in gastrocnemius was attributed to a stiffer gastrocnemius tendon compared to the soleus tendon (Loram et al. 2005a). The opposite is more likely to be true because the gastrocnemius tendon is more compliant than that of soleus because it is shorter and less thick. Our explanation of the gastrocnemius orthodox behaviour is periods of low or no modulation in muscular activity, in particular when the CoG is closer to the ankles. Arguably, the two subjects in which gastrocnemius was predominantly orthodox have access to better quality registration of standing sway.
It is likely that these observed differences in behaviour of soleus and gastrocnemius are related to differences in properties of these muscles. Soleus has 433 spindles, 0.94 spindles per gram, while gastrocnemius has 390 spindles, 0.4 spindles per gram (Voss, 1971). Traditionally, this has supported the view that soleus is the more important proprioceptive muscle (Fitzpatrick et al. 1992a, 1994). However, because soleus was more modulated in activity, more spindles are needed to distinguish the changes in muscle length due to joint rotation from the fluctuations in activity (authors’ unpublished observations). On the other hand, gastrocnemius had more periods of un-modulated activity, therefore fewer muscle spindles are required to register joint rotation (authors’ unpublished observations). The two muscles differ also by fibre type composition: soleus has a higher proportion of slow muscle fibres, more suited for tonic activity; gastrocnemius is mainly composed of fast muscle fibres, which are more fatiguing. Gastrocnemius is better suited for extended periods of un-modulated behaviour.
Our results leave two questions open for further investigation. (i) Is standing possible with soleus predominantly orthodox in addition to gastrocnemius and tibialis anterior? (ii) Are soleus and gastrocnemius recruited to an agonist role in an orderly fashion predicted by the position of the CoG?
(iv) Difference between the deep and superficial compartment of tibialis anterior
In normal standing, the deep compartment shortened orthodoxly during forward sway, while, counter-intuitively, at the same time the un-modulated superficial compartment lengthened (Fig. 3D vs. E). How can this difference be explained?
Tibialis anterior is distally inserted in the foot and changes in foot configuration may affect the changes in muscle length of the compartments. By visual observation, the arch of the foot flattens during forward sway and becomes more curved during backward sway. This may explain why the distal aponeuroses of both compartments moved distally during forwards sway (Fig. 2G, continuous line). Combined, these observations demonstrate that the centre of rotation of the movement during standing is not simply the ankle joint as assumed by the inverted pendulum model. Unlike the prediction of the inverted pendulum model, anterior movement of the axis of rotation of humans during forward sway may provide a small region rather than a single point where passive stability is possible.
The differences in behaviour of the proximal aponeuroses (Fig. 2G, dashed and dotted lines) may be explained by the following: (i) as the EMG was recorded superficially we cannot totally exclude the possibility of an active shortening; (ii) the superficial compartment may attach the tibia superiorly via longer aponeuroses; the deep compartment may attach to the upper two-thirds of the external surface of the shaft of the tibia via short aponeuroses; (iii) the passive action of extensor longus digitorum may influence the change in muscle length of the deep compartment of tibialis anterior via the intermuscular septum; (iv) it has been demonstrated that the central aponeurosis of tibialis anterior is very compliant (Maganaris & Paul, 2000) and therefore the two compartments may be mechanically decoupled from each other; (v) muscle fibres in the two compartments may have a different orientation: movement of muscle fibres out of the plane of the probe would have been invisible to us and not incorporated in the calculation of changes in muscle length.
Conclusions
From the observations of changes in muscle length, this paper predicts, contrary to expectations, that spindles in un-modulated muscles crossing the ankle joint might provide better proprioceptive information in standing than spindles in the actively modulated agonists. We have identified the deep compartment of tibialis anterior as the best mechanical source of proprioceptive information among the ankle muscles. Following this principle, every muscle that is passive across the ankle and the foot may provide good registration of standing sway. These results suggest a general principle of motor control: the best proprioceptive information may come from un-modulated muscles crossing the joint in parallel with the active agonist.
Furthermore, if a peripheral feedback mechanism regulates agonist muscle activity to maintain posture, we predict that reciprocal inhibition from tibialis anterior onto soleus and gastrocnemius is more likely to be effective than an autogenic pathway such as the stretch reflex.
Acknowledgments
We thank all the anonymous participants for their generous support in time, interest and patience during the operation of these experiments. We also thank our colleagues John Howell, Tom McKee, Rob Perkins and Desney Richards for their dedication and technical support.
References
- Aniss AM, Diener H-C, Hore J, Gandevia SC, Burke D. Behaviour of human muscle receptors when reliant on proprioceptive feedback during standing. J Neurophysiol. 1990;64:661–670. doi: 10.1152/jn.1990.64.2.661. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, De Luca CJ. Muscles Alive. Their Functions Revealed by Electromyography. Baltimore: Williams & Wilkins; 1964. [Google Scholar]
- Carlsoo S. Influence of frontal and dorsal loads on muscle activity and on weight distribution in the feet. Acta Orthop Scand. 1964;34:299–309. doi: 10.3109/17453676408989326. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Rogers DK, McCloskey DI. Stable human standing with lower-limb muscle afferents providing the only sensory input. J Physiol. 1994;480:395–403. doi: 10.1113/jphysiol.1994.sp020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Gorman RB, Burke D, Gandevia SC. Postural proprioceptive reflexes in standing human subjects: bandwidth of response and transmission characteristics. J Physiol. 1992a;458:69–83. doi: 10.1113/jphysiol.1992.sp019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Taylor JL, McCloskey DI. Ankle stiffness of standing humans in response to imperceptible perturbation: reflex and task-dependent components. J Physiol. 1992b;454:533–547. doi: 10.1113/jphysiol.1992.sp019278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Kinaestesia: roles for afferent signals and motor commands. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple System. New York: Oxford University Press; 1996. [Google Scholar]
- Gatev P, Thomas S, Kepple T, Hallett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514:915–928. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom A, Johnson M, Schuster J, Heckman CJ. Movement-related receptive fields of spinal motoneurones with active dendrites. J Physiol. 2008;586:1581–1593. doi: 10.1113/jphysiol.2007.149146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeka J, Kiemel T, Creath R, Horak F, Peterka R. Controlling human upright posture: velocity information is more accurate than position or acceleration. J Neurophysiol. 2004;92:2369–2379. doi: 10.1152/jn.00983.2003. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll J-P. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiemel T, Oie KS, Jeka JJ. Multisensory fusion and the stochastic structure of postural sway. Biol Cybern. 2002;87:262–277. doi: 10.1007/s00422-002-0333-2. [DOI] [PubMed] [Google Scholar]
- Lakie M, Caplan N, Loram ID. Human balancing of an inverted pendulum with a compliant linkage: neural control by anticipatory intermittent bias. J Physiol. 2003;551:357–370. doi: 10.1113/jphysiol.2002.036939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakie M, Loram ID. Manually controlled human balancing using visual, vestibular and proprioceptive senses involves a common, low frequency neural process. J Physiol. 2006;577:403–416. doi: 10.1113/jphysiol.2006.116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Direct measurement of human ankle stiffness during quiet standing: the intrinsic mechanical stiffness is insufficient for stability. J Physiol. 2002;545:1041–1053. doi: 10.1113/jphysiol.2002.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Rapid report. Paradoxical muscle movement in human standing. J Physiol. 2004;556:683–689. doi: 10.1113/jphysiol.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Active, nonspring-like muscle movements in human postural sway: how might paradoxical changes in muscle length be produced? J Physiol. 2005a;564:281–293. doi: 10.1113/jphysiol.2004.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Human postural sway results from frequent, ballistic bias impulses by soleus and gastrocnemius. J Physiol. 2005b;564:295–311. doi: 10.1113/jphysiol.2004.076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Use of ultrasound to make noninvasive in vivo measurement of continuous changes in human muscle contractile length. J Appl Physiol. 2006;100:1311–1323. doi: 10.1152/japplphysiol.01229.2005. [DOI] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. The passive, human calf muscles in relation to standing: the short range stiffness lies in the contractile component. J Physiol. 2007;584:677–692. doi: 10.1113/jphysiol.2007.140053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Paradoxical muscle movement during postural control. Med Sci Sports Exerc. 2009;41:198–204. doi: 10.1249/MSS.0b013e318183c0ed. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. Load-elongation characteristics of in vivo human tendon and aponeurosis. J Exp Biol. 2000;203:751–756. doi: 10.1242/jeb.203.4.751. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol. 1981;320:1–30. doi: 10.1113/jphysiol.1981.sp013931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res. 1976;26:59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Paloski WH, Wood SJ, Feiveson AH, Black FO, Hwang EY, Reschke MF. Destabilization of human balance control by static and dynamic head tilts. Gait Posture. 2006;23:315–323. doi: 10.1016/j.gaitpost.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Proske U. Kinesthesia: the role of muscle receptors. Muscle Nerve. 2006;34:545–558. doi: 10.1002/mus.20627. [DOI] [PubMed] [Google Scholar]
- Proske U, Gregory JE. Signalling properties of muscle spindles and tendon organs. Adv Exp Med Biol. 2002;508:5–12. doi: 10.1007/978-1-4615-0713-0_1. [DOI] [PubMed] [Google Scholar]
- Roberts TDM. Neurophysiology of Postural Mechanism. London: The Butterworth & Co (Publishers) Ltd; 1978. [Google Scholar]
- Rothwell J. Control of Human Voluntary Movement. London: Chapman & Hall; 1986. [Google Scholar]
- Sasaki O, Usami S-i, Gagey P-M, Martinerie J, Le Van Quyen M, Arranz P. Role of visual input in nonlinear postural control system. Exp Brain Res. 2002;147:1–7. doi: 10.1007/s00221-002-1170-1. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Group II spindle afferent fibers in humans: their possible role in the reflex control of stance. Prog Brain Res. 1999;123:461–472. doi: 10.1016/s0079-6123(08)62882-4. [DOI] [PubMed] [Google Scholar]
- Voss H. Tabelle der absoluten und relativen Muskelspindelzahlen der menschlichen Skelettmuskulatur. Anat Anz. 1971;129:562–572. [PubMed] [Google Scholar]
- Winter DA, Patla AE, Rietdyk S, Ishac MG. Ankle muscle stiffness in the control of balance during quiet standing. J Neurophysiol. 2001;85:2630–2633. doi: 10.1152/jn.2001.85.6.2630. [DOI] [PubMed] [Google Scholar]
- Wise AK, Gregory JE, Proske U. The responses of muscle spindles to small, slow movements in passive muscle and during fusimotor activity. Brain Res. 1999;821:87–94. doi: 10.1016/s0006-8993(99)01071-9. [DOI] [PubMed] [Google Scholar]